Abstract
Purpose
To investigate the effects of salvianolic acid B (SAB) on tumor necrosis factor α (TNF-α) induced alterations of cerebral microcirculation with a bone-abrading model.
Methods
The influences of craniotomy model and bone-abrading model on cerebral microcirculation were compared. The bone-abrading method was used to detect the effects of intracerebroventricular application of 40 μg/kg·bw TNF-α on cerebral venular leakage of fluorescein isothiocyanate (FITC)-albulmin and the rolling and adhesion of leukocytes on venules with fluorescence tracer rhodamine 6G. The therapeutical effects of SAB on TNF-α induced microcirculatory alteration were observed, with continuous intravenous injection of 5 mg/kg·h SAB starting at 20 min before or 20 min after TNF-α administration, respectively. The expressions of CD11b/CD18 and CD62L in leukocytes were measured with flow cytometry. Immunohistochemical staining was also used to detect E-selectin and ICAM-1 expression in endothelial cells.
Results
Compared with craniotomy method, the bone-abrading method preserved a higher erythrocyte velocity in cerebral venules and more opening capillaries. TNF-α intervention only caused responses of vascular hyperpermeability and leukocyte rolling on venular walls, without leukocyte adhesion and other hemodynamic changes. Pre- or post-SAB treatment attenuated those responses and suppressed the enhanced expressions of CD11b/CD18 and CD62L in leukocytes and E-selectin and ICAM-1 in endothelial cells induced by TNF-α.
Conclusions
The pre- and post-applications of SAB during TNF-α stimulation could suppress adhesive molecular expression and subsequently attenuate the increase of cerebral vascular permeability and leukocyte rolling.
Keywords: Microcirculation, Tumor necrosis factor-alpha, Salvianolic acid B
Introduction
For the observation of brain microcirculation, craniotomy is often used by stripping off the parietal bone to expose microvessels on pia mater. In order to protect the local microcirculation from the impact of the changed physical conditions, coverslip and resin glue are used to close the local observation window, catheters and infusion pumps to produce an artificial thermostatic cerebral fluid circulation.1, 2 However, the unavoidable change of intracranial pressure after craniotomy disturbs the hemodynamic function of cerebral microcirculation, even under so-called normal or quiescent condition, possibly leading to the misinterpretation of microcirculatory functional alteration. The primary purpose of this study was to establish a microinvasion model to preserve an intact cerebral microcirculation.
As a typical pro-inflammatory mediator, tumor necrosis factor α (TNF-α) can trigger a serious inflammatory response, including the activation of leukocytes and endothelial cells, adhesion of leukocytes on endothelium and the increase of vascular permeability, etc.1 It is also suggested that TNF-α induced inflammation can play a crucial role in secondary insult of traumatic brain injury.3, 4 In this study, the early-phase change of brain microcirculation induced by TNF-α was observed in a microinvasion model and compared with that in craniotomy.
Salvia miltiorrhiza (SM) has been popularly used for the treatment of a variety of cardiovascular disorders such as coronary heart disease, hyperlipidemia and cerebrovascular disease. The major water soluble components of SM are dihydroxylphenyl lactic acid and salvianolic acid B (SAB).5, 6, 7 SAB is found to suppress neutrophil-endothelial adhesion and inhibit the expression of intercellular adhesion molecule 1 (ICAM-1) induced by TNF-α in human umbilical venous endothelial cells.8 Using the microinvasive bone-abrading method, this study aimed to observe the effect of SAB on TNF-α-induced alteration of leukocyte rheology and permeability in cerebral venules. The expressions of adhesive molecules in leukocytes and endothelial cells were detected to explore the mechanisms of rheological and permeability changes in cerebral venules.
Methods
Reagents
SAB was purchased from the Feng-Shan-Jian Medical Company, Kunming, China. Fluorescein isothiocyanate (FITC)-labeled rat anti-mouse monoclonal antibodies against CD11b, CD18 and CD62L were purchased from BD Biosciences Pharmingen, USA. Mouse TNF-α, FITC-albumin and Rhodamine 6G was from Sigma–Aldrich, Inc., USA. Goat anti-mouse antibodies against E-selectin and ICAM-1 were purchased from Santa Cruz Biotechnology, Inc., USA. Biotin-free horseradish peroxidase (HRP)-labeled anti-goat secondary antibody and 3, 3′-diaminobenzidine tetrahydrochloride (DAB) substrate kit were from Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China. All other chemicals used were of the highest grade available commercially.
Animals
C57BL mice weighing 18–22 g were purchased from the Animal Center, Health Science Center, Peking University (Beijing, certificate No. SCXK 2006-0025). The mice were housed individually in cages at 22 °C and the humidity of 40% under a 12-h light/dark cycle with free access to tap water and pellet food. The animals were fasted for 12 h before experiment but allowed free access to water. All animals were handled according to the guidelines of Peking University Animal Research Committee.
At 20 min after being anesthetized with urethane (2.0 g/kg body weight, intraperitoneal route), the mice were tracheotomized and mechanically ventilated with small animal ventilator (ALC-V8, Alcott Biotech Co., Shanghai, China) in room air. A left side incision was made on the neck, and a custom-designed occluder (a small noose consisting of a fine polyethylene catheter in a plastic tube) was implanted in left lateral common carotid artery for the detection of mean artery pressure.9 To evaluate the possible influence of systemic circulation on cerebral venular rheology and permeability, mean arterial pressure (MAP) was measured by left common carotid artery cannulation. The femoral vein was cannulated for intravenous infusion of drugs and intraperitoneal administration of FITC-albumin or rhodamine 6G.
The animal head was secured in a stereotactic frame and the skull was exposed through a midline incision. In the craniotomy model, a high-speed portable dental drill (Strong-90, Saeshin, Korea) was used to cut open a 3 mm × 3 mm cranial window in the left parietal bone and the dura mater was exposed and kept moist by dripping 37 °C normal saline. In the bone-abrading model, the dental drill was used to abrade the left parietal bone until the thickness of the bone was about 0.1 mm and there was no obvious bump on the surface. The target vessel could be clearly seen while the bone was not broken and the dura mater was not exposed. Saline at 37 °C was intermittently dripped to maintain the moisture of parietal bone surface and to avoid grinding-induced local high temperature.
Intracerebroventricular injection of TNF-α to C57BL mice
A small hole was drilled with a dental drill 2 mm from right side of the cranial raphe on a line drawn through the anterior base of the ears. A microinjector with 26-gauge needle was inserted 2 mm perpendicular to the skull and TNF-α was slowly injected at the dose of 40 μg/kg·bw (0.2 mg/ml, 4 μl for a 20 g mouse) in 30 s and then the skull pinhole was closed with bone wax. For ascertaining the areas in ventricular system of the brain where the drug was given, 1% (w/v) Evans Blue (4 μl per mice) was injected into the cerebroventricle, and the brain was taken out to observe the spread of Evan Blue under a stereomicroscope. At 20 min after injection, the whole brain surface was stained mildly. At 2 h after injection, the staining in right periventricle was reduced and the whole brain surface was further stained (images not shown). This indicated that intraventricular injection method used in the experiment could ensure the direct role of TNF-α on the pial surface.
Application of SAB before or after TNF-α intervention
The mice were randomly divided into 4 groups: control group with intravenous infusion of saline (6 ml/kg·h), beginning at 20 min before controlled intracerebro-ventricular injection of saline (200 μl/kg·bw) and lasting for 120 min without any TNF-α or SAB intervention, TNF-α treated group with 120 min continuing intravenous infusion of saline and intracerebroventricular injection of 40 μg/kg·bw TNF-α at 20 min, SAB + TNF-α treated group with 120 min continuing intravenous infusion of SAB (0.83 mg/ml, 5 mg/kg·h), beginning at 20 min before TNF-α administration, and TNF-α + SAB treated group with 120 min continuing intravenous infusion of SAB at 20 min after TNF-α administration.
Erythrocyte velocity in venules
The cerebral microcirculation was observed using a fluorescence microscope (Leica DM-LFS; Leica Microsystems, Wetzlar, Germany) equipped with a color monitor (TCL J2118A; TCL, Huizhou, China), a video timer (VTG-55B; FOR-A, Tokyo, Japan) and a DVD recorder (DVR-R25; Malata, Xiamen, China). Venules ranging from 30 to 50 μm in diameter and 200 μm in length were selected for the study. The microvessel images were recorded through a high-speed video camera system at a rate of 1000 frames per second (FASTCAM-ultima APX; Photron, San Diego, USA), and the recordings were replayed at a rate of 25 frames per second from the stored images.5, 10 The erythrocyte velocity was measured with Image-Pro Plus 5.0 software (Media Cybernetics, USA) before (baseline) and at 0, 20, 40, 60, 80, 100, and 120 min after TNF-α treatment, respectively.
Leukocyte rolling and adhesion in venules
The fluorescence tracer rhodamine 6G was administrated (5 mg/kg·bw) to the animal via the femoral vein 10 min before TNF-α administration. The cerebral cortex venules were observed using a camera (USS-301, UNIQ, USA) mounted to an intravital microscope (BX51WI, Olympus, Japan) illuminated with a mercury lamp and fluorescence videos were stored and processed. The rolling leukocytes were identified as cells rolling obviously slower than erythrocytes in the venules. The adherent leukocytes were identified as cells attached to the venular wall for more than 30 s. The number of rolling and adherent leukocytes was counted in a selected venule of 200 μm length per mouse under the basal condition and 0, 20, 40, 60, 80, 100, and 120 min after TNF-α treatment, respectively.
Venular diameter and albumin leakage
FITC-albumin was infused (50 mg/kg·bw) through the femoral vein 10 min before TNF-α application. Intravital microscopy was used to acquire fluorescent venular images. Using Image-Pro Plus 5.0 software, the inner diameter of cerebral venules (venular diameter, Dv) was measured before TNF-α treatment and 0, 20, 40, 80, 100, and 120 min after treatment. The diameter was presented as the mean of three measurements at one location. The fluorescence intensities of FITC-albumin inside the lumen of selected venules (Iv) and in the surrounding interstitial area (Ii) were estimated. The ratio Ii/Iv was calculated and compared with the baseline as an indicator of albumin leakage.
Venular blood flow and intravascular shear rate and left parietal lobe blood flow
Venular blood flow was calculated from the product of mean erythrocyte velocity () and microvascular cross-sectional area (π·Dv2/4), assuming cylindrical geometry of the blood vessels. Venular wall shear rate (WSR) was calculated on the basis of the Newtonian definition:11
Left parietal lobe blood flow was measured using laser Doppler perfusion imager (PeriScan PIM3; PERIMED, Stockholm, Sweden) in other mice of each group. The left parietal lobe of brain was exposed by bone-abrading as described above. A computer-controlled optical scanner directed a low-powered He–Ne laser beam over the exposed cortex. The scanner head was positioned in parallel to the cerebral cortex at a distance of 18.0 cm. The scanning procedure took 4 s for a measurement covering an area of 24 mm × 12 mm. At each measuring site, the beam illuminated the tissue to a depth of 0.5 mm. A color-coded image to denote specific relative perfusion levels was displayed on a video monitor.12 The images were acquired before (baseline) and 0, 20, 40, 60, 80, 100, and 120 min after TNF-α administration. Relative perfusion values for each area were normalized and expressed as percentage of the baseline.
Surface expression of CD11b/CD18 and CD62L in leukocyte membrane
To determine the expression of CD11b, CD18 and CD62L in leukocytes, blood was collected from other mice (n = 6) of all groups 2 h after treatment of TNF-α via the abdominal aorta and anti-coagulated with heparin (20 unit/ml). The samples were then labeled with 1 μg/ml FITC-conjugated mouse anti-rat antibody of CD11b, CD18, or CD62L, respectively, for 20 min at room temperature. Afterwards, erythrocytes were lysed using hemolysin as described by the manufacturer (BD Biosciences, USA), and the remaining cells were washed twice with phosphate-buffered saline. Leukocytes were then sorted by FACS Calibur Flow cytometry (BD Biosciences, USA) based on characteristic forward-/side-scatter expression, and 5000 leukocytes were evaluated from each sample for determination of mean fluorescence intensity.13
Immunohistochemical observation of E-selectin and ICAM-1 in cerebral vascular endothelial cells
Treated mice were sacrificed by cervical dislocation 2 h after intraventricular injection of TNF-α and the brain was removed and washed with saline and kept in 10% neutral buffered formalin for at least 24 h at room temperature. The brain was embedded in paraffin and 6 μm slides were cut on a sliding microtome (RM2255, LEICA, Gemany) for immunohistochemistry. All immunostaining was carried out by standard protocols using anti-E-selectin or anti-ICAM-1 as the primary antibody and a biotin-free HRP-labeled antibody as the secondary antibody. HRP complex was identified with diaminobenzidine (DAB). An upright microscope (BH-2, Olympus, Japan) connected with a color camera (Digital sight DS-5M-U1, Nikon, Japan) was used for the observation and recording.
Statistical analysis
All parameters were averaged for six animals (n = 6) and expressed as mean ± SE. Statistical analysis was performed using ANOVA followed by the Bonferroni test for multiple comparison, and p < 0.05 is considered statistically significant.
Results
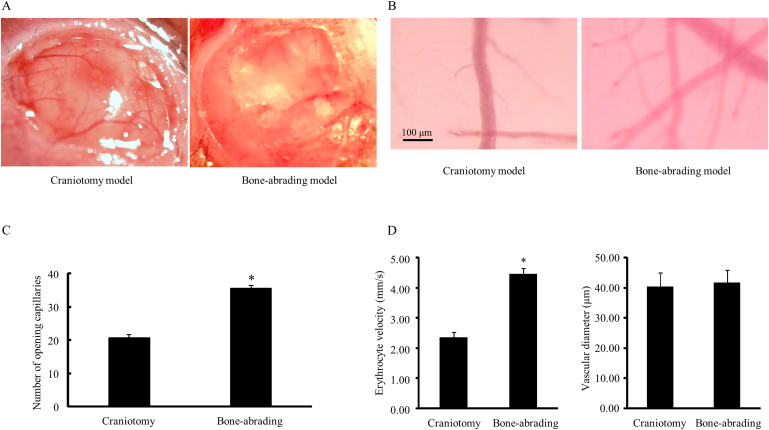
Although the image of venules and capillaries was a little blurred in a bone-abrading model, more capillaries were seen, with the number of opening capillaries significantly elevated from 20.75 ± 1.65 in the craniotomy model to 35.50 ± 1.73 in the bone-abrading model (p < 0.05, Fig. 1B, 1C). The velocity of erythrocytes in venules was also increased from (2.35 ± 0.17) mm/s in the craniotomy model to (4.45 ± 0.21) mm/s in the bone-abrading model (p < 0.05), while the venular diameters between these two models showed no significant difference (Fig. 1D). These results indicated that the bone-abrading method could maintain the microcirculation better than craniotomy method by keeping a more matched physiological microenvironment.
Fig. 1.
Effects of craniotomy method and bone-abrading method on cerebral venular microcirculation. A: The calvarium; B: The pia mater microcirculation; C: The numbers of opening capillaries in pia mater in two groups; D: The erythrocyte velocity (left panel) and diameters (right panel) in two groups. Data are expressed as means ± SEM (n = 3 in the craniotomy group, n = 6 in the bone-abrading group). * p < 0.05, compared with the craniotomy group.
The results showed that there were no changes at different time points in all groups (data not shown), suggesting that cerebral intraventricular injection of TNF-α, as well as continuous intravenous infusion of SAB, did not affect the systemic pressure of the animal. TNF-α and SAB-induced pial microcirculatory alterations in the follow-up experiments were not due to the changes of systemic blood pressure.
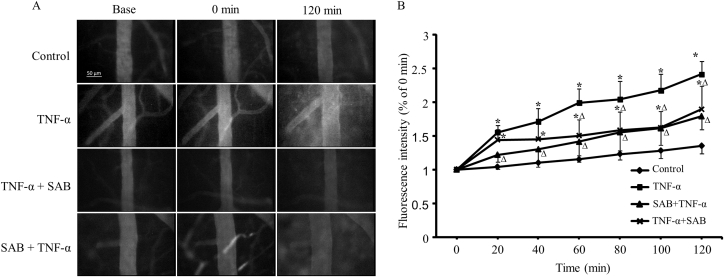
The cerebral venular permeability was detected by intravenous injection of FITC-labeled albumin through femoral vein. The images of tracing protein showed that TNF-α evoked a leakage of albumin from cerebral venules, while the intensity increased (155 ± 5) % at 20 min and (241 ± 13)% at 120 min after TNF application (Fig. 2A). Both pre- and post-administration of SAB attenuated this leakage with decreased fluorescence intensity significantly lower than that in TNF-α treated group, with the fluorescence intensity in pre-treated group only increased (122 ± 4)% and (179 ± 8)% at 20 min and 120 min after TNF-α application. In TNF-α + SAB treated group, the fluorescence intensity increased (144 ± 6)% and (189 ± 14)% at 20 min and 120 min after TNF-α application (p < 0.05, Fig. 2B). The pre-administration of SAB preserved the venular barrier function better than the post-administration of SAB while the fluorescence intensity in SAB + TNF-α treated group presented no significant difference compared with control, but it was still higher in TNF-α + SAB treated group compared with control.
Fig. 2.
Effects of pre- or post-SAB treatment on TNF-α induced hyper-permeability in cerebral venules. A: The images of FITC-labeled albumin in and out of the venules; B: The changes of venular permeability quantified with the ratio of the fluorescence intensity in and out of the venules. Data are expressed as means ± SEM (n = 3 in all groups). * p < 0.05, compared with control group. Δp < 0.05, compared with TNF-α group.
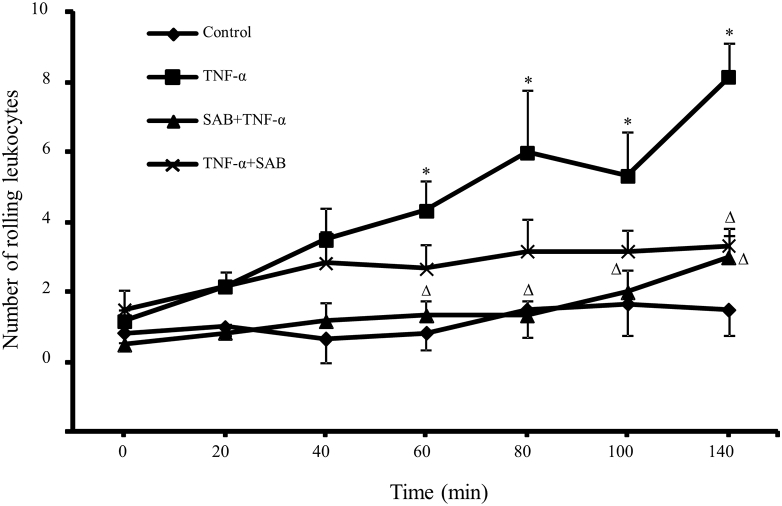
The number of rolling leukocytes on 200 μm length for 30 s was calculated by labeling the leukocytes with Rhodamine 6G. Compared with control group, TNF-α treated group had an significantly increased number of rolling leukocytes from 0.83 ± 1.17 to 4.33 ± 2.07 at 60 min, and from 1.50 ± 1.87 to 8.17 ± 2.40 at 120 min after TNF-α treatment, respectively. The pre-administration of SAB remarkably reduced the rolling leukocyte number on venular wall, with the rolling number was 1.33 ± 0.82 and 3.00 ± 1.26 at 60 min and 120 min after TNF-α treatment, significantly lower than that in TNF-α treated group (p < 0.05). The post-administration of SAB had less effect on attenuating the leukocyte rolling, only significantly decreased the number to 3.33 ± 1.21 at 120 min after TNF-α treatment (Fig. 3).
Fig. 3.
Effects of pre- or post-SAB treatment on TNF-α induced leukocyte rolling on cerebral venules. The number of rolling leukocytes was counted by staining the leukocytes with fluorescence tracer rhodamine 6G through femoral vein injection. Data are expressed as means ± SEM (n = 3 in all groups) * p < 0.05, compared with control group. Δ p < 0.05, compared with TNF-α group.
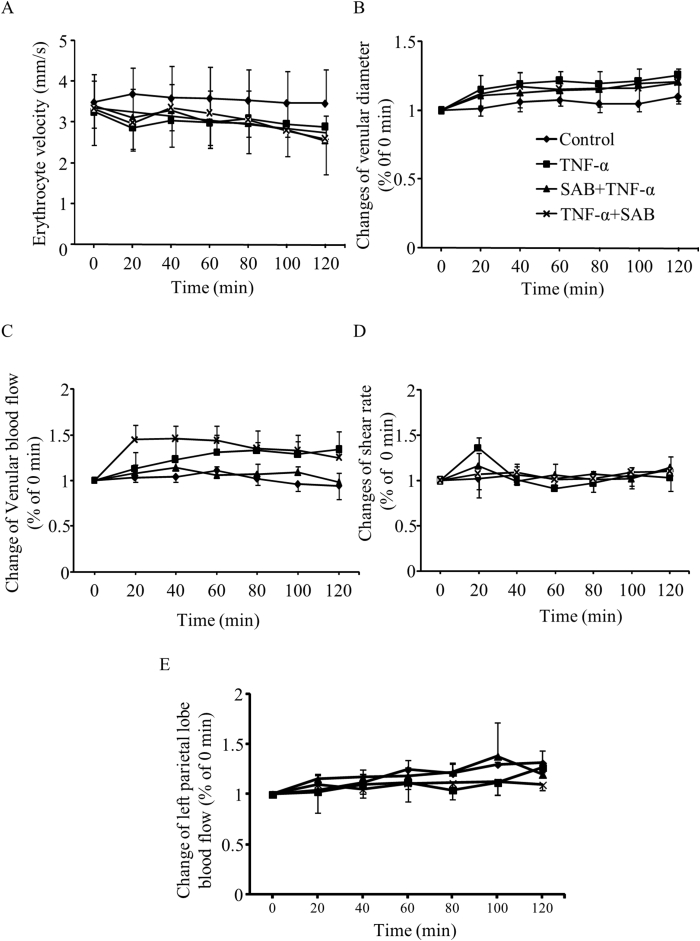
The venular diameter and erythrocyte velocity were measured in all groups and the venular blood flow and WSR were calculated accordingly. The observed venules ranged 20–40 μm in diameter with erythrocyte velocity of (3.37 ± 0.66) mm/s. The baseline venular blood flow and WSR were 1.50 × 106–1.30 × 107 μm3/s and 700–2600/s, respectively. The results showed that, although there was a trend of vessel dilatation and slowing down of the erythrocyte velocity, the intervention of TNF-α and the pre- and post-treatment of SAB plus TNF-α did not significantly change these hemodynamic parameters in cerebral venules (Fig. 4A–D), indicating that vascular hemodynamics plays little role in higher vascular permeability and increased rolling leukocytes. Since TNF-α was injected to the intracerebroventricle and left parietal lobe bone was abraded, the left parietal lobe blood flow was measured with a laser Doppler perfusion imager through the abraded bone. There was no significant alteration in left parietal lobe blood flow in mice in each group during the experiment processes (Fig. 4E).
Fig. 4.
Alteration of cerebral venular hemodynamics in TNF-α and/or SAB treated groups. A: The erythrocyte velocity of the venules; B: The diameters of the venules; C: The blood flow of the venules; D: The intravascular shear rates of the venules; E: The cerebral blood flow of left parietal lobe. Data are expressed as means ± SEM (n = 3 in all groups).
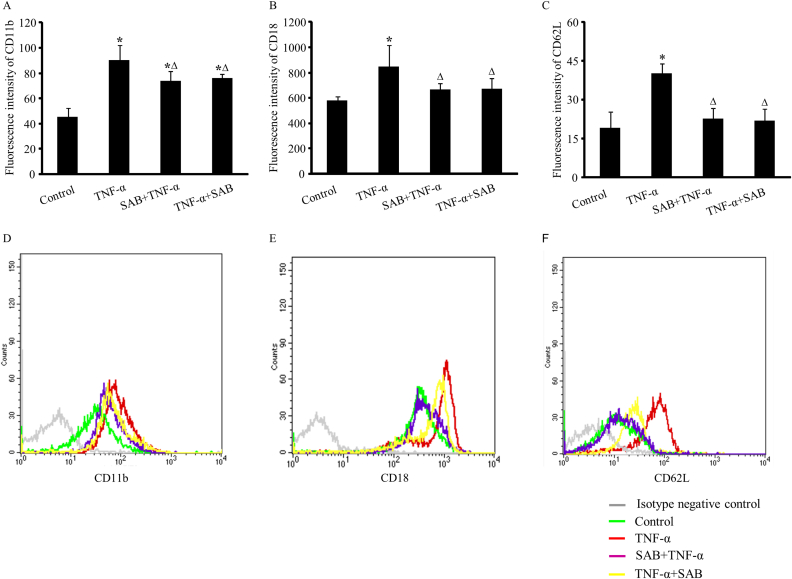
To explore the real reason for this early-phase vascular hyper-permeability and leukocyte rolling, the expressions of CD11b/CD18 and CD62L 2 h after TNF-α were detected in leukocyte membrane by isolating white blood cells from treated mice. The result presented an obvious increase in CD11b, CD18 and CD62L expressions in leukocyte membrane after TNF-α treatment, with the relative fluorescence intensity changed from 45.57 ± 7.20 to 90.41 ± 11.55, 580.46 ± 33.54 to 846.80 ± 167.47, and 19.02 ± 6.40 to 40.17 ± 3.69, respectively (p < 0.05, Fig. 5). Considering the time needed for the transcription and translation of protein, this increase was mostly due to the release and translocation of CD11b/CD18 and CD62L from previously existing vesicles. Both the pre- and post-application of SAB in TNF-α treated mice could attenuate these enhancements of adhesive molecular expressions in leukocyte membrane. The suppression on CD18 and CD62L was more thorough since their expressions were resumed close to control level. The fluorescence intensity of CD18 in pre-treated and post-treated groups was 666.76 ± 49.17 and 670.43 ± 82.65, respectively, and the fluorescence intensity of CD62L was 22.81 ± 3.95 and 21.96 ± 4.43 (p > 0.05 compared with control group). The pre- or post-administration of SAB only partially inhibited the release of CD11b, since their expression levels were still higher than those in control mice. The fluorescence intensity of CD11b was 73.91 ± 7.59 and 75.87 ± 3.66, respectively (p < 0.05 compared with control group), even it was lower than that in TNF-α treated mice (Fig. 5).
Fig. 5.
Effects of pre- or post-SAB treatment on TNF-α induced expression of adhesion molecules on leukocyte membrane. A: The expression of CD11b; B: The expression of CD18; C: The expression of CD62L. Data are represented as the geometric mean of the fluorescence intensity (GMFI ± SEM) (n = 6 in all groups). * p < 0.05, compared with control group. Δ p < 0.05, compared with TNF-α group. Representative flow plots of CD11b, CD18 and CD62L on neutrophils in different groups are also showed in D, E and F. Histograms show the distribution of immunofluorescence labeling intensity of CD11b, CD18 and CD62L expressions in each group. Ordinate indicates cell counts and abscissa represents florescent intensity.
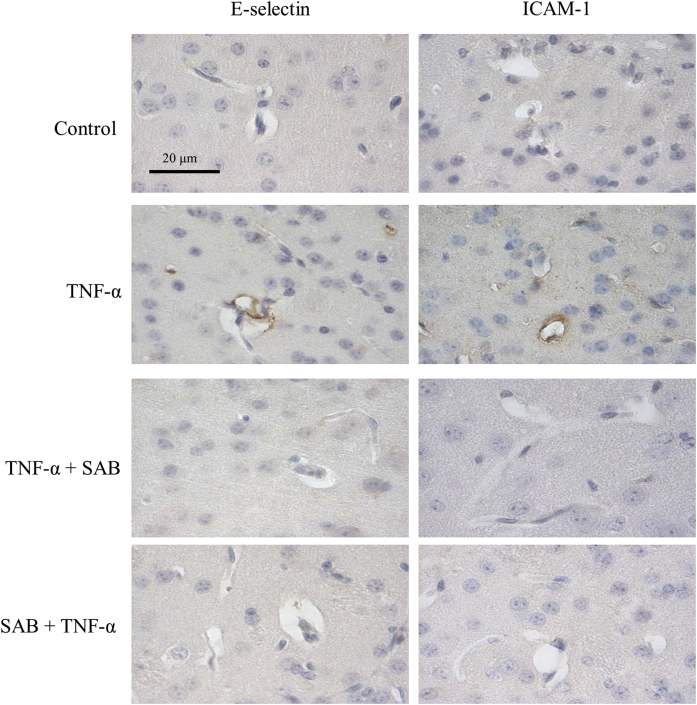
The expressions of endothelial adhesive molecules E-selectin and ICAM-1 were detected by immunohistochemistry in slices of brain tissue. Remarkable brown stainings of E-selectin and ICAM-1 in cerebral microvascular walls were both seen in TNF-α treated mice, indicating the release of these molecules from vesicles after TNF-α stimulation. The pre- or post-application of SAB abolished these molecular expression, suggesting that SAB could inhibit their release and translocation to the endothelial membrane (Fig. 6).
Fig. 6.
Immunohistochemical expressions of endothelial adhesive molecules E-selectin and ICAM-1 in cerebral microvessels of each group. All data are from at least three independent experiments.
Discussion
To establish a micro-invasive method for the cerebral microcirculation observation, a high-speed drill was used to abrade the left parietal bone until the target vessel could be clearly seen. Since the bone was not stripped off, the cerebral dura mater was not exposed and was kept intact without disturbing the intracranial pressure, so as to create a more matched physiological condition for the observation of microcirculation. It is supposed that the opening of dura mater and the subsequent alteration of intracranial pressure will disturb the intracranial circulation.14 The result in this study showed that the traditional craniotomy method significantly slowed down the erythrocyte velocity in cerebral venules of the same diameters and decreased the number of opening capillaries, compared with those in bone-abrading preparation (Fig. 1). It indicated that compared with the craniotomy method, this bone-abrading method could preserve the relatively normal microcirculation much better and the observation of cerebral microcirculation with intact dura mater would correspond with the clinical situation better.
In this study, as the advantage of bone-abrading model, intracerebroventricular injection by the subdural cavity was used in order to investigate the direct role of TNF-α in brain microcirculation. Evan's tracing observation showed that by means of cerebrospinal fluid circulation, TNF-α was quickly delivered to the entire surface of the pia mater of the brain (data not showed). This intraventricular injection minimized TNF-α dosage, enabled local distribution and directly acted on cerebral microcirculation of TNF-α.
On the basis of the above-mentioned model, the data showed that the microcirculation alterations at 2 h after TNF-α treatment were not severe, only with the responses of hyperpermeability and leukocyte rolling on venular walls, without leukocyte adhesion and other hemodynamic changes, while the dosage of TNF-α (800 ng per mouse) was already much higher than ordinary usage (100 ng per mouse),15 in addition to ICV injection as local application instead of intravenous injection. These results suggested that the early microcirculatory alteration under inflammatory stimulation observed in traditional craniotomy model might be a little aggrandized.
It would be interesting to explore the mechanism of enhanced vascular permeability and leukocyte rolling in early stage of TNF-α interference, while the hemodynamic parameters were still kept normal and there was no significant leukocyte adhesion. The result showed that 2 h TNF-α treatment enhanced the surface expressions of CD11b/CD18 and CD62L in leukocytes (Fig. 5), and E-selectin and ICAM-1 in endothelial cells (Fig. 6).
As one of the selectin family members, CD62L (L-selectin) mediates the tethering and rolling of leukocytes (especially lymphocytes) on blood vessel wall by interacting with ligands on endothelia. The molecular basis of this highly dynamic adhesion (rolling) is the low affinity and rapid kinetics of selectin interactions between leukocytes and endothelia.16 Although it is believed that adhesion molecule interaction is the structural basis of leukocyte adhesion on vascular endothelial cell surface,17 Jung et al18 performed a experiment in CD18 knockout mice and found that the interaction of CD18 and E-selectin was also involved in leukocyte rolling process.19 Although the expression of CD11b/CD18 in leukocytes was increased in this present study, no leukocyte adhesion was seen in 2 h observation time in this experiment. One possible explanation is that the expression of adhesion molecules has not yet reached its peak at early stage of TNF-α stimulation. And well-preserved hemodynamics with a high blood vessel WSR observed in experiments might also help to prevent the adhesion of leukocytes. It is also recognized that CD11b/CD18 might be pre-formed in leukocytes inside secretary granules under resting conditions, and would be translocated to the cell surface upon stimulation.20 The upregulation of CD62L expression within a short period after stimulation is also found in different types of leukocytes, although the detailed mechanism is not known yet.21, 22
It has been reported that E-selectin is not stored in the cell and has to be transcribed, translated, and transported to the cell surface. It takes about 2 h, after cytokine recognition, for E-selectin to be expressed on the endothelial cell's surface.23 It has been further characterized that, in response to TNF-α treatment, E-selectin is activated in endothelial cells with a peaking time of 2–4 h.15 The present result of TNF-α-enhanced expression of E-selectin in endothelial cells was consistent with Griffin et al's report in which TNF-α alone caused a remarkable increase in E-selectin expression in 2 h in a mouse model. It is also well known that E-selectin governs the rolling of leukocytes on venular endothelial cells during the process of leukocyte activation.24 In this study, TNF-α treatment enhanced expression of E-selectin in cerebral vascular endothelial cells in just 2 h matched the increasing rolling leukocytes in venular wall in this present study.
As a typical inflammatory mediator, TNF-α can directly target the endothelial cells and result in the activation and functional alteration. One of the most important response is the morphological adaptation of endothelial cells and the opening of inter-endothelial junctions.25 In this micro-invasive model, the administration of TNF-α caused a rapid increase in cerebral venular permeability in 20 min and this hyperpermeability response happened even quicker than the increase of leukocyte rolling (Fig. 2, Fig. 3), indicating the direct effect of TNF-α on endothelial cells.
It is well-accepted that activated leukocytes can alter vascular permeability to macromolecules by releasing various secretion mediators that target vascular endothelial cells. Recently, it is reported that the increase of microvascular permeability could be induced directly by the clustering of ICAM-1 in leukocyte.26 It is speculated that the activation and clustering of ICAM-1 in endothelial cells would have the same effects. Adherens junctional structures were disorganized directly by TNF-α and the crosstalk between endothelial adherens junctional molecule and adhesion molecule altered the endothelial barrier function.27 The present in situ study further confirms that the cerebral venular hyperpermeability could be enhanced by the increased ICAM-1 expression in endothelial cells without the adhesion of leukocyte to microvessels 2 h after TNF-α treatment.
With no exceptions, the results showed that the application of salvianolic acid B before and after TNF-α stimulation could both attenuate all the above-mentioned cerebral microcirculatory alterations induced by 2 h TNF-α intervention. Pre-treated SAB presented a better efficiency than post-treated SAB in alleviating the increase of vascular permeability and leukocyte rolling (Fig. 2, Fig. 3). TNF-α-induced increased expressions of CD11b/CD18 and CD62L in leukocytes were suppressed by SAB treatment (Fig. 6) and E-selectin and ICAM-1 staining were also weakened by SAB application. As a multi-target agent, SAB protects the cardiovascular function by multiple mechanisms.12, 28, 29 Guo et al5 demonstrated that SAB attenuates LPS-induced enhancement of CD11b and CD18 expressions in leukocytes. It was also reported that SAB attenuated the expression of endothelial cell ICAM-1 and vascular cell adhesion molecule 1 induced by TNF-α by inhibiting the activation of nuclear factor-κB.30 It was also found that SAB reduced the permeability and attenuated the disorganization of VE-cadherin induced by TNF-α in endothelial cells.31
In summary, this study modified the cerebral microcirculatory observation model with intact cerebral dura mater using bone-abrading method, resulting in a preserved intracranial pressure and better cerebral microcirculation than the craniotomy method. In this model, 2 h TNF-α intervention only caused responses of vascular hyperpermeability and leukocyte rolling on venular walls, without leukocyte adhesion and other hemodynamic changes. These responses might be triggered by enhanced expressions of CD11b/CD18 and CD62L in leukocytes and E-selectin and ICAM-1 in endothelial cells. The pre- and post-applications of SAB during TNF-α stimulation could suppress those molecular expressions and subsequently attenuate the increase of cerebral vascular permeability and leukocyte rolling.
Fund
This study was supported by the General Program from the Natural Science Foundation of China (30971201, 81170297) and Southern Medical University Research Program for Young Scientists Training.
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
References
- 1.Carvalho-Tavares J., Hickey M.J., Hutchison J. A role for platelets and endothelial selectins in tumor necrosis factor-alpha-induced leukocyte recruitment in the brain microvasculature. Circ Res. 2000;87:1141–1148. doi: 10.1161/01.res.87.12.1141. [DOI] [PubMed] [Google Scholar]
- 2.Xu X.S., Ma Z.Z., Wang F. The antioxidant cerebralcare granule attenuates cerebral microcirculatory disturbance during ischemia-reperfusioninjury. Shock. 2009;32:201–209. doi: 10.1097/SHK.0b013e3181996d61. [DOI] [PubMed] [Google Scholar]
- 3.Csuka E., Morganti-Kossmann M.C., Lenzlinger P.M. IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury:relationship to IL-6, TNF-alpha, TGF-beta1 and blood-brain barrier function. J Neuroimmunol. 1999;101:211–221. doi: 10.1016/s0165-5728(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 4.Yan E.B., Satgunaseelan L., Paul E. Post-traumatic hypoxia is associated with prolonged cerebral cytokine production, higher serum biomarker levels, and poor outcome in patients with severe traumatic brain injury. J Neurotrauma. 2014;31:618–629. doi: 10.1089/neu.2013.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo J., Sun K., Wang C.S. Protective effects of dihydroxylphenyl lactic acid and salvianolic acid B on LPS-induced mesenteric microcirculatory disturbance in rats. Shock. 2008;29:205–211. doi: 10.1097/SHK.0b013e318070c61a. [DOI] [PubMed] [Google Scholar]
- 6.Han J.Y., Fan J.Y., Horie Y. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol Therher. 2008;117:280–295. doi: 10.1016/j.pharmthera.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Xu M., Guo H., Han J. Structural characterization of metabolites of salvianolic acid B from Salvia miltiorrhiza in normal and antibiotic-treated rats by liquid chromatography-mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2007;858:184–198. doi: 10.1016/j.jchromb.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 8.Ren D.C., Du G.H., Zhang J.T. Inhibitory effect of the water-soluble extract of Salvia miltiorrhiza on neutrophil-endothelial adhesion. Jpn J Pharmacol. 2002;90:276–280. doi: 10.1254/jjp.90.276. [DOI] [PubMed] [Google Scholar]
- 9.Choki J., Greenberg J., Sclarsky D. Correlation between brain surface potassium and glucose utilization after bilateral cerebral ischemia in the gerbil. Stroke. 1984;15:851–857. doi: 10.1161/01.str.15.5.851. [DOI] [PubMed] [Google Scholar]
- 10.Han J.Y., Miura S., Akiba Y. Chronic ethanol consumption exacerbates microcirculatory damage in rat mesentery after reperfusion. Am J Physiol Gastrointest Liver Physiol. 2001;280:G939–G998. doi: 10.1152/ajpgi.2001.280.5.G939. [DOI] [PubMed] [Google Scholar]
- 11.Russell J., Cooper D., Tailor A. Low venular shear rates promote leukocyte-dependent recruitment of adherent platelets. Am J Physiol Gastrointest Liver Physiol. 2003;284:G123–G129. doi: 10.1152/ajpgi.00303.2002. [DOI] [PubMed] [Google Scholar]
- 12.Wang M.X., Liu Y.Y., Hu B.H. Total salvianolic acid improves ischemia-reperfusion-induced microcirculatory disturbance in rat mesentery. World J Gastroenterol. 2010;16:5306–5316. doi: 10.3748/wjg.v16.i42.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun K., Wang C.S., Guo J. Effect of Panax notoginseng saponins on lipopolysaccharide-induced adhesion of leukocytes in rat mesenteric venules. Clin Hemorheol Microcirc. 2006;34:103–108. [PubMed] [Google Scholar]
- 14.Schwarzmaier S.M., Kim S.W., Trabold R. Temporal profile of thrombogenesis in the cerebral microcirculation after traumatic brain injury in mice. J Neurotrauma. 2010;27:121–130. doi: 10.1089/neu.2009.1114. [DOI] [PubMed] [Google Scholar]
- 15.Griffin G.K., Newton G., Tarrio M.L. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol. 2012;188:6287–6299. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholson M.W., Barclay A.N., Singer M.S. Affinity and kinetic analysis of L-selectin (CD62L) binding to glycosylation-dependent cell-adhesion molecule-1. J Biol Chem. 1998;273:763–770. doi: 10.1074/jbc.273.2.763. [DOI] [PubMed] [Google Scholar]
- 17.Simon S.I., Burns A.R., Taylor A.D. L-selectin (CD62L) cross-linking signals neutrophil adhesive functions via the Mac-1 (CD11b/CD18) beta 2-integrin. J Immunol. 1995;155:1502–1514. [PubMed] [Google Scholar]
- 18.Jung U., Norman K.E., Scharffetter-Kochanek K. Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J Clin Invest. 1998;102:1526–1533. doi: 10.1172/JCI119893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunne J.L., Ballantyne C.M., Beaudet A.L. Control of leukocyte rolling velocity in TNF-alpha-induced inflammation by LFA-1 and Mac-1. Blood. 2002;99:336–341. doi: 10.1182/blood.v99.1.336. [DOI] [PubMed] [Google Scholar]
- 20.Zen K., Utech M., Liu Y. Association of BAP31 with CD11b/CD18. Potential role in intracellular trafficking of CD11b/CD18 in neutrophils. J Biol Chem. 2004;279:44924–44930. doi: 10.1074/jbc.M402115200. [DOI] [PubMed] [Google Scholar]
- 21.Cocks R.A., Chan T.Y., Rainer T.H. Leukocyte L-selectin is up-regulated after mechanical trauma in adults. J Trauma. 1998;45:1–6. doi: 10.1097/00005373-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Rainer T.H., Lam N., Cocks R.A. Adrenaline upregulates monocyte L-selectin in vitro. Resuscitation. 1999;43:47–55. doi: 10.1016/s0300-9572(99)00121-5. [DOI] [PubMed] [Google Scholar]
- 23.Leeuwenberg J.F., Smeets E.F., Neefjes J.J. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology. 1992;77:543–549. [PMC free article] [PubMed] [Google Scholar]
- 24.Wiese G., Barthel S.R., Dimitroff C.J. Analysis of physiologic E-selectin-mediated leukocyte rolling on microvascular endothelium. J Vis Exp. 2009 doi: 10.3791/1009. pii:1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar P., Shen Q., Pivetti C.D. Molecular mechanisms of endothelial hyperpermeability:implications in inflammation. Expert Rev Mol Med. 2009;11:e19. doi: 10.1017/S1462399409001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sumagin R., Kuebel J.M., Sarelius I.H. Leukocyte rolling and adhesion both contribute to regulation of microvascular permeability to albumin via ligation of ICAM-1. Am J Physiol Cell Physiol. 2011;301:C804–C813. doi: 10.1152/ajpcell.00135.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Martin L., Marcos-Ramiro B., Bigarella C.L. Crosstalk between reticular adherens junctions and platelet endothelial cell adhesion molecule-1 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol. 2012;32:e90–e102. doi: 10.1161/ATVBAHA.112.252080. [DOI] [PubMed] [Google Scholar]
- 28.Wang F., Liu Y.Y., Liu L.Y. The attenuation effect of 3, 4-dihydroxy-phenyl lactic acid and salvianolic acid B on venular thrombosis induced in rat mesentery by photochemical reaction. Clin Hemorheol Microcirc. 2009;42:7–18. doi: 10.3233/CH-2009-1180. [DOI] [PubMed] [Google Scholar]
- 29.Ho J.H., Hong C.Y. Salvianolic acids:small compounds with multiple mechanisms for cardiovascular protection. J Biomed Sci. 2011;18:30. doi: 10.1186/1423-0127-18-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y.H., Lin S.J., Ku H.H. Salvianolic acid B attenuates VCAM-1 and ICAM-1 expression in TNF-alpha-treated human aortic endothelial cells. J Cell Biochem. 2001;82:512–521. doi: 10.1002/jcb.1176. [DOI] [PubMed] [Google Scholar]
- 31.Ding M., Zhao G.R., Yuan Y.J. Aqueous extract of Salvia miltiorrhoza regulates adhesion molecule expression of tumor necrosis factor alpha-induced endothelial cells by blocking activation of nuclear factor kappaB. J Cardiovasc Pharmacol. 2005;45:516–524. doi: 10.1097/01.fjc.0000159643.82641.e9. [DOI] [PubMed] [Google Scholar]