Abstract
Male fitness depends on the expression of costly traits involved in obtaining mates (pre-copulatory) and fertilization (post-copulatory). However, very little is known about the nutrient requirements for these traits and whether males compromise their diet to maximize one trait at the expense of another. Here we used Nutritional Geometry to investigate macronutrient requirements for pre- and post-copulatory traits in Drosophila, when males were the first or second to mate with females. We found no significant effects of male diet on sperm competitiveness. However, although males self-regulate their macronutrient intake at a protein-to-carbohydrate ratio (“P:C ratio”) of 1:1.5, this ratio does not coincide with their optima for several key reproductive traits: both the short-term (~24 hr) rate of offspring production after a female’s first mating, as well as the total offspring number sired when males were second to mate were maximized at a P:C ratio of 1:9, whereas male attractiveness (latency to mate), were maximised at a P:C ratio of 1:1. These results suggest a compromised optimum diet, and no single diet that simultaneously maximizes all male reproductive traits. The protein intake of first males also negatively affected female offspring production following remating, suggesting a long-term intersexual effect of male nutrition.
In most species, females are polyandrous (females copulate with multiple males) and, as a result, males are likely to encounter both competition over access to mates (pre-copulation) and fertilization (post-copulation)1,2. These two competitive episodes strongly influence male fitness3,4. However, the expression of male sexual traits involved in both pre- and post-copulatory competition is thought to be costly, meaning that male fitness is expected to depend on resource availability, including nutrients5,6.
Macronutrients (i.e. protein, carbohydrates and fats) are vital in the molecular homeostasis of the organism and radically shape the physiology, behaviour and the expression of life-history traits7,8,9,10. In insects, the intake of specific macronutrients can affect both lifespan and reproduction in a sex-specific fashion (e.g.11,12,13,14). For instance, in both Drosophila melanogaster11,15 and Telleogrylus commodus12 female lifespan increases under high-carbohydrate and low-protein diets whereas maximal female reproduction generally requires high-protein diets. On the other hand, male lifespan and reproduction can align: in addition to longer lifespan, high carbohydrate intake also increases male sexual performance in the cockroach Nauphoeta cinerea16,17, in the broad-horned beetle Gnatocerus cornutus18 and in D. melanogaster15. However, male nutrition has to integrate the needs of both pre- and post-copulatory episodes, and imbalanced diets can impose constraints on male allocation to sexual traits19. Although recent studies have attempted to investigate dietary effects on the interrelation between pre- and post-copulatory traits (e.g.19,20,21), few have attempted to characterise the specific macronutrient requirements that maximize male expression of these traits12,15,16,17,18,22,23. Most studies in this field have focused primarily on pre-copulatory traits (e.g.12,17,23), and only recently have researchers attempted to address the effects of macronutrient balance on male post-copulatory traits15,16,22,24. For instance, recent work indicates that a protein:carbohydrate ratio of 1:2 maximises sperm number and fertility in the cockroach Nauphoeta cinerea23. Although sperm number can be important for male sperm competitiveness, it does not necessarily predict the outcome of post-copulatory competition (see25). Positive linear relationships between carbohydrate intake and fitness have been demonstrated in male fruitflies D. melanogaster15,22. Yet, it is not clear whether carbohydrate is beneficial to males in pre- or post-copulatory sexual selection, or both. This raises important questions: what are the specific nutritional requirements for competitive male pre- and post-copulatory traits? Can male diet maximize pre- and post-copulatory traits simultaneously or do different traits require different diets, meaning that males have to compromise in their dietary choices?
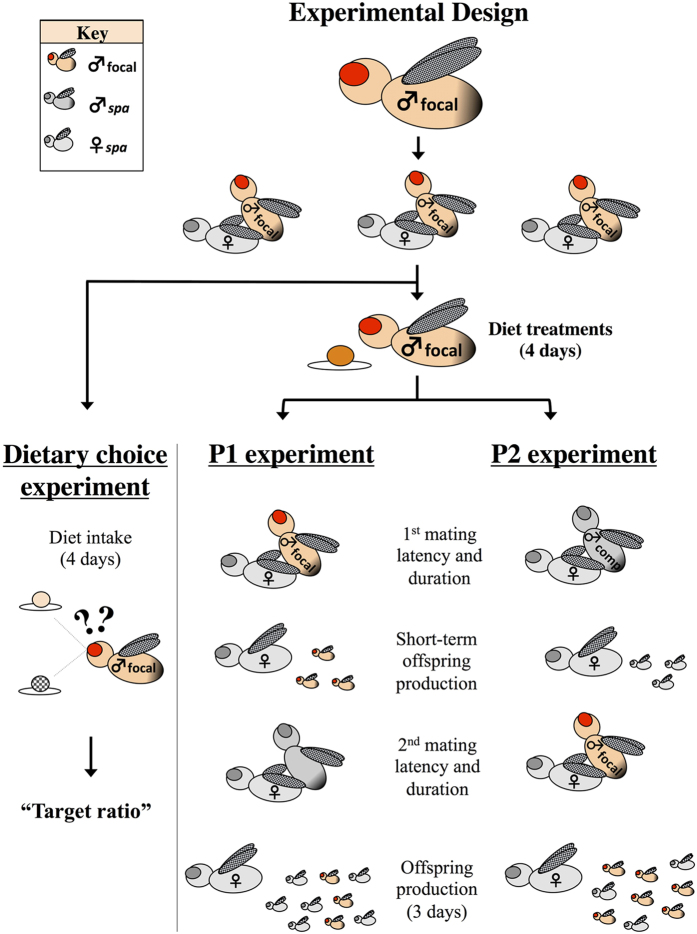
To address these questions, we used the geometric framework of nutrition (Nutritional Geometry26) to experimentally investigate the nutritional requirements across male Drosophila melanogaster sexual selection episodes. Nutritional geometry is a state-space model to investigate the effects of multiple nutrients on fitness (reviewed in5). We had two main motivations for this study: i) to investigate the specific nutritional requirement for male success in both pre- and post-copulatory sexual selection and ii) to determine the nutritional balance sought by males when given a choice of diets. We first depleted male ejaculate reserves (e.g. accessory gland products) by mating males 3 times in succession (see27,28,29,30) - which we expected to place males in a condition in which macronutrient acquisition is necessary to replenish their ejaculate reserves. We gave a subset of these males a choice between complementary diets with different concentrations of macronutrient and measured the protein-to-carbohydrate ratio (P:C ratio) males aimed to achieve by self-regulating their diet intake – the “target ratio”. The remaining males were allocated to one of 15 defined diets (“no choice”), which varied in both the P:C ratio and in the concentration of macronutrients5. Males in these no-choice diet treatments then assumed either one of two roles: 1) the first male to mate with a virgin female, in which the female was subsequently mated with a competitor male (P1 experiment) or 2) the second male to mate with a non-virgin female who had previously mated with a competitor male (P2 experiment). This design allowed us to scrutinise male whether male dietary self-regulation matches the macronutrient requirements of both pre- and post-copulatory episodes of sexual selection.
Methods
Fly stocks and culture
We used a wild-type D. melanogaster stock collected from Dahomey (now Benin) in 1970, and maintained in large outbred populations with overlapping generations31. We also used a recessive sparklingpoliert mutation (spa) strain, which produces a rough-looking eye phenotype when homozygous32. The spa strain was back-crossed into Dahomey for >5 generations to ensure a standard genetic background33. Using wild-type focal males, and homozygous spa females and competitor males, allowed us to assign paternity through the progeny’s eye phenotype33. All fly stocks were maintained, and all experiments conducted, at 25 °C on a 12:12 light:dark cycle in a non-humidified room (i.e. natural humidity). We used standard sugar-yeast-maize-molasses medium with ad libitum yeast to feed all spa females and spa male competitors (see Supplemental Information for standard fly food recipe). We controlled larval density to avoid effects on adult phenotypes34: all experimental flies were raised at a density of ~200 eggs in 75 ml bottles with ~45 ml of standard maize-molasses fly food (see Supplementary Information). Virgin flies were collected on ice anaesthesia within 8 hours of eclosion and kept in single-sex vials of 15–20 individuals for 3 days with ad libitum yeast prior to experiments.
Depletion of male ejaculate reserves
The first steps of all experiments were identical and therefore are explained together until the point in which the experimental procedures diverge (Fig. 1). We allowed virgin wild-type males to mature in standard maize-molasses fly food with ad libitum yeast for 3d after emerging from pupae in same sex groups of 15 individuals. We then transferred focal males to fresh vials also with standard food and ad libitum yeast and conduced 3 matings to deplete male ejaculate reserves, as follows. We placed each male with one wild-type virgin female and allowed him to mate. After the first mating, we substituted the female for a fresh wild-type virgin female. This process was repeated until our focal males had mated exactly 3 times. Males were given up to 10 h to complete the 3 matings. Three consecutive matings can dramatically reduce the amount of seminal fluid transferred to females due to ejaculate depletion27. Therefore our treatment was likely place males in a condition in which nutrient acquisition is necessary to replenish male’s ejaculate reserves, and possibly also energy reserves for courtship. Males that successfully mated with 3 females (i.e. henceforth “focal males”) at the end of 10 h were randomly allocated to one of the three experiments (see below). Males that failed to mate with 3 females were discarded. For the P1 and P2 experiments, focal males were randomly allocated to one of the 15 diet treatments described above and maintained on these diets for 4 days before the experimental matings.
Figure 1. Schematic overview of the experimental design.
Virgin wild-type males were kept 3d in standard food with ad libitum yeast for maturation, and then mated with three virgin females consecutively to deplete their ejaculate reserves (i.e. “focal males”). Focal males were then assigned to either a Choice experiment or to 15 distinct defined diets for the P1 and P2 experiments (see Methods for details).
Diet preparation
Focal males were maintained in vials containing agar/water/nipagin medium sealed with Parafilm into which a 5 μL capillary with liquid diet was placed (adapted from the CAFE assay11,35). To prepare the diets, we used sucrose as source of carbohydrate (MP Biomedicals, cat. 194018) and hydrolysed yeast as source of protein (cat. 103304). We used sterilized distilled water, filtered in micro-filter of Merck Millipore with pores of 0.22 μm in a sterilized flow, and replaced from the capillaries every ~18 h to minimize bacteria contamination. Diet intake was measured using a digital calliper.
Experimental design
We performed 3 experiments: (1) a dietary choice experiment - where focal males, held singly, were given a choice between complementary diets, and dietary choice was assessed. This experiment allowed us to investigate the P:C ratio males choose when given the opportunity to balance their diet; (2) P1 experiment - where focal males were the first to mate with a virgin spa female, and females were subsequently given the opportunity to remate with a competitor spa male; (3) P2 experiment - where spa females were first mated to a spa male and subsequently given the opportunity to mate with a focal male (Fig. 1)5.
Dietary choice experiment
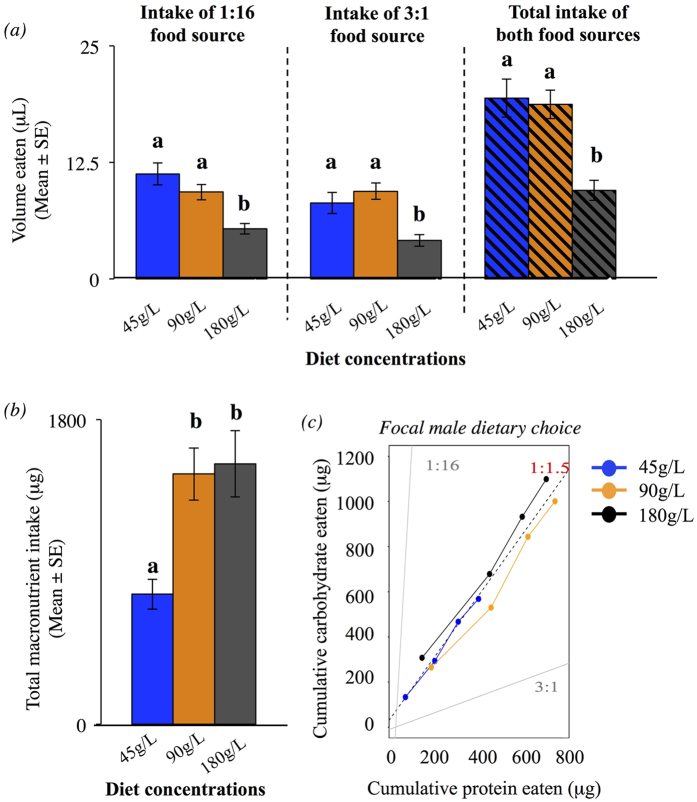
Focal males were given a choice between two capillaries with diets with P:C ratios of 1:16 and 3:1, both diets at one of the three concentrations (45 g/L, 90 g/L and 180 g/L). The cumulative intake of both diets were measured every ~18 h for 4d, after which focal males were discarded. The experiment was conducted in two replicates (total N = 68 males). The P:C ratio that males aimed to reach is referred to as ‘the target ratio’ (see5 for a review of the method).
P1 experiment
We used P:C ratios of: 1:16, 1:9, 1:3, 1:1 and 3:1, each with 3 different concentrations (45 g/L, 90 g/L and 180 g/L) for a total of 15 diets prepared as described above (see “Dietary choice Experiment”). The average intake of protein and carbohydrate in all diets is given in Table S1. After 4 days on one of the 15 the diet treatments, focal males were transferred to fresh vials containing one virgin spa female on standard maize-molasses food with ad libitum yeast. Flies were allowed to interact until mating was observed. Immediately after a single mating, or if no mating occurred in 4 hours, focal males were discarded. The latency of females to mate with the focal male – a measure of male pre-copulatory attractiveness36 and copulation duration were recorded. Mated females were retained and allowed to lay eggs until the following day. 24 hrs after their initial mating the spa females were moved to fresh vials with ad libitum yeast granules, and 1 spa male (competitor male) was added to each vial. The pair were allowed to interact for up to 8 hours or until a single copulation was observed. spa males, and females that failed to remate, were discarded. Females that mated with spa males (89 out of the initial 210) were immediately transferred into fresh vials with standard maize-molasses and yeast ad libitum for egg-laying every 24 h for 3 days, after which females were then discarded. Females that did not remate to spa males were discarded. The offspring produced from eggs laid in vials in which females spent the period between matings were used as an estimate of short-term offspring production (including offspring produced by females that did not remate), which is an important component of both male and female reproductive success, since both sexes potentially benefit from a high rate of offspring production soon after the first mating37. We also measured the total offspring produced by females that successfully mated with both the 1st and 2nd males, as well the total offspring sired by the focal male (i.e. the wild-type-eyed offspring) over the total of 4 days (i.e. the “short term” offspring, plus those offspring produced after the female remated). We calculated P1, the proportion of offspring sired by the focal males relative to all offspring produced by females after their second mating (see24) as an measure of focal male success in sperm competition. All adult offspring were counted 15–17 days after oviposition to allow ample time for development and eclosion at 25 °C.
P2 experiment
The design of the P2 experiment was conduced identically to the P1 experiment (see above), except that the spa competitor male was the 1st to mate with the female and the focal male was the 2nd to mate with the female. 105 out of 221 females mated with both the spa and the focal male. We counted the number of wild-type eyed offspring (sired by the focal male) and spa offspring (sired by the spa male) produced by females that copulated with focal males, and calculated P2 - the proportion of offspring sired by focal males as the second male to mate - as a measure of sperm competitiveness. For all experiments, the evaporation rate was measured as the average loss of diet in three empty vials (i.e. no flies) per diet per concentration.
Data analysis
We first standardised the intake measure of both macronutrients as follows:
 |
where Ix is the individual intake of the macronutrient (protein or carbohydrate), 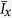 is the mean male intake of that macronutrient and sd(Ix) is the standard deviation of the male intake of that macronutrient16. For the P1 experiment we tested latency of females to mate with focal males, duration of mating with the focal males, the short-term offspring production (between the first and second matings), the proportion of females that remated, P1 paternity share, total offspring sired by the focal male, and total offspring production of the females. We analysed the same factors for the P2 experiment except without the short-term offspring production, and P2 paternity share instead of P1.
is the mean male intake of that macronutrient and sd(Ix) is the standard deviation of the male intake of that macronutrient16. For the P1 experiment we tested latency of females to mate with focal males, duration of mating with the focal males, the short-term offspring production (between the first and second matings), the proportion of females that remated, P1 paternity share, total offspring sired by the focal male, and total offspring production of the females. We analysed the same factors for the P2 experiment except without the short-term offspring production, and P2 paternity share instead of P1.
We used a general linear model for the analysis of duration of the focal male matings. We used a generalized model (GLM) with quasipoisson errors for the analysis of count data such as the short-term offspring production, total offspring sired by the focal male, total offspring production of female (P1 experiment), and offspring production of females after the 2nd mating (P2 experiment). For proportion data (i.e. P1 and P2 paternity share, the proportion of females that remated), we used a GLM with quasibinomial errors. We fitted a GLM with a gamma error to evaluate the effects of latency of females to mate with focal males; latency was square-root transformed to improve model fit in the P2 experiment. For the P1 latency analysis we included all females (including those that subsequently failed to remate). We Box-Cox transformed the mating duration data for the focal male mating in the P1 experiment to best approximate normality of residuals. In addition to the main effects of P and C intake, we controlled for confounding variables by adding the following covariates in models where appropriate: intermating period (i.e. period between the 1st and 2nd mating), duration of 1st and 2nd mating, latency to 1st mating, short-term productivity, and total female productivity (see Supplemental Tables for details). We provide the output of the analyses in Tables S2–S7 together with the raw data of the reproductive traits measured in this study (Figure S1 and S2) in the Supplementary Information. To visualise the nutritional landscapes, we used the functions ‘Tps’ and ‘surface’ of the ‘fields’ package in R, which is a standard method for visualization of nutritional geometry data (e.g.5,11,15). All nutritional landscapes are of raw (non-transformed) data. For the dietary choice experiment, we measured male total volume intake and total macronutrient intake using ANOVA with diet concentration (i.e. 45 g/L, 90 g/L and 180 g/L) controlling for experimental replicate, followed by a post-hoc Student-Newman-Keuls test (α = 0.05) using the ‘agricolae’ package in R. We confirmed that the ANOVA residuals approximated normality. We used paired t-test to compare the observed and the predicted intake of carbohydrates. The predicted carbohydrate intake was calculated as the total individual macronutrient intake – i.e. sum of the intakes of protein and carbohydrates - multiplied by the P:C ratio of interest (e.g. predicted carbohydrate intake in a P:C ratio of 1:1 = total male macronutrient intake * 0.5). All analyses were performed using R version 3.2.238.
Results
Dietary choice experiment
We first investigated the P:C ratio regulated by males when given the opportunity to balance their macronutrient intake. Despite having a significantly higher volume intake in the 45 g/L diets (1:16 food source: ANOVAConcentration: F2,64 = 11.459, p < 0.001; 3:1 food source: ANOVAConcentration: F2,64 = 9.240, p < 0.001; Total volume intake: ANOVAConcentration: F2,64 = 12.267, p < 0.001, Fig. 2a), the total macronutrient intake was still lower when males fed on a 45 g/L diet (ANOVAConcentration: F2, 64 = 10.17, p < 0.001, Fig. 2b). In diet concentrations of 90 g/L and 180 g/L, males reached similar macronutrient intake, but compensated the difference in diet concentration by eating a higher volume of food when fed a 90 g/L diet (Fig. 2a,b); this volume was similar to the volume eaten by males in 45 g/L diets (Fig. 2a). Together, these results suggest that males can compensate the low food concentration by eating more, but there is a point beyond which the food is too diluted for compensation to be attained (i.e. males are not physically or physiologically able to eat more). Importantly, across all concentrations males consumed a P:C ratio ~1:1.5 (45 g/L, P:C = 1:1.57; 90 g/L, P:C = 1:1.3; 180 g/L, P:C = 1:1.58; Mean P:C = ~1:1.5; Fig. 2c) suggesting that males biased their macronutrient intake to high carbohydrates. In total, males ate an average of approximately 590 μg of carbohydrates and 400 μg of protein. Males had a higher carbohydrate intake than expected in a 1:1 ratio (t67 = 6.544, p < 0.001), and a lower carbohydrate intake than expected in a 1:9 ratio (t67 = −12.784, p < 0.001), confirming that male dietary choice tends to have a slightly higher carbohydrate intake than a balanced diet of P:C ratio 1:1. Knowing the P:C ratio males aimed to achieve, we hypothesized that this ratio maximized either pre- or post-copulatory traits, or both. We then fed males on 15 diets with fixed P:C ratios and performed P1 and P2 experiments (see Methods).
Figure 2. Male dietary choice experiment.
(a) Solid bars: Male volume intake (in μL) of the 1:16 and 3:1 food sources across the three experimental concentrations (i.e. 45 g/L, 90 g/L and 180 g/L). Striped bards: The sum of the intake of both food sources (“total intake of both food sources”). Note that male volume intake is higher in 45 g/L and 90 g/L diets (post-hoc SNK-test (α = 0.05)). Blue bars – diet concentration of 45 g/L; Orange bars – diet concentration of 90 g/L; Dark grey bars – diet concentration of 180 g/L. (b) Total male macronutrient intake (in μg) across the three different concentrations (i.e. 45 g/L, 90 g/L and 180 g/L) in the dietary choice experiment. Post-hoc SNK-test (α = 0.05). Blue bar – diet concentration of 45 g/L; Orange bar – diet concentration of 90 g/L; Dark grey bar – diet concentration of 180 g/L. (c) Cumulative intake of protein and carbohydrate (in μg) and the target ratio of 1:1.5 of male in our experiment. Dark-grey solid lines represent the two dietary choices available for males (i.e. 1:16 and 3:1 P:C ratios). Black dashed line – Target ratio of 1:1.5. Blue line – diet concentration of 45 g/L, Orange line – diet concentration of 90 g/L and Black line – diet concentration of 180 g/L.
P1 experiment – focal male 1st to mate
Dietary effects on pre-copulatory traits
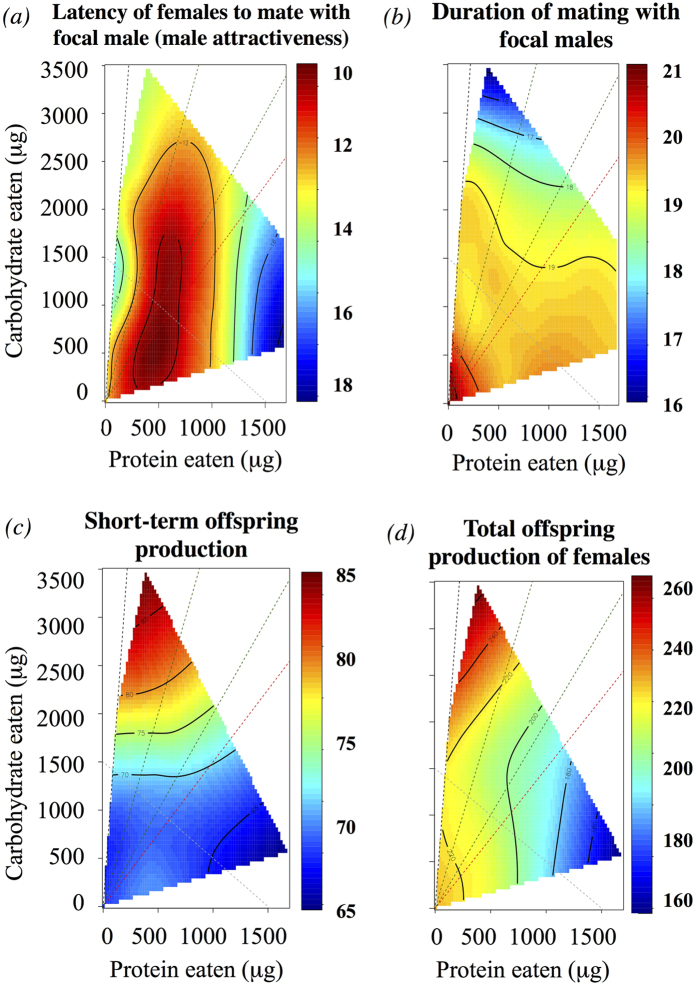
There was a marginally non-significant quadratic effect of focal male protein intake on our measure of male attractiveness to virgin females (the latency of virgin females to mate with the focal male), in which the nutritional landscape peaked at an intermediate protein intake of ~500 μg and a low-to-intermediate carbohydrate consumption (~150 μg to ~1500 μg; Dispersion: 0.218; Protein*Protein: F1,206 = 3.454, p-value = 0.064; Table S2) with P:C ratios lying between 3:1 and 1:3 (Fig. 3a).
Figure 3. Nutritional landscapes of the P1 experiment, in which male mated with virgin females.
(a) Female latency to mate with the focal male (min) – i.e. focal male attractiveness. Note that higher male attractiveness (low latency) is indicated by hotter colours. (b) Duration of the focal male mating. (c) Short-term (~24 h) offspring production after mating with the focal male. (d) Total offspring production of females (including offspring of the 1st and 2nd males). For guidance, we included reference lines in the graph: Grey dashed line is the caloric isocline (i.e. line in which the calories are the same); Black dashed line represents the most carbohydrate-rich diet used in our experiment (i.e. P:C ratio of 1:16); Red dashed line stress the P:C ratio of 1:1.5 or the “target ratio” and green dashed lines show the P:C ratio of 1:4 and 1:2.
Dietary effects on mating duration and post-copulatory traits
We found that mating duration was negatively influenced by carbohydrate intake (Dispersion: 62.455; Carbohydrate: F1,102 = 4.134, p-value = 0.043; Table S3, Fig. 3b). Maximum mating durations corresponded to the low carbohydrate and protein intakes and P:C ratios between 1:4 and 1:9. We found no significant effect of macronutrients on the latency of females to mate with the 2nd male or proportion of females that subsequently remated with the 2nd male (Tables S2 and S4). Increasing carbohydrate intake had a significant effect on short-term offspring production (offspring produced before remating; Dispersion: 6.38; Carbohydrate: F1,86 = 4.293, p-value = 0.041; Table S5), and the nutritional landscape peaked at a high carbohydrate intake (~3500 μg) and a P:C ratio of ~1:9 (Fig. 3c). We did not find a significant effect of macronutrient intake on the total offspring sired by males over the entire period of the experiment (Table S5). However, the total number of offspring produced by females (which includes both those sired by the focal and competitor male) was negatively influenced by focal (1st male) dietary protein intake (Dispersion: 14.24; Protein, F1,87 = 6.830, p-value = 0.010; Table S5), and there was a marginally non-significant quadratic trend for a carbohydrate effect (Carbohydrate* Carbohydrate: F1,84 = 3.595, p-value = 0.061; see Table S5); inspection of the nutritional landscape showed that the total number of offspring produced by females was the greatest at high male carbohydrate intake (~3250 μg) and low male protein intake (~360 μg) also in a P:C ratio of ~1:9 (Fig. 2d). However, neither protein nor carbohydrate significantly influenced P1 (i.e. the proportion of offspring sired by focal males as the first male to mate; Table S6).
P2 experiment – focal male 2nd to mate
Dietary effects on pre-copulatory traits
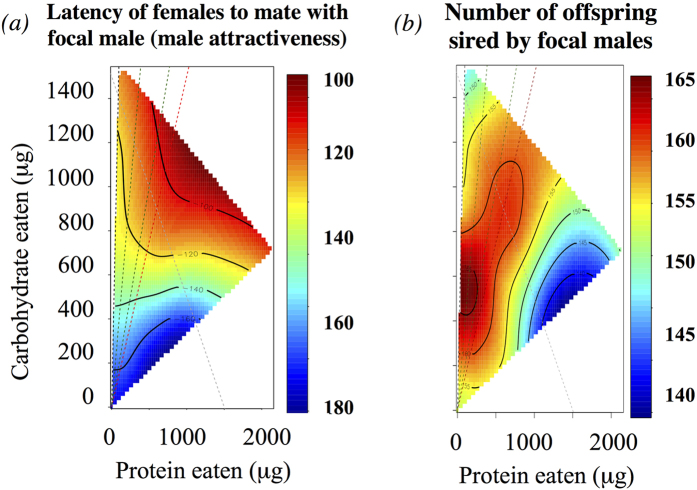
We found a non-significant trend for increased carbohydrate intake to reduce the latency of females to mate with the focal male (i.e. an increase in male attractiveness; Dispersion: 0.333; Carbohydrate: F1,104 = 3.044, p-value = 0.084; Table S2), which measures male attractiveness when focal males encounter an unreceptive females; there was also a borderline non-significant interaction protein*carbohydrate on the latency of females to mate with the focal male (Protein*Carbohydrate: F1,99 = 3.799, p-value = 0.054, Table S2). Together, these results showed that the nutritional landscape for male attractiveness as a 2nd male to mate with unreceptive females peaked at relatively high macronutrient intakes (~1100 μg of each of the macronutrients) and a P:C ratio of ~1:1 (Fig. 4a). We found no effect of focal male macronutrient intake on the proportion of females that remated with the focal male (Table S4).
Figure 4. Nutritional landscapes of the P2 experiment, in which males mated with non-virgin females.
(a) Female latency to mate with the focal male (min) – i.e. focal male attractiveness. Note that higher male attractiveness (low latency) is indicated by hotter colours. (b) The number of offspring sired by the focal male. For guidance, we included reference lines in the graph: Grey dashed line is the caloric isocline (i.e. line in which the calories are the same); Black dashed line represents the most carbohydrate-rich diet used in our experiment (i.e. P:C ratio of 1:16); Red dashed line stress the P:C ratio of 1:1.5 or the “target ratio” and green dashed lines show the P:C ratio of 1:4 and 1:2.
Dietary effects on mating duration and post-copulatory traits
Focal male macronutrient intake had no significant effect on the duration of the focal male mating (Table S3). There was a significant interaction between protein and carbohydrate intake on the number of offspring sired by the focal male (Dispersion: 25.03; Protein*Carbohydrate: F1,97 = 4.598, p-value = 0.034, Table S7), in which the number of offspring sired by the focal male was the greatest at a low protein intake (~60 μg) but at an intermediate carbohydrate intake (~550 μg) in a P:C ratio of ~1:9 (Fig. 4b). However, there was no significant effect of focal male macronutrient intake on the total number of offspring produced by females (including those sired by both the spa 1st male and the focal 2nd male; Table S7), or on the proportion of wild-type offspring sired (P2) by the focal males (Table S6).
Discussion
Our results reveal that macronutrient intake, particularly of carbohydrates, can influence male pre and post-copulatory reproductive traits. The data suggest that there is a mismatch between male dietary choice and the dietary requirement for specific reproductive traits. When given the opportunity to self-regulate their diet, males seek a P:C ratio of ~1:1.5. However, this ratio is different from the P:C ratios that maximized offspring production (~1.9; Fig. 3c) and male attractiveness (~1.1; Fig. 3a). These results show that males may be compromising their dietary intake to maximize different pre- and post-copulatory traits since there is not a single diet that maximizes all male reproductive traits. We also found that increasing male protein intake significantly reduced the total offspring productivity of their mates, suggesting an intersexual effect of male diet on female offspring production. Below, we discuss the main findings of this study.
Male dietary choice
The data show that males compensate the macronutrient dilution in the diet by eating more food, as found previously for female D. melanogaster11. However, there is likely either a physical or physiological constraint (or both) that constrains male maximum food intake. If diets are too diluted, as in the case of our 45 g/L concentration, males may not be able to consume sufficient liquid to reach their optimum macronutrient intake (Fig. 2a,b). Despite this, males in our experiment still balanced their nutrient intake to a ~1:1.5 P:C ratio even when they failed to reach their optimum total macronutrient intake (Fig. 2c). This carbohydrate-rich diet is likely to have fitness benefits but it does not coincide exactly with the peaks of the nutritional landscapes of the reproductive traits measured in this study (see ‘Results’). These results, together with the findings of15,22 in D. melanogaster, provide evidence that males may have to compromise their diet balance intake, because there is not a unique diet capable of maximizing all male traits.
There have been several explanations for why males do not balance their diet to maximize their fitness traits (e.g.12,15). First, males might maximize a trait that has not been measured in the study15 or regulate nutrient intake to maximize multiple, competing traits16,17. However, males might also be constrained in their dietary balance for the expression of fitness traits because of their shared genome and dietary preferences with females (“sexual conflict over nutrition”), which might shift male choice to a diet balance that is not optimal for male’s fitness (e.g. see discussion in12). Sexual conflict over nutrition was proposed as one of the explanation for male non-optimal dietary balance in D. melanogaster15 (but see14), T. commodus12 and Gryllodes sigillatus23.
Dietary effects on the production of offspring
The data show that male carbohydrate intake in a P:C ratio of ~1:9 significantly maximizes both short-term offspring productivity after mating with virgin females, and the number wild-type offspring sired by males after mating with non-virgin females (Figs 3c and 4b). These results are consistent with the broader results of15, in which male D. melanogaster lifetime offspring siring increases with male carbohydrate intake. The mechanisms underlying the effects of macronutrient on offspring siring are not known, although several potential routes for this effect are possible. For instance, in adult male cockroaches Nauphoeta cinerea, carbohydrate intake in a P:C ratio of ~1:2 maximizes male sperm number16, showing that adult male carbohydrate can influence ejaculate components. In D. melanogaster, sex-peptide (SP), a seminal fluid protein present in the male ejaculate and known for increasing egg laying and reducing female receptivity39,40,41,42,43, binds to and is gradually released from sperm40,42,44,45. Therefore, it is possible that the effects found in our study might be linked directly to SP or sperm, or to an interaction between sperm number and SP transference and release. Other seminal fluid proteins could also be affected by male macronutrient intake. For instance, D. melanogaster ovulin is a peptide present in the male ejaculate that controls female egg laying in the first day after mating46,47, and a suite of other seminal proteins are required for long-term female post-mating responses48,49. If the expression of any of these proteins is under dietary regulation then diet will inevitably have consequences for male reproductive success.
We also found that high protein intake has a significantly strong negative effect on the total offspring productivity of their mates in the P1, but not P2, experiment. Although there are several mechanisms that can underlie intersexual effects of macronutrient intake, a change in ejaculate traits is again a likely candidate50. For instance, if male macronutrient intake alters the production or transference of SP, ovulin, or other proteins required for full female post-mating responses, this could ultimately affect female productivity (see discussion above). In addition, a surplus of protein intake has been hypothesised to be toxic for both males and females, even though it maximises female egg production11,12,13,15,51. Thus, high protein intake might impair male ejaculate production or reduce ejaculate quality, which in turn could reduce offspring production of females. These results also suggest an intersexual effect of male nutrition on the offspring production of females. Future studies should address whether male macronutrient intake can also influence offspring traits as well as number, and the potential underlying ejaculate mechanisms.
Dietary effects on sperm competitiveness
We found no evidence that the intake of macronutrient affected male sperm competitive ability, which was measured as the proportion of offspring sired by the focal males (i.e. P1 or P2) (see24 for similar approach). This is consistent with the previous findings in adult male D. melanogaster, which showed that yeast concentration (i.e. varying from 20% up to 200% of dietary yeast) did not have any effect on either P1 or P224. Nonetheless, diet can affect the proportion of offspring sired by males if changes in the availability of macronutrients occur during development. For instance, showed that larvae D. melanogaster reared in low yeast (i.e. 10% of the normal yeast content) developed into smaller adult males that had significantly lower P152. This may suggest a critical window for the development or maturation of male traits involved in sperm competition, in which the lack of essential macronutrients at this critical point affects male adult success in sperm competition, whereas adult dietary changes have a lesser influence on sperm competitiveness.
It should also be noted that in our study both the sperm competition and offspring production measures following female remating were neccesarily taken from females that successfully remated under controlled conditions (i.e. on the day following the initial mating), in both the P1 and P2 experiments. Thus, these females may be particularly susceptible to fast remating. It will be important in future studies to investigate the influences on overall male reproductive success when all females (not just those that remate the following day) are given opportunities to remate multiply, in free-mating conditions and over longer periods, to more closely reflect the conditions under which sexual selection acts in nature (as well as our lab-adapted populations).
Dietary effects on male pre-copulatory success and mating duration
The results suggest that male dietary requirements to maximize pre-copulatory success are different from the requirements for offspring siring and short-term productivity. We found that male carbohydrate intake has a significant negative effect on the duration of the 1st mating (with the focal male) in the P1 experiment, and that mating duration was maximized at low macronutrient intake (Fig. 3b). In addition, there was a marginal non-significant trend for the interaction of carbohydrate and protein to affect male pre-copulatory attractiveness to previously mated, unreceptive females, whereby males performed better under a high macronutrient with a P:C ratio of ~1:1 (Fig. 4a). Encountering unreceptive females is likely common to males D. melanogaster as females in this species are polyandrous53. Therefore, male attractiveness to unreceptive females is expected to play a significant role in determining male fitness. This is consistent with findings for the cockroach, Nauphoeta cinerea, for which a positive effect of carbohydrate intake on pheromone production and male attractiveness has been reported17. In males D. melanogaster, sexual attractiveness and female recognition are influenced by volatile pheromones and cuticular hydrocarbons54,55,56, and future studies will reveal whether male carbohydrate intake increases male sexual attractiveness by modulating the production of these compounds. Carbohydrate intake might also contribute to male energetic supply during courtship. For instance, male carbohydrate intake is positively associated with male calling effort in Teleogryllus commodus, which is a trait used by males to attract females, suggesting that dietary carbohydrate may be used as a direct source of energy for courtship12.It is important to note, however, that we only detected a marginally non-significant trend in this study, that the macronutrient effects on mating latency in the P2 experiment are for females that were willing to remate in the experiment (as with the sperm competition measures – see discussion above), and that we detected no significant effects of macronutrients on the proportion of females that remated (Table S4). Thus, the overall importance to male reproductive success of macronutrient intake effects on mating latency with previously mated females requires further investigation. We also found a non-significant trend for intermediate intake of protein (~500 μg) to increase male attractiveness to receptive (virgin) females in P:C ratios from 1:3 and 3:1. This result is consistent with the idea that male diet can influence pre-copulatory attractiveness to both virgin and previously mated females, and the data from both experiments are consistent with the idea that macronutrient intake has different effects on male pre- and post-copulatory traits.
It is important to note that the data in our experiment was taken from males under a specific set of conditions. Males were 8 days old at the time of measurements, and had been experimentally depleted of ejaculate prior to treatments. The effect of previous mating history on dietary effects and dietary choices remains to be tested, although there is currently no evidence that age alters the P:C ratio chosen by male D. melanogaster (see15). More generally it will be interesting for future studies to test whether flies make dynamic choices throughout their lives based on social experience, and whether there are affects of different types of social interactions (e.g. mating, male-male aggression) Furthermore, it will be important to determine whether the ability to choose diets per se influences male performance, by comparing individuals given diet choice versus no-choice.
The specific mechanisms that link male diet to the reproductive traits measured in this study remain to be elucidated. Some effects may be part of a more general behavioural and physiological response to diet. For example, non-optimal diets in general should reduce the pool of resources available to males to allocate to specific traits. This could potentially lead to an overall change in male locomotor activity which might explain the effects on copulation duration and the trends in latency to mating. Similarly, changes to post-copulatory traits might map onto a more general physiological response to diet. However, that fact that our results show macronutrient intake having effects on different traits (or no effects on some traits) suggests that the link between macronutrients and male reproductive traits are more complex than simply a non-specific loss of optimal condition under non-optimal diets. Our results support the idea that some traits are much more sensitive to diets than other traits, and that different diets maximise different traits.
Conclusions
Overall, our results show male dietary compromise, whereby male dietary choice does not coincide with specific male nutritional requirements for traits involved in pre- and post-copulatory sexual selection. Our study also supports previous findings on the strong effects of carbohydrate intake on male reproduction, which seems to be consistent across insect species12,15,16,17,23. This taxonomic consistency suggests a tentative possibility of a widespread effect of carbohydrate on male reproduction in invertebrates. It will be important to know how male nutrition affects the abundance of seminal fluid proteins in the ejaculate and its implications to male fitness, which in turn will likely affect the intensity and operation of sexual selection and sexual conflict. Most species of vertebrates also undergo pre- and post-copulatory sexual selection57,58,59, and future investigations should seek to reveal whether macronutrients have similar effects in these taxa.
Additional Information
How to cite this article: Morimoto, J. and Wigby, S. Differential effects of male nutrient balance on pre- and post-copulatory traits, and consequences for female reproduction in Drosophila melanogaster. Sci. Rep. 6, 27673; doi: 10.1038/srep27673 (2016).
Supplementary Material
Acknowledgments
We thank Fleur Ponton and Stephen J. Simpson for the help during the early stages of the experiment, and Eleanor Bath and Irem Sepil for the help during the experiment. JM is funded by a DPhil scholarship from the Brazilian National Council for Scientific and Technological Development (CNPq) and SW is funded by NERC (NE/J018937/1) and BBSRC (BB/K014544/1) fellowships.
Footnotes
Author Contributions J.M. and S.W. designed and performed the experiment, analysed the data and wrote the manuscript. J.M. prepared the figures and tables.
References
- Pizzari T. & Wedell N. The polyandry revolution. Philosophical Transactions of the Royal Society B: Biological Sciences 368, 10.1098/rstb.2012.0041 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G. A. & Pizzari T. In Current Perspectives on Sexual Selection 119–163 (Springer, 2015). [Google Scholar]
- Kvarnemo C. & Simmons L. W. Polyandry as a mediator of sexual selection before and after mating. Philosophical Transactions of the Royal Society B: Biological Sciences 368, 10.1098/rstb.2012.0042 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons L. W. Sperm Competition and its Evolutionary Consequences in the Insects. Monographs in Behavior and Ecology (2001). [Google Scholar]
- Simpson S. J. & Raubenheimer D. The nature of nutrition: a unifying framework from animal adaptation to human obesity. (Princeton University Press, 2012). [Google Scholar]
- Rowe L. & Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proceedings of the Royal Society B-Biological Sciences 263, 1415–1421, 10.1098/Rspb.1996.0207 (1996). [DOI] [Google Scholar]
- Powell J. D., Pollizzi K. N., Heikamp E. B. & Horton M. R. Regulation of immune responses by mTOR. Annu. Rev. Immunol. 30, 39, 10.1146/annurev-immunol-020711-075024 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantranupong L., Wolfson R. L. & Sabatini D. M. Nutrient-Sensing Mechanisms across Evolution. Cell 161, 67–83, 10.1016/j.cell.2015.02.041 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A., Comb W. C. & Sabatini D. M. Nutrient-sensing mechanisms and pathways. Nature 517, 302–310, 10.1038/nature14190 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns S. C. The evolution of life histories. Vol. 249 (Oxford University Press Oxford, 1992). [Google Scholar]
- Lee K. P. et al. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proceedings of the National Academy of Sciences 105, 2498–2503, 10.1073/pnas.0710787105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklakov A. A. et al. Sex-Specific Fitness Effects of Nutrient Intake on Reproduction and Lifespan. Curr. Biol. 18, 1062–1066, doi: http://dx.doi.org/10.1016/j.cub.2008.06.059 (2008). [DOI] [PubMed] [Google Scholar]
- Fanson B. G., Weldon C. W., Pérez-Staples D., Simpson S. J. & Taylor P. W. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni). Aging Cell 8, 514–523, 10.1111/j.1474-9726.2009.00497.x (2009). [DOI] [PubMed] [Google Scholar]
- Lee K. P., Kim J.-S. & Min K.-J. Sexual dimorphism in nutrient intake and life span is mediated by mating in Drosophila melanogaster. Anim. Behav. 86, 987–992, 10.1016/j.anbehav.2013.08.018 (2013). [DOI] [Google Scholar]
- Jensen K., McClure C., Priest N. K. & Hunt J. Sex-specific effects of protein and carbohydrate intake on reproduction but not lifespan in Drosophila melanogaster. Aging Cell, 10.1111/acel.12333 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunning H. et al. Protein and carbohydrate intake influence sperm number and fertility in male cockroaches, but not sperm viability. Proceedings of the Royal Society B: Biological Sciences 282, 20142144, 10.1098/rspb.2014.2144 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- South S. H., House C. M., Moore A. J., Simpson S. J. & Hunt J. Male cockroaches prefer a high carbohydrate diet that makes them more attractive to females: implications for the study of condition dependence. Evolution 65, 1594–1606, 10.1111/j.1558-5646.2011.01233.x (2011). [DOI] [PubMed] [Google Scholar]
- House C. M. et al. Macronutrient balance mediates the growth of sexually selected weapons but not genitalia in male broad‐horned beetles. Funct. Ecol., 10.1111/1365-2435.12567 (2015). [DOI] [Google Scholar]
- Mehlis M., Rick I. P. & Bakker T. C. M. Dynamic resource allocation between pre- and postcopulatory episodes of sexual selection determines competitive fertilization success. Proceedings of the Royal Society of London B: Biological Sciences 282, 10.1098/rspb.2015.1279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke T., David P. & Chapuis E. Environment-Dependent Sexual Selection: Bateman’s Parameters under Varying Levels of Food Availability. American Naturalist 185, 756–768, 10.1086/681128 (2015). [DOI] [PubMed] [Google Scholar]
- Devigili A., Evans J. P., Di Nisio A. & Pilastro A. Multivariate selection drives concordant patterns of pre-and postcopulatory sexual selection in a livebearing fish. Nature communications 6, 10.1038/ncomms9291 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddiex A. J., Gosden T. P., Bonduriansky R. & Chenoweth S. F. Sex-specific fitness consequences of nutrient intake and the evolvability of diet preferences. American Naturalist 182, 91–102, 10.1086/670649 (2013). [DOI] [PubMed] [Google Scholar]
- Rapkin J. et al. Macronutrient intake regulates sexual conflict in decorated crickets. J. Evol. Biol., 10.1111/jeb.12794 (2015). [DOI] [PubMed] [Google Scholar]
- Fricke C., Bretman A. & Chapman T. Adult Male Nutrition and Reproductive Success in Drosophila Melanogaster. Evolution 62, 3170–3177, 10.2307/25483551 (2008). [DOI] [PubMed] [Google Scholar]
- Snook R. R. Sperm in competition: not playing by the numbers. Trends Ecol. Evol. 20, 46–53, 10.1016/j.tree.2004.10.011 (2005). [DOI] [PubMed] [Google Scholar]
- Simpson S. & Raubenheimer D. A multi-level analysis of feeding behaviour: the geometry of nutritional decisions. Philosophical Transactions of the Royal Society B-Biological Sciences 342, 381–402, 10.1098/rstb.1993.0166 (1993). [DOI] [Google Scholar]
- Sirot L., Buehner N., Fiumera A. & Wolfner M. Seminal fluid protein depletion and replenishment in the fruit fly, Drosophila melanogaster: an ELISA-based method for tracking individual ejaculates. Behav. Ecol. Sociobiol. 63, 1505–1513, 10.1007/s00265-009-0806-6 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiblich A. et al. Bone morphogenetic protein- and mating-dependent secretory cell growth and migration in the Drosophila accessory gland. Proceedings of the National Academy of Sciences 109, 19292–19297, 10.1073/pnas.1214517109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linklater J. R., Wertheim B., Wigby S. & Chapman T. Ejaculate depletion patterns evolve in response to experimental manipulation of sex ratio in Drosophila melanogaster. Evolution 61, 2027–2034, 10.1111/j.1558-5646.2007.00157.x (2007). [DOI] [PubMed] [Google Scholar]
- Hihara F. Effects of the male accessory gland secretion on oviposition and remating in females of Drosophila melanogaster. Zoological Magazine 90, 307–316 (1981). [Google Scholar]
- Wigby S. et al. Seminal Fluid Protein Allocation and Male Reproductive Success. Curr. Biol. 19, 751–757, 10.1016/J.Cub.2009.03.036 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W., Duan H., Frei E. & Noll M. shaven and sparkling are mutations in separate enhancers of the Drosophila Pax2 homolog. Development 125, 2943–2950 (1998). [DOI] [PubMed] [Google Scholar]
- Fricke C., Martin O. Y., Bretman A., Bussière L. F. & Chapman T. Sperm competitive ability and indices of lifetime reproductive success. Evolution 64, 2746–2757, 10.1111/j.1558-5646.2010.01022.x (2010). [DOI] [PubMed] [Google Scholar]
- Clancy D. J. & Kennington W. J. A simple method to achieve consistent larval density in bottle cultures. Drosophila Information Service 84, 168–169 (2001). [Google Scholar]
- Ja W. W. et al. Prandiology of Drosophila and the CAFE assay. Proceedings of the National Academy of Sciences 104, 8253–8256, 10.1073/pnas.0702726104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretman A., Fricke C. & Chapman T. Plastic responses of male Drosophila melanogaster to the level of sperm competition increase male reproductive fitness. Proceedings of the Royal Society B-Biological Sciences 276, 1705–1711, 10.1098/Rspb.2008.1878 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edward D. A., Fricke C., Gerrard D. T. & Chapman T. Quantifying the Life-History Response to Increased Male Exposure in Female Drosophila Melanogaster. Evolution 65, 564–573, 10.1111/J.1558-5646.2010.01151.X (2011). [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R. Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ (2015).
- Avila F. W., Ravi Ram K., Bloch Qazi M. C. & Wolfner M. F. Sex Peptide Is Required for the Efficient Release of Stored Sperm in Mated Drosophila Females. Genetics 186, 595–600, 10.1534/genetics.110.119735 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T. et al. The sex peptide of Drosophila melanogaster: Female post-mating responses analyzed by using RNA interference. Proc. Natl. Acad. Sci. USA 100, 9923–9928, 10.1073/Pnas.1631635100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli E. Sex-peptides: seminal peptides of the Drosophila male. Cell. Mol. Life Sci. 60, 1689–1704, 10.1007/S00018-003-3052 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. F. & Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proceedings of the National Academy of Sciences 100, 9929–9933, 10.1073/Pnas.1631700100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigby S. & Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 15, 316–321, 10.1016/j.cub.2005.01.051 (2005). [DOI] [PubMed] [Google Scholar]
- Kubli E. & Bopp D. Sexual Behavior: How Sex Peptide Flips the Postmating Switch of Female Flies. Curr. Biol. 22, R520–R522, 10.1016/j.cub.2012.04.058 (2012). [DOI] [PubMed] [Google Scholar]
- Peng J. et al. Gradual Release of Sperm Bound Sex-Peptide Controls Female Postmating Behavior in Drosophila. Curr. Biol. 15, 207–213, doi: http://dx.doi.org/10.1016/j.cub.2005.01.034 (2005). [DOI] [PubMed] [Google Scholar]
- Herndon L. A. & Wolfner M. F. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proceedings of the National Academy of Sciences 92, 10114–10118 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz Y., Lung O., Frongillo E. A. Jr & Wolfner M. F. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr. Biol. 10, 99–102, doi: http://dx.doi.org/10.1016/S0960-9822(00)00288-8 (2000). [DOI] [PubMed] [Google Scholar]
- Ram K. R. & Wolfner M. F. Sustained Post-Mating Response in Drosophila melanogaster Requires Multiple Seminal Fluid Proteins. PLoS Genetics 3, e238, 10.1371/journal.pgen.0030238 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gligorov D., Sitnik J. L., Maeda R. K., Wolfner M. F. & Karch F. A novel function for the Hox gene Abd-B in the male accessory gland regulates the long-term female post-mating response in Drosophila. PLoS Genetics 9, e1003395, 10.1371/journal.pgen.1003395 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean A. J., Kopps A. M. & Bonduriansky R. Revisiting telegony: offspring inherit an acquired characteristic of their mother's previous mate. Ecol. Lett. 17, 1545–1552, 10.1111/ele.12373 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanson B. G. & Taylor P. W. Protein:carbohydrate ratios explain life span patterns found in Queensland fruit fly on diets varying in yeast:sugar ratios. Age (Dordr) 34, 1361–1368, 10.1007/s11357-011-9308-3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw L. A. et al. Larval rearing environment affects several post-copulatory traits in Drosophila melanogaster. Biol. Lett. 3, 607–610, 10.1098/Rsbl.2007.0334 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markow T. Evolution of Drosophila mating systems. Evol. Biol. 29, 73–106 (1996). [Google Scholar]
- Kurtovic A., Widmer A. & Dickson B. J. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446, 542–546, 10.1038/nature05672 (2007). [DOI] [PubMed] [Google Scholar]
- Datta S. R. et al. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature 452, 473–477, 10.1038/nature06808 (2008). [DOI] [PubMed] [Google Scholar]
- Ferveur J.-F. Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav. Genet. 35, 279–295 (2005). [DOI] [PubMed] [Google Scholar]
- Birkhead T. R. & Pizzari T. Postcopulatory sexual selection. Nature Reviews Genetics 3, 262–273, 10.1038/nrg774 (2002). [DOI] [PubMed] [Google Scholar]
- Birkhead T. Promiscuity: an evolutionary history of sperm competition. (Harvard University Press, 2000). [Google Scholar]
- Andersson M. Sexual Selection. Princeton University Press (1994). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.