Abstract
Herbal supplements are not governed by the same regulations as prescription drugs, we hypothesize that the content of their active ingredients may vary largely among different manufacturers. This may produce variable therapeutic outcomes. This study aims to examine this hypothesis on commonly used herbal supplements among cancer patients. CYP1A2 has been implicated in the activation of many carcinogens and alteration in its activity may be a mechanism associated with the protective effect of herbal products. Activity of human CYP1A2 was used to determine the effect of four herbal supplements of different brands, namely, black cohosh (BC), ginseng, grape seed extract (GSE) and green tea extract (GTE). The herbal content was extracted with methanol, and extract aliquots were used to determine their effect on CYP1A2. Human liver microsomes, the CYP1A2 probe (7-ethoxyresorufin) and NADPH in buffer were incubated with and without herbal extract. Metabolite (resorufin) formation was monitored by HPLC. Seven BC products caused a mild inhibition of CYP1A2, ranging from 2.4 % by GNC Plus to 21.9 % by Nature's Resource. Among nine ginseng products tested, the inhibitory effect varied from 4.2 % by Imperial to 44.6 % by Solarays. The effect of nine GSE brands also varied, ranging from 1.7 % (Country Life) to 26.5 % (Veg Life). Of twelve GTE products, the inhibitory effect varied from 2.9 % by Henry's to 46.6 % by GNC Plus. It appears that the inhibition of selected herbal supplements on CYP1A2 activity varies considerably among different brands of the products. This may be due to variations in the herbal products' active ingredients content.
Keywords: dietary supplements, CYP1A2, black cohosh, ginseng, grape seed extract, green tea extract, drug interaction
Introduction
Dietary supplements are commonly consumed by patients worldwide. In the United States, approximately 25 % of people use dietary supplements (Gurley et al. 2005[22]). Many of these supplements are herbal in nature (Wargovich, 2001[51]). It is worthy of note that the rate of herbal usage is much greater in cancer patients, in some cases, up to 50 % of patients treated in cancer centers (Pierce et al., 2002[40]; Richardson et al., 2000[41]; Rock et al., 2004[42]; Wargovich, 2001[51]; Wargovich et al., 2010[52]).
Cytochrome P450 (CYP) enzymes are responsible for detoxification of a wide range of foreign compounds including drugs, environmental pollutants, and carcinogens. Most chemical carcinogens require metabolic activation by phase I enzymes (e.g., CYP) and detoxification by conjugation phase II enzymes (e.g., glutathione S-transferase and glucuronosyl transferase). The coordinated expression and regulation of phase I and phase II metabolizing enzymes and their metabolic balance may be an important host factor in determining susceptibility to cancer (Guengerich 1999[19]; Sato et al., 2000[44]). There may be several underlying mechanisms of chemoprevention by herbal supplements. The herbal supplements not only possess good reactive oxidative free-radical scavenging abilities, but they may also be capable of inhibiting or inducing phase I and phase II enzymes. CYP1A1 plays an important role in the activation of carcinogens such as polycyclic aromatic hydrocarbons (PAHs), heterocyclic amines, nitrosamines and mycotoxins. CYP1A2, which is induced in the human liver by cigarette smoking, has been implicated in the activation of a number of carcinogens (Guengerich, 1999[19]; Sato et al., 2000[44]). CYP1A2 has been strongly involved in the activation of heterocylic aromatic amine cooked-food carcinogens, such as 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) and 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) (Murray et al., 1993[37]). It has been shown that various flavonoids (e.g., apigenin, chrysin and quercetin) are capable of reducing the formation of DNA adducts from PAH by inhibition of CYP1A1 (Lautraite et al., 2002[33]). Alterations of CYP activity by phytochemical ingredients of herbal products may be one of the mechanisms associated with a protective effect against cancer.
Black cohosh (Cimicifuga racemosa) is a shrub-like plant native to the eastern forests of North America. It has been used by Native Americans for menopausal symptoms, pre-menstrual discomfort, dysmenorrheas and for many other indications. Several preparations of black cohosh are highly recommended as a safe and effective natural remedy for menopausal symptoms. Women who have been advised by their physicians to avoid hormone replacement therapy (HRT), who are at high risk for breast cancer or have discontinued HRT after a diagnosis of breast cancer currently use black cohosh as treatment (Rockwell et al., 2005[43]). The root of ginseng (Panax ginseng C.A. Meyer) has traditionally been used in East Asia over 2000 years for the treatment of cancer, cardiovascular diseases, hypertension, diabetes mellitus and liver dysfunction (Helms, 2004[27]). Ginseng was the second highest selling herbal supplement in the United States in 2000, with gross retail sales of $ US 62 million (Blumenthal, 2001[6]). Grape seed extract is one of the top-selling herbal supplements in the United States (Sparreboom et al., 2004[48]). Commercial preparations of grape seed polyphenols, widely referred to as “grape seed extract (GSE)”, are standardized to contain 95 % procyanidins. GSE preparations are marketed in the USA as a dietary supplement, due to their health benefits, particularly the strong antioxidant activity. There are several studies reporting that GSE could be potential cancer chemopreventive agents (Agarwal et al., 2002[1]; Chen et al., 2005[10]; Singletary and Meline, 2001[47]; Zhao et al., 1999[57]) and can prevent heart attack and skin aging (Bagchi et al., 1997[5], 1998[4]; Maffei et al., 1996[35]). Consumption of green tea (Camellia sinensis) has been claimed to have potential health benefits, such as the prevention of cancer and cardiovascular diseases (Cabrera et al., 2006[9]; Yang and Landau, 2000[53]; Yang et al., 2002[54], 2006[55]). Green tea extracts are widely used as dietary supplements. Green tea and tea polyphenols have been investigated extensively because tea polyphenols possess strong antioxidant properties and show inhibitory activity against carcinogenesis (Anger et al., 2005[3]; Maliakal et al., 2011[36]; Yang et al., 2006[55]). Since tea is the most popular beverage in the world and because of the absence of toxicity, tea is an excellent candidate for cancer prevention (Yang et al., 2002[54], 2006[55]).
Because dietary herbal supplements are not subject to the same FDA regulations as prescription drugs and over-the-counter medications, herbal products generally lack quality control and the regulatory oversight of therapeutic products. With these reasons we hypothesize that the content of active ingredients in herbal supplements may vary among different manufacturers. This difference in the active ingredients may produce variation in therapeutic outcomes and the extent of herbal drug interactions. This hypothesis is based on the following observations. First, it has been reported with a few herbal supplements, including St. John's wort, ginseng and ephedra, that the content of active ingredients varied widely between brands and in some cases the content variation was also found between batches of the same herbal products (Draves and Walker 2003[14]; Gurley et al., 2000[20]; Harkey et al., 2001[26]). Second, in relation to antioxidant activity of grape polyphenols, a correlation existed between the antioxidant activity and the content of polyphenols in grape seed extracts (Zhao et al., 1999[57]). Therefore, the present study aimed to investigate this hypothesis with regard to commonly used herbal supplements, black cohosh, ginseng, grape seed extract and green tea extract. Activity of human CYP1A2 was used as a parameter to examine the effect of these herbal supplements purchased from many manufacturers.
Materials and Methods
Chemicals and dietary supplements
All chemicals and reagents used were of analytical grade. Resorufin, 7-ethoxy-resorufin and NADPH were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Four different commercial herbal supplements namely black cohosh (BC), ginseng, grape seed extract (GSE) and green tea extract (GTE) were randomly purchased from pharmacy stores in California, USA, during the year 2005. There were seven brands of BC, nine brands of ginseng, nine brands of GSE and twelve brands of GTE products. Details of manufacturers and content of each capsule are listed in Table 1(Tab. 1).
Table 1. Characteristics of herbal supplements used.
Preparation of human liver microsomes
A human liver used was obtained from a Caucasian male donor aged 50 years who had met traumatic death. He was neither taking medication nor had significant past medical history. The use of human liver was approved by the Southern Regional Health Authority (Otago) Ethics Committee, Dunedin, New Zealand. Human liver microsomes were prepared by a standard differential ultracentrifugation as previously described (Zhang et al., 1997[56]).
Preparation of herbal supplement methanolic extract
The content of herbal supplement studied was determined by weighing the actual content in each capsule. This was performed with six replicates (n = 6 capsules). The contents from these six capsules were combined. The content of an herbal product, equivalent to the average content in one capsule was extracted with approximately 80 mL methanol using ultra sonication for 1 hour. The mixture was adjusted to a final volume of 100 mL with methanol. An aliquot of this mixture was then centrifuged at 2500 g for 15 min. The supernatant was collected and referred as methanolic extract.
CYP1A2 assay procedure
Aliquots (5 µL) of the herbal methanolic extract were tested for their ability to inhibit the metabolism of a CYP1A2 marker substrate using an in vitro liver microsomal technique. 7-Ethoxyresorufin was used as a specific probe substrate for human CYP1A2 (Burke et al., 1994[8]). Incubation mixtures (0.5 mL) containing human liver microsomes (0.1 mg/mL), 7-ethoxyresorufin (500 nM) and NADPH (1 mM) in phosphate buffer (0.067 M, pH 7.4) were incubated with or without (i.e. as control) herbal extract (5 µL) at 37 C for 10 min. All experiments were performed in four replicates. After incubation, the reaction was terminated by addition of 1 mL cold methanol. The mixture was centrifuged and the aliquots (30 µL) of supernatant were injected onto an HPLC column. Formation of the metabolite (resorufin) generated by the CYP1A2-mediated reaction was assayed by a reversed-phase HPLC method (Hanioka et al., 2000[25]) with fluorescence detection. The detection limit of this assay was 0.1 nM. The inter- and intra-assay coefficient of variation was < 6 % over the concentration range of 0.1 to 100 nM.
Statistical analysis
Results were expressed as mean and standard deviation (SD). Data was analyzed by a one-way ANOVA, followed by multiple comparisons utilizing Tukey's test (SPSS version 18.0, SPSS Inc., Chicago, IL, USA). A p < 0.05 was considered to be statistically significant.
Results
Our preliminary study had demonstrated that the methanolic extracts of herbal supplements investigated did not have any compounds that interfered with the HPLC assay for resorufin (CYP1A2 assay). This was confirmed by the results obtained from incubation of each herbal supplement extract with human liver microsomes under the same experimental conditions used, without CYP1A2 substrate (7-ethoxyresorufin). After incubation, none of the herbal supplement extracts had peaks interfered with resorufin in the HPLC analysis.
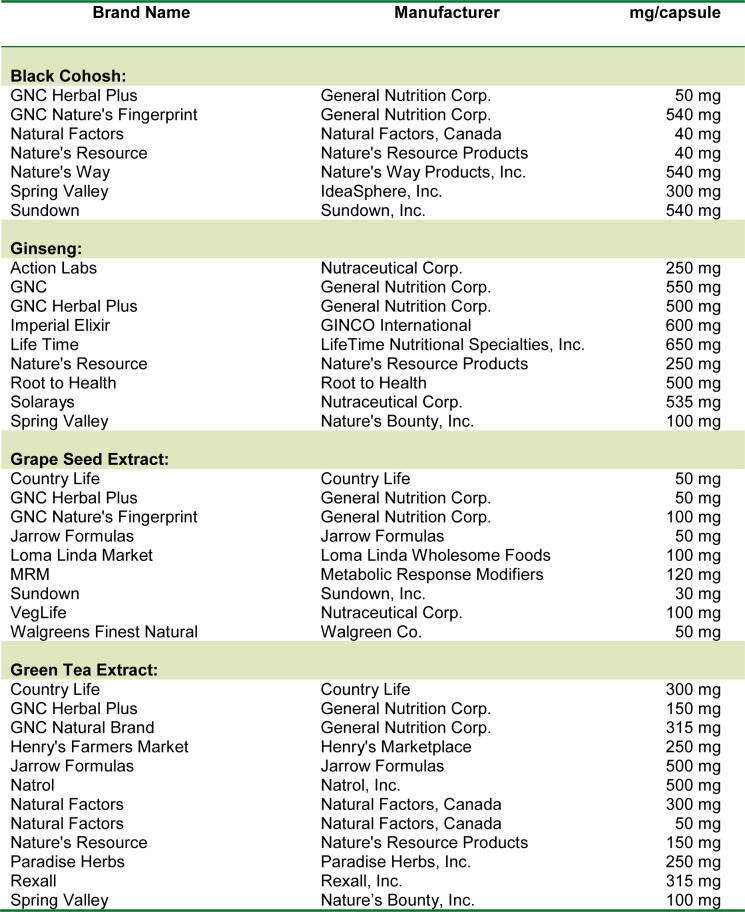
Results with the seven brands of BC product have shown that there was very little to mild inhibition of the activity of CYP1A2 caused by BC products (Figure 1(Fig. 1)). The inhibitory effect varied from 2.4 % inhibition by GNC Plus brand to 21.9 % inhibition by the Natures Resource product. The actual activities of CYP1A2 caused by all (except GNC Plus brand) BC herbal extracts were significantly less than the control value (p < 0.05). The percentage of CYP1A2 inhibition produced by BC products did not correlate with the BC herbal content claimed in these products (r = 0.035, p > 0.5).
Figure 1. Effects of different brands of black cohosh dietary supplement on the activity of CYP1A2. Each bar represents mean (and SD) % of inhibition of human CYP1A2 activity, obtained from 4 different measurements.
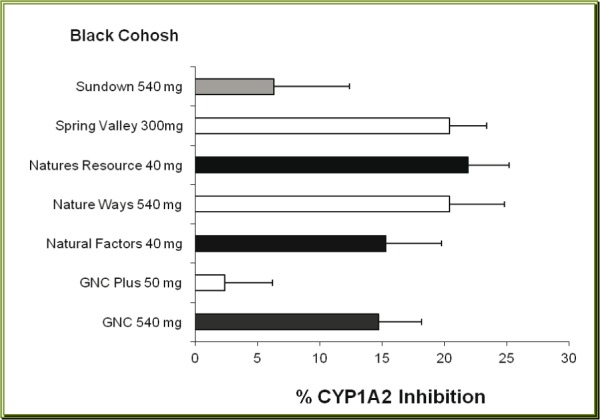
Nine different brands of ginseng pro-ducts were tested, two products were shown to inhibit the activity of CYP1A2 moderately while the other seven brands had very little effect on the activity of CYP1A2 (Figure 2(Fig. 2)). The two ginseng products producing moderate inhibition were 100 mg Spring Valley (34.1 % inhibition) and 535 mg Solarays (44.6 % inhibition) brands. Overall, the inhibitory effect on CYP1A2 varied from 4.2 % by Imperial (600 mg) to 44.6 % by Solarays (535 mg) brand. There was no significant correlation between the % CYP1A2 inhibition and the contents of ginseng (r = -0.234, p > 0.4). For example, the Spring Valley ginseng product containing 100 mg caused CYP1A2 inhibition of 31.4 %, while GNC Plus (500 mg) ginseng inhibited the activity of CYP1A2 by only 14.2 %.
Figure 2. Effects of different brands of ginseng dietary supplement on the activity of CYP1A2. Each bar represents mean (and SD) % of inhibition of human CYP1A2 activity, obtained from 4 different measurements.
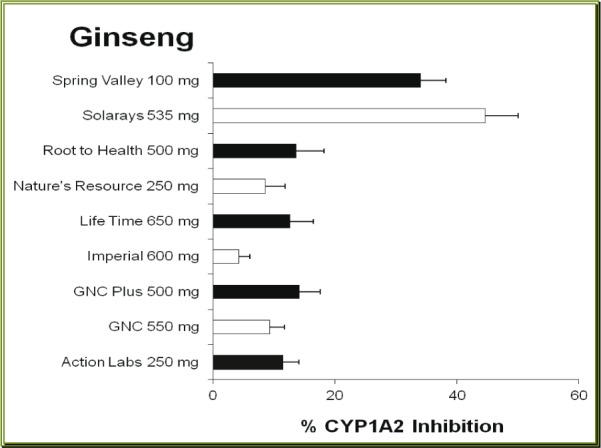
Of nine brands of GSE tested, six brands caused moderate inhibition of CYP1A2 activity, the other three brands produced very little inhibition of CYP1A2 (Figure 3(Fig. 3)). The inhibitory effect caused by GSE ranged from 1.7 % by Country Life brand to 26.5 % by VegLife product. Similar to the other two herbal products mentioned above, the inhibitory effects produced by GSE did not appear to correlate with the content of GSE (r = 0.454, p > 0.1). For instance, among the products containing 50 mg GSE, the effect varied from very little effect (1.7 %) by Country Life to moderate inhibition of 23.2 % by GNC Plus brand.
Figure 3. Effects of grape seed extract dietary supplements with different brands on the activity of human CYP1A2. Each bar represents mean (and SD) % of inhibition of the CYP1A2, obtained from 4 different measurements.
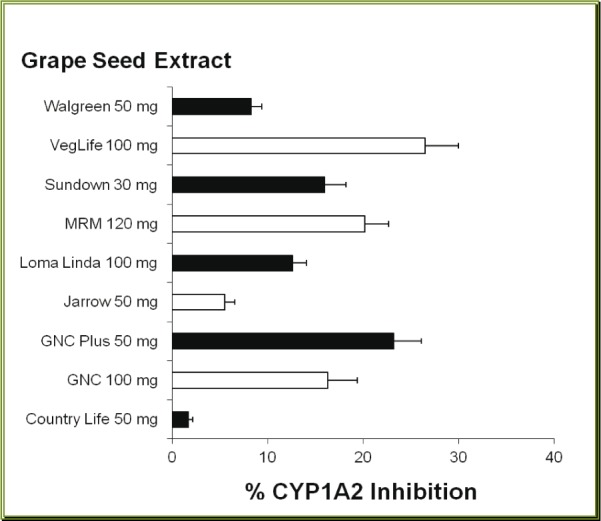
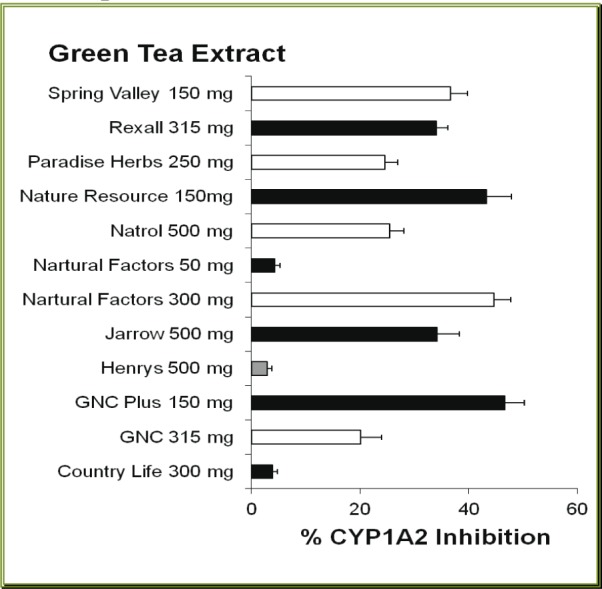
Most of green tea extract (GTE) products caused some degree of inhibition of CYP1A2. Of twelve brands of GTE studied, nine brands produced moderate inhibition of human CYP1A2. However, the inhibitory effect produced by GTE varied from 2.9 % by Henry's to 46.6 % by GNC Plus GTE (Figure 4(Fig. 4)). The actual CYP1A2 activities in the presence of all (except Henry's) GTE extracts were significantly less than the control value (p < 0.05). However, the % inhibition of CYP1A2 produced by these products did not correlate (r = -0.191, p > 0.5) with the content specified in the GTE capsules.
Figure 4. Effects of green tea dietary supplements on human CYP1A2 activity. Each bar represents mean (and SD) % inhibition of the CYP1A2, obtained from 4 different measurements.
Discussion
Even though the use of herbal supplements is very common in recent years, there is limited information on the safety and efficacy of herbal products used alone or in combination with prescription drugs. Additional studies are needed to illustrate the safety and efficacy of herbal products in humans (Green et al., 2001[18]; Haller et al, 2004[23]; Hamburger et al., 2001[24]; Shi and Klotz, 2012[46]). Meanwhile, caution must be taken in prescribing drugs to patients who take herbal supplements. Also risk and benefit of taking herbal supplements should be considered. Patients and health care professionals must be encouraged to discuss the use of herbal products and be well-informed about the potential interactions between herbs and prescription drugs.
The goal of this study was to clarify whether the four herbal supplements, black cohosh, ginseng, grape seed extract and green tea extract would affect human CYP1A2 activity. These herbal supple-ments are commonly used among cancer patients (Pierce et al., 2002[40]; Richardson et al., 2000[41]; Rock et al., 2004[42]; Wargovich, 2001[51]; Wargovich et al., 2010[52]). Of these supplements studied, black cohosh and ginseng caused very mild inhibition of CYP1A2 whereas green tea and grape seed extracts produced considerable inhibition of CYP1A2 activity. It has been shown that black cohosh exhibited a potent inhibition of CYP3A4 (Sevior et al., 2010[45]; Tsukamoto et al., 2005[49]). A recent in vitro study has also reported that the active constituents of black cohosh triterpene glycosides were weakly, while fukinolic acid and cimi-cifugic acids A and B strongly inhibited CYP1A2 and CYP3A4 isozymes (Huang et al., 2010[30]). Interactions between black cohosh and CYP3A are rather complicated. It has been demonstrated that induction of mouse Cyp3a11 is species-specific and involved only mouse PXR, not the human counterpart (Pang et al., 2011[39]). Thus, the incidence of herbal drug interaction in patients taking black cohosh may not be mediated by human PXR and CYP3A4. A study in healthy volunteers examined the effects of black cohosh root extract on CYP1A2 metabolism using caffeine as a probe drug (Gurley et al., 2005[22]). They found that black cohosh did not inhibit CYP1A2 activity. Results on BC obtained from this study have shown that BC caused very weak to mild inhibition of human CYP1A2 activity. Contradiction between our results and those from Gurley and co-workers (Gurley et al., 2005[22]) could be related to the fact that their study was done in vivo (healthy volunteers) whereas ours was an in vitro study. Also the inhibition of human CYP1A2 activity detected in this study was mild (< 25 %) and this may not be seen as a significant effect in an in vivo study. These may suggest that taking black cohosh concomitantly with prescription drugs metabolized by CYP1A2 is unlikely to cause herbal drug interaction. Con-suming black cohosh as dietary supplement may not have a benefit for protection of cancer if its cause resulted from bioactivation via CYP1A2.
Effect of ginseng on the activity of human CYP1A2 was investigated; the results obtained from the present study show little to moderate inhibitory effect. Of nine brands tested, only two products Spring Valley (34.1 % inhibition) and Solarays (44.6 % inhibition) caused mode-rate inhibition of CYP1A2. These results suggest that ginseng herbal supplements have little effect on human CYP1A2 activity. However, a moderate inhibitory effect can be produced by some specific brands of ginseng products. This may be resulted from variation in active ingredients of ginseng in different brand products. Our findings showing little inhibitory effect of ginseng on the activity of human CYP1A2 are in agreement with those reported previously (Gurley et al., 2005[21]). In their study, 28 days of ginseng supplementations in elderly volunteers did not appear to affect the activity of CYP1A2. In addition, an in vitro study was carried out to determine the effect of ginseng's active components ginsenosides and eleuthe-rosides on the catalytic activity of c-DNA expressed CYP isoforms. It was found that these active components of ginseng are not likely to inhibit the metabolism of drugs in which the primary route of elimination is mediated by P450 enzymes including CYP1A2 (Henderson et al., 1999[28]). A recent study in rats has shown that after pretreatment with ginseng for 1 week, an induction effect on CYP1A2 was observed as there was a significant increase in clearance of the CYP1A2 probe drug caffeine, compared with that of the control group (Liu et al., 2012[34]). Results with Western blot analysis also revealed the upregulation of the CYP1A2 protein expression induced by ginseng pretreatment.
Similar to ginseng, the effect of GSE dietary supplements on human CYP1A2 activity was mild. Six of nine GSE brands investigated have shown to inhibit CYP1A2 activity moderately (< 27 % inhibition). These results are similar to those observed in an in vitro experiment showing that low concentrations of GSE (normalized to 1 mM catechin) slightly activated, whereas high concentration of GSE (10 mM catechin) moderately inhibited (approximately 24 % inhibition) the activity of human CYP1A2 (Etheridge et al., 2007[15]). An in vitro study has demonstrated that GSE and other grape phytochemicals were strong inhibitors of human CYP1A2 enzyme (Kowalczyk et al., 2009[32]). This is inconsistent with our results and may be explained by differences in experimental conditions such as concentrations of GSE and in vitro systems used.
Our finding with green tea extract (GTE) suggests that most of GTE products inhibited the activity of human CYP1A2 as nine of twelve brands studied have demonstrated to cause moderate to strong inhibition of CYP1A2 (Figure 4(Fig. 4)). These results are in agreement with those observed previously in which it has been shown that green tea polyphenols (e.g. epicatechin, epi-catechin gallate and epigallocatechin) appeared to inhibit the activity of CYP1A2 (Dhawan et al., 2002[13]). Green tea had complex effects upon CYP1A1 activity in rat liver microsomes, which consisted of an initial activation of the enzyme at low concentrations followed by inhibition at higher concentrations (Anger et al., 2005[3]). This observation may explain our results with variable inhibitory effect on human CYP1A2 produced by different brands of green tea supplement products. These products are likely to contain different contents of active ingredients of green tea. Thus, different concentrations of green tea's active constituents were presented in the green tea extracts obtained from different brand products. A recent report has found that the most abundant component in green tea, i.e., (-) epigallocatechin-3-gallate (EGCG) did not affect the activity of CYP1A2 (Bothe et al., 2011[7]). An in vivo study in rats has demonstrated that after 4 days pretreatment with GTE, there was an induction of CYP1A2 as the hepatic CYP1A2 levels were increased by approximately 2-fold, while the enzyme activity increased slightly i.e., by 10 % of control (Jang et al., 2005[31]). Also a previous study in healthy volunteers found that a 4 weeks pretreatment with green tea catechin did not alter the phenotypic index of CYP1A2 (Chow et al., 2006[11]). It was likely that EGCG and epicatechin-3-gallate would be the major components responsible for the inhibitory effect of GTE (Anderson et al., 2003[2]; Chow et al., 2006[11]). The role of green tea in cancer prevention, meaning the ability to delay the onset of the carcinogenic process, has become an intense area of research over the past year (Netsch et al., 2006[38]). The drinking of green tea is particularly popular in Asian cultures, and its connection with human health benefits has resulted in the inclusion of GTEs as common botanical ingredients in dietary supplements, nutraceuticals, and functional foods (Henning et al., 2006[29]).
The inhibitory effect produced by each herbal supplement appeared to vary between brands of the products. The differences in the inhibitory effect of herbal supplementary products investigated on human CYP1A2 could be associated with/or likely to be due to the variation in active ingredients in these herbal supplements. This finding is in accordance with our previous study showing a similar observation in which there was a large variation in inhibitory effect of herbal supplements on human CYP3A4 (Wanwimolruk et al., 2009[50]). Of note is that human CYP3A4 is the most abundant isoform and metabolizes more than 40 % of prescription drugs, thus drug-drug interactions with CYP3A4 is extremely important and this enzyme activity is also affected by herbal and plant extracts (Guengerich 1999[19]; Shi and Klotz, 2012[46]). Although the active ingredients in each herbal supplement was not measured in this study (because of complexity and difficulty of the analytical assays), our findings are in harmony with earlier studies which have demonstrated a great variation in the active ingredients in many herbal products. These examples include the following findings. Inconsistencies between actual product composition and labeled content have been reported with ginseng, echinacea, St. John's wort, Ma Huang and androstenedione (Cui et al., 1994[12]; Draves and Walker, 2003[14]; Green et al., 2001[18]; Haller et al., 2004[23]; Harkey et al., 2001[26]). Of 25 commercial ginseng preparations analyzed, the content of active ingredients (ginsenosides and eleutherosides) differed significantly from labeled amounts (Harkey et al., 2001[26]). The contents were found to range from 11 % to 328 % of the labeled content. There was also significant product-to-product variability. Testing of 28 brands of St. John's wort capsules revealed large variations in the total content of active ingredients (sum of hypericin and pseudohypericin). The percentage of label claim varied from 0 % to 109 % (Draves and Walker, 2003[14]). Likewise, the Good Housekeeping Institute has also found a 17-fold difference in the hypericin content and a 13-fold difference in pseudohypericin content present in 10 St. John's wort preparations tested (Good Housekeeping Institute, 1998[17]). Our findings suggest that the benefit of taking these herbal supplements as antioxidants will be dependent upon the brand of product consumed. Without exclusive quality control such as standardizing active ingredients in dietary supplement products, consumers are not likely to gain benefits from taking these natural dietary supplement products.
In summary, the results in the current study demonstrate that there are small and large variations of the inhibitory effect of herbal dietary supplements on human CYP1A2. Particularly for grape seed extract (even though its effect was less pronounced than that of green tea extract) and green tea extract supplements, the variations (15-fold to 16-fold) among different brands were extraordinary. This questions the quality of the dietary supplements that many patients take presuming these dietary supplement products to be safe. In addition, it supports the recommendation earlier suggested by other investigators (Cui et al., 1994[12]; Draves and Walker, 2003[14]; Foster et al., 2005[16]; Good Housekeeping Institute, 1998[17]; Green et al., 2001[18]; Henning et al., 2006[29]) that trustworthy labeling information and standardized manufacturing practices by means of biological and phytochemical assays be put in place for the quality control of herbal/botanical dietary supplements. Moreover, lack of inhibitory effects of black cohosh, grape seed extract and ginseng on CYP1A2 activity implies that there is no clinically significant herbal drug interaction as a result of enzyme inhibition to be expected upon coadministration of these herbal supplements with prescription drugs which are metabolized by CYP1A2. However, from our results we cannot exclude that coadministration of green tea extract supplements with CYP1A2 drugs may cause an increase in the concentrations of such drugs. Clinical studies are warranted to refine this issue. Conversely, a possible in vivo inhibition of CYP1A2 by green tea extract might provide a beneficial protection against cancer, caused by the metabolic activation of CYP1A2.
Notes
This work was presented in part at the American Association of Pharmaceutical Scientists (AAPS) Annual Meeting, October 29-November 2, 2006, San Antonio, Texas, USA.
Acknowledgements
This study was supported by research grants from the Office of the Higher Education Commission, Mahidol University under the National Research Universities Initiative and School of Pharmacy, Loma Linda University, California, USA.
References
- 1.Agarwal C, Singh RP, Agarwal R. Grape seed extract induces apoptotic death of human prostate carcinoma DU145 cells via caspases activation accompanied by dissipation of mitochondrial membrane potential and cytochrome C reductase. Carcinogenesis. 2002;23:1869–1876. doi: 10.1093/carcin/23.11.1869. [DOI] [PubMed] [Google Scholar]
- 2.Anderson GD, Rosito G, Mohustsy MA, Elmer GW. Drug interaction potential of soy extract and Panax ginseng. J Clin Pharmacol. 2003;43:643–648. [PubMed] [Google Scholar]
- 3.Anger DL, Petre MA, Crankshaw DJ. Hete-roactivation of cytochrome P450 1A1 by teas and tea polyphenols. Br J Pharmacol. 2005;145:926–933. doi: 10.1038/sj.bjp.0706255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagchi D, Garg A, Krohn RL, Bagchi M, Bagchi DJ, Balmoori J, et al. Protective effects of grape seed proanthocyanidins and selected antioxidants against TPA-induced hepatic and brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen Pharmacol. 1998;30:771–776. doi: 10.1016/s0306-3623(97)00332-7. [DOI] [PubMed] [Google Scholar]
- 5.Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamin C and E, and a grape seed proanthocyanidin extract in vitro. Res Commun Mol Pathol Pharmacol. 1997;95:179–189. [PubMed] [Google Scholar]
- 6.Blumenthal M. Herb sales down 15 percent in mainstream market. Herbalgram. 2001;51:69. [Google Scholar]
- 7.Bothe H, Gassmann K, Götz C, Fritsche E, Abel J, Haarmann-Stemmann T. Epigallocatechin-3-gallate does not affect the activity of enzymes involved in metabolic activation and cellular excretion of benzo[a]pyrene in human colon carcinoma cells. Toxicol Lett. 2011;203:258–264. doi: 10.1016/j.toxlet.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Burke MD, Thompson S, Weaver RJ, Wolf CR, Mayer RT. Cytochrome P450 specificities of alkoxyresorufin O-dealkylation in human and rat liver. Biochem Pharmacol. 1994;48:923–936. doi: 10.1016/0006-2952(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 9.Cabrera C, Artacho R, Gimez R. Beneficial efforts of green tea – a review. J Am Coll Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 10.Chen D, Daniel KG, Chen MS, Kuhn DJ, Landis-Piwowar KR, Dou QP. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem Pharmacol. 2005;69:1421–1432. doi: 10.1016/j.bcp.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Chow HH, Hakim IA, Vining DR, Crowell JA, Cordova CA, Chew WM, et al. Effects of repeated green tea catechin administration on human cytochrome P450 activity. Cancer Epidemiol Biomarkers Prev. 2006;15:2473–2476. doi: 10.1158/1055-9965.EPI-06-0365. [DOI] [PubMed] [Google Scholar]
- 12.Cui J, Garle M, Eneroth P, Bjorkem I. What do commercial ginseng preparations contain? Lancet. 1994;344:134. doi: 10.1016/s0140-6736(94)91322-6. [DOI] [PubMed] [Google Scholar]
- 13.Dhawan A, Anderson D, de Pascual-Teresa S, Santos-Buelga C, Clifford MN, Ioannides C. Evaluation of the antigenotoxic potential of monomeric and dimeric flavanols, and black tea polyphenols against heterocyclic amine-induced DNA damage in human lymphocytes using the Comet assay. Mutat Res. 2002;515:39–56. doi: 10.1016/s1383-5718(01)00347-3. [DOI] [PubMed] [Google Scholar]
- 14.Draves AH, Walker SE. Analysis of the hypericin and pseudohypericin content of commercially available St. John’s wort preparations. Can J Clin Pharmacol. 2003;10:114–118. [PubMed] [Google Scholar]
- 15.Etheridge AS, Black SR, Patel PR, So J, Mathews JM. An in vitro evaluation of cytochrome P450 inhibition and P-gly-coprotein interaction with goldenseal, Ginkgo biloba, grape seed, milk thistle, and ginseng extracts and their constituents. Planta Med. 2007;73:731–741. doi: 10.1055/s-2007-981550. [DOI] [PubMed] [Google Scholar]
- 16.Foster BC, Arnason JT, Briggs CJ. Natural health products and drug disposition. Annu Rev Pharmacol Toxicol. 2005;45:203–296. doi: 10.1146/annurev.pharmtox.45.120403.095950. [DOI] [PubMed] [Google Scholar]
- 17.Good Housekeeping Institute. New Good Housekeeping Institute study finds drastic discrepancy in potencies of popular herbal supplements. Consumer Safety Symposium on Dietary Supplements and Herbs, New York City, New York, March 3, 1998 (News release). [Google Scholar]
- 18.Green GA, Catlin DH, Starcevic B. Analysis of over-the-counter dietary supplements. Clin J Sport Med. 2001;11:254–259. doi: 10.1097/00042752-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Gurley BJ, Gardner SF, Hubbard MA. Content versus label claims in ephedra-containing dietary supplements. Am J Health Syst Pharm. 2000;57:963–969. doi: 10.1093/ajhp/57.10.963. [DOI] [PubMed] [Google Scholar]
- 21.Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Cui Y, et al. Clinical assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly: St John's wort, garlic oil, Penax ginseng and Ginkgo biloba. Drugs Aging. 2005;22:525–539. doi: 10.2165/00002512-200522060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Khan IA, et al. In vivo effects of goldenseal, kava kava, black cohosh, and valerian on human cytochrome P450 1A2, 2D6, 2E1, and 3A4/5 phenotypes. Clin Pharmacol Ther. 2005;77:415–426. doi: 10.1016/j.clpt.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haller CA, Duan M, Benowitz NL, Jacob P., III Concentrations of ephedra alkaloids and caffeine in commercial dietary supplements. J Anal Toxicol. 2004;28:145–151. doi: 10.1093/jat/28.3.145. [DOI] [PubMed] [Google Scholar]
- 24.Hamburger M, Webner C, Benthin B. Cycloartane glycosides from Cimicifuga racemosa. Pharm Pharmacol Lett. 2001;11:98–100. [Google Scholar]
- 25.Hanioka N, Tatarazako N, Jinno H, Arizono T, Ando M. Determination of cytochrome P450 1A activities in mammalian liver microsomes by high-performance liquid chromatography with fluorescence detection. J Chromatogr B. 2000;744:399–406. doi: 10.1016/s0378-4347(00)00278-4. [DOI] [PubMed] [Google Scholar]
- 26.Harkey MR, Henderson GL, Gershwin ME, Stern JS, Hackman RM. Variability in commercial ginseng products: an analysis of 25 preparations. Am J Clin Nutr. 2001;73:1101–1106. doi: 10.1093/ajcn/73.6.1101. [DOI] [PubMed] [Google Scholar]
- 27.Helms S. Cancer prevention and therapeutic: Panax Ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- 28.Henderson GL, Harkey MR, Gershwin ME, Hackman RM, Stern JS, Stresser DM. Effects of ginseng components on c-DNA-expressed cytochrome P450 enzyme catalytic activity. Life Sci. 1999;65:PL209–PL214. doi: 10.1016/s0024-3205(99)00407-5. [DOI] [PubMed] [Google Scholar]
- 29.Henning SM, Aronson W, Niu Y, Conde F, Lee NH, Seeram NP, et al. Tea polyphenols and theaflavins are present in prostate tissue of humans and mice after green and black tea consumption. J Nutr. 2006;136:1839–1843. doi: 10.1093/jn/136.7.1839. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Jiang B, Nuntanakorn P, Kennelly EJ, Shord S, Lawal TO, et al. Fukinolic acid derivatives and triterpene glycosides from black cohosh inhibit CYP isozymes, but are not cytotoxic to Hep-G2 cells in vitro. Curr Drug Saf. 2010;5:118–124. doi: 10.2174/157488610790936150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang EH, Choi JY, Park CS, Lee SK, Kim CE, Park HJ, et al. Effects of green tea extract administration on the pharmacokinetics of clozapine in rats. J Pharm Pharmacol. 2005;57:311–316. doi: 10.1211/0022357055687. [DOI] [PubMed] [Google Scholar]
- 32.Kowalczyk MC, Walaszek Z, Kowalczyk P, Kinjo T, Hanausek M, Slaga TJ. Differential effects of several phytochemicals and their derivatives on murine keratinocytes in vitro and in vivo: implications for skin cancer prevention. Carcinogenesis. 2009;30:1008–1015. doi: 10.1093/carcin/bgp069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lautraite S, Musonda AC, Doehmer J, Edwards GO, Chipman JK. Flavonoids inhibit genetic toxicity produced by carcinogens in cells expressing CYP1A2 and CYP1A1. Mutagenesis. 2002;17:45–53. doi: 10.1093/mutage/17.1.45. [DOI] [PubMed] [Google Scholar]
- 34.Liu R, Qin M, Hang P, Liu Y, Zhang Z, Liu G. Effects of Panax notoginseng Saponins on the activities of CYP1A2, CYP2C9, CYP2D6 and CYP3A4 in rats in vivo. Phytother Res. 2012;26:1113–1118. doi: 10.1002/ptr.3688. Available from: http://dx.doi.org/10.1002/ptr.3688. [DOI] [PubMed] [Google Scholar]
- 35.Maffei FR, Carini M, Aldini G, Berti F, Rossoni G, Bombardelli E, et al. Procyanidins from Vitis vinifera seeds protect rabbit heart from ischemia/reperfusion injury: antioxidant intervention and/or iron and copper sequestering ability. Planta Med. 1996;62:495–502. doi: 10.1055/s-2006-957956. [DOI] [PubMed] [Google Scholar]
- 36.Maliakal P, Sankpal UT, Basha R, Maliakal C, Ledford A, Wanwimolruk S. Relevance of drug metabolizing enzyme activity modulation by tea polyphenols in the inhibition of esophageal tumorigenesis. Med Chem. 2011;7:480–487. doi: 10.2174/157340611796799096. [DOI] [PubMed] [Google Scholar]
- 37.Murray BP, Edwards RJ, Murray S, Singleton AM, Davies DS, Boobis AR. Human hepatic CYP1A1 and CYP1A2 content, determined with specific anti-peptide antibodies, correlates with the mutagenic activation of PhIP. Carcinogenesis. 1993;14:589–592. doi: 10.1093/carcin/14.4.585. [DOI] [PubMed] [Google Scholar]
- 38.Netsch MI, Gutmann H, Schmidlin CB, Aydogan C, Drewe J. Induction of CYP1A by green tea extract in human intestinal cell lines. Planta Med. 2006;72:514–520. doi: 10.1055/s-2006-931537. [DOI] [PubMed] [Google Scholar]
- 39.Pang X, Cheng J, Krausz KW, Guo DA, Gonzalez FJ. Pregnane X receptor-mediated induction of Cyp3a by black cohosh. Xenobiotica. 2011;41:112–123. doi: 10.3109/00498254.2010.527021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pierce JP, Faerber S, Wright FA, Rock CL, Newman V, Flatt SW, et al. A randomized trial of the effect of plant-based dietary pattern on additional breast cancer events and survival: the Women’s Healthy Eating and Living (WHEL) study. Contr Clin Trials. 2002;23:728–756. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 41.Richardson MA, Ramirez T, Palmer JL, Greisinger A, Singletary SE. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000;18:2505–2514. doi: 10.1200/JCO.2000.18.13.2505. [DOI] [PubMed] [Google Scholar]
- 42.Rock CL, Newman VA, Neuhouser ML, Major J, Barnett MJ. Antioxidant supplement use in cancer survivors and the general population. J Nutr. 2004;Suppl:3155S–3156S. doi: 10.1093/jn/134.11.3194S. [DOI] [PubMed] [Google Scholar]
- 43.Rockwell S, Liu Y, Higgin SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90:233–239. doi: 10.1007/s10549-004-4260-x. [DOI] [PubMed] [Google Scholar]
- 44.Sato M, Sato T, Izumo T, Amagasa T. Genetically high susceptibility of oral squamous cell carcinoma in terms of combined genotyping of CYP1A1 and GSTM1 genes. Oral Oncol. 2000;36:267–271. doi: 10.1016/s1368-8375(99)00090-1. [DOI] [PubMed] [Google Scholar]
- 45.Sevior DK, Hokkanen J, Tolonen A, Abass K, Tursas L, Pelkonen O, et al. Rapid screening of commercially available herbal products for the inhibition of major human hepatic cytochrome P450 enzymes using the N-in-one cocktail. Xenobiotica. 2010;40:245–254. doi: 10.3109/00498251003592683. [DOI] [PubMed] [Google Scholar]
- 46.Shi S, Klotz U. Drug interactions with herbal medicines. Clin Pharmacokinet. 2012;51:77–104. doi: 10.2165/11597910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 47.Singletary KW, Meline B. Effect of grape seed proanthocyanidins in colon aberrant crypts and breast tumors in a rat dual-organ tumor model. Nutr Cancer. 2001;39:252–258. doi: 10.1207/S15327914nc392_15. [DOI] [PubMed] [Google Scholar]
- 48.Sparreboom A, Cox MC, Acharya MR, Figg WD. Herbal remedies in the United States: Potential adverse reactions with anticancer drugs. J Clin Oncol. 2004;22:2489–2503. doi: 10.1200/JCO.2004.08.182. [DOI] [PubMed] [Google Scholar]
- 49.Tsukamoto S, Aburatani M, Ohta T. Isolation of CYP3A4 inhibitors from the Black Cohosh (Cimicifuga racemosa) Evid Based Complement Alternat Med. 2005;2:223–226. doi: 10.1093/ecam/neh086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wanwimolruk S, Wong K, Wanwimolruk P. Variable inhibitory effect of commercial herbal supplements with different brands on human cytochrome P450 CYP3A4. Drug Metabol Drug Interact. 2009;24:17–35. doi: 10.1515/dmdi.2009.24.1.17. [DOI] [PubMed] [Google Scholar]
- 51.Wargovich MJ. Colon cancer chemoprevention with ginseng and other botanicals. J Korean Med Sci. 2001;16(Suppl):S81–S86. doi: 10.3346/jkms.2001.16.S.S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wargovich MJ, Morris J, Brown V, Ellis J, Logothetis B, Weber R. Nutraceutical use in late-stage cancer. Cancer Metastasis Rev. 2010;29:503–510. doi: 10.1007/s10555-010-9240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang CS, Landau JM. Effects of tea consumption on nutrition and health. J Nutr. 2000;130:2409–2412. doi: 10.1093/jn/130.10.2409. [DOI] [PubMed] [Google Scholar]
- 54.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 55.Yang CS, Sang S, Lambert JD, Hou Z, Ju J, Lu G. Possible mechanisms of the cancer-preventive activities of green tea. Mol Nutr Food Res. 2006;50:170–175. doi: 10.1002/mnfr.200500105. [DOI] [PubMed] [Google Scholar]
- 56.Zhang H, Coville PF, Walker RJ, Miners JO, Birkett DJ, Wanwimolruk S. Evidence for an involvement of human CYP3A in the 3-hydroxylation of quinine. Br J Clin Pharmacol. 1997;43:245–252. doi: 10.1046/j.1365-2125.1997.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao J, Wang J, Chen Y, Agarwal R. Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation-promotion protocol and identification of procyanidins B5-3′-gallate as the most effective antioxidant constituent. Carcinogenesis. 1999;20:1737–1745. doi: 10.1093/carcin/20.9.1737. [DOI] [PubMed] [Google Scholar]