Version Changes
Revised. Amendments from Version 1
The statement in the abstract saying that the findings “demonstrate the limitations in the culture-based regulations” was removed. We added a brief mention of the limited literature relating to Cannabis microbiomes to the introduction. Clarification as to the scope of the study including only analysis of fungal microbiomes was added. We revised paragraph 2 of the introduction to focus on the numerous literature reports of pulmonary aspergillosis associated with cannabis use instead of potential dangers of mycotoxin toxicity. We provided additional background on the overlap of cannabinoid and mycotoxin metabolism via cytochrome P450 system. Figure 2 was revised and the figure legend expanded. We removed spurious hits to non-fungal species and reported only high-confidence species detected with 10 reads or more. This resulted in a higher number of species reported for some samples and fewer for others. The first paragraph of the discussion section was expanded to describe the additional findings. We added a sentence to the end of the second paragraph of the discussion mentioning the two existing publications on endophytic fungi in Cannabis. We removed the comment on potential growth inhibition relating to terpenoids. With respect to potential paxilline contamination, we de-emphasized the concern based solely on the detection of P. paxilli, and stated instead that if the results were verified by tests indicating high levels of paxilline then it may be a cause for concern. The comments relating to the sensitivity of ELISA assays were deleted. Some clarification was added to the concluding paragraph to emphasize the need to ensure that all species of potential concern can be detected, and also the need for additional studies to characterize a broader diversity samples, including measurements of toxin levels where relevant.
Abstract
The Center for Disease Control estimates 128,000 people in the U.S. are hospitalized annually due to food borne illnesses. This has created a demand for food safety testing targeting the detection of pathogenic mold and bacteria on agricultural products. This risk extends to medical Cannabis and is of particular concern with inhaled, vaporized and even concentrated Cannabis products . As a result, third party microbial testing has become a regulatory requirement in the medical and recreational Cannabis markets, yet knowledge of the Cannabis microbiome is limited. Here we describe the first next generation sequencing survey of the fungal communities found in dispensary based Cannabis flowers by ITS2 sequencing, and demonstrate the sensitive detection of several toxigenic Penicillium and Aspergillus species, including P. citrinum and P. paxilli, that were not detected by one or more culture-based methods currently in use for safety testing.
Keywords: Cannabis, Microbiome, Mycotoxins, Cannabidiol, Paxilline, Citrinin, qPCR, Culture, Next generation sequencing
Introduction
Our knowledge of the natural microbiome of field-grown Cannabis in terms of rhizosphere bacteria, and endophytic fungi is limited to just a few focused studies 1– 3. Very little is known about the potential for bacterial and fungal contamination on medicinal Cannabis. Nevertheless, many states in the U.S. are now crafting regulations for detection of microbial contamination on Cannabis in the absence of any comprehensive survey of actual samples. A few of these regulations are inducing growers to “heat kill” or pasteurize Cannabis flowers to lower microbial content. While this seems a harmless suggestion, we must remain aware of how these drying techniques may create false negatives in culture-based safety tests used to monitor colony-forming units (CFU). Even though pasteurization may be effective at sterilizing some of the microbial content, it does not eliminate various pathogenic toxins or spores. Aspergillus spores and mycotoxins are known to resist pasteurization 4, 5. Similar thermal resistance has been reported for E. coli produced Shiga toxin 6. While pasteurization may reduce CFU’s used in petri-dish or plating based safety tests, it does not reduce the microbial toxins, spores or DNA encoding these toxins.
Monitoring for mycotoxic fungi in cannabis preparations has been recommended as part of routine safety testing by the Cannabis Safety Institute. A major driver for this recommendation has been numerous reported cases of serious or fatal pulmonary Aspergillosis associated with marijuana smoking in immunocompromised patients 7– 9. The major cannabinoids have been shown to be potent inhibitors of several cytochrome P450 enzymes at therapeutic concentrations, including 1A1, 1A2, 1B1 2B6, 2C19, 2D6, 3A4 and 3A5 10. Some of these enzymes have been implicated in the metabolism of the fungal toxins aflatoxin and ochratoxin 11– 13. This raises questions about potential interactions and appropriate safety tolerances for mycotoxins in patients being treated with cannabinoid therapeutics. In addition, some Fusarium species that produce toxins have proven to be difficult to selectively culture with tailored media 14– 16. This is a common problem associated with culture-based systems as carbon sources are not exclusive to certain microbes and only 1% of microbial species are believed to be culturable 17.
While the risks of mycotoxic fungal contamination have been well studied in the food markets, the presence of the fungal populations present on Cannabis flowers has never been surveyed with next generation sequencing techniques 18– 23. With the publication of the Cannabis genome 24, 25 and many other pathogenic microbial genomes, quantitative PCR assays have been developed that can accurately quantify fungal DNA present in Cannabis samples 26. Here, we analyze the yeast and mold species present in 10 real world, dispensary-derived Cannabis samples by quantitative PCR and sequencing, and demonstrate the presence of several mycotoxin producing fungal strains that are not detected by widely used culture-based assays.
Methods
Culture-based methods
The culture-based methods selected for testing here represent those currently in use by established medicinal Cannabis safety testing laboratories. 3.55ml of tryptic soy broth (TSB) was used to wet 250mg of homogenized flower in a whirlpack bag. TSB was aspirated from the reverse side of the 100μm mesh filter and placed into a Biolumix TM growth vial and spread onto a 3M Petri Film TM and a SimPlate TM (3M Petrifilm TM 3M Microbiology, St. Paul, MN, USA; SimPlates TM Biocontrol Systems, Bellevue, WA, USA; BioLumix TM Neogen, Lansing MI, USA) according to the respective manufacturers’ recommendations. Biolumix TM vials were grown and monitored for 48 hours while Petri-films TM and SimPlates TM were grown for 5 days. Petri-films TM and SimPlates TM were colony counted manually by three independent observers. Samples were tested on total coliform, total entero, total aerobic, and total yeast and mold. Only total yeast and mold discrepancies were graduated to sequencing.
DNA purification
Plant DNA was extracted with SenSATIVAx according to manufacturers’ instructions (Medicinal Genomics part #420001). DNA was eluted with 50μl ddH20.
Primers used for PCR and sequencing
PCR was performed using 5μl of DNA (3ng/μl) 12.5μl 2X LongAmp (NEB) with 1.25μl of each 10μM MGC-ITS3 and MGC-ITS3 primer (MGC-ITS3; TACACGACGTTGTAAAACGACGCATCGATGAAGAACGCAGC) and (MGC-ITS3R; AGGATAACAATTTCACACAGGATTTGAGCTCTTGCCGCTTCA) with 10μl ddH20 for a 25μl total reaction. An initial 95°C 5 minute denaturization was performed followed by 40 cycles of 95°C for 15s and 65°C for 90s. Samples were purified with 75μl SenSATIVAx, washed twice with 100μl 70% EtOH and bench dried for 5 minutes at room temperature. Samples were eluted in 25μl ddH20.
Total Yeast and Mold assay and ITS amplification
A commercially available total yeast and mold qPCR assay (TYM-PathogINDICAtor, Medicinal Genomics, Woburn MA) was used to screen for fungal DNA in a background of host Cannabis DNA. The TYM qPCR assay targets the ribosomal DNA Internal Transcribed Spacer region 2 (ITS2) using modified primers described previously 27, 28. Fungal DNA amplified using these primers may also be subjected to next generation sequencing to identify the contributing yeast and mold species. ITS sequencing has been widely used to identify and enumerate fungal species present in a given sample 29.
Tailed PCR cloning and sequencing
DNA libraries were constructed with 250ng DNA using New England Biolabs (Ipswich, MA) NEBNext Quick ligation module (NEB # E6056S). End repair used 3μl of enzyme mix, 6.5μl of reagent mix, 55.5μl of DNA + ddH20. Reaction was incubated at 30°C for 20 minutes. After end repair, ligation was performed directly with 15μl of blunt end TA mix, 2.5μl of Illumina adaptor (10μM) and 1μl of ligation enhancer (assumed to be 20% PEG 6000). After 15 minute ligation at 25°C, 3μl of USER enzyme was added to digest the hairpin adaptors and prepare for PCR. The USER enzyme was tip-mixed and incubated at 37°C for 20 minutes. After USER digestion, 86.5μl of SenSATIVAx was added and mixed. The samples were placed on a magnet for 15 minutes until the beads cleared and the supernatant could be removed. Beads were washed twice with 150μl of 70% EtOH. Beads were left for 10 minute to air dry and then eluted in 25μl of 10mM Tris-HCl.
Library PCR
25μl 2X Q5 polymerase was added to 23μl of DNA with 1μl of i7 index primer (25μM) and 1μl universal primer (25μm). After an initial 95°C for 10s, the library was amplified for 15 cycles of 95°C 10s, 65°C 90s. Samples were purified by mixing 75μl of SenSATIVAx into the PCR reaction. The samples were placed on a magnet for 15 minutes until the beads cleared and the supernatant could be removed. Beads were washed twice with 150μl of 70% EtOH. Beads were left for 10 minute to air dry and then eluted in 25μl of 10mM Tris-HCl. Samples were prepared for sequencing on the MiSeq version 2 chemistry according to the manufacturers’ instructions. 2×250bp reads were selected to obtain maximal ITS sequence information.
PaxP verification PCR
Primers described by Shirazi-zand et al. were utilized to amplify a segment of the 725bp PaxP gene from Penicillium paxilli. 25μl LongAmp (NEB) 4μl 10μM primer, 1μl DNA (14ng/μl), 20μl ddH20 to make a 50μl PCR reaction. Cycling conditions were slightly modified to accommodate a different polymerase. 95°C for 30s followed by 28 cycles of 95°C 15s, 55°C for 30s, 65°C 2.5 minutes. Samples were purified with 50μl of SenSATIVAx as described above. 1μl of purified PCR product was sized on Agilent HS 2000 chip. Nextera libraries and sequencing were performed according to instructions from Illumina using 2×75bp sequencing on a version 2 MiSeq.
Penicillium Citrinum verification PCR
Citrinum forward GATTTTCCAAAATGCCGTCT and Citrinum reverse GCTCAAGCATTAATCTAGCTA primers were used with identical PCR conditions as above with the exception using 35 cycles of PCR. Samples were purified with 50μl of SenSATIVAx as described above. 1μl of purified PCR product was sized on Agilent HS 2000 chip. Nextera libraries and sequencing were performed according to instructions from Illumina using 2×75bp sequencing on a version 2 MiSeq. Reads were mapped to Genbank accession number LKUP01000000. Mappings were confirmed using BLAST to NCBI to ensure the strongest hits were to P. citrinum.
Analysis
Reads were demultiplexed and trimmed with Casava 1.8.2 and trim_galore v0.4.1 ( http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/). FLASH v1.2.11 30 was used to merge the reads using max_overlap 150. The reads were aligned to microbial references using MG-RAST v3.2 31. Alignments and classifications were confirmed with a second software tool from One Codex ( https://onecodex.com/) and critical pathways identified for further evaluation with PCR of toxin producing genes. Reads are deposited in NCBI under SRA accession: SRP065410. Nextera 2×75bp sequencing of the PaxP gene was mapped to accession number HM171111.1 with CLCbio Workstation V4 at 98% identity over 80% of the read. One Codex analysis was put into Public mode under the following public URLs:
Australian Bastard:
https://app.onecodex.com/analysis/public/201e7f1642e04a3c
https://app.onecodex.com/analysis/public/58f1e03c10434bfa
KD4:
https://app.onecodex.com/analysis/public/2e86e262817246c4
https://app.onecodex.com/analysis/public/1abd5b60446140a0
KD6:
https://app.onecodex.com/analysis/public/a92d3dff5485499d
https://app.onecodex.com/analysis/public/8d72e2514e564ecd
KD8:
https://app.onecodex.com/analysis/public/8d72e2514e564ecd
https://app.onecodex.com/analysis/public/d6e2e0bcfba3469f
Liberty Haze:
https://app.onecodex.com/analysis/public/7bcd650fa5544f2c
https://app.onecodex.com/analysis/public/7f0feb6cb0a94d56
Girls Scout Cookie:
https://app.onecodex.com/analysis/public/a71b1ce8331c461d
https://app.onecodex.com/analysis/public/8d6f10c7ee684f93
Jakes Grape:
https://app.onecodex.com/analysis/public/bc8af5ed19e5407a
https://app.onecodex.com/analysis/public/99d7a4a2f7af486b
RECON:
https://app.onecodex.com/analysis/public/8a22a16cc2e24731
https://app.onecodex.com/analysis/public/0af6ae26a01f48d5
GreenCrack:
https://app.onecodex.com/analysis/public/6114843d2eb3425e
https://app.onecodex.com/analysis/public/3eee642786c54a88
LA Confidential:
https://app.onecodex.com/analysis/public/01e8aefb0d4f4f62
https://app.onecodex.com/analysis/public/b74c2988fcd84e38
NYC Diesel:
Results
We purified DNA from Cannabis samples obtained from two different geographic regions (Amsterdam and Massachusetts) several years apart (2011 and 2015). The majority of samples purified and screened with ITS qPCR were negative for amplification signal implying reagents clean of fungal contamination . Six of the 17 dispensary-derived Cannabis samples tested positive for yeast and mold in the TYM qPCR assay. These results were compared with the results derived from three commercially available culture-based detection systems for each of the 17 samples (3M Petrifilm TM 3M Microbiology, St. Paul, MN, USA; SimPlates TM Biocontrol Systems, Bellevue, WA, USA; BioLumix TM Neogen, Lansing MI, USA; Figure 1). Of the 6 qPCR positive samples, two tested negative in all 3 culture-based assays and four tested negative in 1 or 2 of the culture-based assays ( Table 1). None of the qPCR negative samples tested positive in any of the culture-based assays. Each of the 6 discordant samples was subjected to ITS sequencing to precisely identify the collection of microbes present. Four additional samples from a different geographic origin (Amsterdam) were also subjected to ITS sequencing, for a total of 10 Cannabis samples.
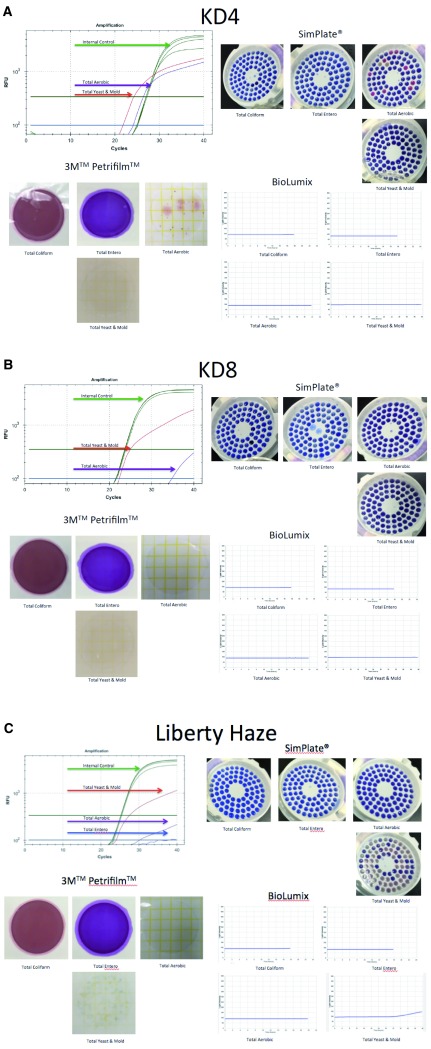
Figure 1. Comparison of 4 different microbial detection technologies.
Figure 1A. qPCR signal from TYM (red line) test run concurrently (multiplexed) with a plant internal control marker (green line). This marker targets a conserved region in the Cannabis genome and should show up in every assay (upper left). SimPlates count the number of discolored wells (purple to pink) as a proxy for CFU/gram. Only total aerobic show growth (upper right). Petrifilm only demonstrate colonies on total aerobic platings (lower left). Biolumix demonstrate no signal across all 4 tests (lower right). Figure 1B. Sample KD8 fails to culture any total yeast and mold yet demonstrates significant TYM qPCR signal. Sample was graduated to ITS based next generation sequencing. Figure 1C. Sample Liberty Haze was tested with 3 culture based methods and compared to qPCR. Sample was graduated to ITS based next generation sequencing.
Table 1. Samples were cultured with 3 different techniques and compared to quantitative PCR (qPCR).
Biolumix had the lowest sensitivity failing to pick up 4/17 samples detected with other culture-based platforms. qPCR identified 2 samples that were not picked up by any other method. Positive qPCR samples were sequenced to identify the contributing signal. Highlighted samples fail the 10,000 CFU/g cutoffs which equates to a Cq of 26 on the qPCR assay according to the manufacturers’ instructions. (f) is fail or over 10,000 CFU/g. (p) is pass or under 10,000 CFU/g. The raw CFU numbers can be deduced by dividing the CFU number by the 1,000 fold dilution factor used in this study.
| Samples | Total Yeast and Mold (10,000 CFU/g = fail) | |||
|---|---|---|---|---|
| Simplate ® (CFU/g) | 3M ® (CFU/g) | BioLumix
®
(CFU/g) |
Cq | |
| KD4 | 0 | 0 | pass | 21.71 (f) |
| KD8 | 0 | 0 | pass | 22.5 (f) |
| PC3 | 0 | 0 | pass | >40 (p) |
| White Widow | 0 | 0 | pass | >40 (p) |
| KD1 | 0 | 0 | pass | 29.33 (p) |
| KD2 | 0 | 0 | pass | >40 (p) |
| KD3 | 0 | 0 | pass | 30.16 (p) |
| KD5 | 1000 (p) | 6000 (p) | pass | 27.76 (p) |
| KD6 | 3000 (p) | 19000 (f) | pass | 24.72 (f) |
| KD7 | 0 | 0 | pass | >40 (p) |
| Liberty Haze | 172000 (f) | 89000 (f) | pass | 24.02 (f) |
| Blueberry Kush | 0 | 0 | pass | 37.99 (p) |
| Blueberry Kush -spiked | >738,000 (f) | TNTC (f) | fail | 15.71 (f) |
| Girl Scout Cookies | >738,000 (f) | TNTC (f) | pass | 19.66 (f) |
| Jake's Grape | >738,000 (f) | TNTC (f) | pass | 24.56 (f) |
| Serious Happiness | 0 | 0 | pass | >40 (p) |
| White Rhino | 0 | 3000 (p) | pass | >40 (p) |
TNTC = Too Numerous To Count
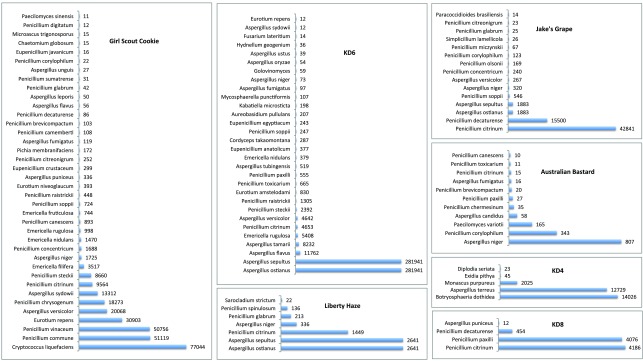
Each discordant sample presented with an array of microbial species, as shown in Figure 2. No sample presented with a single dominant species, and each sample displayed multiple species of interest. Of particular concern were the identified DNA sequences from toxin producing species: Aspergillus versicolor 32– 36, Aspergillus terreus 37, Penicillium citrinum 38– 40, Penicillium paxilli 41, 42.
Figure 2. Detection of fungal species by ITS2 sequencing and MG-RAST analysis.
Histograms are provided for each of the Cannabis samples that tested negative by at least one culture based method and positive using a qPCR-based total yeast and mold test. The number of reads corresponding to each detected fungal species is indicated to the right of each bar. Species detected with less than 10 reads are not included. The overall read counts per sample are more a reflection of sample normalization for sequencing than of the absolute fungal DNA levels.
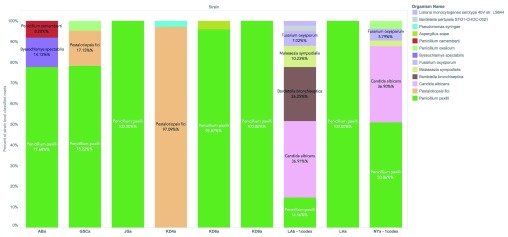
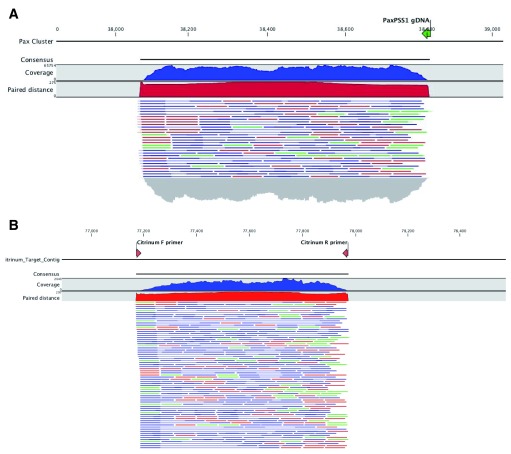
We further analyzed the ITS sequence alignments using the whole genome shotgun based microbiome classification software known as One Codex 43. Nine of the ten samples sequenced showed the presence of P. paxilli ( Figure 3). To verify the accuracy of this ITS phylotyping, a gene involved in the paxilline toxin biosynthesis pathway of P. paxilli was amplified with PaxPss1 and PaxPss2 primers described by Saikia et al. 44. The resulting 725bp amplicon (expected size) was sequenced to confirm the presence of the P. paxilli biosynthesis gene in the Cannabis sample KD8 ( Figure 4). This was successfully repeated with primers designed to target genes in the citrinin pathway of P. citrinum. There were some discrepancies between the results derived from the two software platforms (One Codex and MG-RAST). The MG-RAST analysis, using merged, paired reads correlated better with the PCR results. While One Codex predicted and confirmed KD8 as having the highest P. paxilli content, the One Codex platform is optimized for whole genome shotgun data and may not be able to differentiate the 18S sequence differences (391/412 aligned bases) between these two species with a K-mer based approach.
Figure 3. One Codex classification of ITS reads.
P. paxilli is the most frequently found contaminant in Cannabis flowers. P. citrinum is not in the One Codex database at this time. One Codex utilizes a fast k-mer based approach for whole genome shotgun classification and can be influenced by read trimming and database content. The reads provided to MG-RAST were trimmed and FLASH’d (paired end reads merged when overlapping) prior to classification. K-mer based approaches can significantly differ from longer word size methods and this underscores the importance of confirmatory PCR in microbiome analysis.
Figure 4. PCR of genes encoding Paxilline and Citrinin demonstrates amplification of the expected size.
Citrinum primers we designed from Genbank accession number LKUP01000753. Paxilline primers were used as described in Saikia et al. PCR products were made into shotgun libraries with Nextera and sequenced on an Illumina MiSeq with 2×75bp reads to over 10,000X coverage. Reads were mapped with CLCbio 4 to NCBI accession number HM171111.1 ( A) and LKUP01000000 respectively ( B). Paired reads are displayed as blue lines, green and red lines are unpaired reads. Read coverage over the amplicons are depicted in a blue histogram over the cluster while paired end read distance is measured in a red histogram over the region. Off target read mapping is limited. P. paxilli mappings are displayed on top ( A) and P. citrinum mappings are displayed on bottom ( B). Alignment of PCR primers to P. paxilli reference shows a 5 prime mismatch that is a result of the primers being designed to target spliced RNA according to Saikia et al.
With the confirmed presence of P. paxilli, we are curious to find out whether the toxin, paxilline, is present in the samples. Development of monoclonal antibodies to paxilline has recently been described 45, but commercial ELISA assays with sensitivity under 50ppb do not appear to be available at this time. A >50ppb multiplexed ELISA assay is available from Randox Food Diagnostics (Crumlin, UK). Detection with LC-MS/MS has also been described 46, 47, however, and experiments are underway to determine whether paxilline can be identified in the background of cannabinoids and terpenes present in Cannabis samples.
Discussion
This study demonstrates detection of numerous fungal species by molecular screening of ITS2 in several dispensary-derived Cannabis samples. These included the toxigenic Penicillium species: P. paxilli, P. citrinum, P. commune, P. chrysogenum, P. corylophilum, Aspergillus species: A. terreus, A. niger, A. flavus, A. versicolor and Eurotium repens. In addition, a pathogenic species Cryptococcus liquefaciens was detected. The fungal microbiomes of the different samples differed significantly in the number and diversity of species present. Two samples contained a large diversity of species, similar to previous studies that used field-grown samples and culture-based outgrowth methods 2, 3, 48. Other samples contained only a few species at significant levels. This is perhaps not surprising given the prevalence of indoor culture methods using artificial growth media for medicinal Cannabis. However, we do not have any knowledge of the specific growth conditions that were used for the samples analyzed.
Three different culture-based assays failed to detect all of the positive samples and one, BioLumix TM, detected only one out of 7 positive samples. A review of the literature suggests that Penicillium microbes can be cultured on CYA media, but some may require colder temperatures (21-24C) and 7 day growth times 49. Of the Penicillium, only P. citrinum has been previously reported to culture with 3M Petri-Film 50. It is possible the different water activity of the culture assay compared to the natural flower environment is contributing to the false negative test results.
Quantitative PCR is agnostic to water activity and can be performed in hours instead of days. The specificity and sensitivity provides important information on samples that present risks invisible to culture based systems. The drawback to qPCR is the method’s indifference to living or non-living DNA. While techniques exist to perform live-dead qPCR, the live status of the microbes is unrelated to toxin potentially produced while the microbes were alive. ELISA assays exist to screen for some toxins 51. Current state-recommended ELISA’s do not detect citrinin or paxilline, the toxins produced by P. citrinum and P. paxilli, respectively. The predominance of these Penicillium species in a majority of the samples tested is interesting. Several Penicillium species are known to be endophytes on various plant species, including P. citrinum 18, and this raises the question of whether they may be common Cannabis endophytes. Indeed, P. citrinum and a species identified as P. copticola (a member of the citrinun section 51) have previously been identified as Cannabis endophytes, along with several Aspergillus species 2, 3.
Paxilline is a tremorgenic and ataxic potassium channel blocker and has been shown to attenuate the anti-seizure properties of cannabidiol in certain mouse models 52– 54. Paxilline is reported to have tremorgenic effects at nanomolar concentrations and is responsible for Ryegrass-staggers disease 55. Cannabidiol is often used at micromolar concentrations for seizure reduction and contamination with paxilline, if confirmed, would be a cause for concern. Citrinin is a mycotoxin that disrupts Ca2+ efflux in the mitochondrial permeability transition pore (mPTP) 56– 63. Ryan et al. demonstrated that cannabidiol affects this pathway suggesting a similar potential cause for concern regarding CBD-citrinin interaction 64. Considering the hydrophobicity of these mycotoxins and the growing interest in the use of extracted oils from CBD-rich Cannabis strains for treatment of drug resistant epilepsy 65– 70, more precise molecular screening of fungal toxins in these products might be warranted.
ITS amplification and sequencing offers a hypothesis-free testing approach that can be employed to identify a broad range of fungal species present in a given sample. Appropriate primer design can survey a broad spectrum of fungal genomes while affording rapid iteration of design. Quantitative PCR has also demonstrated single molecule sensitivity and linear dynamic range over 5 orders of magnitude offering a very sensitive approach for detection of microbial risks. Our survey of Cannabis flowers in this study was limited, however. Further studies are required to survey a broader range of samples, and to determine whether paxilline, citrinin, aflatoxin or ochratoxin can be detected at concentrations that represent a clinical risk in Cannabis samples or extracts derived from plants that test positive for the fungi known to produce those toxins.
Conclusions
Several toxigenic fungi were detected in dispensary-derived Cannabis samples using molecular amplification and sequencing techniques. These microbes were not detected using traditional culture-based platforms. These results suggest that culture based techniques borrowed from the food industry should be re-evaluated for Cannabis testing to ensure that they are capable of detecting the prevalent species detected by molecular methods with adequate sensitivity. We recommend that additional sequencing studies be performed to characterize the fungal and bacterial microbiomes of a more diverse selection of Cannabis samples. Such sampling should include dispensary-derived samples from both indoor and outdoor crops, as well as samples from police seizures from well-provenanced foreign sources, such as Mexico. Finally, further studies should be performed to measure toxin levels in strains that test positive for toxigenic species.
Acknowledgments
John McPartland, Cindy Orser, Brad Douglass, Joost Heeroma, Nick Greenfield, Rebecca McKernan and Kellie Dodd for thoughtful advice.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; referees: 2 approved]
References
- 1. Winston ME, Hampton-Marcell J, Zarraonaindia I, et al. : Understanding cultivar-specificity and soil determinants of the cannabis microbiome. PLoS One. 2014;9(6):e99641. 10.1371/journal.pone.0099641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kusari P, Kusari S, Spiteller M, et al. : Endophytic fungi harbored in Cannabis sativa L.: diversity and potential as biocontrol agents against host plant-specific phytopathogens. Fungal Divers. 2013;60(1):137–151. 10.1007/s13225-012-0216-3 [DOI] [Google Scholar]
- 3. Gautam A, Kant M, Thakur Y: Isolation of endophytic fungi from Cannabis sativa and study their antifungal potential. Archives Of Phytopathology And Plant Protection. 2013;46(6):627–635. 10.1080/03235408.2012.749696 [DOI] [Google Scholar]
- 4. Fujikawa H, Itoh T: Tailing of thermal inactivation curve of Aspergillus niger spores. Appl Environ Microbiol. 1996;62(10):3745–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kabak B, Dobson AD: Biological strategies to counteract the effects of mycotoxins. J Food Prot. 2009;72(9):2006–2016. [DOI] [PubMed] [Google Scholar]
- 6. Rasooly R, Do PM: Shiga toxin Stx2 is heat-stable and not inactivated by pasteurization. Int J Food Microbiol. 2010;136(3):290–294. 10.1016/j.ijfoodmicro.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 7. Ruchlemer R, Amit-Kohn M, Raveh D, et al. : Inhaled medicinal cannabis and the immunocompromised patient. Support Care Cancer. 2015;23(3):819–822. 10.1007/s00520-014-2429-3 [DOI] [PubMed] [Google Scholar]
- 8. Gargani Y, Bishop P, Denning DW: Too many mouldy joints - marijuana and chronic pulmonary aspergillosis. Mediterr J Hematol Infect Dis. 2011;3(1):e2011005. 10.4084/MJHID.2011.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bal A, Agarwal AN, Das A, et al. : Chronic necrotising pulmonary aspergillosis in a marijuana addict: a new cause of amyloidosis. Pathology. 2010;42(2):197–200. 10.3109/00313020903493997 [DOI] [PubMed] [Google Scholar]
- 10. Zendulka O, Dovrtělová G, Nosková K, et al. : Cannabinoids and Cytochrome P450 Interactions. Curr Drug Metab. 2016;17(3):206–226. 10.2174/1389200217666151210142051 [DOI] [PubMed] [Google Scholar]
- 11. Dohnal V, Wu Q, Kuča K: Metabolism of aflatoxins: key enzymes and interindividual as well as interspecies differences. Arch Toxicol. 2014;88(9):1635–1644. 10.1007/s00204-014-1312-9 [DOI] [PubMed] [Google Scholar]
- 12. de Groene EM, Jahn A, Horbach GJ, et al. : Mutagenicity and genotoxicity of the mycotoxin ochratoxin A. Environ Toxicol Pharmacol. 1996;1(1):21–26. 10.1016/1382-6689(95)00005-4 [DOI] [PubMed] [Google Scholar]
- 13. Lewis CW, Smith JE, Anderson JG, et al. : Increased cytotoxicity of food-borne mycotoxins toward human cell lines in vitro via enhanced cytochrome p450 expression using the MTT bioassay. Mycopathologia. 1999;148(2):97–102. 10.1023/A:1007130923558 [DOI] [PubMed] [Google Scholar]
- 14. Bragulat MR, Martínez E, Castellá G, et al. : Selective efficacy of culture media recommended for isolation and enumeration of Fusarium spp. J Food Prot. 2004;67(1):207–211. [DOI] [PubMed] [Google Scholar]
- 15. Castellá G, Bragulat MR, Rubiales MV, et al. : Malachite green agar, a new selective medium for Fusarium spp. Mycopathologia. 1997;137(3):173–178. 10.1023/A:1006886529511 [DOI] [PubMed] [Google Scholar]
- 16. Desjardins AE, Proctor RH: Molecular biology of Fusarium mycotoxins. Int J Food Microbiol. 2007;119(1–2):47–50. 10.1016/j.ijfoodmicro.2007.07.024 [DOI] [PubMed] [Google Scholar]
- 17. Stewart EJ: Growing unculturable bacteria. J Bacteriol. 2012;194(16):4151–4160. 10.1128/JB.00345-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kusari PKS, Kusari S, Spiteller M, et al. : Endophytic fungi harbored in Cannabis sativa L.: diversity and potential as biocontrol agents against host plant-specific phytopathogens. Fungal Divers. 2013;60(1):137–151. 10.1007/s13225-012-0216-3 [DOI] [Google Scholar]
- 19. McPartland: Fungal pathogens of Cannabis sativa in Illinois. Phytopathology. 1983;72:797. [Google Scholar]
- 20. McPartland: Common names for diseases of Cannabis sativa L. Plant Dis. 1991;75:226–227. [Google Scholar]
- 21. McPartland JM: Microbiological contaminants of marijuana. J Int Hemp Assoc. 1994;1:41–44. Reference Source [Google Scholar]
- 22. McPartland JM: Cannabis pathogens X: Phoma, Ascochyta and Didymella species. Mycologia. 1994;86(6):870–878. 10.2307/3760600 [DOI] [Google Scholar]
- 23. McPartland JM: A review of Cannabis diseases. J Int Hemp Assoc. 1996;3(1):19–23. Reference Source [Google Scholar]
- 24. van Bakel H, Stout JM, Cote AG, et al. : The draft genome and transcriptome of Cannabis sativa. Genome Biol. 2011;12(10):R102. 10.1186/gb-2011-12-10-r102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McKernan: The Cannabis sativa Genome.2011. Reference Source [Google Scholar]
- 26. McKernan:2015. Reference Source [Google Scholar]
- 27. Borneman J, Hartin RJ: PCR primers that amplify fungal rRNA genes from environmental samples. Appl Environ Microbiol. 2000;66(10):4356–4360. 10.1128/AEM.66.10.4356-4360.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. White TJ, Bruns T, Lee S, et al. : Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: A Guide to Methods and Applications. 1990;315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI] [Google Scholar]
- 29. Mason M, Cheung I, Walker L: Creating a geospatial database of risks and resources to explore urban adolescent substance use. J Prev Interv Community. 2009;37(1):21–34. 10.1080/10852350802498391 [DOI] [PubMed] [Google Scholar]
- 30. Magoč T, Salzberg SL: FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–2963. 10.1093/bioinformatics/btr507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Glass EM, Wilkening J, Wilke A, et al. : Using the metagenomics RAST server (MG-RAST) for analyzing shotgun metagenomes. Cold Spring Harb Protoc. 2010;2010(1): pdb.prot5368. 10.1101/pdb.prot5368 [DOI] [PubMed] [Google Scholar]
- 32. Abd Alla EA, Metwally MM, Mehriz AM, et al. : Sterigmatocystin: incidence, fate and production by Aspergillus versicolor in Ras cheese. Nahrung. 1996;40(6):310–313. 10.1002/food.19960400604 [DOI] [PubMed] [Google Scholar]
- 33. Aly SA, Elewa NA: The effect of Egyptian honeybee propolis on the growth of Aspergillus versicolor and sterigmatocystin biosynthesis in Ras cheese. J Dairy Res. 2007;74(1):74–78. 10.1017/S002202990600207X [DOI] [PubMed] [Google Scholar]
- 34. Engelhart S, Loock A, Skutlarek D, et al. : Occurrence of toxigenic Aspergillus versicolor isolates and sterigmatocystin in carpet dust from damp indoor environments. Appl Environ Microbiol. 2002;68(8):3886–3890. 10.1128/AEM.68.8.3886-3890.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kocić-Tanackov S, Dimić G, Lević J, et al. : Effects of onion ( Allium cepa L.) and garlic ( Allium sativum L.) essential oils on the Aspergillus versicolor growth and sterigmatocystin production. J Food Sci. 2012;77(5):M278–284. 10.1111/j.1750-3841.2012.02662.x [DOI] [PubMed] [Google Scholar]
- 36. Song F, Ren B, Chen C, et al. : Three new sterigmatocystin analogues from marine-derived fungus Aspergillus versicolor MF359. Appl Microbiol Biotechnol. 2014;98(8):3753–3758. 10.1007/s00253-013-5409-5 [DOI] [PubMed] [Google Scholar]
- 37. El-Sayed Abdalla A, Zeinab Kheiralla M, Sahab A, et al. : Aspergillus terreus and its toxic metabolites as a food contaminant in some Egyptian Bakery products and grains. Mycotoxin Res. 1998;14(2):83–91. 10.1007/BF02945097 [DOI] [PubMed] [Google Scholar]
- 38. Ames DD, Wyatt RD, Marks HL, et al. : Effect of citrinin, a mycotoxin produced by Penicillium citrinum, on laying hens and young broiler chicks. Poult Sci. 1976;55(4):1294–1301. 10.3382/ps.0551294 [DOI] [PubMed] [Google Scholar]
- 39. Mazumder PM, Mazumder R, Mazumder A, et al. : Antimicrobial activity of the mycotoxin citrinin obtained from the fungus Penicillium citrinum. Anc Sci Life. 2002;21(3):191–197. [PMC free article] [PubMed] [Google Scholar]
- 40. Park SY, Kim R, Ryu CM, et al. : Citrinin, a mycotoxin from Penicillium citrinum, plays a role in inducing motility of Paenibacillus polymyxa. FEMS Microbiol Ecol. 2008;65(2):229–237. 10.1111/j.1574-6941.2008.00492.x [DOI] [PubMed] [Google Scholar]
- 41. Itoh Y, Johnson R, Scott B: Integrative transformation of the mycotoxin-producing fungus, Penicillium paxilli. Curr Genet. 1994;25(6):508–513. 10.1007/BF00351670 [DOI] [PubMed] [Google Scholar]
- 42. Shibayama M, Ooi K, Johnson R, et al. : Suppression of tandem-multimer formation during genetic transformation of the mycotoxin-producing fungus Penicillium paxilli by disrupting an orthologue of Aspergillus nidulans uvsC. Curr Genet. 2002;42(1):59–65. 10.1007/s00294-002-0330-y [DOI] [PubMed] [Google Scholar]
- 43. Minot SS, Krumm N, Greenfield NB: One Codex: A Sensitive and Accurate Data Platform for Genomic Microbial Identification. bioRxiv. 2015. 10.1101/027607 [DOI] [Google Scholar]
- 44. Saikia S, Parker EJ, Koulman A, et al. : Defining paxilline biosynthesis in Penicillium paxilli: functional characterization of two cytochrome P450 monooxygenases. J Biol Chem. 2007;282(23):16829–16837. 10.1074/jbc.M701626200 [DOI] [PubMed] [Google Scholar]
- 45. Maragos CM: Development and Evaluation of Monoclonal Antibodies for Paxilline. Toxins (Basel). 2015;7(10):3903–3915. 10.3390/toxins7103903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vishwanath V, Sulyok M, Labuda R, et al. : Simultaneous determination of 186 fungal and bacterial metabolites in indoor matrices by liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem. 2009;395(5):1355–1372. 10.1007/s00216-009-2995-2 [DOI] [PubMed] [Google Scholar]
- 47. Uhlig S, Egge-Jacobsen W, Vrålstad T, et al. : Indole-diterpenoid profiles of Claviceps paspali and Claviceps purpurea from high-resolution Fourier transform Orbitrap mass spectrometry. Rapid Commun Mass Spectrom. 2014;28(14):1621–1634. 10.1002/rcm.6938 [DOI] [PubMed] [Google Scholar]
- 48. Gzebenyuk NV: The occurrence of fungi on hemp stems. Mikologiya i Fitopathologiya. 1984;18(4):322–326. Reference Source [Google Scholar]
- 49. Houbraken J, Frisvad JC, Samson RA: Taxonomy of Penicillium section Citrina. Stud Mycol. 2011;70(1):53–138. 10.3114/sim.2011.70.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. 3M. Reference Source [Google Scholar]
- 51. Labs R:2015. Reference Source [Google Scholar]
- 52. Shirazi-zand Z, Ahmad-Molaei L, Motamedi F, et al. : The role of potassium BK channels in anticonvulsant effect of cannabidiol in pentylenetetrazole and maximal electroshock models of seizure in mice. Epilepsy Behav. 2013;28(1):1–7. 10.1016/j.yebeh.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 53. Sabater-Vilar M, Nijmeijer S, Fink-Gremmels J: Genotoxicity assessment of five tremorgenic mycotoxins (fumitremorgen B, paxilline, penitrem A, verruculogen, and verrucosidin) produced by molds isolated from fermented meats. J Food Prot. 2003;66(11):2123–2129. [DOI] [PubMed] [Google Scholar]
- 54. Sánchez-Pastor E, Andrade F, Sánchez-Pastor JM, et al. : Cannabinoid receptor type 1 activation by arachidonylcyclopropylamide in rat aortic rings causes vasorelaxation involving calcium-activated potassium channel subunit alpha-1 and calcium channel, voltage-dependent, L type, alpha 1C subunit. Eur J Pharmacol. 2014;729:100–106. 10.1016/j.ejphar.2014.02.016 [DOI] [PubMed] [Google Scholar]
- 55. Imlach WL, Finch SC, Dunlop J, et al. : The molecular mechanism of "ryegrass staggers," a neurological disorder of K + channels. J Pharmacol Exp Ther. 2008;327(3):657–664. 10.1124/jpet.108.143933 [DOI] [PubMed] [Google Scholar]
- 56. Chagas GM, Campello AP, Klüppel ML: Mechanism of citrinin-induced dysfunction of mitochondria. I. Effects on respiration, enzyme activities and membrane potential of renal cortical mitochondria. J Appl Toxicol. 1992;12(2):123–129. 10.1002/jat.2550120209 [DOI] [PubMed] [Google Scholar]
- 57. Chagas GM, Oliveira BM, Campello AP, et al. : Mechanism of citrinin-induced dysfunction of mitochondria. II. Effect on respiration, enzyme activities, and membrane potential of liver mitochondria. Cell Biochem Funct. 1992;10(3):209–216. 10.1002/cbf.290100311 [DOI] [PubMed] [Google Scholar]
- 58. Chagas GM, Oliveira MA, Campello AP, et al. : Mechanism of citrinin-induced dysfunction of mitochondria. IV--Effect on Ca 2+ transport. Cell Biochem Funct. 1995;13(1):53–59. 10.1002/cbf.290130110 [DOI] [PubMed] [Google Scholar]
- 59. Chagas GM, Oliveira MB, Campello AP, et al. : Mechanism of citrinin-induced dysfunction of mitochondria. III. Effects on renal cortical and liver mitochondrial swelling. J Appl Toxicol. 1995;15(2):91–95. 10.1002/jat.2550150206 [DOI] [PubMed] [Google Scholar]
- 60. Da Lozzo EJ, Oliveira MB, Carnieri EG: Citrinin-induced mitochondrial permeability transition. J Biochem Mol Toxicol. 1998;12(5):291–297. [DOI] [PubMed] [Google Scholar]
- 61. Jeswal P: Citrinin-induced chromosomal abnormalities in the bone marrow cells of Mus musculus. Cytobios. 1996;86(344):29–33. [PubMed] [Google Scholar]
- 62. Ribeiro SM, Campello AP, Chagas GM, et al. : Mechanism of citrinin-induced dysfunction of mitochondria. VI. Effect on iron-induced lipid peroxidation of rat liver mitochondria and microsomes. Cell Biochem Funct. 1998;16(1):15–20. [DOI] [PubMed] [Google Scholar]
- 63. Ribeiro SM, Chagas GM, Campello AP, et al. : Mechanism of citrinin-induced dysfunction of mitochondria. V. Effect on the homeostasis of the reactive oxygen species. Cell Biochem Funct. 1997;15(3):203–209. [DOI] [PubMed] [Google Scholar]
- 64. Ryan D, Drysdale AJ, Lafourcade C, et al. : Cannabidiol targets mitochondria to regulate intracellular Ca 2+ levels. J Neurosci. 2009;29(7):2053–2063. 10.1523/JNEUROSCI.4212-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Devinsky O, Whalley BJ, Di Marzo V: Erratum to: Cannabinoids in the Treatment of Neurological Disorders. Neurotherapeutics. 2015;12(4):910. 10.1007/s13311-015-0392-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Devinsky O, Whalley BJ, Di Marzo V: Cannabinoids in the Treatment of Neurological Disorders. Neurotherapeutics. 2015;12(4):689–691. 10.1007/s13311-015-0388-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Friedman D, Devinsky O: Cannabinoids in the Treatment of Epilepsy. N Engl J Med. 2015;373(11):1048–1058. 10.1056/NEJMra1407304 [DOI] [PubMed] [Google Scholar]
- 68. Rosenberg EC, Tsien RW, Whalley BJ, et al. : Cannabinoids and Epilepsy. Neurotherapeutics. 2015;12(4):747–768. 10.1007/s13311-015-0375-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cilio MR, Thiele EA, Devinsky O: The case for assessing cannabidiol in epilepsy. Epilepsia. 2014;55(6):787–790. 10.1111/epi.12635 [DOI] [PubMed] [Google Scholar]
- 70. Devinsky O, Cilio MR, Cross H, et al. : Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55(6):791–802. 10.1111/epi.12631 [DOI] [PMC free article] [PubMed] [Google Scholar]