Abstract
The only generally accepted serological marker currently used for the diagnosis of autoimmune pancreatitis (AIP) is IgG4. Our aim was mainly to determine whether hybrid κ\λ antibody can help to diagnose AIP and to differentiate it from pancreatic cancer. We established an enzyme-linked immunosorbent assay (ELISA) system to measure the levels of hybrid κ\λ antibodies in human sera. Sera were obtained from 338 patients, including 61 with AIP, 74 with pancreatic cancer, 50 with acute pancreatitis, 40 with ordinary chronic pancreatitis, 15 with miscellaneous pancreatic diseases, and 98 with normal pancreas. Our study showed levels of hybrid κ\λ antibodies in the AIP group were significantly higher than in the non-AIP group (P < 0.001). The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for the diagnosis of AIP were 80.3%, 91%, 66.2% and 95.5% respectively. Furthermore, the combined measurement of serum hybrid κ\λ antibody and IgG4 tended to increase the sensitivity although the difference was not statistically significant (90.2% vs. 78.7%, P = 0.08), compared to measurement of IgG4 alone. Our findings suggest that hybrid κ\λ antibody could be a new serological marker to diagnose AIP and differentiate it from pancreatic cancer.
Autoimmune pancreatitis (AIP) is a form of chronic pancreatitis characterized by an autoimmune inflammatory process in which prominent lymphoplasmacytic infiltrates with associated fibrosis of the pancreas cause organ dysfunction1. Historically, AIP was used to describe the clinical profiles associated with lymphoplasmacytic sclerosing pancreatitis (LPSP), which is now called type 1 AIP2. Accordingly, type 2 AIP is used to describe idiopathic duct-centric chronic pancreatitis (IDCP)3. Considering the well-known and long-standing association with serum IgG4 elevation, some experts suggested using “AIP” solely for type 1 AIP and referring to type 2 AIP as IDCP4. Steroid therapy often leads to the rapid and sustained resolution of pancreatic mass lesions, pancreatic-duct strictures, and biliary obstruction1, and therefore the diagnosis of AIP has a significant impact on the prognosis and treatment of the patient. The characteristic features of dense lymphoplasmacytic infiltration and fibrosis in the pancreas are the gold standard for the diagnosis of AIP. However, it is usually difficult to obtain specimens from the pancreas for histological confirmation5.
In 2001, Hamano et al.6 first reported that serum IgG4 concentrations were highly sensitive and highly specific for AIP. In 2006, IgG4 was first added to the serological criteria for the diagnosis of AIP by the Japan Pancreas Society7. The HISORt criteria8 and the International Consensus Diagnostic Criteria (ICDC)2 allow the use of serum IgG4 as a serological criterion. However, although serum IgG4 is useful for screening, it is not reliable as a single diagnostic marker for AIP. Studies have shown that 4–10% of both healthy controls and controls with other diseases have high serum IgG4 concentrations9,10. In addition, about 20% of patients with AIP have normal serum IgG4 concentrations at presentation11,12. The most important disease that should be differentiated from AIP is pancreatic cancer13,14. Both diseases tend to occur in elderly men, presenting with similar clinical features, such as painless obstructive jaundice, weight loss, and recent-onset diabetes15,16,17. However, many studies reported that moderate elevations in serum IgG4 cannot be used alone to distinguish AIP from pancreatic cancer due to its low sensitivity and specificity9,18. CA19-9 is considered as a biomarker in pancreatic cancer, but this can also be elevated in other pancreatic diseases such as chronic pancreatitis, or in other pathological states19. So far, a simple serological test for the diagnosis and differentiation of AIP from pancreatic cancer is still lacking.
The classic antibody paradigm is that a single mature plasma cell produces symmetrical antibodies composed of one type of immunoglobulin heavy chain and one type of immunoglobulin light chain, either kappa (κ) or lambda (λ)20. Within the last 10 years, it has been shown that human IgG4 is a dynamic antibody, which is involved in a continuous process of half-molecule exchange. This process, also referred to as “Fab-arm exchange”, can result in asymmetric antibodies with two different antigen-combining sites21,22,23,24. Recently, one report showed that hybrid κ/λ antibodies compose a substantial portion of IgG4 in normal human serum25. These molecules are formed by two IgG4 heavy chains plus one κ and one λ light chain. Because AIP is characterized by an elevated serum IgG4 concentration, we attempted to investigate the diagnostic utility of hybrid κ/λ antibody in the diagnosis of AIP and its differentiation from pancreatic cancer.
The principal aim of this study was to establish a light-chain capture sandwich ELISA system to quantify the relative hybrid κ\λ antibody concentrations in patients with a variety of pancreatic diseases. We aimed to determine the sensitivity, specificity and predictive values of serum hybrid κ\λ antibody for the diagnosis of AIP, and compare them with those of serum IgG4. Because the principal differential diagnosis of AIP is pancreatic cancer, we also aimed to evaluate the use of serum hybrid κ\λ antibody and combined measurement with serum IgG4 in differentiating between the two diseases.
Results
High prevalence of serum hybrid κ\λ antibodies in patients with AIP in comparison with other study groups
After the four-parameter equation of the standard serum was determined, the arbitrary units in test sera were calculated from their O.D. values. Using the double sandwich ELISA system and the standard reference curve we determined the prevalence of hybrid κ\λ antibody in all groups (Fig. 1). The median of hybrid κ\λ antibody in patients with AIP, acute pancreatitis, ordinary chronic pancreatitis, pancreatic cancer, miscellaneous pancreatic diseases and normal pancreas were 6.75 AU/mL (range, 1.53–55.5 AU/mL), 2.06 AU/mL (range, 0.26–4.97 AU/mL), 2.06 AU/mL (range, 0.86–4.94 AU/mL), 2.04 AU/mL (range, 0.93–6.1 AU/mL), 1.61 AU/mL (range, 0.94–4.87 AU/mL) and 2.05 AU/mL (range, 0.42–7.2 AU/mL), respectively. Levels of hybrid κ\λ antibody in AIP group were significantly higher than in the non-AIP groups (P < 0.001). There was no significant difference between the non-AIP groups (P = 0.66).
Figure 1. Arbitrary units (AU) of hybrid κ\λ antibody in study groups.
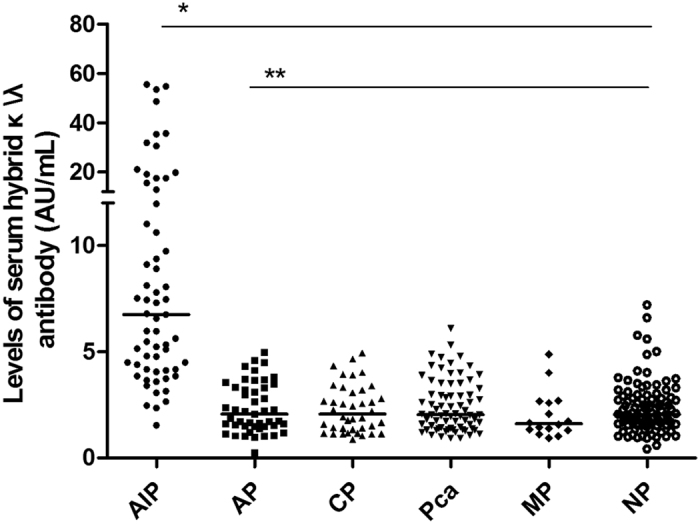
Arbitrary units (AU) of hybrid κ\λ antibody in patients with autoimmune pancreatitis (AIP, n = 61), acute pancreatitis (AP, n = 50), ordinary chronic pancreatitis (CP, n = 40), pancreatic cancer (Pca, n = 74), miscellaneous pancreatic diseases (MP, n = 15) and normal pancreas (NP, n = 98). Each horizontal bar represents the median value of serum hybrid κ\λ antibody in each study group. Significance: *P < 0.001 for AIP with respect to each non-AIP group, **P = 0.66 between groups of non-AIP.
Sensitivity, specificity, and predictive values of hybrid κ\λ antibody in the diagnosis of AIP
In order to evaluate the utility of hybrid κ\λ antibody in the diagnosis of AIP, cutoff values were established to determine the sensitivity and specificity. The sensitivity and specificity of hybrid κ\λ antibody differed at various cutoff values. Using the cutoff value of 4.04 AU/mL from the ROC curve (Fig. 2), we calculated that the sensitivity and specificity were 80.3% (49/61) and 91% (252/277), respectively. The area under the ROC curve was 0.93 (P < 0.001). The PPV and NPV of serum hybrid κ\λ antibody for diagnosis of AIP were 66.2% (49/74) and 95.5% (252/264) when calculated using the 61 AIP patients diagnosed during the study period (Table 1).
Figure 2. ROC curve evaluating the diagnostic utility for AIP.
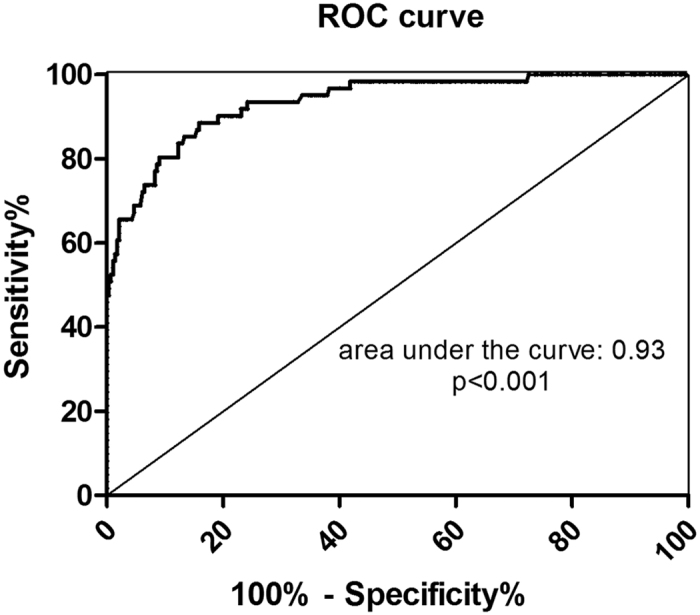
Table 1. Sensitivity, specificity and predictive value of hybrid κ\λ antibodies in patients with autoimmune pancreatitis (AIP) and controls (non-AIP).
| Levels of serum hybrid κ\λ antibody | AIP (n = 61) | Non-AIP (n = 277) | Total | Predictive value (%) |
|---|---|---|---|---|
| ≥4.04 AU/mL | 49 | 25 | 74 | Positive 66.2% |
| <4.04 AU/mL | 12 | 252 | 264 | Negative 95.5% |
| Total | 61 | 277 | 338 | |
| Sensitivity 80.3% | Specificity 91% |
Sensitivity, specificity and predictive values of serum IgG4 in the diagnosis of AIP
The median of serum IgG4 level was 3340 mg/L (range, 164–21100 mg/L), 616 mg/L (range, 22–2594 mg/L), 653 mg/L (range, 81–1409 mg/L), 557.5 mg/L (range, 68–2470 mg/L), 746.5 mg/L (range, 81–1452 mg/L), and 460.5 mg/L (range, 21–2930 mg/L) in patients with AIP, acute pancreatitis, ordinary chronic pancreatitis, pancreatic cancer, miscellaneous pancreatic diseases, and normal pancreas, respectively. Serum IgG4 concentration was significantly higher in patients with AIP than in the other study groups (P < 0.001 for each comparison, Fig. 3). When a serum IgG4 concentration of 1350 md/L was used as a cut-off value, the sensitivity was calculated at 78.7% (48/61) with a specificity of 87.7% (243/277). The sensitivity, specificity, PPV and NPV of serum IgG4 were not significantly different to those of serum hybrid κ\λ antibody (78.7% vs. 80.3%, P = 0.823; 87.7% vs. 91%, P = 0.215; 58.5% vs. 66.2%, P = 0.323; 94.9% vs. 95.5%, P = 0.777, Table 2).
Figure 3. Levels of serum IgG4 in study groups.
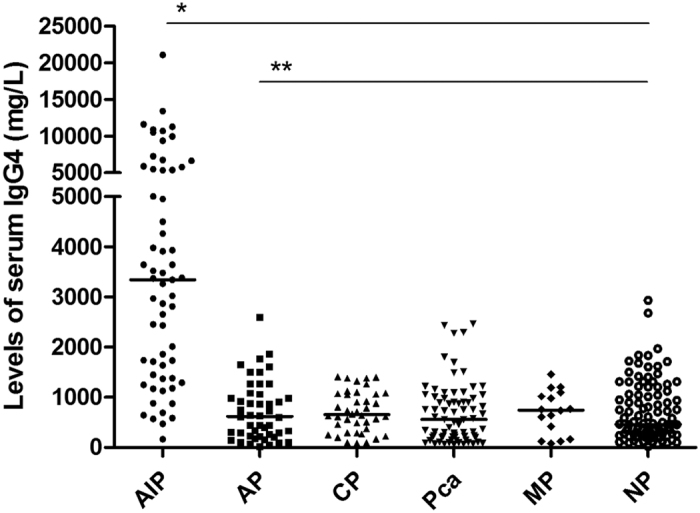
Levels of serum IgG4 in patients with autoimmune pancreatitis (AIP, n = 61), acute pancreatitis (AP, n = 50), ordinary chronic pancreatitis (CP, n = 40), pancreatic cancer (Pca, n = 74), miscellaneous pancreatic diseases (MP, n = 15) and normal pancreas (NP, n = 98). Each horizontal bar represents the median value of serum IgG4 in each study group. Significance: *P < 0.001 for AIP with respect to each non-AIP group, **P = 0.78 between groups of non-AIP.
Table 2. Sensitivity, specificity and predictive value of IgG4 in patients with autoimmune pancreatitis (AIP) and controls (non-AIP).
| Levels of serum IgG4 | AIP (n = 61) | Non-AIP (n = 277) | Total | Predictive value (%) |
|---|---|---|---|---|
| ≥1350 mg/L | 48 | 34 | 82 | Positive 58.5% |
| <1350 mg/L | 13 | 243 | 256 | Negative 94.9% |
| Total | 61 | 277 | 338 | |
| Sensitivity 78.7% | Specificity 87.7% |
The combined measurement of hybrid κ\λ antibody and IgG4 in the diagnosis of AIP
Seropositivity was defined as the elevation of serum IgG4 or hybrid κ\λ antibody levels. For the AIP group, seven patients (7/61, 11.5%) showed elevation of serum hybrid κ\λ antibody with normal serum IgG4, whereas six patients (6/61, 9.8%) showed elevation of serum IgG4 in spite of normal hybrid κ\λ antibody levels (Table 3). As a result, the combined measurement could increase the diagnostic sensitivity from 78.7% to 90.2% (55/61) compared with serum IgG4 alone, although this difference was not statistically significant (P = 0.08). The specificity of the combined measurement was not sacrificed significantly (85.9% vs. 87.7%, P = 0.53) .
Table 3. The elevation of serum hybrid κ\λ antibody and IgG4 in AIP.
| IgG4 elevation (≥1350 mg/L) | |||
|---|---|---|---|
| Hybrid κ\λ antibody elevation (≥4.04 AU/mL) | Yes | No | |
| Yes | 42 (68.8%) | 7 (11.5%) | |
| No | 6 (9.8%) | 6 (9.8%) | |
Serum hybrid κ\λ antibody and IgG4 in the differentiation of AIP from pancreatic cancer
Among patients with pancreatic cancer, nine patients had elevated levels of hybrid κ\λ antibodies (9/74), and eight patients had elevated levels of serum IgG4 (8/74). Hybrid κ\λ antibody (≥4.04 AU/mL) showed a specificity of 87.8%. In the case of IgG4 (≥1350 mg/L), the specificity was 89.2%. There was no significant difference between the two tests (P = 0.797, Table 4).
Table 4. Sensitivity and specificity of hybrid κ\λ antibody and IgG4 in the differentiation of AIP from pancreatic cancer.
| AIP (n = 61) | Pancreatic cancer (n = 74) | Sensitivity (%) | Specificity (%) | ||
|---|---|---|---|---|---|
| Hybrid κ\λ antibody elevation (≥4.04 AU/mL) | Yes | 49 | 9 | 80.3% | 87.8% |
| No | 12 | 65 | |||
| IgG4 elevation (≥1350 mg/L) | Yes | 48 | 8 | 78.7% | 89.2% |
| No | 13 | 66 | |||
| Hybrid κ\λ antibody or IgG4 elevation | Yes | 55 | 9 | 90.2% | 87.8% |
| No | 6 | 65 |
Analogous to the sensitivity in the diagnosis of AIP from all non-AIP groups, the combined measurement showed a specificity of 87.8% (65/74) in the differentiation of AIP from pancreatic cancer, which was almost the same as that (89.2%, 66/74) of IgG4 alone (P = 0.797, Table 4).
Discussion
In a large cohort of patients with a wide variety of pancreatic diseases, we showed that levels of hybrid κ\λ antibodies in patients with AIP were significantly higher than in patients with non-AIP conditions. The sensitivity and specificity for hybrid κ\λ antibody in the diagnosis of AIP were 80.3% and 91% respectively. A proportion (12/61, 19.7%) of AIP patients had normal hybrid κ\λ antibody levels, and a proportion “25/277, 9%” of non-AIP patients had elevated hybrid κ\λ antibody levels. For serum IgG4 levels, the sensitivity and specificity were 78.7% and 87.7%, respectively, which are not as high as the initial study by Hamano et al.26, but are close to the reports by Ghazale et al.9 and other previous reports10,11,12. The differences in the diagnostic values of serum IgG4 for AIP could be attributed to differences in the patients enrolled in the studies, or differences in the disease activity of the cases selected27,28.
Our study showed that the sensitivity and specificity of serum IgG4 was not significantly different from serum hybrid κ\λ antibody in the diagnosis of AIP. In view of this, we wondered whether the combined measurement could increase the diagnostic performance for AIP. Seropositivity was defined as the elevation of serum IgG4 or of hybrid κ\λ antibody. Interestingly, seven AIP patients (7/61, 11.5%) showed elevated levels of serum hybrid κ\λ antibodies despite normal serum IgG4, and six AIP patients (6/61, 9.8%) showed elevation of serum IgG4 with normal levels of hybrid κ\λ antibodies. As a result, the combined measurement of serum IgG4 and hybrid κ\λ antibody could strongly increase the diagnostic sensitivity to 90.2% in comparison with using serum IgG4 alone. More importantly, with the combined measurement the specificity was not significantly sacrificed. This finding is similar to that of a previous report by Song T et al.29. They showed that the combined measurement of total serum IgG and IgG4 might increase diagnostic sensitivity without compromising the specificity. However, the sensitivity of the combined measurement in their study was only 68.3% (56/82), which was significantly lower than our result (56/82 vs. 55/61, P = 0.002).
In our study of 61 patients with AIP, only 12 patients (19.7%) had hybrid κ\λ antibody levels below the cut-off value of 4.04 AU/mL. Therefore, the negative predictive value of hybrid κ\λ antibody levels for AIP was very high (95.5%) and the likelihood of AIP in a patient without hybrid κ\λ antibody elevation is very low, which could help rule out AIP from non-AIP diseases. The positive predictive value of a test is closely related the prevalence of the disease of interest in the studied population. AIP is uncommon with an estimated prevalence of <1/100,000 in the general population30. Despite the high specificity of serum hybrid κ\λ antibody for AIP, 91% in our study, it had a low PPV (66.2%). Even so, if more patients with nonspecific abdominal pain were included in our research, the proportion of AIP would be lower and so would the PPV.
When a cutoff value of 4.04 AU/mL of serum hybrid κ\λ antibody was used to differentiate AIP from pancreatic cancer, in contrast to the high prevalence of hybrid κ\λ antibody in patients with AIP, only nine (9/74, 12.1%) patients with pancreatic cancer had elevated levels of hybrid κ\λ antibodies. The specificity was 87.8%, which was almost the same as that for serum IgG4 (89.2%). In keeping with the result for the diagnosis of AIP from all non-AIP groups, the specificity of the combined measurement of serum IgG4 and hybrid κ\λ antibody in the differentiation of AIP from pancreatic cancer was not significantly sacrificed, when compared with that of serum IgG4 alone.
More recently, we reported hybrid κ/λ antibody was a novel biomarker related to disease activity and inflammation in rheumatoid arthritis (RA). Levels of serum hybrid κ/λ antibody were markedly elevated in the patients with RA compared with osteoarthritis (OA) and healthy controls31. Nevertheless, the levels of hybrid κ/λ antibody in patients with AIP were significantly higher than those with RA (data not shown). In the present study, all the patients enrolled were suspected to have pancreatic diseases, with obstructive jaundice and abdominal pain, or a pancreatic mass. Hybrid κ/λ antibody is valuable in diagnosing or differentiating AIP from pancreatic cancer based on the clinical findings of suspected pancreatic disorders. Considering the elevation of hybrid κ/λ antibody in RA, or other possible autoimmune diseases, elevated hybrid κ\λ antibody concentrations should be interpreted in conjunction with a thorough evaluation of the clinical, radiological and histological findings for AIP diagnosis, especially for patients who have autoimmune diseases.
Our study has two main limitations. First, the results are limited by a shortage of type 2 AIP patients. Because type 2 AIP is not part of the spectrum of IgG4-related diseases, and does not have definitive serological autoimmune markers3, we can speculate that hybrid κ\λ antibody concentrations may be normal in these patients. Nevertheless, this does not change its significance in clinical practice because most cases of AIP in Asia fit the profile of type 1 AIP2,32,33 and patients with type 2 AIP differ significantly in their demography, other organ involvement, and disease relapse3. Second, the relatively small number of AIP patients are included that may not be sufficient to allow for a decisive conclusion regarding our results because AIP is an uncommon pancreatic disease. To strengthen the research and pave the way for clinical use of the new biomarker, a prospective study in a different and large patient population is needed in the future.
In summary, this is the first report showing that the level of hybrid κ\λ antibody is markedly elevated in AIP. The high sensitivity and specificity indicated that it could be a new serological marker to diagnose AIP, and to differentiate it from pancreatic cancer. The combined measurement of serum hybrid κ\λ antibody and IgG4 tended to have a higher sensitivity without sacrificing specificity, compared with serum IgG4 alone. Further studies are necessary to clarify its characteristics, and to evaluate the immunoregulatory role in disease activity.
Methods
Samples and materials
All 338 patients studied were referred to Peking Union Medical College Hospital for suspected pancreatic disease, with obstructive jaundice and abdominal pain, or a pancreatic mass. Among them, 61 patients (51 men and 10 women) were diagnosed with type 1 AIP and the remaining patients were classified into the five diagnostic groups summarized in Table 5: acute pancreatitis, ordinary chronic pancreatitis, pancreatic cancer, miscellaneous pancreatic diseases, and normal pancreas. Miscellaneous conditions included other pancreatic abnormalities such as pancreatic cyst, pancreatic neuroendocrine tumors, and insulinoma. The diagnosis of AIP was based on the International Consensus Diagnostic Criteria2, on the basis of clinical data, imaging, and histopathological findings. All diagnostic cases are definitive cases. Diagnosis of pancreatic cancer was confirmed by pancreatic histology and/or cytology. Patients who had no identifiable pancreatic abnormality were classified as “normal pancreas”. All patients were enrolled randomly between May 2014 and January 2016. Serum samples were stored at −80 C until performing the assay. In addition, a pooled serum sample from 20 healthy subjects (10 men and 10 women) with normal findings on abdominal ultrasonography and without autoimmunity was used as a standard reference serum to determine the standard curve, arbitrarily assigned to contain two units of hybrid κ\λ antibodies per milliliter (AU/mL). The standard reference serum was aliquoted and stored at −80 °C until required. The study was approved by the Ethics Committee of the National Center for Clinical Laboratories and conducted in accordance with the guidelines of the Declaration of Helsinki. Written informed consent for the use of their samples in research was obtained from all subjects.
Table 5. Demographic characteristics of study groups.
| Study group | n | Age (years) | Sex (male/female) |
|---|---|---|---|
| AIP | 61 | 57 (27–83) | 51/10 |
| Pancreatic cancer | 74 | 58 (29–79) | 58/16 |
| Acute pancreatitis | 50 | 42 (14–78) | 22/28 |
| Ordinary chronic pancreatitis | 40 | 52 (8–78) | 28/12 |
| Miscellaneous pancreatic diseasesa | 15 | 46 (26–77) | 6/9 |
| Normal pancreas | 98 | 46 (33–56) | 57/41 |
aMiscellaneous pancreatic diseases included 4 patients with pancreatic cysts, 7 patients with pancreatic neuroendocrine tumors, and 4 patients with insulinoma.
Enzyme-linked immunosorbent assay for the detection of serum hybrid κ\λ antibody
The optimum concentrations for each component of the hybrid κ\λ antibody ELISA were determined by a chessboard titration, in which 96-well microplates (Nunc Maxisorp, Denmark) were coated with anti-human λ light-chain antibody (Abcam, China) in 0.05 M sodium carbonate buffer (pH 9.6; 100 μL/ well at 5 μg/mL) and left overnight at 4 °C in a moist box. After a series of washes with PBST (0.5% v/v Tween 20 in PBS), the plates were blocked with 1% w/v BSA in PBST for 2 h at 37 °C.
Next, 100 μl aliquots of test serum at a dilution of 1:10,000 in serum diluent (1% w/v BSA in PBST) were added to all wells. All serum samples were tested in duplicate. Following five washes, the plates were probed with 100 μL/well of mouse anti-κ light-chain HRP-conjugated antibodies (1:2000; Abcam, China) for 1 hour at 37 °C. After extensive washing, the plates were developed with 100 μL/well tetramethyl benzidine (Sigma-Aldrich, USA) for 10 min. The reaction was stopped with 50 μL/well 0.5 M sulfuric acid. Plates were read with a Thermo Multiskan EX plate reader at a wavelength of 450 nm with a 620 nm reference (OD450/620 nm).
The reference standard was used on each ELISA plate to make a standard curve. A twofold serial dilution (1:1000 to 1:128000) of the standard reference serum in dilution buffer was examined in parallel with all samples on each individual plate as described above. The four-parameter logistic-log curve fitting method was used to generate standard curves. Hybrid κ\λ antibody units of the serum samples were then calculated from their O.D. values using the parameters estimated from the standard curve. Linearity and precision (intra- and inter-assay) were carried out to ensure the ELISA performance. Serum samples needed to be re-assayed at a higher dilution when a test sample’s absorbance value fell outside the linear portion of the standard curve.
Assay of serum IgG4 in all groups
The serum concentrations of IgG4 in all groups were measured using an automated immunonephelometry (BN-II nephelometer, Dade Behring, Germany). Human IgG4 latex reagents (N Latex IgG4, Siemens) were used to quantify the concentrations of serum IgG4. The sensitivity and specificity of serum IgG4 for the diagnosis of AIP were estimated at a concentration of 1350 mg/L, which is the cut-off value used by the Japan Pancreas Society8.
Statistical analysis
ELISA results were analyzed by a four-parameter logistic-log curve fitting program (ELISA v. 2.15; Centers for Disease Control and Prevention, USA) and expressed in arbitrary units (units per milliliter). Sensitivity, specificity, and predictive values were calculated using conventional formulae, and were compared using the χ2 test. According to data distribution, values were represented as median and range. Comparisons were performed using the Mann-Whitney U test between AIP and non-AIP group. The Kruskal-Wallis nonparametric analysis is used to compare differences between non-AIP groups. Receiver operator characteristic (ROC) curves were used to judge the diagnostic utility. Statistical analyses were performed with SPSS 19.0 (SPSS Inc., USA) and GraphPad Prism version 6.0 (GraphPad, USA) as appropriate. Two-sided p-values of less than 0.05 were regarded as statistically significant.
Additional Information
How to cite this article: Hao, M. et al. Hybrid kappa\lambda antibody is a new serological marker to diagnose autoimmune pancreatitis and differentiate it from pancreatic cancer. Sci. Rep. 6, 27415; doi: 10.1038/srep27415 (2016).
Acknowledgments
This work was supported by National Natural Science Foundation of China Grant 81271915 and National High Technology Research and Development Program of China Grant 2011AA02A116.
Footnotes
Author Contributions M.H., W.L. and L.Y. designed of the experiments and wrote the main manuscript text. They contributed equally to this work; S.Y., G.F., T.L. and X.Y. collected the specimen and analyzed the data; G.W., D.Z. and J.D. prepared all figures; K.Z., G.L., R.Z., Y.H. and L.W. supplied technical, or material support; J.L. obtained funding and supplied critical revision of the manuscript. All authors reviewed the manuscript.
References
- Finkelberg D. L., Sahani D., Deshpande V. & Brugge W. R. Autoimmune pancreatitis. N Engl J Med 355, 2670–2676 (2006). [DOI] [PubMed] [Google Scholar]
- Shimosegawa T. et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas 40, 352–358 (2011). [DOI] [PubMed] [Google Scholar]
- Chari S. T. et al. Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreas 39, 549–554 (2010). [DOI] [PubMed] [Google Scholar]
- Hart P. A., Zen Y. & Chari S. T. Recent Advances in Autoimmune Pancreatitis. Gastroenterology 149, 39–51 (2015). [DOI] [PubMed] [Google Scholar]
- Kamisawa T. & Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol 41, 613–625 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aithal G. P., Breslin N. P. & Gumustop B. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 345, 147–148 (2001). [DOI] [PubMed] [Google Scholar]
- Okazaki K. et al. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol 41, 626–631 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari S. T. et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol 4, 1010–1016; quiz 934 (2006). [DOI] [PubMed] [Google Scholar]
- Ghazale A. et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol 102, 1646–1653 (2007). [DOI] [PubMed] [Google Scholar]
- Sadler R. et al. The diagnostic significance of serum IgG4 levels in patients with autoimmune pancreatitis: a UK study. Eur J Gastroenterol Hepatol 23, 139–145 (2011). [DOI] [PubMed] [Google Scholar]
- Sah R. P. & Chari S. T. Serologic issues in IgG4-related systemic disease and autoimmune pancreatitis. Curr Opin Rheumatol 23, 108–113 (2011). [DOI] [PubMed] [Google Scholar]
- Tabata T. et al. Serum IgG4 concentrations and IgG4-related sclerosing disease. Clin Chim Acta 408, 25–28 (2009). [DOI] [PubMed] [Google Scholar]
- Morselli-Labate A. M. & Pezzilli R. Usefulness of serum IgG4 in the diagnosis and follow up of autoimmune pancreatitis: A systematic literature review and meta-analysis. J Gastroenterol Hepatol 24, 15–36 (2009). [DOI] [PubMed] [Google Scholar]
- Kamisawa T. et al. Strategy for differentiating autoimmune pancreatitis from pancreatic cancer. Pancreas 37, e62–e67 (2008). [DOI] [PubMed] [Google Scholar]
- Yoshida K. et al. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci 40, 1561–1568 (1995). [DOI] [PubMed] [Google Scholar]
- Okazaki K. & Chiba T. Autoimmune related pancreatitis. Gut 51, 1–4 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara L. P. & Chari S. T. Autoimmune pancreatitis. Curr Gastroenterol Rep 7, 101–106 (2005). [DOI] [PubMed] [Google Scholar]
- Ngwa T., Law R., Hart P., Smyrk T. C. & Chari S. T. Serum IgG4 elevation in pancreatic cancer: diagnostic and prognostic significance and association with autoimmune pancreatitis. Pancreas 44, 557–560 (2015). [DOI] [PubMed] [Google Scholar]
- Chang M. C. et al. Adiponectin as a potential differential marker to distinguish pancreatic cancer and chronic pancreatitis. Pancreas 35, 16–21 (2007). [DOI] [PubMed] [Google Scholar]
- Bernier G. M. & Cebra J. J. Polypeptide Chains of Human Gamma-Globulin: Cellular Localization by Fluorescent Antibody. Science 144, 1590–1591 (1964). [DOI] [PubMed] [Google Scholar]
- van der Neut Kolfschoten M. et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 317, 1554–1557 (2007). [DOI] [PubMed] [Google Scholar]
- Aalberse R. C., Stapel S. O., Schuurman J. & Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy 39, 469–477 (2009). [DOI] [PubMed] [Google Scholar]
- Rispens T., Ooijevaar-de Heer P., Bende O. & Aalberse R. C. Mechanism of Immunoglobulin G4 Fab-arm Exchange. Journal of the American Chemical Society 133, 10302–10311 (2011). [DOI] [PubMed] [Google Scholar]
- Labrijn A. F. et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol 27, 767–771 (2009). [DOI] [PubMed] [Google Scholar]
- Young E. et al. Estimation of polyclonal IgG4 hybrids in normal human serum. Immunology 142, 406–413 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano H. et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 344, 732–738 (2001). [DOI] [PubMed] [Google Scholar]
- Ohara H., Nakazawa T., Ando T. & Joh T. Systemic extrapancreatic lesions associated with autoimmune pancreatitis. J Gastroenterol 42 Suppl 18, 15–21 (2007). [DOI] [PubMed] [Google Scholar]
- Kaji R. et al. Serum immunoglobulin G4 associated with number and distribution of extrapancreatic lesions in type 1 autoimmune pancreatitis patients. J Gastroenterol Hepatol 27, 268–272 (2012). [DOI] [PubMed] [Google Scholar]
- Song T. J. et al. The combined measurement of total serum IgG and IgG4 may increase diagnostic sensitivity for autoimmune pancreatitis without sacrificing specificity, compared with IgG4 alone. Am J Gastroenterol 105, 1655–1660 (2010). [DOI] [PubMed] [Google Scholar]
- Uchida K., Masamune A., Shimosegawa T. & Okazaki K. Prevalence of IgG4-Related Disease in Japan Based on Nationwide Survey in 2009. Int J Rheumatol 2012, 358371 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L. et al. Increased Kappa/Lambda Hybrid Antibody in Serum Is a Novel Biomarker Related to Disease Activity and Inflammation in Rheumatoid Arthritis. Mediators of Inflammation 2016, 2953072 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. et al. Review of 43 patients with autoimmune pancreatitis based on the international consensus diagnostic criteria in China. Pancreas 43, 810–811 (2014). [DOI] [PubMed] [Google Scholar]
- Meng Q. et al. Diagnosis and Treatment of Autoimmune Pancreatitis in China: A Systematic Review. PLos One 10, e0130466 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


