Abstract
RAGE is a multiligand receptor able to bind advanced glycation end-products (AGEs), amphoterin, calgranulins, and amyloid-beta peptides, identified in many tissues and cells, including neurons. RAGE stimulation induces the generation of reactive oxygen species (ROS) mainly through the activity of NADPH oxidases. In neuronal cells, RAGE-induced ROS generation is able to favor cell survival and differentiation or to induce death through the imbalance of redox state. The dual nature of RAGE signaling in neurons depends not only on the intensity of RAGE activation but also on the ability of RAGE-bearing cells to adapt to ROS generation. In this review we highlight these aspects of RAGE signaling regulation in neuronal cells.
1. Introduction
The receptor for advanced glycation end-products (RAGE) is a multiligand receptor able to bind not only the advanced glycation end-products (AGEs) but also amphoterin, calgranulins, and amyloid-beta peptides (Aβ) [1]. It is normally expressed at low levels in many adult tissues but its activation induces a positive feedback favoring its expression and enhancing cell responses [2]. Through the imbalance of redox state, RAGE activation is involved in the onset and progression of proinflammatory or proapoptotic cell responses [3]. However, more recently the role of RAGE in physiological processes such as cell differentiation has been demonstrated [4]. In this review we focused our attention on the role of RAGE and the associated reactive oxygen species (ROS) production in neuronal differentiation or death.
2. RAGE: Structure and Functions
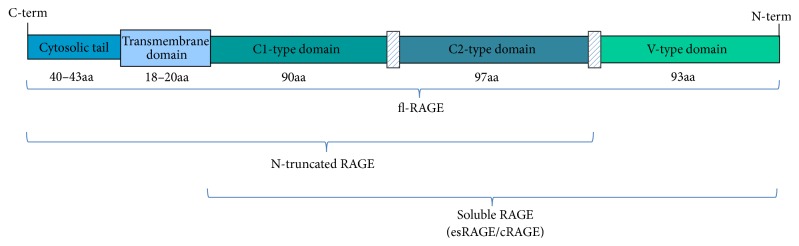
RAGE is a member of the immunoglobulin superfamily [5–7]. In humans RAGE gene is localized on chromosome 6, near the major histocompatibility complex III. The gene encodes for a ~55 kDa protein of 404 amino acids [8], the full-length RAGE (fl-RAGE), composed of three structural regions: an extracellular region comprising a V-type domain and two C-types domains, a short transmembrane region, and a cytoplasmic tail [5–7]. The V-type and C1-type domains are the binding sites for the ligands while the 40–43 amino acid cytoplasmic tail is critical for the intracellular signal transduction [2]. In addition, truncated RAGE isoforms have been described. The N-truncated RAGE variant is lacking the N-terminal V-type domain and is localized on the membrane. On the contrary, other variants lacking C-terminal domain but containing all of the immunoglobulin domains are soluble forms of RAGE and secreted extracellularly. Among these soluble RAGE variants, the endogenous secretory RAGE (esRAGE) results from an alternative splicing of RAGE mRNA [9–11], while the cleaved RAGE (cRAGE) derives from fl-RAGE proteolytic cleavage by the metalloproteinase ADAM10 and MMP9 [12, 13] (Figure 1). Soluble forms of RAGE are known to prevent RAGE binding to ligands, acting as a decoy [2].
Figure 1.
Schematic representation of full-length RAGE and its variants. fl-RAGE is composed of a V-type domain, two C-type domains, a transmembrane domain, and an intracellular tail. The N-truncated form is lacking the N-terminal V-type domain. The soluble form of RAGE is lacking the C-terminal domain but containing all of the immunoglobulin domains. Soluble RAGE may derive from alternative splicing of RAGE mRNA (endogenous secretory esRAGE) or from fl-RAGE proteolytic cleavage from the cell surface (cleaved cRAGE) [13].
RAGE expression is dependent on cell type and developmental stage. In general, RAGE is constitutively expressed during embryonic development and downregulated in adult life [14, 15]. Indeed, except for skin and lung where RAGE is highly expressed throughout life, in physiological conditions RAGE is expressed at low levels in a wide range of adult cells such as endothelial cells, cardiomyocytes, neutrophils, monocytes/macrophages, lymphocytes, dendritic cells [15, 16], and, in the adult central nervous system (CNS), glia and neurons [16–19]. However, it has been well shown that RAGE is upregulated in presence of its ligands.
2.1. RAGE Ligands
As explained by its name, RAGE has been firstly identified in consequence to its ability to bind to advanced glycation end-products (AGEs) [5–7]. AGEs are a heterogeneous group of compounds, characteristic of aging process and diabetes, which are formed in prooxidant environments, in a time-dependent way, through the nonenzymatic reaction between reducing sugars and free amino residues of proteins [20–22]. The contribution of oxidation is so important that all the process can be referred to as glycoxidative reaction [23]. The glycoxidative damage is a typical hallmark of diabetic sequelae such as nefropathy, neuropathy, or micro- and macrovasculopathies in which the high concentration of blood sugars obviously favors glycative reactions [24]. However, glycative damage plays a key role also in end-stage renal disease associated with uremia and hemodialysis and in different age-related pathologies, especially for the crucial contribution of chronic oxidative damage [25–28]. Later on, several ligands have been found to be able to interact with RAGE, highlighting its multiligand nature [29, 30]. Indeed, HMGB1 (amphoterin), S100/calgranulins, and amyloid-β peptides have been identified as ligands of RAGE as well [31].
HMGB1, a highly conserved ubiquitous protein normally expressed in the nucleus is released by necrotic cells and is known to act as a signal of cell damage [32–34]. However, it has been demonstrated that HMGB1 is also secreted by living cells in the central nervous system where it is involved in a number of neuronal functions such as differentiation, cell survival, and neurite outgrowth [14, 17, 35, 36].
S100/calgranulins are a family of calcium-binding polypeptides involved in the regulation of protein phosphorylation, cell cycle, and enzyme activity that accumulate extracellularly in sites of chronic inflammation and act as a proinflammatory stimulus [37, 38].
Aβ peptides derive from amyloid precursor protein (APP) processing and accumulate in Alzheimer's disease, forming the amyloid plaques [39].
Moreover, surface molecules on bacteria, prions, and leukocytes have been demonstrated to be able to interact with RAGE in immune response and chronic inflammation [40–42].
Therefore, the accumulation of all the mentioned ligands leads to the activation of RAGE which not only is involved in the pathogenesis and complications of many aging-related diseases such as diabetes, osteoarthritis, cardiovascular, and Alzheimer's diseases, but also regulates several cellular processes of primary importance such as inflammation, apoptosis, autophagy, and proliferation, playing a crucial role in tissue homeostasis and regeneration [2, 3, 16, 43, 44].
2.2. RAGE Signaling
RAGE interaction with its ligands induces different pathways making the RAGE-mediated cellular signaling extremely complex. The activation of a wide array of signaling pathways has been demonstrated: ERK1/2 (p44/p42), p38 and SAPK/JNK MAP kinases, rho-GTPases, phosphoinositol-3-kinase, JAK/STAT, and different PKC isoforms have been shown to play a role in RAGE-mediated cellular responses [2, 45–47]. RAGE-dependent signaling pathway activation directly induces ROS production mainly through NADPH oxidase (NOX) activation, as detailed below. Moreover, it is important to underline that RAGE signaling leads to the activation of the transcription factor NF-κB that in turn induces RAGE expression, making a positive loop that enhances cell response [2]. However, other transcription factors such as SP-1, AP-2, and NF-IL6 have been shown to regulate RAGE expression [2].
2.2.1. RAGE and Oxidative Stress
It is well known that ROS can modulate signal transduction pathways until they are balanced by adequate antioxidant responses but are able to severely damage cells and tissues when redox balance is lost and oxidative stress is induced. In vitro studies with cultured capillary endothelial cells and in vivo infusion studies have shown that AGE interaction with RAGE leads to oxidative stress, revealed by the appearance of malondialdehyde in the vessel wall and thiobarbituric acid-reactive substances in the tissue [48] and this has been well characterized as one of the crucial mechanism of damage in endothelial cells during diabetes [49].
It has been shown that AGE-RAGE-derived ROS generation is due, at least in part, to the activation of NOX that is able to generate anion superoxide as the main product of its reaction [50, 51]. NOX is a multimeric complex, identified in phagocytes where ROS overproduction leads to bacteria killing [52]. So far, different isoenzymes of NOX have been identified in nonphagocytic cells, active in the generation of ROS for signaling purpose [53, 54] and in neurons NOX1 and NOX2 have been identified [55, 56]. NOX activation leads to NF-κB-mediated iNOS expression favoring the generation of highly toxic peroxynitrite, as shown in vascular smooth muscle cells (VSMC) [34, 57, 58]. However, the controlled ROS production derived from NOX is able to modulate signaling molecules such as p21 contributing to the activation of NF-κB in rat pulmonary artery smooth muscle cells exposed to AGEs [59]. These findings highlight a central role of NOX in the molecular mechanisms involved in RAGE-mediated cell responses.
In addition, the mitochondrial respiratory chain is implicated in ROS generation induced by RAGE activation, as shown with regard to VCAM-1 expression in endothelial cells treated with AGEs [60].
3. RAGE and Neuronal Differentiation
RAGE expression in neurons was observed for the first time in adult bovine nervous system mainly in motor and cortical areas [15]. Its identification in normal, nonpathological tissues led the authors to hypothesize a physiological, even though not clear, role played by RAGE. Later on, several studies demonstrated that HMGB1 and S100B, identified as RAGE ligands in the nervous system, are centrally involved in neuronal differentiation and the implication of RAGE in embryonic and adult neuronal differentiation, in peripheral nerve regeneration, and in neurite outgrowth/elongation has been demonstrated [14, 17, 35, 36, 61, 62].
In particular, experimental findings show a functional role of the HMGB1-RAGE-NF-κB axis in the modulation of adult neurogenesis. Indeed, RAGE has been found expressed in vivo in the neural stem/progenitor cells (NS/PCs) in the neurogenic Subventricular Zone region of the adult mouse brain where it is coexpressed with Sox2 [63, 64]. However, RAGE expression in mature Tbr1 positive neurons has not been demonstrated, suggesting that RAGE is primarily involved in the early events of adults neurogenesis. In addition, it has been clearly shown that HMGB1 released from reactive astrocytes promotes NS/PCs proliferation through the activation of RAGE and the phosphorylation of JNK [65]. Other studies confirm the crucial importance of RAGE in mediating brain repair and nerve regeneration favoring the crosstalk with inflammatory pathways, as showed by using transgenic mice [61] or in neuronal regeneration induced by S100B [66].
Studies in adult sensory neurons exposed to HMGB1, S100B, or human glycated albumin (HGA) demonstrate that RAGE signaling mediates neurotrophin-dependent neurite outgrowth through the activation of JAK-STAT, ERK, and NF-κB pathways [67]. The RAGE-driven activation of NF-κB in neuronal differentiation and neurite outgrowth has been demonstrated also in Retinoic Acid- (RA-) induced P19 neuronal cell differentiation [68] and in the survival of N18 neuroblastoma and in C6 glioma cells [17]. Furthermore, RAGE, HMGB1, and S100B progressively increase during neuronal differentiation of teratocarcinoma-derived NT2/D1 cells: RAGE is expressed only in cells committed to a neuronal phenotype and directly involved in cellular morphological changes, and S100B seems to be the principal ligand [4]. However, other studies on teratocarcinoma cells and primary neurons show that, although RAGE ectopic overexpression, in absence of RA, is not sufficient to drive neuronal differentiation, cell exposure to RA promotes neurite outgrowth through the activation of RAGE and Rac1/Cdc42 [68]. Moreover, the functional inactivation of RAGE in neuroblastoma cells demonstrates its crucial role in the elongation of neurites rather than in neurite outgrowth [69]. In agreement, our recent study underlines that RA-induced neuroblastoma differentiation promotes RAGE-dependent neurite elongation [70]. In particular, during cell differentiation, Aβ 1-42 production is increased and, through the binding to RAGE, enhances the expression of the amphoterin-induced gene and open reading frame-1 (AMIGO-1) suggesting its involvement in neurite elongation [70], as also reported by other authors [71–74]. Importantly, we showed that monomeric but no oligomeric Aβ 1-42 is responsible for this effect, in line with data in the literature sustaining that monomeric Aβ 1-42 can exert physiological functions while the toxic properties of the peptide are due to its aggregation in oligomers or fibrils [75]. However, the involvement of the oligomeric form of Aβ 1-42 in neuronal differentiation cannot be ruled out, as shown on hippocampal neuronal progenitors [64] which has been also demonstrated to be dependent on S100B-RAGE interaction [76].
In addition, RAGE activation is able to induce prosurvival signals in neurons. Indeed, HMGB1 and the two S100 family proteins, S100B and S100A1, increase the expression of the antiapoptotic protein Bcl-2, in a RAGE-dependent way, favoring neuroblastoma cell survival [17, 29]. In a similar way, other authors observed that, during RA-induced neurodifferentiation, HMGB1-RAGE interaction is involved in Bcl-2 production [69].
Furthermore, the HMGB1-RAGE interaction induces phosphorylation and nuclear localization of cyclic AMP response element-binding protein (CREB) in ERK1/2 dependent manner, increasing the expression of chromogranins [77] and regulating neuronal differentiation and survival [78].
In addition, several studies clearly show that, in the differentiation of neuroblastoma cells, RA-treatment induces a prooxidative status and modifies gene expression leading to changes in redox environment. In particular, cell exposure to RA increases NOX activity and the mitochondrial membrane potential and, at the same time, induces SOD gene expression, Nrf2 protein synthesis, NF-κB gene expression, and glycolytic pathway upregulation [79–83]. The involvement of ROS in neurite outgrowth and differentiation has been found also in other cell types as primary neurons and pheochromocytoma PC12 cells [84, 85]. Our unpublished results have demonstrated that neuroblastoma cells treated with monomeric Aβ 1-42 are able to activate NOX favoring neurite elongation (Nitti et al., unpublished).
4. RAGE and Neuronal Damage
In addition to its positive effects in neurite outgrowth and neuronal differentiation, RAGE activation can be involved in neuronal damage due to the overproduction of toxic ROS, cytokines and pro-inflammatory molecules [86]. The accumulation of RAGE ligands promotes oxidative stress, progressive neuronal dysfunctions and neurodegeneration. Thus, RAGE-mediated effects are observed in diabetic neuropathy [87] and in the pathogenesis of Alzheimer's [88], Parkinson's [89], Huntington's diseases [90] and amyotrophic lateral sclerosis [91].
Indeed, AGE accumulation and their RAGE-dependent toxic effects on neurons are considered to play a role of primary importance in the pathogenetic mechanism of the diabetic neuropathy and therapeutic approaches against AGE-RAGE have also been proposed [92]. AGEs have been found to accumulate in senile plaques and in neurofibrillary tangles [93] and their ability to activate RAGE contributes to trigger neuronal death during Alzheimer's disease. More recent studies, have shown that genetic deficiency of neuronal RAGE protects against the synaptic injury induced by AGEs in transgenic mice [94]. The direct binding of RAGE to Aβ, mainly to its aggregated forms, is considered important in mediating amyloid toxicity [95] and RAGE activation by HMGB1 has recently been considered to have a crucial role in favoring neurodegeneration contributing to the development of amyotrophic lateral sclerosis [86].
Three main signaling pathways, activated by RAGE in neurodegeneration have been identified: (i) NOX-dependent signaling, leading to ROS production, activating NF-κB and increasing cytokine and chemokine expression; (ii) RAS-dependent signaling, activating MAP kinases (JUN, ERK1/2 or p38) and modulating NF-κB; (iii) JAK/STAT signaling, leading to the induction of interleukin expression. In all cases, RAGE activation favors the generation of ROS from mitochondria, induces protein aggregation and increases the release of pro-inflammatory molecules [86]. It is important to note that RAGE is also expressed on microglial cells, where it plays a crucial role enhancing cytokine production, oxidative stress and neuroinflammation [96–98].
In addition, it has been demonstrated that RAGE mediates Aβ transport via endocytosis and transcytosis across the blood-brain barrier (BBB), promoting Aβ pathologic accumulation in brain parenchyma [99, 100]. Interestingly, RAGE can act as a carrier for Aβ also on neuronal cell surface: RAGE-dependent p38 MAPK activation promotes the internalization of the whole Aβ-RAGE complex into the cytosolic compartment, leading to mitochondrial dysfunctions, oxidative stress, and neuronal damage [101].
Moreover, RAGE activation can induce neuronal loss triggering the apoptotic process and, in some cases, inducing ER-stress, or favoring autophagy. Indeed, it has been demonstrated that HMGB1-RAGE interaction induces neuronal apoptosis in mixed neuron-glia cultures via p38 MAPK and ERK signaling activation [102]. Similarly, RAGE-dependent apoptosis has been described also in neuroblastoma cells exposed to S100B or AGEs [17, 103]. Moreover, S100B production and subsequent RAGE expression can lead to neuronal apoptosis mediated by ER-stress in infantile neuronal ceroid lipofuscinosis (INCL) and palmitoyl-protein thioesterase-1- (PPT1-) KO mice [104–106]. Furthermore, Aβ-RAGE interaction can increase intracellular Ca2+, that, activating CaMKKβ-AMPK, leads to autophagosome formation in neuroblastoma cells, hypothesizing the involvement of autophagy in Aβ-dependent neurodegeneration [107].
5. Conclusions
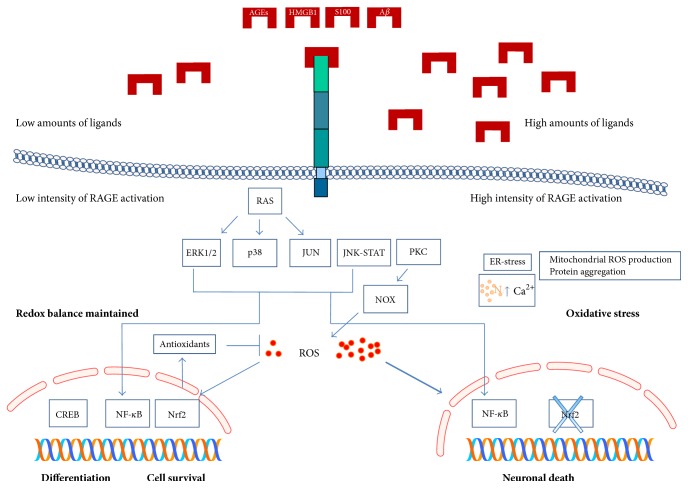
In this review we have shown the double nature of RAGE in neuronal cells: on the one hand, it is able to increase cell survival and favor neuronal differentiation; on the other hand, its activation induces neuronal death. These contradictory effects seem not to be related to the ligand of RAGE, as we provided evidence that the different ligands are able to induce both kinds of cell response but probably depend on the intensity and duration of RAGE activation and, crucially, on specific features of RAGE-bearing cells. Indeed, it has clearly shown that low-level of RAGE activation, induced by low concentration of ligands, has prosurviving differentiating effects, while, in the presence of high amounts of ligands, RAGE induces neuronal death [17]. Interestingly, it has been proved that neurons can be preconditioned by low-level RAGE stimulation increasing their resistance to the toxic effects of high concentrations of RAGE ligands. Indeed, ROS derived by RAGE activation seem to play a crucial role due to their ability to activate prosurviving NF-κB-dependent pathways when they are generated in low amounts [108, 109]. However, in order to balance ROS production and counteract oxidative stress, the production of molecules with antioxidant and detoxifying activities, such as glutathione or heme oxygenase-1, becomes a key point in neuronal response to RAGE activation. Therefore, when RAGE-expressing cells are able to induce a balanced antioxidant response (e.g., in undifferentiated cells) ROS generation can be kept at low levels acting as molecular mediators of cell growth and differentiation. On the contrary, when cells are unable to properly adapt to ROS generation (e.g., in fully differentiated cells or in aging neurons), RAGE activation induces oxidative stress leading to neuronal death (Figure 2). Our previous studies demonstrated that neuroblastoma cells, basically resistant to AGE exposure, become sensitive to AGEs only after cell differentiation [110] and also fully differentiated NT2 neurons, unable to react to RAGE-dependent ROS generation, are sensitive to glycated serum [45]. It is conceivable that the transcription factor Nrf2, master regulator of antioxidant and adaptive response, plays a role in the neuron response (differentiation or death) after RAGE activation. Indeed, it has recently shown that Nrf2-dependent responses are necessary to complete the differentiation program, whilst in terminally differentiated neurons the impairment of Nrf2 signaling is involved in the enhancement of neuronal sensitivity to oxidative stress [111]. This becomes particularly important in aging which is known to further impair the function of Nrf2 [112] and seems to be important in neuronal response to RAGE activation. It has been recently clearly demonstrated that pharmacological approaches able to inhibit RAGE activation and to stimulate Nrf2 activity, reducing oxidative stress, improve learning and memory in AD mice [113]. These findings underline that the ability to adapt to ROS generation is a crucial point in defining neuronal response to RAGE activation.
Figure 2.
Schematic representation of RAGE signaling in neurons. Differences between prodifferentiating pathways and death signals are highlighted.
Acknowledgments
This work was funded by MIUR-PRIN20125S38FA (M. Nitti) and Genoa University (M. Nitti).
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Stern D., Yan S. D., Yan S. F., Schmidt A. M. Receptor for advanced glycation endproducts: a multiligand receptor magnifying cell stress in diverse pathologic settings. Advanced Drug Delivery Reviews. 2002;54(12):1615–1625. doi: 10.1016/s0169-409x(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 2.Bierhaus A., Humpert P. M., Morcos M., et al. Understanding RAGE, the receptor for advanced glycation end products. Journal of Molecular Medicine. 2005;83(11):876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 3.Ramasamy R., Vannucci S. J., Yan S. S. D., Herold K., Yan S. F., Schmidt A. M. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15(7):16R–28R. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- 4.Kim J., Wan C. K., O'Carroll S. J., Shaikh S. B., Nicholson L. F. B. The role of receptor for advanced glycation end products (RAGE) in neuronal differentiation. Journal of Neuroscience Research. 2012;90(6):1136–1147. doi: 10.1002/jnr.23014. [DOI] [PubMed] [Google Scholar]
- 5.Neeper M., Schmidt A. M., Brett J., et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. The Journal of Biological Chemistry. 1992;267(21):14998–15004. [PubMed] [Google Scholar]
- 6.Schmidt A. M. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. Journal of Biological Chemistry. 1992;267(21):14987–14997. [PubMed] [Google Scholar]
- 7.Schmidt A. M., Mora R., Cao R., et al. The endothelial cell binding site for advanced glycation end products consists of a complex: an integral membrane protein and a lactoferrin-like polypeptide. The Journal of Biological Chemistry. 1994;269(13):9882–9888. [PubMed] [Google Scholar]
- 8.Sugaya K., Fukagawa T., Matsumoto K.-I., et al. Three genes in the human MHC class III region near the junction with the class II: gene for receptor of advanced glycosylation end products, PBX2 homeobox gene and a notch homolog, human counterpart of mouse mammary tumor gene int-3. Genomics. 1994;23(2):408–419. doi: 10.1006/geno.1994.1517. [DOI] [PubMed] [Google Scholar]
- 9.Malherbe P., Richards J. G., Gaillard H., et al. cDNA cloning of a novel secreted isoform of the human receptor for advanced glycation end products and characterization of cells co-expressing cell-surface scavenger receptors and Swedish mutant amyloid precursor protein. Molecular Brain Research. 1999;71(2):159–170. doi: 10.1016/S0169-328X(99)00174-6. [DOI] [PubMed] [Google Scholar]
- 10.Yonekura H., Yamamoto Y., Sakurai S., et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochemical Journal. 2003;370(3):1097–1109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlueter C., Hauke S., Flohr A. M., Rogalla P., Bullerdiek J. Tissue-specific expression patterns of the RAGE receptor and its soluble forms—a result of regulated alternative splicing? Biochimica et Biophysica Acta (BBA)—Gene Structure and Expression. 2003;1630(1):1–6. doi: 10.1016/j.bbaexp.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L., Bukulin M., Kojro E., et al. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. Journal of Biological Chemistry. 2008;283(51):35507–35516. doi: 10.1074/jbc.M806948200. [DOI] [PubMed] [Google Scholar]
- 13.Chuah Y. K., Basir R., Talib H., Tie T. H., Nordin N. Receptor for advanced glycation end products and its involvement in inflammatory diseases. International Journal of Inflammation. 2013;2013:15. doi: 10.1155/2013/403460.403460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hori O., Brett J., Slattery T., et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. The Journal of Biological Chemistry. 1995;270(43):25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 15.Brett J., Schmidt A. M., Yan S. D., et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. American Journal of Pathology. 1993;143(6):1699–1712. [PMC free article] [PubMed] [Google Scholar]
- 16.Ott C., Jacobs K., Haucke E., Navarrete Santos A., Grune T., Simm A. Role of advanced glycation end products in cellular signaling. Redox Biology. 2014;2(1):411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huttunen H. J., Kuja-Panula J., Sorci G., Agneletti A. L., Donato R., Rauvala H. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. The Journal of Biological Chemistry. 2000;275(51):40096–40105. doi: 10.1074/jbc.m006993200. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt A., Kuhla B., Bigl K., Münch G., Arendt T. Cell cycle related signaling in Neuro2a cells proceeds via the receptor for advanced glycation end products. Journal of Neural Transmission. 2007;114(11):1413–1424. doi: 10.1007/s00702-007-0770-0. [DOI] [PubMed] [Google Scholar]
- 19.Qin J., Goswami R., Dawson S., Dawson G. Expression of the receptor for advanced glycation end products in oligodendrocytes in response to oxidative stress. Journal of Neuroscience Research. 2008;86(11):2414–2422. doi: 10.1002/jnr.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt A. M., Yan S. D., Stern D. M. The dark side of glucose. Nature Medicine. 1995;1(10):1002–1004. doi: 10.1038/nm1095-1002. [DOI] [PubMed] [Google Scholar]
- 21.Vlassara H., Bucala R., Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Laboratory Investigation. 1994;70(2):138–151. [PubMed] [Google Scholar]
- 22.Brownlee M. Negative consequences of glycation. Metabolism: Clinical and Experimental. 2000;49(2, supplement 1):9–13. doi: 10.1016/s0026-0495(00)80078-5. [DOI] [PubMed] [Google Scholar]
- 23.Fu M.-X., Wells-Knecht K. J., Blackledge J. A., Lyons T. J., Thorpe S. R., Baynes J. W. Glycation, glycoxidation, and cross-linking of collagen by glucose. Kinetics, mechanisms, and inhibition of late stages of the Maillard reaction. Diabetes. 1994;43(5):676–683. doi: 10.2337/diab.43.5.676. [DOI] [PubMed] [Google Scholar]
- 24.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circulation Research. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moh A., Sakata N., Takebayashi S., et al. Increased production of urea hydrogen peroxide from Maillard reaction and a UHP-Fenton pathway related to glycoxidation damage in chronic renal failure. Journal of the American Society of Nephrology. 2004;15(4):1077–1085. doi: 10.1097/01.asn.0000121841.82093.09. [DOI] [PubMed] [Google Scholar]
- 26.Münch G., Deuther-Conrad W., Gasic-Milenkovic J. Glycoxidative stress creates a vicious cycle of neurodegeneration in Alzheimer's disease—a target for neuroprotective treatment strategies? Journal of Neural Transmission, Supplement. 2002;(62):303–307. doi: 10.1007/978-3-7091-6139-5_28. [DOI] [PubMed] [Google Scholar]
- 27.Kizer J. R., Benkeser D., Arnold A. M., et al. Advanced glycation/glycoxidation endproduct carboxymethyl-lysine and incidence of coronary heart disease and stroke in older adults. Atherosclerosis. 2014;235(1):116–121. doi: 10.1016/j.atherosclerosis.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schleicher E., Friess U. Oxidative stress, AGE, and atherosclerosis. Kidney International. Supplement. 2007;(106):S17–S26. doi: 10.1038/sj.ki.5002382. [DOI] [PubMed] [Google Scholar]
- 29.Bucciarelli L. G., Wendt T., Rong L., et al. RAGE is a multiligand receptor of the immunoglobulin superfamily: Implications for homeostasis and chronic disease. Cellular and Molecular Life Sciences. 2002;59(7):1117–1128. doi: 10.1007/s00018-002-8491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt A. M., Yan S. D., Yan S. F., Stern D. M. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. Journal of Clinical Investigation. 2001;108(7):949–955. doi: 10.1172/JCI200114002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt A. M., Yan S. D., Yan S. F., Stern D. M. The biology of the receptor for advanced glycation end products and its ligands. Biochimica et Biophysica Acta. 2000;1498(2-3):99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- 32.Wang H., Bloom O., Zhang M., et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 33.Andersson U., Tracey K. J. HMGB1 in sepsis. Scandinavian Journal of Infectious Diseases. 2003;35(9):577–584. doi: 10.1080/00365540310016286. [DOI] [PubMed] [Google Scholar]
- 34.Treutiger C. J., Mullins G. E., Johansson A.-S. M., et al. High mobility group 1 B-box mediates activation of human endothelium. Journal of Internal Medicine. 2003;254(4):375–385. doi: 10.1046/j.1365-2796.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 35.Huttunen H. J., Fages C., Rauvala H. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-κB require the cytoplasmic domain of the receptor but different downstream signaling pathways. Journal of Biological Chemistry. 1999;274(28):19919–19924. doi: 10.1074/jbc.274.28.19919. [DOI] [PubMed] [Google Scholar]
- 36.Chou D. K. H., Zhang J., Smith F. I., McCaffery P., Jungalwala F. B. Developmental expression of receptor for advanced glycation end products (RAGE), amphoterin and sulfoglucuronyl (HNK-1) carbohydrate in mouse cerebellum and their role in neurite outgrowth and cell migration. Journal of Neurochemistry. 2004;90(6):1389–1401. doi: 10.1111/j.1471-4159.2004.02609.x. [DOI] [PubMed] [Google Scholar]
- 37.Hofmann M. A., Drury S., Fu C., et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97(7):889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 38.Marenholz I., Heizmann C. W., Fritz G. S100 proteins in mouse and man: From evolution to function and pathology (including an update of the nomenclature) Biochemical and Biophysical Research Communications. 2004;322(4):1111–1122. doi: 10.1016/j.bbrc.2004.07.096. [DOI] [PubMed] [Google Scholar]
- 39.Selkoe D. J. Alzheimer's disease. In the beginning. Nature. 1991;354(6353):432–433. doi: 10.1038/354432a0. [DOI] [PubMed] [Google Scholar]
- 40.Chapman M. R., Robinson L. S., Pinkner J. S., et al. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science. 2002;295(5556):851–855. doi: 10.1126/science.1067484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasaki N., Takeuchi M., Chowei H., et al. Advanced glycation end products (AGE) and their receptor (RAGE) in the brain of patients with Creutzfeldt-Jakob disease with prion plaques. Neuroscience Letters. 2002;326(2):117–120. doi: 10.1016/S0304-3940(02)00310-5. [DOI] [PubMed] [Google Scholar]
- 42.Chavakis T., Bierhaus A., Al-Fakhri N., et al. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. Journal of Experimental Medicine. 2003;198(10):1507–1515. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie J., Méndez J. D., Méndez-Valenzuela V., Aguilar-Hernández M. M. Cellular signalling of the receptor for advanced glycation end products (RAGE) Cellular Signalling. 2013;25(11):2185–2197. doi: 10.1016/j.cellsig.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 44.Sorci G., Riuzzi F., Giambanco I., Donato R. RAGE in tissue homeostasis, repair and regeneration. Biochimica et Biophysica Acta—Molecular Cell Research. 2013;1833(1):101–109. doi: 10.1016/j.bbamcr.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 45.Nitti M., D'Abramo C., Traverso N., et al. Central role of PKCδ in glycoxidation-dependent apoptosis of human neurons. Free Radical Biology and Medicine. 2005;38(7):846–856. doi: 10.1016/j.freeradbiomed.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Xu Y., Wang S., Feng L., Zhu Q., Xiang P., He B. Blockade of PKC-beta protects HUVEC from advanced glycation end products induced inflammation. International Immunopharmacology. 2010;10(12):1552–1559. doi: 10.1016/j.intimp.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Downs C. A., Kreiner L. H., Johnson N. M., Brown L. A., Helms M. N. Receptor for advanced glycation end-products regulates lung fluid balance via protein kinase C-gp91phox signaling to epithelial sodium channels. American Journal of Respiratory Cell and Molecular Biology. 2015;52(1):75–87. doi: 10.1165/rcmb.2014-0002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan S. D., Schmidt A. M., Anderson G. M., et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. The Journal of Biological Chemistry. 1994;269(13):9889–9897. [PubMed] [Google Scholar]
- 49.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 50.Yamagishi S.-I., Maeda S., Matsui T., Ueda S., Fukami K., Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochimica et Biophysica Acta. 2012;1820(5):663–671. doi: 10.1016/j.bbagen.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 51.Koulis C., Watson A. M. D., Gray S. P., Jandeleit-Dahm K. A. Linking RAGE and Nox in diabetic micro- and macrovascular complications. Diabetes and Metabolism. 2015;41(4):272–281. doi: 10.1016/j.diabet.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Cross A. R., Segal A. W. The NADPH oxidase of professional phagocytes—prototype of the NOX electron transport chain systems. Biochimica et Biophysica Acta (BBA)—Bioenergetics. 2004;1657(1):1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bedard K., Krause K.-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological Reviews. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 54.Bokoch G. M., Knaus U. G. NADPH oxidases: not just for leukocytes anymore! Trends in Biochemical Sciences. 2003;28(9):502–508. doi: 10.1016/s0968-0004(03)00194-4. [DOI] [PubMed] [Google Scholar]
- 55.Sorce S., Krause K.-H. NOX enzymes in the central nervous system: from signaling to disease. Antioxidants and Redox Signaling. 2009;11(10):2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 56.Nayernia Z., Jaquet V., Krause K.-H. New insights on NOX enzymes in the central nervous system. Antioxidants & Redox Signaling. 2014;20(17):2815–2837. doi: 10.1089/ars.2013.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong A., Dukic-Stefanovic S., Gasic-Milenkovic J., et al. Anti-inflammatory antioxidants attenuate the expression of inducible nitric oxide synthase mediated by advanced glycation endproducts in murine microglia. European Journal of Neuroscience. 2001;14(12):1961–1967. doi: 10.1046/j.0953-816X.2001.01820.x. [DOI] [PubMed] [Google Scholar]
- 58.San Martin A., Foncea R., Laurindo F. R., Ebensperger R., Griendling K. K., Leighton F. Nox1-based NADPH oxidase-derived superoxide is required for VSMC activation by advanced glycation end-products. Free Radical Biology and Medicine. 2007;42(11):1671–1679. doi: 10.1016/j.freeradbiomed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 59.Lander H. M., Tauras J. M., Ogiste J. S., Hori O., Moss R. A., Schmidt A. M. Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. The Journal of Biological Chemistry. 1997;272(28):17810–17814. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- 60.Basta G., Lazzerini G., Del Turco S., Ratto G. M., Schmidt A. M., De Caterina R. At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(7):1401–1407. doi: 10.1161/01.ATV.0000167522.48370.5e. [DOI] [PubMed] [Google Scholar]
- 61.Rong L. L., Yan S.-F., Wendt T., et al. RAGE modulates peripheral nerve regeneration via recruitment of both inflammatory and axonal outgrowth pathways. The FASEB Journal. 2004;18(15):1818–1825. doi: 10.1096/fj.04-1900com. [DOI] [PubMed] [Google Scholar]
- 62.Rong L. L., Trojaborg W., Qu W., et al. Antagonism of RAGE suppresses peripheral nerve regeneration. The FASEB Journal. 2004;18(15):1812–1817. doi: 10.1096/fj.04-1899com. [DOI] [PubMed] [Google Scholar]
- 63.Meneghini V., Francese M. T., Carraro L., Grilli M. A novel role for the Receptor for Advanced Glycation End-products in neural progenitor cells derived from adult SubVentricular Zone. Molecular and Cellular Neuroscience. 2010;45(2):139–150. doi: 10.1016/j.mcn.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 64.Meneghini V., Bortolotto V., Francese M. T., et al. High-mobility group box-1 protein and β-amyloid oligomers promote neuronal differentiation of adult hippocampal neural progenitors via receptor for advanced glycation end products/nuclear factor-κB Axis: relevance for Alzheimer's disease. Journal of Neuroscience. 2013;33(14):6047–6059. doi: 10.1523/jneurosci.2052-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li M., Sun L., Luo Y., Xie C., Pang Y., Li Y. High-mobility group box 1 released from astrocytes promotes the proliferation of cultured neural stem/progenitor cells. International Journal of Molecular Medicine. 2014;34(3):705–714. doi: 10.3892/ijmm.2014.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Donato R., Sorci G., Riuzzi F., et al. S100B's double life: intracellular regulator and extracellular signal. Biochimica et Biophysica Acta. 2009;1793(6):1008–1022. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 67.Saleh A., Smith D. R., Tessler L., et al. Receptor for advanced glycation end-products (RAGE) activates divergent signaling pathways to augment neurite outgrowth of adult sensory neurons. Experimental Neurology. 2013;249:149–159. doi: 10.1016/j.expneurol.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 68.Wang L., Li S., Jungalwala F. B. Receptor for advanced glycation end products (RAGE) mediates neuronal differentiation and neurite outgrowth. Journal of Neuroscience Research. 2008;86(6):1254–1266. doi: 10.1002/jnr.21578. [DOI] [PubMed] [Google Scholar]
- 69.Sajithlal G., Huttunen H., Rauvala H., Münch G. Receptor for advanced glycation end products plays a more important role in cellular survival than in neurite outgrowth during retinoic acid-induced differentiation of neuroblastoma cells. The Journal of Biological Chemistry. 2002;277(9):6888–6897. doi: 10.1074/jbc.m107627200. [DOI] [PubMed] [Google Scholar]
- 70.Piras S., Furfaro A. L., Piccini A., et al. Monomeric Aβ1–42 and RAGE: key players in neuronal differentiation. Neurobiology of Aging. 2014;35(6):1301–1308. doi: 10.1016/j.neurobiolaging.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y., Aulia S., Li L., Tang B. L. AMIGO and friends: an emerging family of brain-enriched, neuronal growth modulating, type I transmembrane proteins with leucine-rich repeats (LRR) and cell adhesion molecule motifs. Brain Research Reviews. 2006;51(2):265–274. doi: 10.1016/j.brainresrev.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 72.Chen Y., Hor H. H., Tang B. L. AMIGO is expressed in multiple brain cell types and may regulate dendritic growth and neuronal survival. Journal of Cellular Physiology. 2012;227(5):2217–2229. doi: 10.1002/jcp.22958. [DOI] [PubMed] [Google Scholar]
- 73.Kajander T., Kuja-Panula J., Rauvala H., Goldman A. Crystal structure and role of glycans and dimerization in folding of neuronal leucine-rich repeat protein AMIGO-1. Journal of Molecular Biology. 2011;413(5):1001–1015. doi: 10.1016/j.jmb.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 74.Kuja-Panula J., Kiiltomäki M., Yamashiro T., Rouhiainen A., Rauvala H. AMIGO, a transmembrane protein implicated in axon tract development, defines a novel protein family with leucine-rich repeats. The Journal of Cell Biology. 2003;160(6):963–973. doi: 10.1083/jcb.200209074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piccini A., Borghi R., Guglielmotto M., et al. β-Amyloid 1-42 induces physiological transcriptional regulation of BACE1. Journal of Neurochemistry. 2012;122(5):1023–1031. doi: 10.1111/j.1471-4159.2012.07834.x. [DOI] [PubMed] [Google Scholar]
- 76.Kleindienst A., McGinn M. J., Harvey H. B., Colello R. J., Hamm R. J., Bullock M. R. Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. Journal of Neurotrauma. 2005;22(6):645–655. doi: 10.1089/neu.2005.22.645. [DOI] [PubMed] [Google Scholar]
- 77.Huttunen H. J., Kuja-Panula J., Rauvala H. Receptor for advanced glycation end products (RAGE) signaling induces CREB-dependent chromogranin expression during neuronal differentiation. Journal of Biological Chemistry. 2002;277(41):38635–38646. doi: 10.1074/jbc.M202515200. [DOI] [PubMed] [Google Scholar]
- 78.Ortega-Martínez S. A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Frontiers in Molecular Neuroscience. 2015;8, article 46 doi: 10.3389/fnmol.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Bittencourt Pasquali M. A., De Ramos V. M., Albanus R. D., et al. Gene expression profile of NF-κB, Nrf2, glycolytic, and p53 pathways during the SH-SY5Y neuronal differentiation mediated by retinoic acid. Molecular Neurobiology. 2016;53(1):423–435. doi: 10.1007/s12035-014-8998-9. [DOI] [PubMed] [Google Scholar]
- 80.Zhao F., Wu T., Lau A., et al. Nrf2 promotes neuronal cell differentiation. Free Radical Biology and Medicine. 2009;47(6):867–879. doi: 10.1016/j.freeradbiomed.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.da Frota M. L. C., Pires A. S., Zeidán-Chuliá F., et al. In vitro optimization of retinoic acid-induced neuritogenesis and TH endogenous expression in human SH-SY5Y neuroblastoma cells by the antioxidant Trolox. Molecular and Cellular Biochemistry. 2011;358(1-2):325–334. doi: 10.1007/s11010-011-0983-2. [DOI] [PubMed] [Google Scholar]
- 82.Xun Z., Lee D.-Y., Lim J., et al. Retinoic acid-induced differentiation increases the rate of oxygen consumption and enhances the spare respiratory capacity of mitochondria in SH-SY5Y cells. Mechanisms of Ageing and Development. 2012;133(4):176–185. doi: 10.1016/j.mad.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schneider L., Giordano S., Zelickson B. R., et al. Differentiation of SH-SY5Y cells to a neuronal phenotype changes cellular bioenergetics and the response to oxidative stress. Free Radical Biology and Medicine. 2011;51(11):2007–2017. doi: 10.1016/j.freeradbiomed.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suzukawa K., Miura K., Mitsushita J., et al. Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. The Journal of Biological Chemistry. 2000;275(18):13175–13178. doi: 10.1074/jbc.275.18.13175. [DOI] [PubMed] [Google Scholar]
- 85.Munnamalai V., Suter D. M. Reactive oxygen species regulate F-actin dynamics in neuronal growth cones and neurite outgrowth. Journal of Neurochemistry. 2009;108(3):644–661. doi: 10.1111/j.1471-4159.2008.05787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ray R., Juranek J. K., Rai V. RAGE axis in neuroinflammation, neurodegeneration and its emerging role in the pathogenesis of amyotrophic lateral sclerosis. Neuroscience & Biobehavioral Reviews. 2016;62:48–55. doi: 10.1016/j.neubiorev.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 87.Vincent A. M., Perrone L., Sullivan K. A., et al. Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology. 2007;148(2):548–558. doi: 10.1210/en.2006-0073. [DOI] [PubMed] [Google Scholar]
- 88.Origlia N., Righi M., Capsoni S., et al. Receptor for advanced glycation end product-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid-β-mediated cortical synaptic dysfunction. The Journal of Neuroscience. 2008;28(13):3521–3530. doi: 10.1523/jneurosci.0204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Teismann P., Sathe K., Bierhaus A., et al. Receptor for advanced glycation endproducts (RAGE) deficiency protects against MPTP toxicity. Neurobiology of Aging. 2012;33(10):2478–2490. doi: 10.1016/j.neurobiolaging.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma L., Nicholson L. F. B. Expression of the receptor for advanced glycation end products in Huntington's disease caudate nucleus. Brain Research. 2004;1018(1):10–17. doi: 10.1016/j.brainres.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 91.Juranek J. K., Daffu G. K., Wojtkiewicz J., Lacomis D., Kofler J., Schmidt A. M. Receptor for advanced glycation end products and its inflammatory ligands are upregulated in amyotrophic lateral sclerosis. Frontiers in Cellular Neuroscience. 2015;9, article 485 doi: 10.3389/fncel.2015.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hidmark A., Fleming T., Vittas S., et al. A new paradigm to understand and treat diabetic neuropathy. Experimental and Clinical Endocrinology and Diabetes. 2014;122(4):201–207. doi: 10.1055/s-0034-1367023. [DOI] [PubMed] [Google Scholar]
- 93.Takeuchi M., Yamagishi S.-I. Possible involvement of advanced glycation end-products (AGEs) in the pathogenesis of Alzheimer's disease. Current Pharmaceutical Design. 2008;14(10):973–978. doi: 10.2174/138161208784139693. [DOI] [PubMed] [Google Scholar]
- 94.Zhang H., Wang Y., Yan S., et al. Genetic deficiency of neuronal RAGE protects against AGE-induced synaptic injury. Cell Death and Disease. 2014;5(6) doi: 10.1038/cddis.2014.248.e1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Deane R., Singh I., Sagare A. P., et al. A multimodal RAGE-specific inhibitor reduces amyloid γ-mediated brain disorder in a mouse model of Alzheimer disease. The Journal of Clinical Investigation. 2012;122(4):1377–1392. doi: 10.1172/jci58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fang, Lue L.-F., Yan S., et al. RAGE-dependent signaling in microglia contributes to neuroinflammation, Aβ accumulation, and impaired learning/memory in a mouse model of Alzheimer's disease. The FASEB Journal. 2010;24(4):1043–1055. doi: 10.1096/fj.09-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bianchi R., Giambanco I., Donato R. S100B/RAGE-dependent activation of microglia via NF-κB and AP-1. Co-regulation of COX-2 expression by S100B, IL-1β and TNF-α . Neurobiology of Aging. 2010;31(4):665–677. doi: 10.1016/j.neurobiolaging.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 98.Dukic-Stefanovic S., Gasic-Milenkovic J., Deuther-Conrad W., Münch G. Signal transduction pathways in mouse microglia N-11 cells activated by advanced glycation endproducts (AGEs) Journal of Neurochemistry. 2003;87(1):44–55. doi: 10.1046/j.1471-4159.2003.01988.x. [DOI] [PubMed] [Google Scholar]
- 99.Deane R., Yan S. D., Submamaryan R. K., et al. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nature Medicine. 2003;9(7):907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 100.Mackic J. B., Stins M., McComb J. G., et al. Human blood-brain barrier receptors for Alzheimer's amyloid-β 1-40. Asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. The Journal of Clinical Investigation. 1998;102(4):734–743. doi: 10.1172/jci2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takuma K., Fang F., Zhang W., et al. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-β and neuronal dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(47):20021–20026. doi: 10.1073/pnas.0905686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim S.-W., Lim C.-M., Kim J.-B., et al. Extracellular HMGB1 released by NMDA treatment confers neuronal apoptosis via RAGE-p38 MAPK/ERK signaling pathway. Neurotoxicity Research. 2011;20(2):159–169. doi: 10.1007/s12640-010-9231-x. [DOI] [PubMed] [Google Scholar]
- 103.Chen S., Yin L., Xu Z., et al. Inhibiting receptor for advanced glycation end product (AGE) and oxidative stress involved in the protective effect mediated by glucagon-like peptide-1 receptor on AGE induced neuronal apoptosis. Neuroscience Letters. 2016;612:193–198. doi: 10.1016/j.neulet.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 104.Saha A., Kim S.-J., Zhang Z., et al. RAGE signaling contributes to neuroinflammation in infantile neuronal ceroid lipofuscinosis. FEBS Letters. 2008;582(27):3823–3831. doi: 10.1016/j.febslet.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Z., Lee Y.-C., Kim S.-J., et al. Palmitoyl-protein thioesterase-1 deficiency mediates the activation of the unfolded protein response and neuronal apoptosis in INCL. Human Molecular Genetics. 2006;15(2):337–346. doi: 10.1093/hmg/ddi451. [DOI] [PubMed] [Google Scholar]
- 106.Paschen W. Dependence of vital cell function on endoplasmic reticulum calcium levels: implications for the mechanisms underlying neuronal cell injury in different pathological states. Cell Calcium. 2001;29(1):1–11. doi: 10.1054/ceca.2000.0162. [DOI] [PubMed] [Google Scholar]
- 107.Son S. M., Jung E. S., Shin H. J., Byun J., Mook-Jung I. Aβ-induced formation of autophagosomes is mediated by RAGE-CaMKKβ-AMPK signaling. Neurobiology of Aging. 2012;33(5):1006.11–1006.23. doi: 10.1016/j.neurobiolaging.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 108.Kaltschmidt B., Uherek M., Wellmann H., Volk B., Kaltschmidt C. Inhibition of NF-κB potentiates amyloid β-mediated neuronal apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(16):9409–9414. doi: 10.1073/pnas.96.16.9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaltschmidt B., Sparna T., Kaltschmidt C. Activation of NF-κB by reactive oxygen intermediates in the nervous system. Antioxidants & Redox Signaling. 1999;1(2):129–144. doi: 10.1089/ars.1999.1.2-129. [DOI] [PubMed] [Google Scholar]
- 110.Nitti M., Furfaro A. L., Traverso N., et al. PKC delta and NADPH oxidase in AGE-induced neuronal death. Neuroscience Letters. 2007;416(3):261–265. doi: 10.1016/j.neulet.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 111.Kärkkäinen V., Pomeshchik Y., Savchenko E., et al. Nrf2 regulates neurogenesis and protects neural progenitor cells against Aβ toxicity. STEM CELLS. 2014;32(7):1904–1916. doi: 10.1002/stem.1666. [DOI] [PubMed] [Google Scholar]
- 112.Zhang H., Davies K. J. A., Forman H. J. Oxidative stress response and Nrf2 signaling in aging. Free Radical Biology and Medicine. 2015;88, part B:314–316. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hong Y., An Z. Hesperidin attenuates learning and memory deficits in APP/PS1 mice through activation of Akt/Nrf2 signaling and inhibition of RAGE/NF-κB signaling. Archives of Pharmacal Research. 2015 doi: 10.1007/s12272-015-0662-z. [DOI] [PubMed] [Google Scholar]