Abstract
Purpose
CT plays a crucial role in the early assessment of patients with traumatic brain injury (TBI). Marshall and Rotterdam are the mostly used scoring systems, in which CT findings are grouped differently. We sought to determine the values of the scoring system and initial CT findings in predicting the death at hospital discharge (early death) in patients with TBI.
Methods
There were consecutive 634 traumatic neurosurgical patients with mild-to-severe TBI admitted to the emergency department of College of Medical Sciences. Their initial CT and status at hospital discharge (dead or alive) were reviewed, and both CT scores were calculated. We examined whether each score is related to early death; compared the two scoring systems' performance in predicting early death, and identified the CT findings that are independent predictors for early death.
Results
Both imaging score (Marshall) and clinical score (Rotterdam) can be used to reliably predict mortality in patients with acute traumatic brain injury with high prognostic accuracy. Other specific CT characteristics that can be used to predict early mortality are traumatic subarachnoid hemorrhage, midline shift and status of the peri-mesencephalic cisterns.
Conclusions
Marshall CT classification has strong predictive power, but greater discrimination can be obtained if the individual CT parameters underlying the CT classification are included in a prognostic model as in Rotterdam score. Consequently, for prognostic purposes, we recommend the use of individual characteristics rather than the CT classification. Performance of CT models for predicting outcome in TBI can be significantly improved by including more details of variables and by adding other variables to the models.
Keywords: Brain injuries, Tomography computed, Prognosis, Marshall score, Rotterdam score
Introduction
Traumatic brain injury (TBI) is a global burden. Therefore it is prudent to have a classification that correctly diagnose and accurately predict the outcome following TBI. Routinely, patients with TBI are categorized into mild, moderate and severe head injury by the use of Glasgow coma scale (GCS).1 However, in patients with severe head injuries who are intubated for airway protection, paralyzed for medical control of raised intracranial pressure or in those patients who are restless and therefore sedated for compliance for CT head scan, correct assessment of GCS score cannot be undertaken.2, 3, 4, 5 In such scenario, the best and earliest resort would be utilization of the model system that incorporates the morphological criteria based on radiological images. Though magnetic resonance imaging remains a valid option, time frame for its acquisition and hindrance of its utilization in ventilated sick patients limits its role for the same. It is therefore limited for detecting white matter changes in later phases of the disease.6, 7 Therefore, in the armamentarium of current radio imaging, CT remains the ideal imaging option for assessment of acute structural damage following TBI.
CT scan has enabled us to improve ourselves with leaps and bounces when it comes to improving the overall outcome of the patients with TBI. 8, 9, 10 The prognostic value of individual CT variables such as the status of basal cistern,11, 12, 13, 14, 15, 16, 17, 18, 19 midline shift13, 14, 18, 19, 20, 21, 22, 23 traumatic subarachnoid hemorrhage 11, 13, 14, 19, 22, 24, 25, 26, 27, 28, 29, 30 and the types of intracranial lesions 11, 14, 18, 22, 31, 32, 33 have been validated in previous studies.
Materials and methods
This study includes consecutive 634 traumatic neurosurgical patients with mild-to-severe TBI admitted to the Department of Neurosurgery, College of Medical Sciences, Bharatpur, Nepal, from January 2013 to August 2014. Each CT score will be calculated by the resident and consultant on call for the day and then be tallied with the final score in the rounds. This study examines whether each score is related to early death, compares the two scoring systems' performance in predicting early death, and identifies the CT findings that are independent predictors of early death. The results will be formatted, calculated and a P-value will be assessed using the SPSS 20 software.
Results
Relationship between Marshall CT scoring and mortality
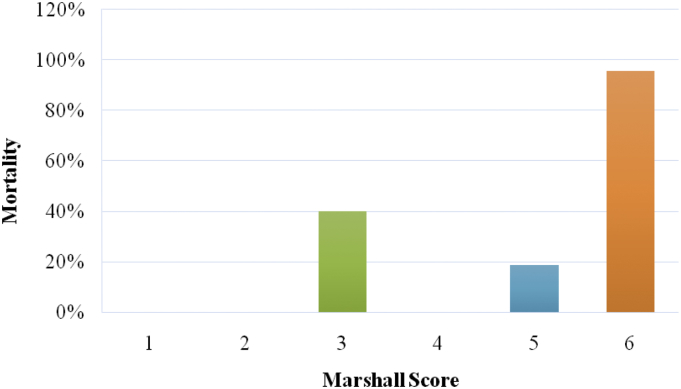
This signifies the importance of Marshall score in predicting mortality in patients with TBI. The mortality in patients with Marshall score 1 and 2 is 0%, for score 3 is 40%, for score 4 is 0%, for score 5 is 18.79% and for score 6 is 95.66%.This clearly proves the value of evacuation of mass lesion (Marshall score 5) in patient with traumatic brain injury in reducing the mortality compared to the patient with compressed cisterns, midline shift and non-evacuated >25 ml blood. Also there are minimal patients in group 4 because most of the patients with midline shift are taken up for operative evacuation regardless of the GCS of the patient. The mortality in patients who had undergone operative evacuation (Marshall score 5), which is the overall operative mortality in cases of TBI, is 18.79%. The mortality is highest (95.66%) for Marshall score 6 (Fig. 1).
Fig. 1.
Relationship between Marshall score and mortality.
Relationship between Rotterdam and mortality of the patients
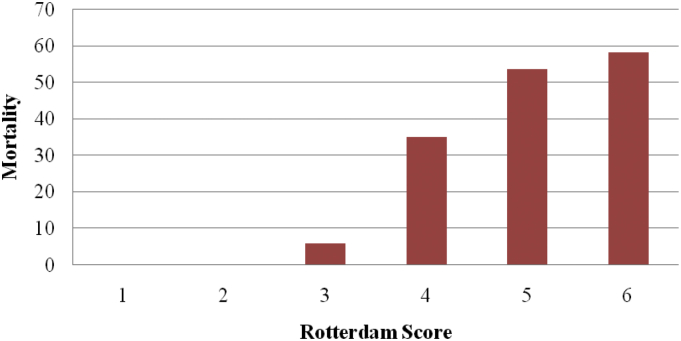
The mortality in patients with Rotterdam score 1 and 2 is 0%, for score 3 is 6%, for score 4 is 35%, for score 5 is 53.65% and for score 6 is 58.33%. This proves that higher Rotterdam score in patients with TBI has added risk of mortality. This shows positive correlation between increasing Rotterdam score and the respective mortality in patients with traumatic brain injury in the respective category (Fig. 2).
Fig. 2.
Relationship between Rotterdam score and mortality.
Marshall score in moderate and severe head injury
When the Marshall score is adjusted only for patients with moderate and severe head injury, then the mortality in patients with score 1 and 2 is 0%, for score 3 is 90%, for score 4 is 31.97%, for score 5 is 31.97% and for score 6 is 100%. This also shows that Marshall Score has positive predictive value in predicting mortality in patients with TBI.
Rotterdam score in moderate and severe head injury
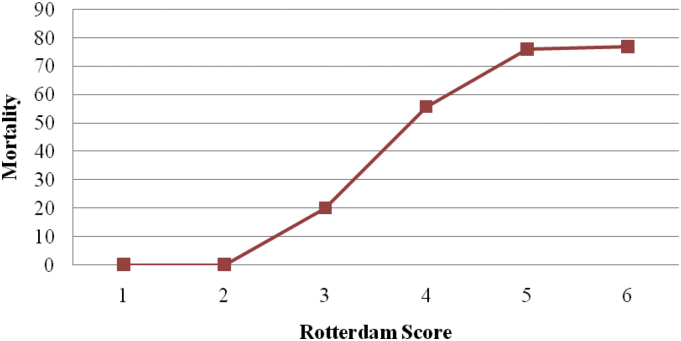
The Rotterdam score adjusted for patients with moderate and severe head injury shows that the mortality for patients with score 1 and 2 is 0%, for score 3 is 20%, for score 4 is 55.85%, for score 5 is 76% and for score 6 is 77% (Fig. 3).
Fig. 3.
Rotterdam score in moderate and severe head injuries.
Significance of other individual variables in the study model
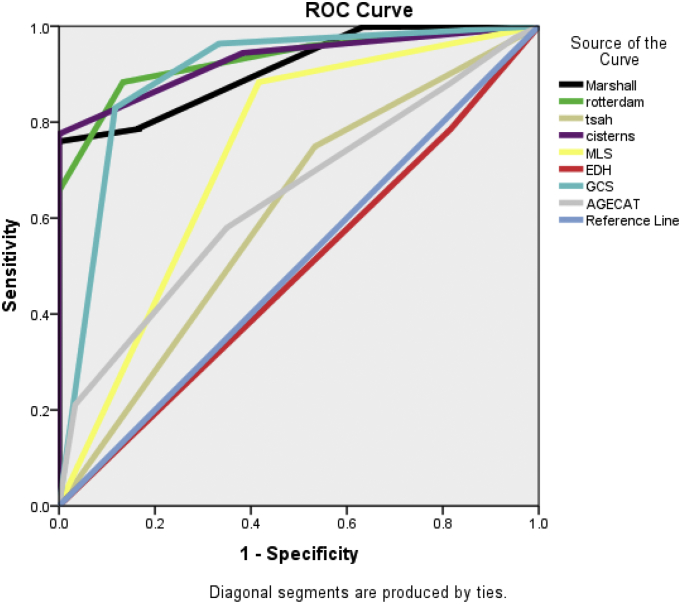
Area under the curve (AUC) as calculated by the binary logistic regression analysis was significant for both the scoring system with 0.912 for Marshall and 0.929 for Rotterdam scores respectively. When we included other variables, then the AUC was 0.929 for cisternal anatomy, 0.897 for GCS score, 0.733 for midline shift and 0.643 for age category respectively in predicting the mortality (Fig. 4).
Fig. 4.
Significance of other individual variables in the study model.
Discussion
A number of limitations of previous studies should be recognized. Firstly, results from a data which had inclusions of the patients with moderate and severe head injuries only cannot be extrapolated to the whole lot of patients with mild head injury who were left out. Secondly, the results were formulated from the analysis of characteristics of the earliest CT scan from the patients. Studies have verified the higher predictive value in better assessment of the outcome from the inclusions of the variables of the worst CT scan.34 Thirdly, outcome analysis was performed taking 6 months mortality into consideration, which may mark multiple confounding bias due to secondary insults from pneumonia, sepsis, pulmonary embolism etc thereby leading to false summations in mortalities. To limit the same, some authors have even suggested taking mortality at 1 week into account.35
Another Achilles heel in the usage of CT scans is the shortcomings of inter-observer error while correctly scoring the scan. One study concluded that even among experienced radiologists, there was inter-rater difference in scoring in at least one variable in every third cases.36 There was also significant difference in defining and categorizing the mass lesions in almost half of the cases.
Predicting outcome following TBI is the Hillary point in our quest to planning and managing our resources in patients with TBI. It bears paramount impact on the economy of developing countries. For prognostication to be clinically relevant, outcomes must provide a mirror impression of future life. In the future, it will be important to develop study models that will incorporate not just the imaging characteristics but also include the clinical parameters that play pivotal role in predicting outcome following TBI.
To summary, this study concludes that the Marshall score has good predictability for assessing the mortality. However, Rotterdam score with its individual CT parameters is a better prognostic model. Therefore, for prognostic purposes, this study recommends the use of individual characteristics rather than the CT classification.
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
References
- 1.Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 2.Malec J.F., Brown A.W., Leibson C.L. The Mayo classification system for traumatic brain injury severity. J Neurotrauma. 2007;24:1417–1424. doi: 10.1089/neu.2006.0245. [DOI] [PubMed] [Google Scholar]
- 3.Balestreri M., Czosnyka M., Chatfield D.A. Predictive value of glasgow coma scale after brain trauma: change in trend over the past ten years. J Neurol Neurosurg Psychiatry. 2004;75:161–162. [PMC free article] [PubMed] [Google Scholar]
- 4.Buechler C.M., Blostein P.A., Koestner A. Variation among trauma centers' calculation of glasgow coma scale score: results of a national survey. J Trauma. 1998;45:429–432. doi: 10.1097/00005373-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Moskopp D., Stähle C., Wassmann H. Problems of the glasgow coma scale with early intubated patients. Neurosurg Rev. 1995;18:253–257. doi: 10.1007/BF00383876. [DOI] [PubMed] [Google Scholar]
- 6.Firsching R., Woischneck D., Klein S. Classification of severe head injury based on magnetic resonance imaging. Acta Neurochir (Wien) 2001;143:263–271. doi: 10.1007/s007010170106. [DOI] [PubMed] [Google Scholar]
- 7.Uchino Y., Okimura Y., Tanaka M. Computed tomography and magnetic resonance imaging of mild head injury – is it appropriate to classify patients with Glasgow coma scale score of 13 to 15 as ‘mild injury’? Acta Neurochir (Wien) 2001;143:1031–1037. doi: 10.1007/s007010170008. [DOI] [PubMed] [Google Scholar]
- 8.Teasdale G., Galbraith S., Murray L. Management of traumatic intracranial haematoma. Br Med J. 1982;285:1695–1697. doi: 10.1136/bmj.285.6356.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel S., Castellan N.J. 2nd ed. McGraw-Hill; New York: 1988. Nonparametric Statistics for the Behavioral Sciences. [Google Scholar]
- 10.Chesnut R.M. Evolving models of neurotrauma critical care: an analysis and call to action. Clin Neurosurg. 2000;46:185–195. [PubMed] [Google Scholar]
- 11.Maas A.I., Hukkelhoven C.W., Marshall L.F. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57:1173–1182. doi: 10.1227/01.neu.0000186013.63046.6b. [DOI] [PubMed] [Google Scholar]
- 12.Cordobés F., Lobato R.D., Rivas J.J. Posttraumatic diffuse axonal brain injury. Analysis of 78 patients studied with computed tomography. Acta Neurochir (Wien) 1986;81:27–35. doi: 10.1007/BF01456261. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg H.M., Gary H.E., Jr., Aldrich E.F. Initial CT findings in 753 patients with severe head injury. A report from the NIH traumatic coma data bank. J Neurosurg. 1990;73:688–698. doi: 10.3171/jns.1990.73.5.0688. [DOI] [PubMed] [Google Scholar]
- 14.Maas A.I., Steyerberg E.W., Butcher I. Prognostic value of computerized tomography scan characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:303–314. doi: 10.1089/neu.2006.0033. [DOI] [PubMed] [Google Scholar]
- 15.Van Dongen K.J., Braakman R., Gelpke G.J. The prognostic value of computerized tomography in comatose head-injured patients. J Neurosurg. 1983;59:951–957. doi: 10.3171/jns.1983.59.6.0951. [DOI] [PubMed] [Google Scholar]
- 16.Selladurai B.M., Jayakumar R., Tan Y.Y. Outcome prediction in early management of severe head injury: an experience in Malaysia. Br J Neurosurg. 1992;6:549–557. doi: 10.3109/02688699209002372. [DOI] [PubMed] [Google Scholar]
- 17.Liu H.M., Tu Y.K., Su C.T. Changes of brainstem and perimesencephalic cistern: dynamic predictor of outcome in severe head injury. J Trauma. 1995;38:330–333. doi: 10.1097/00005373-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Kakarieka A., Braakman R., Schakel E.H. Classification of head injuries based on computerized tomography: prognostic value. Neurologia. 1995;10:159–161. [PubMed] [Google Scholar]
- 19.Servadei F., Nasi M.T., Giuliani G. CT prognostic factors in acute subdural haematomas: the value of the ‘worst’ CT scan. Br J Neurosurg. 2000;14:110–116. doi: 10.1080/02688690050004525. [DOI] [PubMed] [Google Scholar]
- 20.Young B., Rapp R.P., Norton J.A. Early prediction of outcome in head-injured patients. J Neurosurg. 1981;54:300–303. doi: 10.3171/jns.1981.54.3.0300. [DOI] [PubMed] [Google Scholar]
- 21.Quattrocchi K.B., Prasad P., Willits N.H. Quantification of midline shift as a predictor of poor outcome following head injury. Surg Neurol. 1991;35:183–188. doi: 10.1016/0090-3019(91)90069-l. [DOI] [PubMed] [Google Scholar]
- 22.Azian A.A., Nurulazman A.A., Shuaib L. Computed tomography of the brain in predicting outcome of traumatic intracranial haemorrhage in Malaysian patients. Acta Neurochir (Wien) 2001;143:711–720. doi: 10.1007/s007010170051. [DOI] [PubMed] [Google Scholar]
- 23.Pillai S.V., Kolluri V.R., Praharaj S.S. Outcome prediction model for severe diffuse brain injuries: development and evaluation. Neurol India. 2003;51:345–349. [PubMed] [Google Scholar]
- 24.Ono J., Yamaura A., Kubota M. Outcome prediction in severe head injury: analyses of clinical prognostic factors. J Clin Neurosci. 2001;8:120–123. doi: 10.1054/jocn.2000.0732. [DOI] [PubMed] [Google Scholar]
- 25.Okten A.I., Gezercan Y., Ergün R. Traumatic subarachnoid hemorrhage: a prospective study of 58 cases. Ulus Travma Acil Cerrahi Derg. 2006;12:107–114. [PubMed] [Google Scholar]
- 26.Kakarieka A., Braakman R., Schakel E.H. Clinical significance of the finding of subarachnoid blood on CT scan after head injury. Acta Neurochir (Wien) 1994;129:1–5. doi: 10.1007/BF01400864. [DOI] [PubMed] [Google Scholar]
- 27.Mattioli C., Beretta L., Gerevini S. Traumatic subarachnoid hemorrhage on the computerized tomography scan obtained at admission: a multicenter assessment of the accuracy of diagnosis and the potential impact on patient outcome. J Neurosurg. 2003;98:37–42. doi: 10.3171/jns.2003.98.1.0037. [DOI] [PubMed] [Google Scholar]
- 28.Greene K.A., Jacobowitz R., Marciano F.F. Impact of traumatic subarachnoid hemorrhage on outcome in nonpenetrating head injury. Part II: relationship to clinical course and outcome variables during acute hospitalization. J Trauma. 1996;41:964–971. doi: 10.1097/00005373-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Servadei F., Murray G.D., Teasdale G.M. Traumatic subarachnoid hemorrhage: demographic and clinical study of 750 patients from the European brain injury consortium survey of head injuries. Neurosurgery. 2002;50:261–267. doi: 10.1097/00006123-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Hernández A.V., Steyerberg E.W., Butcher I. Adjustment for strong predictors of outcome in traumatic brain injury trials: 25% reduction in sample size requirements in the IMPACT study. J Neurotrauma. 2006;23:1295–1303. doi: 10.1089/neu.2006.23.1295. [DOI] [PubMed] [Google Scholar]
- 31.Narayan R.K., Greenberg R.P., Miller J.D. Improved confidence of outcome prediction in severe head injury. A analysis of the clinical examination, multimodality evoked potentials, CT scanning, and intracranial pressure. J Neurosurg. 1981;54:751–762. doi: 10.3171/jns.1981.54.6.0751. [DOI] [PubMed] [Google Scholar]
- 32.Caroli M., Locatelli M., Campanella R. Multiple intracranial lesions in head injury: clinical considerations, prognostic factors, management, and results in 95 patients. Surg Neurol. 2001;56:82–88. doi: 10.1016/s0090-3019(01)00540-7. [DOI] [PubMed] [Google Scholar]
- 33.Bahloul M., Chelly H., Ben Hmida M. Prognosis of traumatic head injury in South tunisia: a multivariate analysis of 437 cases. J Trauma. 2004;57:255–261. doi: 10.1097/01.ta.0000083004.35231.1e. [DOI] [PubMed] [Google Scholar]
- 34.Servadei F., Murray G.D., Penny K. The value of the worst computed tomographic scan in clinical studies of moderate and severe head injury. Neurosurgery. 2000;46:70–77. doi: 10.1097/00006123-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Mardjono T., Muhammad Z.A., Arwinder S.G. Early mortality predictor of severe traumatic brain injury: a single centre study of prognostic variables based on admission characteristics. Indian J Neurotrauma. 2013;10:3–8. [Google Scholar]
- 36.Havill J.H., Sleigh J.W., Davis G.M. Observer error and prediction of outcome – grading of head injury based on CT. Crit Care Resusc. 2001;3:15–18. [PubMed] [Google Scholar]