Abstract
Purpose
To evaluate midazolam sequential with dexmedetomidine for agitated patients undergoing weaning to implement light sedation in ICU.
Methods
This randomized, prospective study was conducted in Tianjin Third Central Hospital, China. Using a sealed-envelope method, the patients were randomly divided into 2 groups (40 patients per group). Each patient of group A received an initial loading dose of midazolam at 0.3–3 mg/kg·h 24 h before extubation, followed by an infusion of dexmedetomidine at a rate of 0.2–1 μg/kg·h until extubation. Each patient of group B received midazolam at a dose of 0.3–3 mg/kg·h until extubation. The dose of sedation was regulated according to RASS sedative scores maintaining in the range of -2-1. All patients were continuously monitored for 60 min after extubation. During the course, heart rate (HR), mean artery pressure (MAP), extubation time, adverse reactions, ICU stay, and hospital stay were observed and recorded continuously at the following time points: 24 h before extubation (T1), 12 h before extubation (T2), extubation (T3), 30 min after extubation (T4), 60 min after extubation (T5).
Results
Both groups reached the goal of sedation needed for ICU patients. Dexmedetomidine was associated with a significant increase in extubation quality compared with midazolam, reflected in the prevalence of delirium after extubation (20% (8/40) vs 45% (18/40)), respectively (p = 0.017). There were no clinically significant decreases in HR and MAP after infusing dexmedetomidine or midazolam. In the group A, HR was not significantly increased after extubation; however, in the group B, HR was significantly increased compared with the preextubation values (p < 0.05). HR was significantly higher in the group B compared with the group A at 30 and 60 min after extubation (both, p < 0.05). Compared with preextubation values, MAP was significantly increased at extubation in the group B (p < 0.05) and MAP was significantly higher at T3, T4, T5 in the group B than group A (p < 0.05). There was a significant difference in extubation time ((3.0 ± 1.5) d vs (4.3 ± 2.2) d, p < 0.05), ICU stay ((5.4 ± 2.1) d vs (8.0 ± 1.4) d, p < 0.05), hospital stay ((10.1 ± 3.0) d vs (15.3 ± 2.6) d, p < 0.05) between group A and B.
Conclusion
Midazolam sequential with dexmedetomidine can reach the goal of sedation for ICU agitated patients, meanwhile it can maintain the respiratory and circulation parameters and reduce adverse reactions.
Keywords: Dexmedetomidine, Midazolam, Light sedation, Mechanical ventilation
Introduction
Tracheal intubation and extubation may be associated with hypertension and tachycardia. Dexmedetomidine, a highly selective α2-adrenoreceptor agonist, is used for sedation management in various clinical settings and shows an anesthetic-sparing effect.1, 2 Dexmedetomidine provides excellent sedation with minimal cardiovascular instability or respiratory depression and may be a useful adjunct to facilitate smooth tracheal extubation. It has also been reported to decrease the plasma catecholamine responses to intubation and extubation.3, 4 The aim of this study was to compare the effects of dexmedetomidine and midazolam on recovery and hemodynamic responses to tracheal extubation in patients. It was a prospective analysis in 80 patients involved in a randomized, double-blind trial.
Methods
Patients and study design
This study was carried out in 80 patients (40 in each group) at the age range of 38–76 years. Table 1 shows the study group's main demographics. Using a computer-generated randomization scheme, the patients were randomly divided into 2 groups (40 patients per group). Patients with cardiovascular disorders, diabetes, hypertension, morbidly obese patients, difficult airway, medications that affect heart rate (HR) or blood pressure (BP), pregnancy, currently breast feeding, history of sleep apnea and those for emergency procedures were excluded. Patient exclusion criteria were as follows: (1) patient age <18 years or >80 years, (2) mean arterial pressure (MAP) <55 mmHg, (3) HR <55 beats/min, (4) dysrhythmia, (5) those with a history of psychiatric/neurological illness, (6) patients with known allergic reaction to any medication used in this study and (7) patients on recent use of sedatives or analgesics.
Table 1.
Richmond agitation-sedation scale.
| Score | Term | Description |
|---|---|---|
| +4 | Combative | Overtly combative, violent, immediate danger to staff |
| +3 | Very agitated | Pulls or removes tube(s) or catheter(s), aggressive |
| +2 | Agitated | Frequent unpurposed movement, intolerant for ventilator |
| +1 | Restless | Anxious but movement not aggressive or vigorous |
| 0 | Alert and calm | Awake and quiet |
| −1 | Drowsy | Not fully alert, but having sustained awakening (eye opening/eye contact) to voice (>10 s) |
| −2 | Light sedation | Being briefly awake, with eye contact to voice (<10 s) |
| −3 | Moderate sedation | Movement or eye opening to voice (but no eye contact) |
| −4 | Deep sedation | No response to voice, but movement or eye opening to physical stimulation |
| −5 | Unarousable | No response to voice or physical stimulation |
This double-blind, randomized study was conducted in Tianjin Third Central Hospital, Tianjin, China. A total of 80 agitated patients with tracheal intubation in ICU were divided into two groups as midazolam sequential with dexmedetomidine group (group A), midazolam group (group B). Each patient of group A received an initial loading dose of midazolam at 0.3–3 mg/kg·h 24 h before extubation, followed by an infusion of dexmedetomidine at a rate of 0.2–1 mg/kg·h until extubation. Each patient of group B received midazolam at a dose of 0.3–3 mg/kg·h until extubation. The dose of sedation was regulated according to RASS sedative scores maintaining in the range of -2-1. All patients were continuously monitored for 60 min after extubation. During the course, HR, MAP, extubation time, adverse reactions, ICU stay, and hospital stay were observed and recorded continuously at the following time points: 24 h before extubation (T1), 12 h before extubation (T2), extubation (T3), 30 min after extubation (T4), 60 min after extubation (T5).
RASS and extubation index
The Richmond agitation-sedation scale (RASS) is demonstrated in Table 1.
Extubation index
Resolution of the underlying cause of acute respiratory failure; hemodynamic stability, defined as no need for vasoactive/inotropic drugs; preferably absence of fever (defined as temperature <38 °C); adequate gas exchange, as indicated by a partial pressure of oxygen (fraction of inspired oxygen ratio >200 with a positive end-expiratory pressure of 5 cm H2O); partial pressure of carbon dioxide adjusted to bring blood pH value into the normal range.
Statistical analysis
Data were analyzed with SPSS 18 (SPSS Inc, Chicago, IL). Demographic and clinical data were compared using the Mann–Whitney U test for continuous variables and χ2 or Fisher's exact test, where appropriate, for categorical variables. p < 0.05 was considered significantly different.
Results
Baseline characteristics
The demographic data are shown in Table 2. There were no significant differences between two groups.
Table 2.
Clinical characteristics of the study patients.
| Characteristic | Group A | Group B |
|---|---|---|
| Sex (male: female) | 18:22 | 21:19* |
| Age (years) | 64.2 ± 9.9 | 62.5 ± 9.6* |
| APACHEⅡ score | 20.2 ± 5.0 | 19.8 ± 6.2* |
| Weight (kg) | 71.5 ± 10.5 | 70.2 ± 11.2* |
| Height (cm) | 172.8 ± 11.6 | 175.1 ± 12.5* |
| COPD (n) | 25 | 24* |
| ARDS (n) | 11 | 11* |
| Severe asthma (n) | 2 | 2* |
| Others (n) | 2 | 3* |
Note: *p > 0.05, compared with group A; COPD = chronic obstructive pulmonary disease; ARDS = acute respiratory distress syndrome.
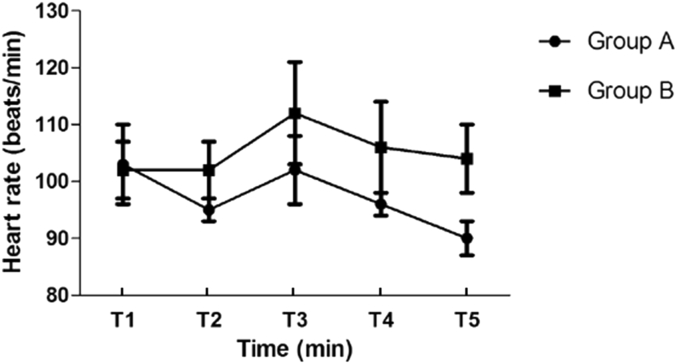
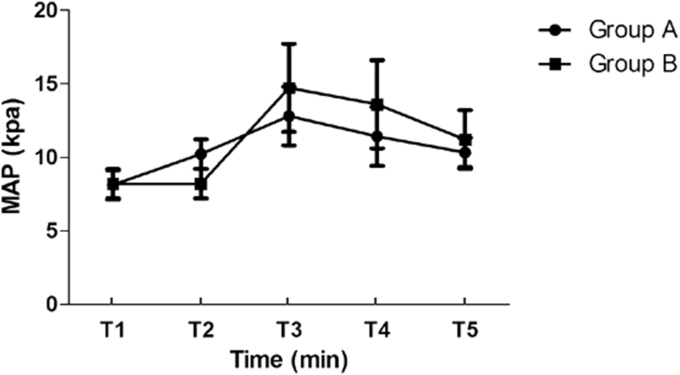
There were no clinically significant decreases in HR and MAP after infusing dexmedetomidine or midazolam. In the group A, HR was not significantly increased after extubation; however, in the group B, HR was significantly increased compared with the preextubation values (p < 0.05). HR was significantly higher in the group B compared with the group A at 30, 60 min after extubation (both, p < 0.05, Fig. 1). Compared with preextubation values, MAP was significantly increased at extubation in the group B (p < 0.05) and MAP was significantly higher at T3, T4, T5 in the group B than group A (p < 0.05, Fig. 2).
Fig. 1.
Trend of heart rate in two groups.
Fig. 2.
Trend of mean arterial pressure in two groups.
Dexmedetomidine was associated with a significant increase in extubation quality compared with midazolam, reflected in the prevalence of delirium after extubation (20% (8/40) vs 45% (18/40)), respectively (p = 0.017). There was a significant difference in extubation time (3.0 ± 1.5 vs 4.3 ± 2.2, p < 0.05), ICU stay (5.4 ± 2.1 vs 8.0 ± 1.4, p < 0.05), hospital stay (10.1 ± 3.0 vs 15.3 ± 2.6, p < 0.05) between group A and B (Table 3).
Table 3.
Recovery parameters.
| Variable | Group A | Group B | p value |
|---|---|---|---|
| Duration of mechanical ventilation (d) | 3.0 ± 1.5 | 4.3 ± 2.2 | <0.05 |
| ICU stay (d) | 5.4 ± 2.1 | 8.0 ± 1.4 | <0.05 |
| Length of hospital stay (d) | 10.1 ± 3.0 | 15.3 ± 2.6 | <0.05 |
| Cases of delirium | 8 | 18 | 0.017 |
Discussion
The presence of delirium in the ICU has been shown to increase the period of mechanical ventilation, ICU and hospital stay, and overall hospitalization cost.5, 6, 7, 8 Several studies reported that prolonged periods of intubation increased the risk of delirium by 1.10–7.90 times as compared to short periods of intubation.9 Consequently, choices of sedatives may be critical for preventing delirium. This study, together with previous work,10 proved dexmedetomidine to be an effective and safe agent for sedation in the ICU. Unlike previous studies, this one sought to compare midazolam sequential with dexmedetomidine with midazolam alone.
Dexmedetomidine has recently attracted considerable attention due to its ability to provide adequate sedation and analgesia without producing excessive hypotension. Unlike conventional sedatives such as midazolam, dexmedetomidine can produce anxiolysis and sedation without provoking significant respiratory depression. Koroglu et al10 found that dexmedetomidine had better quality of sedation and less need for rescue sedation compared with midazolam, without significant adverse effect on hemodynamic or respiratory function. These findings were consistent with our study results, which indicated a significant decrease in HR and MAP. The increases in HR and MAP after extubation were significantly lower with midazolam sequential with dexmedetomidine compared with midazolam used alone (p < 0.05). Thus, because of its sympatholytic activity, dexmedetomidine attenuates various stress responses during extubation and maintains hemodynamic stability.
Several important differences were noted in this study. Midazolam sequential with dexmedetomidine could reduce the length of ICU stay and hospital stay and duration of mechanical ventilation and incidence of delirium. Riker et al11 found that extubation time was significantly shortened in patients sedated with dexmedetomidine compared with those receiving midazolam. In some studies, no intraoperative or post-operative adverse effects were reported in dexmedetomidine group.12, 13 In contrast, few patients in the midazolam group had oxygen desaturation, nausea and vomiting. The patients treated with dexmedetomidine could be aroused easier with adequate sedation and presented less significant respiratory depression.14 Consequently, the patients had a shorter overall ventilation time and an decreased risk of prolonged hospitalization, which may explain the improved outcomes.
There were some limitations in our study: (1) it was conducted in a single medical center; (2) the timing, dosage and target group of dexmedetomidine needed to be validated by future extensive randomized studies to confirm the benefit; (3) the sample size was too small for broad generalization.
In conclusion, midazolam sequential with dexmedetomidine can reach the goal of sedation for ICU agitated patients, meanwhile it can maintain the respiratory and circulation parameters and reduce adverse reactions.
Fund
This research was supported by a grant from Chinese Medical Association (No. 13010090394) and Tianjin Medical Major Project (12KG106, 14KG111).
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
References
- 1.Ngwenyama N.E., Anderson J., Hoernschemeyer D.G. Effects of dexmedetomidine on propofol and remifentanil infusion rates during total intravenous anesthesia for spine surgery in adolescents. Paediatr Anaesth. 2008;18:1190–1195. doi: 10.1111/j.1460-9592.2008.02787.x. [DOI] [PubMed] [Google Scholar]
- 2.Bulow N.M., Barbosa N.V., Rocha J.B. Opioid consumption in total intravenous anesthesia is reduced with dexmedetomidine: a comparative study with remifentanil in gynecologic videolaparoscopic surgery. J Clin Anesth. 2007;19:280–285. doi: 10.1016/j.jclinane.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Jaakola M.L., Ali-Melkkilä T., Kanto J. Dexmedetomidine reduces intraocular pressure, intubation responses and anaesthetic requirements in patients undergoing ophthalmic surgery. Br J Anaesth. 1992;68:570–575. doi: 10.1093/bja/68.6.570. [DOI] [PubMed] [Google Scholar]
- 4.Talke P., Chen R., Thomas B. The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesth Analg. 2000;90:834–839. doi: 10.1097/00000539-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Milbrandt E.B., Deppen S., Harrison P.L. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 6.Lim S.M., Liu C.Y., Wang C.H. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32:2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 7.Shehabi Y., Riker R.R., Bokesch P.M. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38:2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 8.Ely E.W., Shintani A., Truman B. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. J Am Med Assoc. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 9.Burns K.D., Jenkins W., Yeh D. Delirium after cardiac surgery: a retrospective case-control study of incidence and risk factors in a Canadian sample. B C Med J. 2009;51:206–210. [Google Scholar]
- 10.Koroglu A., Demirbilek S., Teksan H. Sedative, haemodynamic and respiratory effects of dexmedetomidine in children undergoing magnetic resonance imaging examination: preliminary results. Br J Anaesth. 2005;94:821–824. doi: 10.1093/bja/aei119. [DOI] [PubMed] [Google Scholar]
- 11.Riker R.R., Shehabi Y., Bokesch P.M. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. J Am Med Assoc. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 12.Abdellatif A.A., Elkabarity R.H., Hamdy T.A. Dexmedetomedine vs midazolam sedation in middle ear surgery under local anesthesia: effect on surgical field and patient satisfaction. Egypt J Anaesth. 2012;28:117–123. [Google Scholar]
- 13.Arain S.R., Ebert T.J. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg. 2002;95:461–466. doi: 10.1097/00000539-200208000-00042. [DOI] [PubMed] [Google Scholar]
- 14.Tellor B.R., Arnold H.M., Micek S.T. Occurrence and predictors of dexmedetomidine infusion intolerance and failure. Hosp Pract (Minneap) 2012;40:186–192. doi: 10.3810/hp.2012.02.959. [DOI] [PubMed] [Google Scholar]