Abstract
Purpose
To evaluate the effect of a hydroethanolic extract of Moltkia coerulea ointment (MCO) on the healing of excision wound in a rat model.
Methods
Circular surgical full thickness excision wound, with 314 mm2 size, was induced in the anterior-dorsal side of each rat. Three different doses of MCO (1%, 3% and 6%) were administrated. On Day 3, 7, 14 and 21, the tissue was sampled and immune cells, fibroblasts and fibrocytes distribution per one mm2 of wound area, collagen density and re-epithelialization were analyzed. Moreover, the total flavnoid, phenols and anti-oxidant potential of the MCO were evaluated. Ultimately, the percentage of wound contraction in different groups was compared with each other.
Results
Hydroethanolic extract of MCO significantly (p < 0.05) increased wound contraction percentage. The animals in medium and high dose MCO-treated groups exhibited remarkably (p < 0.05) higher fibroblast and fibrocyte distribution and significantly (p < 0.05) lower immune cells infiltration. On Day 7 after injury, MCO up-regulated neovascularization in a dose-dependent way.
Conclusion
Our data showed that MCO shortened the inflammation phase by provoking the fibroblast proliferation. Moreover, MCO promoted the healing process by up-regulating the angiogenesis and provoking the structural cells proliferation as well as increasing the collagen synthesis, cross-linking, and deposition.
Keywords: Moltkia coerulea, Excision wound healing, Collagen synthesis, Anti-oxidant, Rats
Introduction
Wound healing, as a normal biological process, can be achieved through four precisely and highly programmed stages including homeostasis, inflammation, proliferation, and remodeling. Indeed, under normal condition, cascade of events occur in order to achieve optimal wound healing such as rapid hemostasis, appropriate inflammation, differentiation and proliferation of mesenchymal cells, and migration to the wound site, appropriate angiogenesis, well organized re-epithelialization (re-growth of epithelial tissue over the wound surface), and finally, proper synthesis, cross-linking, and deposition of collagen to provide strength to the healing tissue.1 Considering the side effects and higher costs of synthetic chemicals in wound therapies, the interest to administrating herbal agents in this field of therapy is raising.
The Boraginaceae family are widespread plants distributed in the tropical and subtropical regions.2 They are comprises of about 131 genera and 2500 species, mainly annual, bi-annual or perennial herbs, sub-shrubs and shrubs, some trees and a few lianes.3 These plants can be subdivided into four main subfamilies, include Cordioideae, Ehretioideae, Heliotrpioideae and Lithospermae. Moltkia coerulea is one of the important plants of Boraginaceae which belongs to Lithospermeae subfamily.2 A range of biological activities, including antioxidant, antibacterial and antiviral effects have been reported for Moltkia petraea.4
M. coerulea contains a large amount of flavnoids and phenols. Flavnoids and phenols are known potent anti-oxidant agents, which are involved in promoting the anti-oxidant capacity of different tissues.4 On the other hand, previous studies indicated that administrating anti-oxidant chemicals result in significant inhibition of inflammation during the wound healing process.5, 6 Therefore, as a preliminary aim, we tried to uncover the anti-oxidant property of the M. coerulea. For this purpose, the total flavnoid, phenol and anti-oxidant power of the M. coerulea were evaluated.
Previous clinical reports showed that sufficient angiogenesis provokes the healing process. In this line, appropriate delivery of the oxygen by extensive and well organized angiogenesis prevents wounds from infection, promotes the proliferative phase and finally enhances the re-epithelialization.7 Moreover, appropriate and rapid proliferation of structural cells, such as fibroblast is considered as an essential factor in the healing process. It is reported that M. coerulea exerts anti-oxidant properties via its free radical scavenging and chelating activity.4 Therefore, here in the present study, we aimed to analyze the effect of M. coerulea hydroethanolic extract on angiogenesis, fibroblasts, fibrocytes proliferation as well as collagen synthesis, deposition and maturation during the healing process.
Methods and materials
Plant and extract preparation
M. coerulea was collected from the central district of the region of Hamedan, Iran in July 2013. The plant was authenticated in the Department of Botany Sciences, Agricultural and Natural Resources Research Center, Hamadan, Iran. Around 180 g of fresh plant material (aerial parts included leaves and flowers) was dried naturally on laboratory benches at room temperature (23 °C–24 °C) for six days until crisp and powdered in an electric blender. Then, 30 g of the plant powder was suspended in 400 ml of hydroethanolic solution (ethanol 70%; the ratio of 3 × 11) for 48 h at room temperature. The mixture was filtered using a fine muslin cloth followed by filter paper (Whatman No 1). The filtrate was placed in an oven to dry at 40 °C. The clear residue obtained was used for the study. The obtained extracts were kept at −15 °C until further use.8
Assessment of 2, 2-di (4-tert-octylphenyl)-1-picrylhydrazyl (DPPH)
DPPH free radical inhibition was assessed as described by Farahpour et al9 with some modification. Micro titer plates (96 wells) were used and 5 different concentrations of each sample were assessed. A solution of 100 mg/ml of DPPH in methanol was used and all experiments were done in triplicates. After a 45 min incubation at 25 °C (heidolph titramax 1000 and incubator 1000, Germany), the absorbances were recorded at 517 nm by using a Powerwave XS Microplate spectrophotometer (Bio-Tek Instruments, Inc., USA). The percent of free radical inhibition (In%) was calculated as follow:
where Ablank was the absorbance of the control reaction (containing all reagents except the test compound), and Asample was the absorbance of the test compound. After that the concentration which could result in 50% inhibition (IC50) was calculated from the graph plotted of inhibition percentage against samples concentration.
Total phenolic content
Total phenolic constituents of samples extracts were determined by modified methods described by Saeed et al,10 and using Folin-Ciocalteu reagent and Gallic acid (ranging from 0 to 1000 mg/L) as standard phenolic compound.
Total flavonoids estimation
The aluminum chloride method was applied for the determination of the total flavonoid content of the extracts. The flavonoid content was expressed as mg of quercetin equivalents per gram of dried extract.10
Animals and study design
Healthy white Wistar male rats weighing approximately 200 g and 9 weeks of age were used in the present study. Two weeks before and during the entire experiments, the animals were housed in individual plastic cages (50 × 40 × 20 cm) with an ambient temperature of 23 °C ± 3 °C, stable air humidity and a natural day/night cycle. Animals were handled on a regular daily basis for 2 weeks prior to the study in order to acclimatize them with testing area and experimental condition. Rats had free access to chew food and fresh water. The procedures were carried out based on the guidelines of the Ethics Committee of the International Association for the Study of pain and the current laws of the Iranian government for laboratory animal care.11 The University Research Council approved all experiments.
Formulation of topical wound application forms
Four variants of the topical application were prepared comprising Eucerin (40%) and Vaseline (60%) as the ointment base formulation for this study. After creation of the surgical wound, all rats were labeled by none toxic color and divided into five groups randomly. Two groups were served as controls: Group 1 (negative control) did not receive any administration and group 2 (positive control) was applied with base formulation (placebo). Groups 3, 4 and 5 were applied with 1 g, 3 g and 6 g of M. coerulea extract mixed with base formulation ointment (MCO), respectively. The ointments were topically applied once a day, starting from the day of operation, on the wound area until the wound was healed completely.
Secondary intention excisional dermal wound model
Each group of animals (n = 16) was anesthetized by intraperitoneal administration of ketamine 5%, 90 mg/kg (Ketaset 5%; Alfasan, Woerden, The Netherlands) and xylazine hydrochloride 2%, 5 mg/kg (Rompun 2%, Bayer, Leverkusen, Germany). The fur was prepared aseptically and the predetermined area was marked on the back of animals. Each rat was fixed on the surgery table in ventral posture. Following surgical preparation a circular surgical full thickness wound was made, 314 mm2 in size, on the anterior-dorsal side of each rat.9, 12 Wound contraction percentage and wound closure time were used to assess wound-healing property.
The wound area was measured by immediate placing of a transparent paper over the wound and tracing it out; the area of this impression was calculated using the graph sheet. The wound healing percentage was calculated by the Walker formula after measuring the wound size.9, 13 The percentage of wound healing was computed at the beginning of experiments and on Day 2, 4, 6, 8, 10, 12, 14, 16, 18 and 20 after test.
Histological study
Following 3, 7, 14 and 21 days after wound induction, cross sectional full thickness of the tissue were dissected out and different phases of the wound healing process were investigated in sampled tissues. Fixed specimens (in 10% buffered formaldehyde) were processed and blocked with paraffin and cut with rotary microtome (5 μm). Then, further special staining for different purposes were done as below.9
Assessment of collagen fibers
Collagen fibers synthesis was estimated by using special staining Masson's trichrome technique (Ayandeh Science Co. kit, Iran). In brief, sections were hydrated to distilled water and re-fixed in Bouin's solution for 1 h at 56 °C. After rinsing in distilled water, the slides were stained with Weigert's iron hematoxylin working solution for 10 min. Then, the slides were rinsed in distilled water, stained in Biebrich scarlet-acid fuchsin solution for 10–15 min and differentiated in phosphomolybdic-phosphotungstic acid solution for 10–15 min. The slides then were transferred directly (without rinse) to aniline blue solution, stained for 5–10 min, rinsed briefly in distilled water and differentiated in 1% acetic acid solution for 2–5 min. Following dehydration, the slides were cleared and coverslipped. One mm2 of the tissue were analyzed by digital analyzer (image pro-insight version 6.00) and graded as mild (+), mild to moderate (++), moderate (+++) and intensive (++++).9
Histomorphometric analyses and assessment of connective tissue and immune cells distribution
Epithelium thickness was analyzed on different days and compared between groups. Immune cells, fibroblasts and fibrocytes distribution were analyzed in one mm2 of the tissue and compared between groups. Edema in all groups was analyzed and graded as negative (−), mild (+), mild to moderate (++), moderate (+++) and intensive (++++).
Statistical analyses
Experimental results were reported as means ± SD. Statistical analyses were performed using PASW 18.0 (SPSS Inc., Chicago, IL, USA). Model assumptions were evaluated by examining the residual plot. Results were analyzed using two-way ANOVA. Dunnett's test for pair-wise comparisons was used to examine the effect of time and treatments. Statistical differences were considered significant when p < 0.05.
Results
Antioxidant activity, total phenol and flavonoid contents
The extract concentrations providing 9.45% inhibition (IC50) of DPPH free radicals were determined and compared with that of butylated hydroxytoluene (BHT), as a standard antioxidant. The phenolic content of the extract was determined based on Folin-Ciocalteu reagent method. Results showed that the assessed extract yield (122.9 ± 1.1) μg/mg for dried extract and (82.5 ± 0.8) μg eq for Rutin/mg dried extract for phenol and flavonoid contents, respectively. All data for flavnoids, phenols and anti-oxidant potential of the MCO are presented in Table 1.
Table 1.
Antioxidant properties, total phenol and total flavonoid contents of hydroethanolic MCO.
| DPPH |
Total phenols |
Total flavonoid |
|
|---|---|---|---|
| IC50 (mg/mL) | μg galic acid/mg extract | μg galic acid/mg extract | |
| M. coerulea | 9.45 | 122.9 ± 1.1 | 82.5 ± 0.8 |
| BHT | 27.03 | – | – |
| Ascorbic acid | – | – | – |
Wound contraction developed in MCO-treated animals
Administration of different doses of MCO in excision wound model significantly (p < 0.05) increased the percentage of wound contraction rate compared with control-negative animals. The wound closure achieved by all doses on 6th post wounding day, approximately close to those contractions from reference drug. Comparing the contraction rate between groups showed that from Day 9 until Day 15, the 6% MCO-treated animals exhibited significantly (p < 0.05) higher percentage of contraction rate (100% on Day 18) in comparison to 3% (98.76% ± 0.32% on Day 18) and 1.5% (89.32% ± 0.67% on Day 18) MCO-treated groups. Meanwhile, the percentage of wound contraction rate in non-treated group was only 79.60% ± 0.49% at 18 days post-injury (Table 2).
Table 2.
Effects of the topical hydroethanolic MCO on circular excision wound contraction area (mm2) and period of epithelialization.
| Groups | Percentage of wound contraction on day (%) |
Epithelialization time (d) | ||||||
|---|---|---|---|---|---|---|---|---|
| Day 3 | Day 6 | Day 9 | Day 12 | Day 15 | Day 18 | Day 21 | ||
| Control | 12.95 ± 1.10 | 27.80 ± 2.24 | 42.07 ± 2.25 | 65.85 ± 1.96 | 76.00 ± 0.73 | 79.60 ± 0.49 | 87.75 ± 0.61 | 22.23 ± 0.62 |
| Placebo | 13.15 ± 1.21 | 30.50 ± 2.12 | 46.49 ± 1.78 | 68.85 ± 1.04 | 78.35 ± 1.56 | 80.50 ± 0.85 | 89.88 ± 0.26 | 21.19 ± 0.90 |
| LD (MCO 1%) | 14.90 ± 1.59 | 34.70 ± 1.76a | 57.87 ± 2.35a | 70.80 ± 1.46a | 84.30 ± 1.43a | 89.32 ± 0.67a | 96.00 ± 0.00b | 18.88 ± 0.71a |
| MD (MCO 3%) | 15.68 ± 1.66 | 39.76 ± 4.88a | 61.77 ± 1.49a | 79.25 ± 1.00a | 86.15 ± 0.41a | 93.76 ± 0.32b | 100.00 ± 0.0b | 18.15 ± 0.62a |
| HD (MCO 6%) | 16.99 ± 1.89 | 44.30 ± 2.32b | 70.30 ± 1.92b | 85.95 ± 1.35b | 90.35 ± 0.16b | 100.00 ± 0.0b | 100.00 ± 0.0b | 17.45 ± 0.79b |
n = 6 in each group. Data are presented as mean ± S.D.
Notes: There are significant differences between groups with different codes (superscript letters a,b; p < 0.05 vs. control).
MCO down-regulated the immune cells infiltration and enhanced the proliferative phase
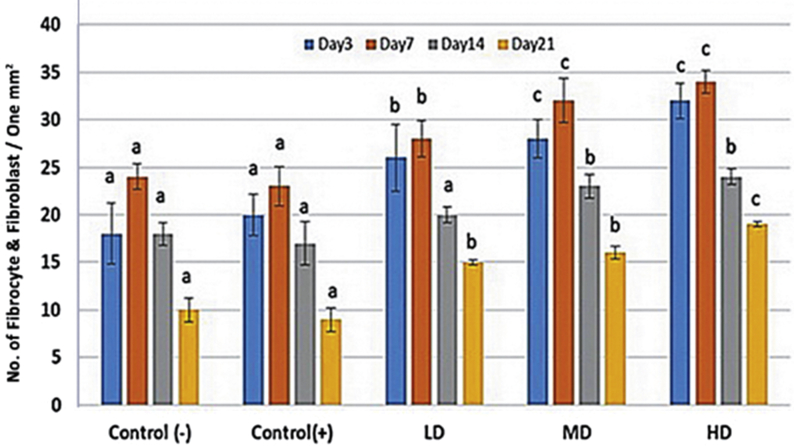
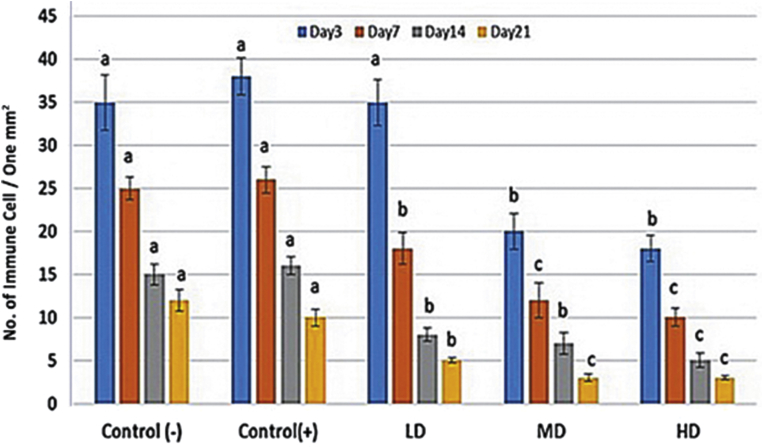
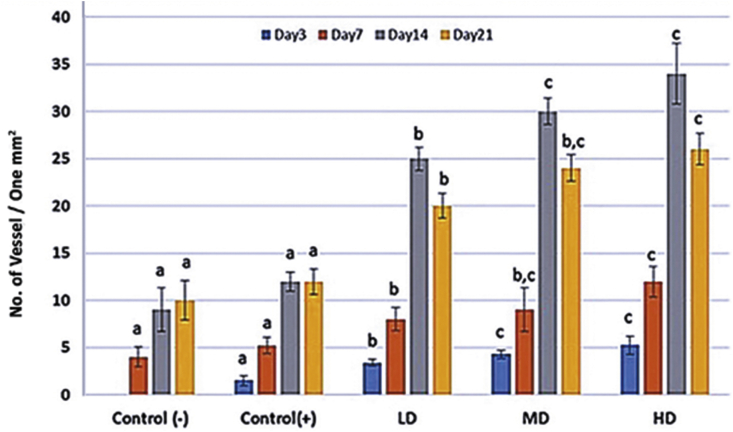
Histological observations demonstrated that administrating different doses of MCO resulted in well-formed granulation tissue associated with appropriate fibrin clots generation (Fig. 1). Moreover, light microscopic analyses showed that MCO, in a dose dependent manner, resulted in significant reduction in immune cells infiltration. Accordingly, the high dose MCO-treated animals exhibited significantly (p < 0.05) lower immune cells infiltration on Day 7 and 14 after wound induction (Fig. 2). Also, the high dose MCO-treated animals exhibited significantly (p < 0.05) higher new vessels formation on Day 7 and 14 after wound induction (Fig. 3). Comparing the fibroblasts and fibrocytes distribution in one mm2 of the wound area showed that MCO, dose dependently, enhanced the fibroblasts and fibrocytes proliferation versus control-negative group on Day 7 and 14 after wound induction (Fig. 4).
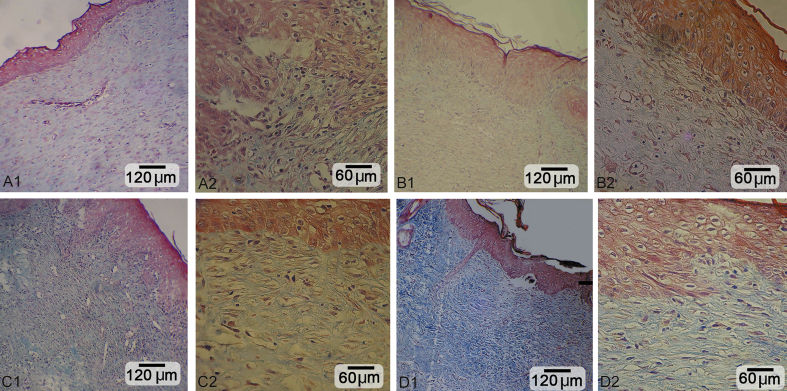
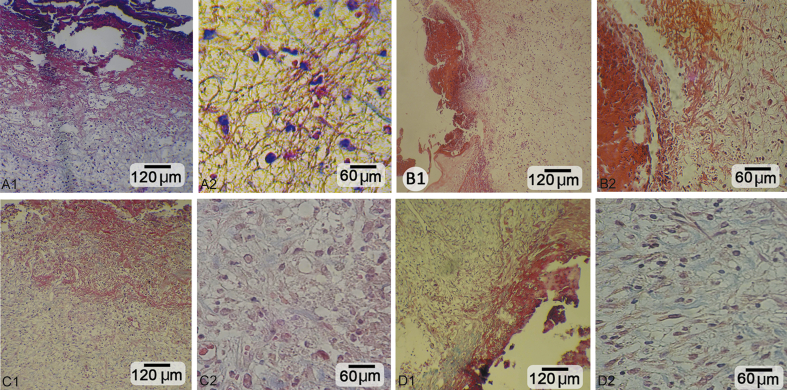
Fig. 1.
Cross section from the skin after 14 days. A1, A2: Negative control. B1, B2: Low dose-treated group: improved epithelium regeneration with well-condensed collagen content and developed angiogenesis. C1, C2: Medium dose-treated group: note ++epithelium regeneration with recovered dermis under epithelium. D1, D2: High dose-treated group: +++ epithelium generation and dermal papillae formation with intensive collagen content. Masson trichrome staining, A1, B1, C1 and D1: 400×; A2, B2, C2 and D2: 800×.
Fig. 2.
Immune cells infiltration in one mm2 of the tissue in different groups. All data are presented as mean ± SD. Note: Control: Negative control; LD: Low dose-treated (1%); MD: Medium dose-treated (3%); HD: High dose-treated (6%). a,b,care presented significant (p < 0.05) differences between marked data.
Fig. 3.
Micro vessels formation per one mm2 of the tissue in different days and different administrated doses. All data are presented as mean ± SD. Note: Control: Negative control; LD: Low dose-treated (1%); MD: Medium dose-treated (3%); HD: High dose-treated (6%). Different signs are presented significant (p < 0.05) differences between marked data.
Fig. 4.
Fibroblast and fibrocytes distribution in one mm2 of the tissue in different groups. All data are presented as mean ± SD. Note: Control: Negative control; LD: Low dose-treated (1%); MD: Medium dose-treated (3%); HD: High dose-treated (6%). a,b,care presented significant (p < 0.05) differences between marked data.
MCO decreased edema and enhanced collagen synthesis and re-epithelialization ratio
Histological demonstrations illustrated that MCO, in a dose dependent manner, reduced the edema in different regions of dermis (Table 3). Moreover, animals in MCO-treated groups exhibited intensive collagen synthesis, bundle distribution versus those in control-negative group (Fig. 5 and Table 3). Similar to collagen deposition the animals in MCO-treated groups showed enhanced re-epithelialization (Fig. 4 and Table 3).
Table 3.
The scores for tissue edema, collagen production and epidermis migration.
| Groups | Edema score | Collagen score | Epidermis thickness |
|---|---|---|---|
| Day 3 | |||
| Negative control | ++++ | − | − |
| Positive control | ++++ | − | − |
| LD (MCO 1%) | +++ | + | − |
| MD (MCO 3%) | ++ | + | − |
| HD (MCO 6%) | ++ | ++ | + |
| Day 7 | |||
| Negative control | ++++ | + | − |
| Positive control | +++ | + | − |
| LD (MCO 1%) | +++ | ++ | + |
| MD (MCO 3%) | ++ | +++ | + |
| HD (MCO 6%) | + | +++ | ++ |
| Day 14 | |||
| Negative control | +++ | ++ | + |
| Positive control | +++ | ++ | + |
| LD (MCO 1%) | ++ | +++ | + |
| MD (MCO 3%) | + | +++ | ++ |
| HD (MCO 6%) | − | +++ | ++++ |
| Day 21 | |||
| Negative control | ++ | +++ | +++ |
| Positive control | ++ | +++ | +++ |
| LD (MCO 1%) | + | +++ | ++++ |
| MD (MCO 3%) | − | ++++ | ++++ |
| HD (MCO 6%) | − | ++++ | ++++ |
Note: The Masson-trichrome staining was scored into ++++: Intensive; +++: Moderate; ++: Mild; +: Faint; −: Negative.
Fig. 5.
Cross section from the skin after 3 days. A1, A2: Negative control group: note the higher magnification for fibrin strands and immune cells infiltration in figure A2. B1, B2: Low dose-treated group. C1, C2: Medium dose-treated: note higher magnification of granulation tissue with collagen content and increased fibroblast distribution and down-regulated leukocytes infiltration (see figure B2, C2). D1, D2: High dose-treated group: note the higher collagen content in dermis under scab (non-continuous line) and well-formed granulation tissue (Fig. 2, Fig. 3) with decreased hemorrhage. The granulation tissue is presented with enhanced fibroblast and decreased number of immune cells versus negative control. Masson trichrome staining, A1, B1, C1, D1: 400×; A2, B2, C2 and D2: 800×.
Discussion
The current study showed that MCO at different administrated doses accelerated the healing process and up-regulated wound contraction rate after 7 days from wound induction. Moreover, the animals treated with MCO exhibited well-formed granulation tissue, enhanced collagen deposition as well as rapid angiogenesis in comparison to non-treated group. Analyzing the fibroblasts and fibrocytes distribution showed that medium (3%) and high dose (5%) MCO-treated animals exhibited massive proliferation of these cells on Day 7 and 14 after injury induction. Moreover, our light microscopic analyses showed that MCO accelerated re-epithelialization.
The first stage of homeostasis begins immediately after wound induction, which is marked with vascular constriction and fibrin clot formation.1 Indeed, sufficient formation of clot plays an important role in controlling the bleeding and infiltration of immune cells (chemotaxis).14 Our light microscopic analyses showed that administrating the MCO (especially in higher doses) resulted in well-formed clot in wound area. The infiltration of immune cells is marked with migration of neutrophils, macrophages, and lymphocytes.1, 14 The role of polymorph nuclear cells and mononuclear immune cells in enhancing the cellular infiltration during wound healing has been reported previously.1, 15 Macrophages and mast cells by synthesis of different growth factors (particularly vascular growth factor and fibroblast growth factor) as well as cytokines accelerate the proliferative phase and up-regulate the mononuclear immune cells infiltration via enhancing the vascular permeability and collagen synthesis.1, 15
Animals in MCO-treated group were demonstrated with intensive immune cells infiltration on Day 3 after injury. Meanwhile, it was diminished on Day 7 after wound induction. However, this situation lasted until Day 14 in non-treated group. Considering this finding, we can conclude that MCO shortened the inflammatory phase. In order to understand the importance of this property, one should note that although the presence of the immune cells is essential for secreting different growth factors, these cells also produce substances such as proteases and reactive oxygen species (ROS), which cause some additional bystander damage.7, 16 Therefore, controlling the inflammatory phase is so important. On the other hand, flavonoids and phenols are also involved in activating anti-inflammatory agents, which are responsible for inhibiting lipid peroxidation. Considering high anti-oxidant potential of the MCO, we can suggest that MCO down-regulates the inflammation by exerting anti-oxidant properties. In corroboration with this hypothesis, previous reports showed that different anti-oxidant agents such as vitamin E and other scavengers maintain and stabilize cellular membrane integrity by providing protection against destruction by oxidation.14, 17, 18
Oxygen is important for cell metabolism, especially by producing sufficient energy by involving in synthesis of ATP. It has been shown that oxygen induces angiogenesis, increases keratinocyte differentiation, migration, and re-epithelialization, enhances fibroblast proliferation and collagen synthesis.7, 16, 19
Our light microscopic analyses showed that animals in MCO-treated groups exhibited increased vascularization versus non-treated group. Thus, it would be more logic to hypothesis that MCO improved the healing process by accelerating the angiogenesis as well as accelerating oxygen delivery.
In the reparative dermis, fibroblasts and endothelial cells are the most prominent cell types that present and support capillary growth.1 Our analyses showed that the distribution/proliferation of fibroblasts and fibrocytes increased in MCO-treated animals (especially in high dose-treated group). Considering that fibroblasts produce collagen as well as glycosaminoglycans and proteoglycans as major components of the extracellular matrix (ECM).1, 14 MCO promoted the collagen synthesis by up-regulating the fibroblasts and fibrocyte cells proliferation.
It is known that well and rapid re-epithelialization decrease the distances and decline the size of the wound.20 For this propose, the contraction is so essential. In present study we showed that MCO shortened the time for re-epithelialization and enhanced the contraction percentage, dose dependently in both kinds of wounds. Thus, we can come close to this fact that MCO by accelerating the healing process shortened the time needed to appropriate contraction.
In conclusion, our data show that MCO exerts beneficial impact, partly by its anti-oxidant properties. Moreover, MCO shortens the inflammatory phase and provokes the fibroblasts, fibrocytes proliferation and enhances the angiogenesis. Ultimately, it accelerates the epithelilization and increases contraction percentage. Therefore, MCO can be considered as an appropriate chemical for wound healing. Albeit more analyses are needed for this purpose.
Conflicts of interest
Authors declare that they have no conflict of interest.
Acknowledgment
Authors wish to thank the Ayandeh Laboratory and Science Company for technical and laboratory helps.
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
References
- 1.Beldon P. Basic science of wound healing. Surg Oxf. 2010;28:409. [Google Scholar]
- 2.Azimova S.S., Glushenkova A.I. Springer Science+ Business Medical LLC; New York: 2012. Lipids, lipophilic components and essential oils from plant sources; p. 163. [Google Scholar]
- 3.Doğu S., Dinç M., Pinar N.M. Anatomical and micromorphological differentiation in the genus moltkia lehm in turkey. Pak J Bot. 2012;44:1083–1090. [Google Scholar]
- 4.Koncić M.Z., Kremer D., Gruz J. Antioxidant and antimicrobial properties of Moltkia petraea (Tratt.) Griseb. flower, leaf and stem infusions. Food Chem Toxicol. 2010;48:1537–1542. doi: 10.1016/j.fct.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Fitzmaurice S.D., Sivamani R.K., Isseroff R.R. Antioxidant therapies for wound healing: a clinical guide to currently commercially available products. Skin Pharmacol Physiol. 2011;24:113–126. doi: 10.1159/000322643. [DOI] [PubMed] [Google Scholar]
- 6.Nayak S.B., Kanhai J., Milne D.M. Experimental evaluation of ethanolic extract of Carapa guianensis L. leaf for its wound healing activity using three wound models. Evid Based Compl Altern Med. 2011;2011:419612. doi: 10.1093/ecam/nep160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sano H., Ichioka S., Sekiya N. Influence of oxygen on wound healing dynamics: assessment in a novel wound mouse model under a variable oxygen environment. PLoS One. 2012 doi: 10.1371/journal.pone.0050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayouni E.A., Miled K., Boubaker S. Hydroalcoholic extract based-ointment from Punica granatum L. peels with enhanced in vivo healing potential on dermal wounds. Phytomedicine. 2011;18:976–984. doi: 10.1016/j.phymed.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Farahpour M.R., Mirzakhani N., Doostmohammadi J. Hydroethanolic Pistacia atlantica hulls extract improved wound healing process; evidence for mast cells infiltration, angiogenesis and RNA stability. Int J Surg. 2015;17:88–98. doi: 10.1016/j.ijsu.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Saeed N., Khan M.R., Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;12:221. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmermann M. Ethical guidelines for investigations of experimental pain inconscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 12.Mekonnen A., Sidamo T., Asres K. In vivo wound healing activity and phytochemical screening of the crude extract and various fractions of Kalanchoe petitiana A. Rich (Crassulaceae) leaves in mice. J Ethnopharmacol. 2013;145:638–646. doi: 10.1016/j.jep.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Walker H.L., Mason A.D., Jr. A standard animal burn. J Trauma. 1968;8:1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Derakhshanfar A., Oloumi M., Mirzaie M. Study on the effect of Peganum harmala extract on experimental skin wound healing in rat: pathological and biomechanical findings. Comp Clin Pathol. 2010;19:169–172. [Google Scholar]
- 15.Younan G.J., Heit Y.I., Dastouri P. Mast cells are required in the proliferation and remodeling phases of microdeformational wound therapy. Plast Reconstr Surg. 2011;128:649e–658e. doi: 10.1097/PRS.0b013e318230c55d. [DOI] [PubMed] [Google Scholar]
- 16.Murphy P.S., Evans G.R. Advances in wound healing: a review of current wound healing products. Plast Surg Int. 2012 doi: 10.1155/2012/190436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamid A.A., Aiyelaagbe O.O., Usman L.A. Antioxidants: Its medicinal and pharmacological applications. Afr J Pure Appl Chem. 2010;4:142–151. [Google Scholar]
- 18.Yip W.L. Influence of oxygen on wound healing. Int Wound J. 2014 doi: 10.1111/iwj.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo S., DiPietro L.A. Factors affecting wound healing. J Dent Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amar M.B., Wu M. Re-epithelialization: advancing epithelium frontier during wound healing. J R Soc Interface. 2014;11:20131038. doi: 10.1098/rsif.2013.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]