Abstract
Objectives. Our previous study has used RNA-seq technology to show that apoptotic molecules were involved in the myocardial protection of electroacupuncture pretreatment (EAP) on the ischemia/reperfusion (I/R) animal model. Therefore, this study was designed to investigate how EAP protects myocardium against myocardial I/R injury through antiapoptotic mechanism. Methods. By using rats with myocardial I/R, we ligated the left anterior descending artery (LAD) for 30 minutes followed by 4 hr of reperfusion after EAP at the Neiguan (PC6) acupoint for 12 days; we employed arrhythmia scores, serum myocardial enzymes, and cardiac troponin T (cTnT) to evaluate the cardioprotective effect. Heart tissues were harvested for western blot analyses for the expressions of pro- and antiapoptotic signaling molecules. Results. Our preliminary findings showed that EAP increased the survival of the animals along with declined arrhythmia scores and decreased CK, LDH, CK-Mb, and cTnT levels. Further analyses with the heart tissues detected reduced myocardial fiber damage, decreased number of apoptotic cells and the protein expressions of Cyt c and cleaved caspase 3, and the elevated level of Endo G and AIF after EAP intervention. At the same time, the protein expressions of antiapoptotic molecules, including Xiap, BclxL, and Bcl2, were obviously increased. Conclusions. The present study suggested that EAP protected the myocardium from I/R injury at least partially through the activation of endogenous antiapoptotic signaling.
1. Introduction
Acute myocardial ischemia (AMI) is the most common cause of mortality and morbidity in the developed countries and rapidly becoming a common malady in the developing countries. Early and fast restoration of blood flow is the most ideal approach to prevent further tissue injury [1]. In fact, thrombolytic therapy via drug administration or primary percutaneous coronary intervention (PCI) is the most effective strategy to reduce the size of myocardial infarct and improve clinical outcome. Unfortunately, however, prompt reperfusion can also induce myocardial injury, and the phenomenon is termed myocardial ischemia/reperfusion injury (MIRI) [2].
The damage from reperfusion is triggered by the increased production of oxygen-free radicals at the time of reperfusion and the impaired antioxidant ability of the heart, leading to cell apoptosis and increased infarct size [3, 4]; this accounts for up to 50% of the final size of a myocardial infarct [5]. To reduce MIRI, various methods and drugs have been used in experimental and clinical studies [6], such as remote ischemic preconditioning [7], exenatide [8], and atorvastatin pretreatment [9]. However, effective therapies to prevent reperfusion injury have proven elusive. Despite an improved understanding of the pathophysiology of this process and encouraging preclinical trials of multiple agents, most of the clinical trials to reduce MIRI have yielded disappointing results [10, 11]. Adjunctive therapies and new treatments to limit reperfusion injury remain an active area of investigation.
Acupuncture has been practiced in China for over two thousand years as an effective approach to improve the symptoms of angina and palpitation by promoting physiologically endogenous protection system [12–15]. According to Chinese medicine theory, Neiguan acupoint (PC6) on the pericardium meridian is considered as the main acupoint for improving heart function and energy metabolism, promoting ischemia tolerance, eliminating free radical, and protecting against cell death [16–24]. Growing evidence, including our previous study, experimentally shows that EAP could alleviate I/R injury of brain and heart tissues [15, 25–27]. Simultaneously, EAP also plays protective roles against cardiac I/R injury in adult patients undergoing heart valve replacement surgery by reducing the level of serum cardiac troponin I, the inotrope score, and by shortening time spent in the intensive care unit [22], and also by reducing PCI-related myocardial injury [28]. However, detailed mechanisms have not been fully elucidated.
The importance of apoptosis in cell death following reperfusion has been demonstrated in in vivo rodent models, which also allows for the evaluation of pharmacological, growth factor-mediated, and genetic interventions [2, 6]. Our previous study has used RNA-seq technology to show that apoptotic molecules were involved in the myocardial protection of EAP at PC6 on the I/R animal model [27], suggesting that cardioprotective effect of EAP may be closely related to antiapoptotic signaling. Therefore, the aims of our present study are to determine, through rat I/R models, whether EAP protects myocardium, along with its antiapoptotic mechanism. The results show that EAP could regulate both pro- and antiapoptotic molecules, suggesting that EAP might be an appropriate alternative treatment for myocardial ischemia patients who will be undergoing a thrombolytic therapy or primary percutaneous coronary intervention.
2. Methods
2.1. Antibodies and Reagents
Antibodies for Cyt c, Smac/Diablo, HtrA2/Omi, Endo G, AIF, caspase 3, cleaved caspase 3, Xiap, BclxL, and Bcl2 were obtained from cell signaling. Antibodies for GAPDH were purchased from Abcam (Cambridge, UK). For assessing apoptosis, the in situ cell death detection kit, POD (TUNEL) was obtained from Roche (Lewes, UK).
2.2. Animals and Grouping
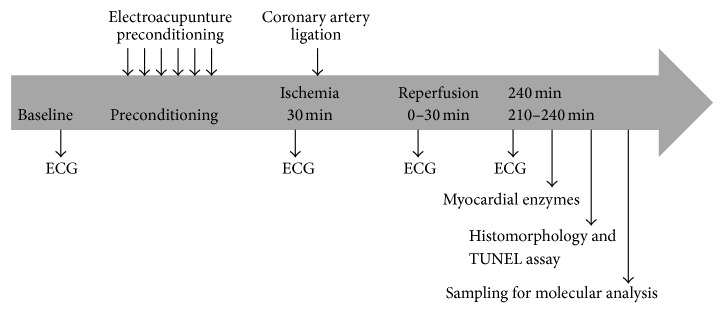
Male adult Sprague-Dawley (SD) rats (280 ± 20 g), supplied by the Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China), were randomly divided into six groups after adaptive feeding for a week: sham operation control group (SO), EAP at PC6 + SO (ES), EAP at nonacupoint + SO (NS), myocardial ischemia/reperfusion model group (I/R), EAP at PC6 + I/R (EA), and EAP at nonacupoint + I/R (NA). A timeline of the study is shown in Figure 1. The study was approved by the Institute for Animal Care and Use Committee at Nanjing University of Chinese Medicine, and all experimental procedures were designed and conducted according to the Guide for the Care and Use of Laboratory Animals published by National Institutes of Health (NIH Publication Number 80-23, revised 1996).
Figure 1.
The timeline of the experimental process. Six preconditioning groups of rats were used (n = 15/each group). ECG indicates electrocardiogram.
2.3. EAP Intervention
Prior to the MIRI experiment, rats among ES, NS, EA, and NA groups were pretreated with electroacupuncture for a total of 12 days in the waking state, with one day of rest after six consecutive days of treatment. Both EA and ES groups were pretreated at the PC6, located in the forelimbs [25]. Two acupuncture needles were separately inserted into the PC6 located on each upper limb which were located at a point 1.5 cm proximal to the palm crease just above the median nerve as previously described [16, 25], and an electrical current was provided to the needles for a period of 20 minutes through an electrical stimulator with a stimulus isolation unit (Han Acuten, WQ1002F, Beijing, China) at 2/15 Hz, with an intensity level of 1 mA [20]. The acupuncture needle, 7 mm long and 0.16 mm in diameter, was inserted 2-3 mm into the subcutis. In the NA and NS groups, the same pretreatments were applied at nonacupoint, located at the junction between the tail and buttock [27, 29]. Rats in SO and I/R groups were restrained in tubes the same as others groups for 20 min daily for 12 days. The electroacupuncture procedure was carried out by a specialized acupuncturist.
2.4. In Vivo MIRI
Rats that had accepted 12 sessions of EAP were anesthetized with mechanical ventilation using 5% isoflurane and maintained by inhalation of 1-2% isoflurane in 100% oxygen with a mixture of 70% N2O and 30% O2 after endotracheal intubation. Tidal volume and respiratory rate were set at 1.0 mL/100 g body weight and 45–60 breaths per minute, respectively. Adequate anesthesia was ensured by monitoring heart rate and the absence of a withdrawal response to a paw pinch. Lead II electrocardiogram (ECG) was monitored and recorded. The left carotid artery was cannulated for blood pressure measurement. Body temperature was maintained with a servo-controlled heating pad at 37°C.
The heart was exposed via a left thoracotomy between the 4th and 5th intercostal space. Following pericardiotomy, the left anterior descending (LAD) coronary artery was ligated with a 6.0 silk suture for 30 min to induce ischemia, followed by 240 min of reperfusion [29]. Successful coronary artery occlusion was confirmed by elevation of the ST segment in the ECG and an immediate 15–30 mmHg fall in arterial blood pressure. The chest was closed 30 min after the LAD was reperfused, and the rats were kept warm and allowed to recover. Buprenorphine HCl (0.05 mg/kg) was employed to minimize pain and distress by intramuscular injection after operation immediately [30].
2.5. Electrocardiogram (ECG) Recording and Scoring of Arrhythmia
A standard limb lead II ECG was successively monitored and recorded before, during myocardial ischemia, and after reperfusion under anesthesia by the use of a computerized PowerLab system (ADInstruments, Australia), and then monitored again from 210 min to 240 min under anesthesia [27]. The arrhythmias were assessed during the 30 min period of ischemia and the 30 min of reperfusion. The arrhythmia scores were accessed by an electrocardiogram specialist physician and assigned as described previously [27] according to the system described by Curtis and Walker [31] as follows: 0 = no arrhythmia; 1 ⩽ 10 s premature ventricular contraction (PVC) and/or ventricular tachycardia (VT); 2 = 11–30 s PVC and/or VT; 3 = 31–90 s PVC and/or VT; 4 = 91–180 s PVC and/or VT or reversible ventricular fibrillation (VF) of <10 s; 5 ⩾ 180 s PVC and/or VT, >10 s reversible VF; and 6 = irreversible VF. In addition, the survival rate of animals was also calculated.
2.6. Myocardial Enzyme Analysis
Following a four-hour reperfusion period, blood was collected from the jugular vein and centrifuged (10,000 g, 10 minutes, and 4°C). Serum lactate dehydrogenase (LDH), creatine kinase (CK) and creatine kinase-MB (CK-MB) levels were analyzed with a biochemistry analyzer. Plasma was extracted and analyzed for cTnT levels by electrochemiluminescence immune-assay method. All the myocardial enzyme detections were performed by the Medical laboratory of Jiangsu Province Hospital (Nanjing, China).
2.7. Histological and Morphological Analyses
After sacrificing the animals through intravenous injection of high-dose (150 mg/kg) of pentobarbitone at the end of the experiment, cardiac tissues were fixed in 4% paraformaldehyde (Sigma-Aldrich, Inc.) and embedded in paraffin. The embedded tissues were sectioned and stained with TUNEL assay kit as described previously [32, 33], which was employed to observe the positive apoptotic cells in myocardial tissue.
2.8. Apoptotic Molecules Analysis
After sacrificing the rats, the hearts were quickly removed and placed into liquid nitrogen. Then, total proteins were extracted for the further western blot (WB) assays. Our study has shown that electroacupuncture at PC6 could change gene expression profiles of I/R heart, and that EAP can markedly modify the expression levels of numerous genes involved in apoptotic pathways [27]. Therefore, in this study, we examined the protein expression of caspase 3, cytochrome c (Cyt c), Smac/Diablo, HtrA2/Omi, Endo G, AIF, Bcl2, BclxL, and Xiap to evaluate the status of apoptotic signaling.
2.9. Western Blot Analysis
WB was performed as described previously [34]. Briefly, after detecting the concentrations by the BCA protein assay (Pierce), equivalent amounts of protein (30 μg/lane) were separated by SDS-polyacrylamide gel electrophoresis and transferred onto PVDF membranes. Each PVDF membranes were incubated with the appropriate primary antibodies anti-Cyt c (1 : 1000), anti-Smac/Diablo (1 : 1000), anti-HtrA2/Omi (1 : 1000), anti-Endo G (1 : 1000), anti-AIF (1 : 1000), anti-caspase 3 (1 : 1000), anti-cleaved caspase 3 (1 : 1000), anti-Xiap (1 : 1000), anti-BclxL (1 : 1000), anti-Bcl2 (1 : 1000), and anti-GAPDH (1 : 2000) and followed by incubation with peroxidase-conjugated secondary antibodies. Proteins were quantified using the SuperSignal West Pico Chemiluminescent substrate (Pierce).
2.10. Statistical Analysis
All values were expressed as a mean ± standard deviation (SD). Statistical analyses were performed using SPSS 17.0. Multiple group comparisons were performed by one-way ANOVA. Tukey's procedure was used for multiple-range tests. Differences were considered significant when P < 0.05.
3. Results
3.1. EAP at PC 6 Protected Rat Myocardium from MIRI
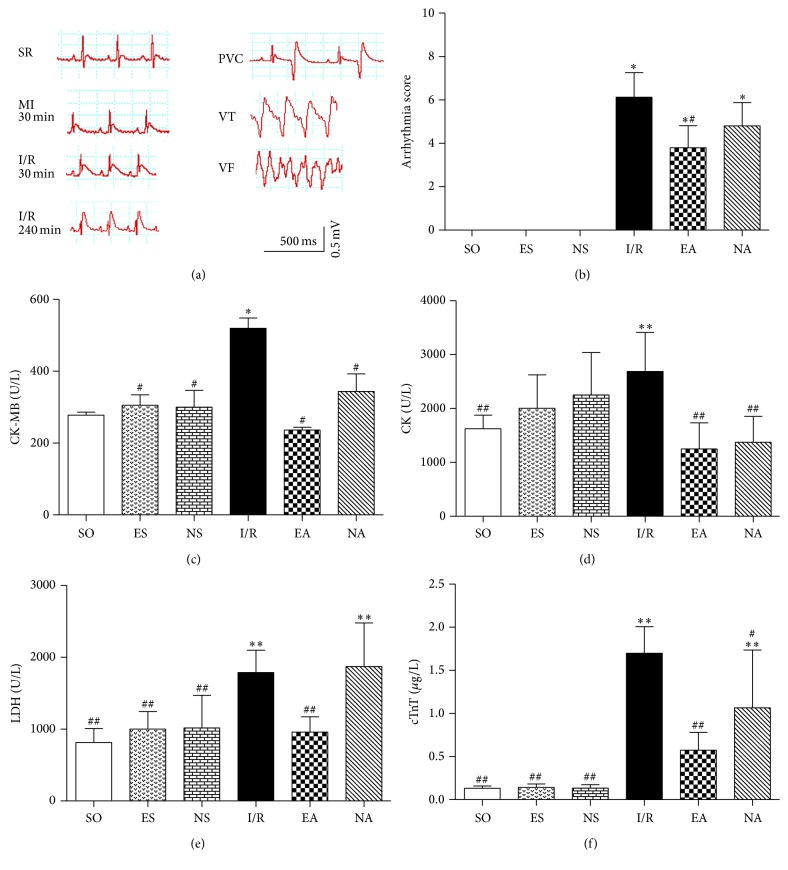
To investigate protective effects of EAP at the PC6 on MIRI, we first observed ECG's ST segments, arrhythmic scores, and serum enzyme levels. The ECG recordings showed that the ST segments were noticeably elevated in I/R, EA, and NA groups during the 30 min after myocardial ischemia (MI) operation, and premature ventricular contraction (PVC), ventricular tachycardia (VT), and ventricular fibrillation (VF) were observed during the four-hour reperfusion period (Figure 2(a)), suggesting a successful I/R model. The arrhythmia scores were decreased in both EA and NA groups, especially in EA group compared to that in the I/R group (Figure 2(b)). The (LDH, levels of serum enzymes CK, CK-Mb, and cTnT), which reflect acute myocardial damage, significantly increased in the I/R group compared to the SO group, but returned to the levels of the SO group after EAP (P < 0.05) (Figures 2(c)–2(f)). Meanwhile, the survival rate in the EA group increased significantly to above 70%, compared to 50% in the I/R group (Figure S1 in Supplementary Material available online at http://dx.doi.org/10.1155/2016/4609784).
Figure 2.
Effects of EAP on MIRI. (a) Representative ECG recording in standard limb lead II. PVC, premature ventricular contraction, VT, ventricular tachycardia, and VF, ventricular fibrillation. (b) Quantitative analysis for arrhythmia sores in each group. (c–f) CK, CK-Mb, LDH, and cTnT levels by biochemistry analyzer after myocardial I/R experiment in each group. Data were expressed as mean ± SD, n = 8–15/each group. ∗ P < 0.05 and ∗∗ P < 0.01 versus SO group; # P < 0.05 and ## P < 0.01 versus I/R group.
3.2. EAP at PC6 Promoted Survival of Cardiomyocytes against I/R Injury
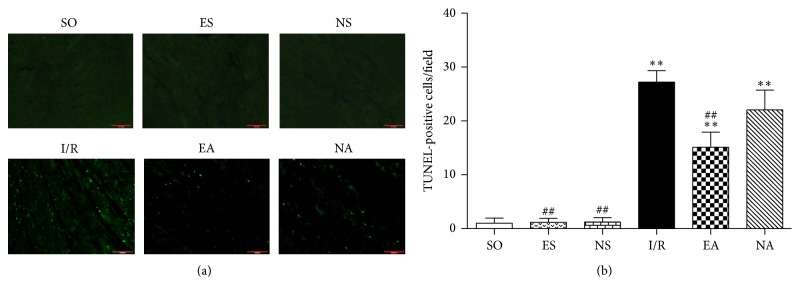
Cardiomyocyte survival is a vital factor for cardiac function. In the face of I/R injury, the myocardium has the ability to develop numerous strategies to evade apoptosis. Expectedly, our study detected noticeably increased TUNEL-positive cells in the I/R heart, but the number of these cells was significantly decreased in the EA group (Figures 3(a) and 3(b)). Meanwhile, we also assessed the extent of myocardial fiber damage by ferroalumen hematoxylin staining [35, 36]. Our results showed that injury of myocardial fibers was obvious in I/R group, in which the cells were stained black. Myocardial cells from the EA group, however, did not pick up the stain (Figure S2).
Figure 3.
EAP promoted cardiomyocyte survival. (a) Representative images of TUNEL staining of each group. Green dots indicate TUNEL-positive cells. (b) Quantification of TUNEL-positive cells in each group. Data were expressed as mean ± SD, n = 8–15/each group. ∗∗ P < 0.01 versus SO group; ## P < 0.01 versus I/R group.
3.3. EAP at PC6 Promotes Antiapoptotic Signaling and Inhibits Proapoptotic Signaling
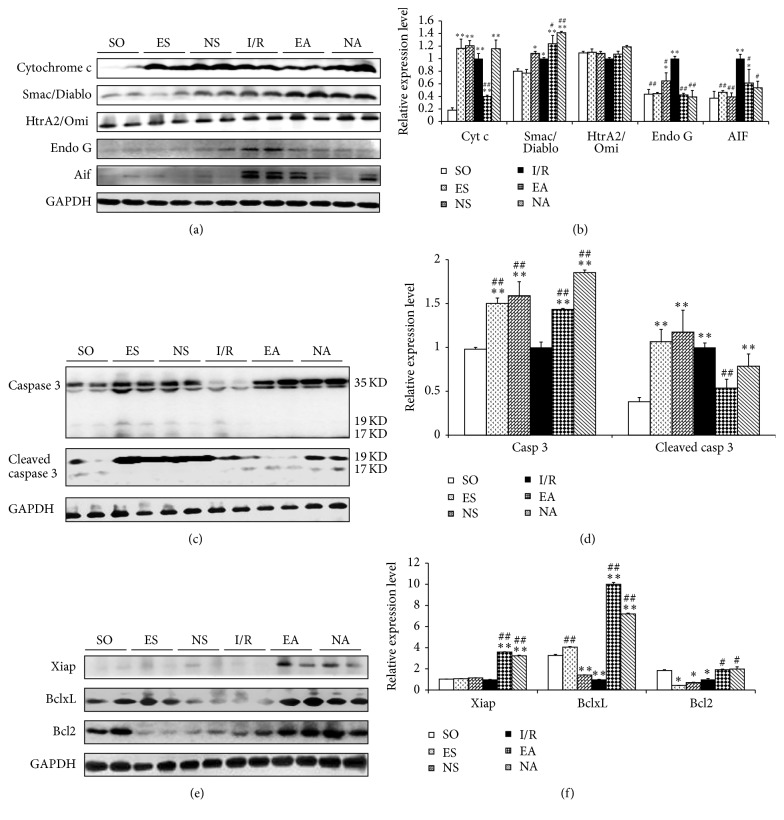
Apoptosis plays an important role in lethal reperfusion injury as indicated by numerous studies in various animal models [6, 37]. To further verify what we observed in the previous study by RNA-seq that apoptosis is a possible mechanism relevant to the myocardioprotective effects of EAP, caspase-dependent and caspase-independent apoptotic signaling in the heart tissues was examined by western blotting for caspase 3, Cyt c, Smac/Diablo, HtrA2/Omi, Endo G, and AIF. As shown in Figures 4(a) and 4(b), the expression of proteins of Cyt c, Endo G, and AIF increased in the I/R group but markedly decreased in the EA group (P < 0.05), suggesting an antiapoptotic mechanism involved in the cardioprotective effects of EAP against I/R injury. Interestingly, though the expression level of total caspases 3 was decreased in the I/R heart and EAP increased it significantly, the level of cleaved caspase 3, a biologically active caspase 3 enzyme, was elevated in the I/R group, and was evidently decreased in the EA group (P < 0.05, Figures 4(c) and 4(d)). Similarly, we detected reduction in antiapoptotic molecules, including Xiap, BclxL, and Bcl2 upon I/R injury, and a significant increase in these proteins after EAP (P < 0.05, Figures 4(e) and 4(f)).
Figure 4.
EAP regulated apoptotic signaling. Representative western blot results of proapoptotic, antiapoptotic, and histone acetylation proteins in each group ((a), (c), and (e)). Quantitative analysis of proapoptotic, antiapoptotic, and histone acetylation proteins in each group ((b), (d), and (f)). Data were expressed as mean ± SD, n = 8–15/each group. ∗ P < 0.05 and ∗∗ P < 0.01 versus SO group; # P < 0.05 and ## P < 0.01 versus I/R group.
4. Discussion
Acupuncture at PC6 is generally prescribed for the treatment of heart and chest disease symptoms, such as palpitation, chest distress, thoracalgia, gastralgia, and nausea and vomiting, based on the theory of Chinese medicine. Previous research has indicated that acupuncture at PC6 acupoint can attenuate cardiac injury, through reducing arrhythmias, apoptosis, and myocardial enzymes [16, 18, 20, 38, 39]. Our previous studies had demonstrated that electroacupuncture could effectively promote angiogenesis and protect myocardial tissue against ischemia injury [33]. EAP, one of acupuncture treatment approaches, has been demonstrated to be protective against I/R injury in brain or heart of the animal models [16, 25, 26, 40, 41]. The cardioprotective effects of EAP in patients undergoing heart valve replacement surgery or PCI operation have also been proved in a randomized controlled trial [22, 28], but the mechanism remains unclear.
As we all know, myocardial injury is often accompanied by changes in ECG and serum enzymes. ST segment elevation and heart rate disorders appear to be the most typical in the ECG detection. And the levels of serum enzymes (LDH, CK, and CK-Mb), especially cTnT [42], reflect acute myocardial damage effectively [43]. Our study, by using rat I/R model, suggested that EAP increased survival rate, reduced the ST segment, the arrhythmia score, and the release of serum enzyme from myocardium, alleviated myocardial fiber damage, and reduced the infarct size of MIRI measurement by TTC staining (the corresponding period results have published) [27], therefore protecting myocardial cells from I/R injury. These findings were consistent with the previous studies [16, 25, 37].
Our previous study and the literature have demonstrated numerous mechanisms that may contribute to MIRI [5, 27, 41–46], such as necrosis, apoptosis, and dysfunction of organelles. Apoptosis has been known to play an important role in the initial stages of reperfusion injury and serve as a mechanism of cellular self-destruction for a variety of processes [36]. Our data indicated a marked decrease in the number of TUNEL-positive cells as a result of EAP, and the extent of myocardial fiber damage was relieved in the EA group compared with the I/R group (Figure S2). The results suggested that EAP can effectively protect the myocardial cells from death, consistent with our previous studies on the reduction of myocardial infarction area by EAP [27]. Apoptosis is initiated by unbalancing pro- and antiapoptotic machineries [47–49]. Many studies have demonstrated the importance of caspase-dependent cell death pathways in injuries or diseases [50]. The most widely studied form of intrinsic apoptosis is stress-mediated release of Cyt c from the mitochondria that results in the formation of the apoptosome, which leads to the activation of the executioner caspase 3 [50]. The process may be regulated by Smac/Diablo and HtrA2/Omi. Meanwhile, it has also been discovered that in response to apoptotic stimuli, mitochondria can release caspase-independent cell death effectors such as apoptosis inducing factor (AIF) and Endonuclease G (Endo G) [51], resulting in caspase-independent apoptosis. In the caspase-dependent pathway, cleaved caspase 3-mediated signaling, such as Cyt c, Smac/Diablo, and HtrA2/Omi, directly activates cell death, whereas caspase-independent pathway, mainly including Endo G and AIF, causes cell death through DNA cleavage [52]. Our results showed that EAP decreased the Cyt c level and indirectly inhibited caspase 3 activation. EAP can also promote the expression of Smac/Diablo but not HtrA2/Omi, which indirectly negatively regulates caspase 3 via the inhibitors of apoptosis proteins (IAPs) [50, 53]. Additionally, this is, to the best of our knowledge, the first report of EAP exerting a protective role on myocardial I/R injury through inhibition of the expression of Endo G and AIF. As reported in other studies, the mitochondrial permeability transition pore (MPTP) is a central player of cell fate and is responsible for mitochondrial swelling with the consequent release of proapoptotic factors (i.e., Cyt c, AIF, and Smac/Diablo) [54]. When a large population of mitochondria is involved, these organelles are subjected to swelling, which leads to the release of the apoptotic factors [55]. In the present study, we demonstrated that EAP significantly decreased the opening of MPTP (Figure S3), therefore reducing the release of proapoptotic factors from the I/R heart. Unexpectedly, increased proteins expression levels of some proapoptotic molecules (Cyt c and cleaved caspase 3) and decreased antiapoptotic proteins (Xiap and Bcl2) were observed in the ES and NS groups, at a similar pattern in the I/R group. This observation needs to be addressed mechanistically in our future study, but it suggests that needling at nonacupoint might be also stimulation for the body to respond to certain extent, though the effectiveness of NA was not significant. This result is consistent with our previous finding [27].
The death of a cell was associated with autophagy and MAPK signaling. Early studies have indicated that apoptosis is caused by the activation of an autophagic process, which could be inhibited by chemical inhibitors of autophagy, and it was shown to depend on the autophagic genes APG5 and beclin [56]. We detected decreased beclin1 protein level in the heart treated by EAP (Figure S4). We also observed activated signal transduction of mitogen-activated protein kinases (MAPKs) in myocardial tissue, which had been reported as an important mediator of ischemic-related events [57]. The levels of p-JNK, p-P38, p-P44/42, and Ras were upregulated, and p-P38 level was downregulated in the I/R group, and all of them were reversed by undergoing EAP (Figure S5). Furthermore, to a certain extent, the EAP at nonacupoint also results in similar changes at molecular level as seen in the EA group (Figure 4), but its cardiprotective effect was inferior to the EA group. This is consistent with our previous observation [27], in which we described that EAP at nonacupoint modified less functional pathways, though it regulated some gene expressions to certain extent. The detailed mechanisms of different gene and protein expression patterns resulting from pretreatment on myocardial I/R injury using acupuncture at acupoint and nonacupoint remain to be investigated.
Interestingly, EAP on the sham operation rats resulted in increased expression of cleaved caspase 3, Cyt c, and Ras. However, these changes were not correlated with any pathological phenotype even though the levels of these proteins were as high as those in the I/R rats. This finding also is consistent with our previous study on ischemia-induced myocardial injury [27]. This phenomenon suggested that EAP might produce a similar stimulation on the heart, as does myocardial injury, mimicking an ischemic preconditioning or remote conditioning [44, 58–60]. A further study might be of significance to confirm this observation and explore its mechanism. Meanwhile, the experimental model is affected by a phenomenon called “nonreflow,” which can influence the results [61]. The nonreflow is a condition where there is a collapse of the blood vessel even after coronary artery opening, and it can limit the results. Therefore, it is necessary to pay more attention to the nonreflow in the follow-up study.
5. Conclusions
In summary, the present study demonstrates that EAP effectively protects the myocardium from I/R injury. And suppression of proapoptosis and promotion of antiapoptosis contribute to this effect. Thus, EAP could be an appropriate alternative treatment for patients with MI who will receive thrombolytic therapy or primary percutaneous coronary intervention.
Supplementary Material
To investigate protective effects of EAP on MIRI, we evaluated rat survival rate and myocardial fiber injury. Our results showed that in the EA group, the survival rate was increased significantly (Fig S1), and the extent of myocardial fiber damage was relieved compared with the I/R group (Fig S2). Meanwhile, to assess the relationship between pro-apoptotic factors and the mitochondrial permeability transition pore (MPTP), we observed that EAP significantly decreased the opening of MPTP (Fig S3), therefore reduced the release of pro-apoptotic factors from the IR heart. In additional, we detected decreased beclin1 protein expression in the heart (Fig S4) and increased activity of signal transduction of mitogen-activated protein kinases (MAPKs) (Fig S5) after EA pre-treatment.
Figure S1 The survival rate of rats in each group (n=15). The survival number of each group was recorded after operation, and the survival rate of each group was calculated with the formula: (survival rat number/the total rat number) ×100%.
Figure S2 EAP at PC6 protected myocardial fibers against I/R injury. These images represent ferroalumen hematoxylin staining for myocardial fibers in each group.
Figure S3 EAP at PC6 decreased the opening of mitochondrial permeability transition pore. Mitochondrial PTP opening was assayed by fluorescence spectrophotometer, and data were expressed as means±SD, n=8-15 /each group. ∗, P<0.05vs. SO group; #, P<0.05 vs. I/R group.
Figure S4 EAP at PC6 decreased autophage-associated beclin1 expression level. A. Representative western blot results of beclin 1 proteins in each group. B. Quantitative analysis of beclin 1 protein in each group. Data were expressed as means±SD, n=8-15/ each group. ∗, P<0.05 vs. SO group; #, P<0.05 vs. I/R group.
Figure S5 EAP at PC6 influenced the expression levels of MAPK signaling. A. Representative western blot results of p-JNK, p-P38, p-P44/42, and Ras proteins in each group. B. Quantitative analysis of p-JNK, p-P38, p-P44/42, and Ras proteins in each group. Data were expressed as means ± SD, n=8-15/ each group. ∗, P<0.05 vs. SO group; #, P<0.05 vs. I/R group.
Acknowledgments
This work was supported by grants from the National Basic Research program of China (973 Program, no. 2012CB518501), the National Natural Science Foundation of China (nos. 81574062, 81574063, 81273838, and 81403478), and Natural Science Foundation of Jiangxi province (no. BK20151569).
Competing Interests
The authors declare that there are no competing interests.
Authors' Contributions
Bing-mei Zhu, Sheng-feng Lu, and Yan Huang conceived and designed the experiments. Yan Huang, Sheng-feng Lu, Wei-xing Shen, Shu-ping Fu, Qian Li, Xia Chen, and Xin-yue Jing performed the experiments. Bing-mei Zhu, Yan Huang, Sheng-feng Lu, and Ning Wang analyzed the data. Sheng-feng Lu, Bing-mei Zhu, Mei-ling Yu, and Wan-xin Liu wrote the paper. All authors read and approved the final paper. Sheng-feng Lu and Yan Huang contributed equally to this work.
References
- 1.Anderson J. L., Marshall H. W., Bray B. E., et al. A randomized trial of intracoronary streptokinase in the treatment of acute myocardial infarction. The New England Journal of Medicine. 1983;308(22):1312–1318. doi: 10.1056/nejm198306023082202. [DOI] [PubMed] [Google Scholar]
- 2.Frank A., Bonney M., Bonney S., Weitzel L., Koeppen M., Eckle T. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Seminars in Cardiothoracic and Vascular Anesthesia. 2012;16(3):123–132. doi: 10.1177/1089253211436350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrosio G., Zweier J. L., Duilio C., et al. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. The Journal of Biological Chemistry. 1993;268(25):18532–18541. [PubMed] [Google Scholar]
- 4.Marczin N., El-Habashi N., Hoare G. S., Bundy R. E., Yacoub M. Antioxidants in myocardial ischemia-reperfusion injury: therapeutic potential and basic mechanisms. Archives of Biochemistry and Biophysics. 2003;420(2):222–236. doi: 10.1016/j.abb.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Yellon D. M., Hausenloy D. J. Myocardial reperfusion injury. The New England Journal of Medicine. 2007;357(11):1121–1135. doi: 10.1056/nejmra071667. [DOI] [PubMed] [Google Scholar]
- 6.Eefting F., Rensing B., Wigman J., et al. Role of apoptosis in reperfusion injury. Cardiovascular Research. 2004;61(3):414–426. doi: 10.1016/j.cardiores.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Thielmann M., Kottenberg E., Kleinbongard P., et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. The Lancet. 2013;382(9892):597–604. doi: 10.1016/s0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- 8.Woo J. S., Kim W., Ha S. J., et al. Cardioprotective effects of exenatide in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33(9):2252–2260. doi: 10.1161/atvbaha.113.301586. [DOI] [PubMed] [Google Scholar]
- 9.Chen M., Li H., Wang Y. Protection by atorvastatin pretreatment in patients undergoing primary percutaneous coronary intervention is associated with the lower levels of oxygen free radicals. Journal of Cardiovascular Pharmacology. 2013;62(3):320–324. doi: 10.1097/FJC.0b013e31829be05b. [DOI] [PubMed] [Google Scholar]
- 10.Bolli R., Becker L., Gross G., Mentzer R., Jr., Balshaw D., Lathrop D. A. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circulation Research. 2004;95(2):125–134. doi: 10.1161/01.res.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 11.Cannon R. O., III Mechanisms, management and future directions for reperfusion injury after acute myocardial infarction. Nature Clinical Practice Cardiovascular Medicine. 2005;2(2):88–94. doi: 10.1038/ncpcardio0096. [DOI] [PubMed] [Google Scholar]
- 12.Xu F. H., Wang J. M. Clinical observation on acupuncture combined with medication for intractable angina pectoris. Zhongguo Zhen Jiu. 2005;25(2):89–91. [PubMed] [Google Scholar]
- 13.Hu J. Acupuncture treatment of palpitation. Journal of Traditional Chinese Medicine. 2008;28(3):228–230. doi: 10.1016/S0254-6272(08)60052-7. [DOI] [PubMed] [Google Scholar]
- 14.Meng J. The effects of acupuncture in treatment of coronary heart diseases. Journal of Traditional Chinese Medicine. 2004;24(1):16–19. [PubMed] [Google Scholar]
- 15.Cao J. P. The basis for acupuncture treatment of coronary heart disease is humoral factors-mediated favorable regulation. Zhen Ci Yan Jiu. 2006;31(3):185–189. [Google Scholar]
- 16.Gao J., Zhang L., Wang Y., et al. Antiarrhythmic effect of acupuncture pretreatment in rats subjected to simulative global ischemia and reperfusion—involvement of adenylate cyclase, protein kinase A, and L-type Ca2+ channel. Journal of Physiological Sciences. 2008;58(6):389–396. doi: 10.2170/physiolsci.rp007108. [DOI] [PubMed] [Google Scholar]
- 17.Li J., Li J., Liang F., et al. Electroacupuncture at PC6 (Neiguan) improves extracellular signal-regulated kinase signaling pathways through the regulation of neuroendocrine cytokines in myocardial hypertrophic rats. Evidence-Based Complementary and Alternative Medicine. 2012;2012:9. doi: 10.1155/2012/792820.792820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lujan H. L., Kramer V. J., DiCarlo S. E. Electroacupuncture decreases the susceptibility to ventricular tachycardia in conscious rats by reducing cardiac metabolic demand. American Journal of Physiology—Heart and Circulatory Physiology. 2007;292(5):H2550–H2555. doi: 10.1152/ajpheart.00979.2006. [DOI] [PubMed] [Google Scholar]
- 19.Ni X., Xie Y., Wang Q., et al. Cardioprotective effect of transcutaneous electric acupoint stimulation in the pediatric cardiac patients: a randomized controlled clinical trial. Paediatric Anaesthesia. 2012;22(8):805–811. doi: 10.1111/j.1460-9592.2012.03822.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsou M.-T., Huang C.-H., Chiu J.-H. Electroacupuncture on PC6 (Neiguan) attenuates ischemia/reperfusion injury in rat hearts. American Journal of Chinese Medicine. 2004;32(6):951–965. doi: 10.1142/S0192415X04002557. [DOI] [PubMed] [Google Scholar]
- 21.Wang S.-B., Chen S.-P., Gao Y.-H., Luo M.-F., Liu J.-L. Effects of electroacupuncture on cardiac and gastric activities in acute myocardial ischemia rats. World Journal of Gastroenterology. 2008;14(42):6496–6502. doi: 10.3748/wjg.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L., Yang J., Wang Q., et al. cardioprotective effects of electroacupuncture pretreatment on patients undergoing heart valve replacement surgery: a randomized controlled trial. Annals of Thoracic Surgery. 2010;89(3):781–786. doi: 10.1016/j.athoracsur.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H., Liu L., Huang G., et al. Protective effect of electroacupuncture at the Neiguan point in a rabbit model of myocardial ischemia-reperfusion injury. Canadian Journal of Cardiology. 2009;25(6):359–363. doi: 10.1016/s0828-282x(09)70095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin Z., Liang J., Wang J., Kolattukudy P. E. Delayed brain ischemia tolerance induced by electroacupuncture pretreatment is mediated via MCP-induced protein 1. Journal of Neuroinflammation. 2013;10, article 63 doi: 10.1186/1742-2094-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao J., Fu W., Jin Z., Yu X. Acupuncture pretreatment protects heart from injury in rats with myocardial ischemia and reperfusion via inhibition of the β1-adrenoceptor signaling pathway. Life Sciences. 2007;80(16):1484–1489. doi: 10.1016/j.lfs.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Li X., Luo P., Wang Q., Xiong L. Electroacupuncture pretreatment as a novel avenue to protect brain against ischemia and reperfusion injury. Evidence-Based Complementary and Alternative Medicine. 2012;2012:12. doi: 10.1155/2012/195397.195397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y., Lu S.-F., Hu C.-J., et al. Electro-acupuncture at Neiguan pretreatment alters genome-wide gene expressions and protects rat myocardium against ischemia-reperfusion. Molecules. 2014;19(10):16158–16178. doi: 10.3390/molecules191016158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q., Liang D., Wang F., et al. Efficacy of electroacupuncture pretreatment for myocardial injury in patients undergoing percutaneous coronary intervention: a randomized clinical trial with a 2-year follow-up. International Journal of Cardiology. 2015;194:28–35. doi: 10.1016/j.ijcard.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 29.Hwang H. S., Kim Y. S., Ryu Y. H., et al. Electroacupuncture delays hypertension development through enhancing NO/NOS activity in spontaneously hypertensive rats. Evidence-Based Complementary and Alternative Medicine. 2011;2011:7. doi: 10.1093/ecam/nen064.130529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birnbaum Y., Ye Y., Rosanio S., et al. Prostaglandins mediate the cardioprotective effects of atorvastatin against ischemia-reperfusion injury. Cardiovascular Research. 2005;65(2):345–355. doi: 10.1016/j.cardiores.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Curtis M. J., Walker M. J. A. Quantification of arrhythmias using scoring systems: an examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovascular Research. 1988;22(9):656–665. doi: 10.1093/cvrese/22.9.656. [DOI] [PubMed] [Google Scholar]
- 32.Labat-Moleur F., Guillermet C., Lorimier P., et al. TUNEL apoptotic cell detection in tissue sections: critical evaluation and improvement. Journal of Histochemistry and Cytochemistry. 1998;46(3):327–334. doi: 10.1177/002215549804600306. [DOI] [PubMed] [Google Scholar]
- 33.Tian Y., Zhang W., Xia D., Modi P., Liang D., Wei M. Postconditioning inhibits myocardial apoptosis during prolonged reperfusion via a JAK2-STAT3-Bcl-2 pathway. Journal of Biomedical Science. 2011;18(1, article 53) doi: 10.1186/1423-0127-18-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu S.-P., He S.-Y., Xu B., et al. Acupuncture promotes angiogenesis after myocardial ischemia through H3K9 acetylation regulation at VEGF gene. PLoS ONE. 2014;9(4) doi: 10.1371/journal.pone.0094604.e94604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y. K., Zhong Z. J., Ban X., Bonser R. S. A compare of three histological stain method for the diagnosis in early myocardial ischemia. Journal of Harbin Medical University. 2002;36(3):238–240. [Google Scholar]
- 36.Shoobridge M. P. K. A new principle in polychrome staining: a system of automated staining, complementary to hematoxylin and eosin, and usable as a research tool. Stain Technology. 1983;58(5):245–258. doi: 10.3109/10520298309066797. [DOI] [PubMed] [Google Scholar]
- 37.Liu S. Q., Roberts D., Kharitonenkov A., et al. Endocrine protection of ischemic myocardium by FGF21 from the liver and adipose tissue. Scientific Reports. 2013;3, article 2767 doi: 10.1038/srep02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao J., Fu W., Jin Z., Yu X. A preliminary study on the cardioprotection of acupuncture pretreatment in rats with ischemia and reperfusion: involvement of cardiac β-adrenoceptors. Journal of Physiological Sciences. 2006;56(4):275–279. doi: 10.2170/physiolsci.rp006606. [DOI] [PubMed] [Google Scholar]
- 39.Zhou W., Ko Y., Benharash P., et al. Cardioprotection of electroacupuncture against myocardial ischemia-reperfusion injury by modulation of cardiac norepinephrine release. American Journal of Physiology—Heart and Circulatory Physiology. 2012;302(9):H1818–H1825. doi: 10.1152/ajpheart.00030.2012. [DOI] [PubMed] [Google Scholar]
- 40.Gao J., Zhao Y., Wang Y., et al. Anti-arrhythmic effect of acupuncture pretreatment in the rats subjected to simulative global ischemia and reperfusion—involvement of intracellular Ca2+ and connexin 43. BMC Complementary and Alternative Medicine. 2015;15(1, article 5) doi: 10.1186/s12906-015-0521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu P.-Y., Tian Y., Xu S.-Y. Mediated protective effect of electroacupuncture pretreatment by miR-214 on myocardial ischemia/reperfusion injury. Journal of Geriatric Cardiology. 2014;11(4):303–310. doi: 10.11909/j.issn.1671-5411.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Novack V., Pencina M., Cohen D. J., et al. Troponin criteria for myocardial infarction after percutaneous coronary intervention. Archives of Internal Medicine. 2012;172(6):502–508. doi: 10.1001/archinternmed.2011.2275. [DOI] [PubMed] [Google Scholar]
- 43.Kemp M., Donovan J., Higham H., Hooper J. Biochemical markers of myocardial injury. British Journal of Anaesthesia. 2004;93(1):63–73. doi: 10.1093/bja/aeh148. [DOI] [PubMed] [Google Scholar]
- 44.Vinten-Johansen J., Jiang R., Reeves J. G., Mykytenko J., Deneve J., Jobe L. J. Inflammation, proinflammatory mediators and myocardial ischemia-reperfusion injury. Hematology/Oncology Clinics of North America. 2007;21(1):123–145. doi: 10.1016/j.hoc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Shi W., Vinten-Johansen J. Endogenous cardioprotection by ischaemic postconditioning and remote conditioning. Cardiovascular Research. 2012;94(2):206–216. doi: 10.1093/cvr/cvs088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hausenloy D. J., Yellon D. M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. The Journal of Clinical Investigation. 2013;123(1):92–100. doi: 10.1172/jci62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Busca A., Saxena M., Kryworuchko M., Kumar A. Anti-apoptotic genes in the survival of monocytic cells during infection. Current Genomics. 2009;10(5):306–317. doi: 10.2174/138920209788920967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziegler D. S., Kung A. L., Kieran M. W. Anti-apoptosis mechanisms in malignant gliomas. Journal of Clinical Oncology. 2008;26(3):493–500. doi: 10.1200/JCO.2007.13.9717. [DOI] [PubMed] [Google Scholar]
- 49.Fulda S. Tumor resistance to apoptosis. International Journal of Cancer. 2009;124(3):511–515. doi: 10.1002/ijc.24064. [DOI] [PubMed] [Google Scholar]
- 50.Portt L., Norman G., Clapp C., Greenwood M., Greenwood M. T. Anti-apoptosis and cell survival: a review. Biochimica et Biophysica Acta—Molecular Cell Research. 2011;1813(1):238–259. doi: 10.1016/j.bbamcr.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Cregan S. P., Dawson V. L., Slack R. S. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene. 2004;23(16):2785–2796. doi: 10.1038/sj.onc.1207517. [DOI] [PubMed] [Google Scholar]
- 52.Wang E., Marcotte R., Petroulakis E. Signaling pathway for apoptosis: a racetrack for life or death. Journal of Cellular Biochemistry. 1999;(supplement 32-33):95–102. doi: 10.1002/(sici)1097-4644(1999)75:32+<95::aid-jcb12>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 53.Kalimuthu S., Se-Kwon K. Cell survival and apoptosis signaling as therapeutic target for cancer: marine bioactive compounds. International Journal of Molecular Sciences. 2013;14(2):2334–2354. doi: 10.3390/ijms14022334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vianello A., Casolo V., Petrussa E., et al. The mitochondrial permeability transition pore (PTP)—an example of multiple molecular exaptation? Biochimica et Biophysica Acta (BBA)—Bioenergetics. 2012;1817(11):2072–2086. doi: 10.1016/j.bbabio.2012.06.620. [DOI] [PubMed] [Google Scholar]
- 55.Grimm S., Brdiczka D. The permeability transition pore in cell death. Apoptosis. 2007;12(5):841–855. doi: 10.1007/s10495-007-0747-3. [DOI] [PubMed] [Google Scholar]
- 56.Shimizu S., Kanaseki T., Mizushima N., et al. Role of Bcl-2 family proteins in a non-apoptopic programmed cell death dependent on autophagy genes. Nature Cell Biology. 2004;6(12):1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 57.King L. A., Toledo A. H., Rivera-Chavez F. A., Toledo-Pereyra L. H. Role of p38 and JNK in liver ischemia and reperfusion. Journal of Hepato-Biliary-Pancreatic Surgery. 2009;16(6):763–770. doi: 10.1007/s00534-009-0155-x. [DOI] [PubMed] [Google Scholar]
- 58.Zhao L., Liu X., Liang J., et al. Phosphorylation of p38 MAPK mediates hypoxic preconditioning-induced neuroprotection against cerebral ischemic injury via mitochondria translocation of Bcl-xL in mice. Brain Research. 2013;1503:78–88. doi: 10.1016/j.brainres.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 59.Sun X.-C., Xian X.-H., Li W.-B., et al. Activation of p38 MAPK participates in brain ischemic tolerance induced by limb ischemic preconditioning by up-regulating HSP 70. Experimental Neurology. 2010;224(2):347–355. doi: 10.1016/j.expneurol.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 60.Zhao T. C., Cheng G., Zhang L. X., Tseng Y. T., Padbury J. F. Inhibition of histone deacetylases triggers pharmacologic preconditioning effects against myocardial ischemic injury. Cardiovascular Research. 2007;76(3):473–481. doi: 10.1016/j.cardiores.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 61.Bouleti C., Mewton N., Germain S. The no-reflow phenomenon: state of the art. Archives of Cardiovascular Diseases. 2015;108(12):661–674. doi: 10.1016/j.acvd.2015.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
To investigate protective effects of EAP on MIRI, we evaluated rat survival rate and myocardial fiber injury. Our results showed that in the EA group, the survival rate was increased significantly (Fig S1), and the extent of myocardial fiber damage was relieved compared with the I/R group (Fig S2). Meanwhile, to assess the relationship between pro-apoptotic factors and the mitochondrial permeability transition pore (MPTP), we observed that EAP significantly decreased the opening of MPTP (Fig S3), therefore reduced the release of pro-apoptotic factors from the IR heart. In additional, we detected decreased beclin1 protein expression in the heart (Fig S4) and increased activity of signal transduction of mitogen-activated protein kinases (MAPKs) (Fig S5) after EA pre-treatment.
Figure S1 The survival rate of rats in each group (n=15). The survival number of each group was recorded after operation, and the survival rate of each group was calculated with the formula: (survival rat number/the total rat number) ×100%.
Figure S2 EAP at PC6 protected myocardial fibers against I/R injury. These images represent ferroalumen hematoxylin staining for myocardial fibers in each group.
Figure S3 EAP at PC6 decreased the opening of mitochondrial permeability transition pore. Mitochondrial PTP opening was assayed by fluorescence spectrophotometer, and data were expressed as means±SD, n=8-15 /each group. ∗, P<0.05vs. SO group; #, P<0.05 vs. I/R group.
Figure S4 EAP at PC6 decreased autophage-associated beclin1 expression level. A. Representative western blot results of beclin 1 proteins in each group. B. Quantitative analysis of beclin 1 protein in each group. Data were expressed as means±SD, n=8-15/ each group. ∗, P<0.05 vs. SO group; #, P<0.05 vs. I/R group.
Figure S5 EAP at PC6 influenced the expression levels of MAPK signaling. A. Representative western blot results of p-JNK, p-P38, p-P44/42, and Ras proteins in each group. B. Quantitative analysis of p-JNK, p-P38, p-P44/42, and Ras proteins in each group. Data were expressed as means ± SD, n=8-15/ each group. ∗, P<0.05 vs. SO group; #, P<0.05 vs. I/R group.