Abstract
Transcription factor E26 transformation-specific sequence-1 (ETS-1) is a transcription factor that regulates the expression of a variety of genes, including growth factors, chemokines, and adhesion molecules. We recently demonstrated that angiotensin II increases the glomerular expression of ETS-1 and that blockade of ETS-1 ameliorates the profibrotic and proinflammatory effects of angiotensin II. The Dahl salt-sensitive rat is a paradigm of salt-sensitive hypertension associated with local activation of the renin–angiotensin system. In these studies, we determined whether: (1) salt-sensitive hypertension is associated with renal expression of ETS-1 and (2) ETS-1 participates in the development of end-organ injury in salt-sensitive hypertension. Dahl salt-sensitive rats were fed a normal-salt diet (0.5% NaCl diet) or a high-salt diet (4% NaCl) for 4 weeks. Separate groups on high-salt diet received an ETS-1 dominant negative peptide (10mg/kg/day), an inactive ETS-1 mutant peptide (10mg/kg/d), the angiotensin II type 1 receptor blocker candesartan (10 mg/kg/d), or the combination high-salt diet/dominant-negative peptide/angiotensin II type 1 receptor blocker for 4 weeks. High-salt diet rats had a significant increase in the glomerular expression of the phosphorylated ETS-1 that was prevented by angiotensin II type 1 receptor blocker. ETS-1 blockade reduced proteinuria, glomerular injury score, fibronectin expression, urinary transforming growth factor-β excretion, and macrophage infiltration. Angiotensin II type 1 receptor blocker reduced proteinuria, glomerular injury score, and macrophage infiltration, whereas concomitant ETS-1 blockade and angiotensin II type 1 receptor blocker had additive effects and reduced interstitial fibrosis. Our studies demonstrated that salt-sensitive hypertension results in increased glomerular expression of phosphorylated ETS-1 and suggested that ETS-1 plays an important role in the pathogenesis of end-organ injury in salt-sensitive hypertension.
Keywords: angiotensins, Dahl salt-sensitive rats, hypertension, kidney
Salt-sensitive (SS) hypertension is a type of hypertension that affects over 50% of humans1 and is associated with a significant risk for the development of hypertensive end-organ damage, including atherosclerosis, left ventricular hypertrophy, and renal injury.2 We and others have shown that salt-sensitive hypertension is characterized by reduced cardiovascular and renal nitric oxide bioavailability and local upregulation of the renin–angiotensin system (RAS).2
Angiotensin II (Ang II) produced as result of intrarenal RAS activation is implicated in the pathogenesis of hypertensive nephrosclerosis and vascular injury by promoting oxidative stress, extracellular matrix deposition, inflammation, and cell proliferation.2–5 However, it is not clear whether these effects occur via independent mechanisms or, alternatively, via common transcriptional mechanisms that mediate the activation of multiple pathways involved in inflammation and fibrosis.
The transcription factor E26 transformation-specific sequence-1 (ETS-1) has been identified as a critical molecule that regulates the vascular expression of a variety of genes, including growth factors, chemokines, and adhesion molecules.6,7 The systemic administration of Ang II results in increased recruitment of inflammatory cells in the vasculature that is markedly reduced in ETS-1 deficient mice7 and associated with reductions in medial hypertrophy.7 Other studies have shown that ETS-1 is induced in the vasculature in response to a variety of stimuli, including Ang II,7 platelet-derived growth factor,8 thrombin,9 interleukin-1,10 and tumor necrosis factor.11
In previous studies, we demonstrated that Ang II increases the expression ETS-1 via the increased generation of reactive oxygen species and that ETS-1 is required for the production of fibronectin in rat mesangial cells.12 In addition, we demonstrated that ETS-1 is up-regulated in the renal cortex after the systemic chronic administration of Ang II and that blockade of ETS-1 using a specific ETS-1 dominant-negative peptide (DN) reduces the renal profibrotic and proinflammatory effects of Ang II.13
Given the large body of experimental evidence demonstrating local activation of RAS14–16 in hypertensive Dahl salt-sensitive (DS) rats, we designed a series of experiments aimed at testing the hypothesis that ETS-1 is up-regulated in salt-sensitive hypertension and that ETS-1 plays a major role as mediator of renal injury in this model of hypertension. In addition, we determined the effects of ETS-1 blockade on renal injury and intrarenal RAS activation expression and the effects of combined RAS and ETS-1 blockade on renal injury in this model of hypertension.
Methods
Experimental Groups
Eight-week-old Dahl/Rapp DS male rats were purchased from Harlan (Indianapolis, IN) and maintained under controlled conditions of light, temperature, and humidity. The animals were housed in facilities accredited by the American Association for Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Sub-Committee at the University of Alabama at Birmingham approved these studies. After 2 weeks of acclimation to the new environment, the rats were divided into 6 groups (n=6 per group) and treated for 4 weeks as follows: normal salt, fed 0.5%, NaCl diet; high salt (HS), fed 4% NaCl diet; HS/DN, fed 4% NaCl diet plus ETS-1 DN (DN, 10 mg/kg/d subcutaneous by osmotic mini pump, Alzet, Cupertino, CA); HS/MU, fed 4% NaCl diet plus ETS-1 mutant peptide (MU, 10 mg/kg/d subcutaneous by osmotic minimum); HS/angiotensin II receptor I blocker (ARB), fed 4% NaCl diet plus the ARB candesartan (10 mg/kg/d in the drinking water); and HS/ARB/DN, fed 4% NaCl diet plus candesartan and ETS-1 DN. Blood pressure was measured by radio telemetry as we have previously described.13 Before euthanasia, the rats were placed in metabolic cages for 16 hours and urine collected for total protein, albumin, angiotensinogen, and transforming growth factor (TGF)-β measurements.
The ETS-1 DN peptide was synthesized (CPC Scientific Inc, San Jose, CA) following the sequences described by Ni et al.17 This peptide competes with ETS-1 for binding to target genes but does not initiate gene transcription. An HIV-1 transactivator of transcription sequence was added to the carboxyl terminus to facilitate intracellular delivery and the amino terminus is biotinylated.18 An inactive peptide ETS-1 mutant (ETS-1 MU) was generated by replacing 2 arginines for glycines as previously described.17,18
Western Blot
Western blot analysis was performed as previously described.19 Briefly, 100 mg of kidney cortex was homogenized in 500 μL lysis buffer (Pro# 78510, Thermo Scientific, Rockford, IL). The resulting lysates were centrifuged for 30 minutes at 10 000g at 4°C, the supernatants collected and protein concentration quantitated by Bio-Rad assay. For immunoblotting, 30 μg of protein was separated by SDS-PAGE (10 or 15% acrylamide gel) and transferred to a polyvinylidene fluoride membrane. The blots were incubated with the primary antibodies against ETS-1 (sc-350, Santa Cruz), phospho-ETS-1(T38; 44-1104G, Invitrogen), and Fibronectin (F3648, Sigma) at 4°C for 24 hours. The blots were washed and incubated with the appropriate secondary antibodies and the signal detected by luminol chemiluminescence.
Immunofluorescence
Five-micrometer-thick kidney sections were prepared from paraffin-embedded tissues. After deparaffinization and antigen retrieval, the sections were rinsed in phosphate-buffered saline. The sections were then incubated with a rabbit antibody to ETS-1 (sc-350, Santa Cruz) or phosphorylated ETS-1 (44-1104G, Invitrogen) and antibodies to cell type–specific markers, including CD31 (ab32457, Abcam) for endothelium, synaptopodin (sc-21537, Santa Cruz) for podocytes, desmin (ab6322, Abcam) for mesangium, or CD68 (MCA341R, Serotec) for macrophages at 4°C overnight. The sections were then washed and incubated with the respective secondary antibodies conjugated with either Alexa Flour 488 (green) or Alexa Flour 594 (red; Invitrogen). Negative controls by omission of primary antibody were included in each experiment. Images were acquired using a Leica DM6000 epifluorescence microscope (Leica Microsystems, Bannockburn, IL) with a Hamamatsu ORCA ER cooled CCD camera and Simple PCI software (Compix, Inc, Cranberry Township, PA). Images were adjusted appropriately to remove background fluorescence.
Immunohistochemistry
The avidin–biotin–peroxidase immunohistochemical technique (ABC kit, Vector) was used to detect macrophage infiltration, using primary antibodies against CD68 as a specific marker for macrophages.20 After deparaffinization and heat-mediated antigen retrieval, CD68-positive areas were immunolocalized by incubation with respective primary antibody, followed by application of a biotinylated goat anti-rabbit secondary antibody (1:200) for 30 minutes. An observer unaware of the experimental conditions measured the number of CD68-positive cells (Image-Pro, Media Cybernetics, Bethesda, MD).
Morphometric Analysis for Glomerular Injury Score and Renal Fibrosis
Light microscopic pictures of Periodic Acid Schiff and trichrome stained kidney sections from the different experimental groups were used for morphometric analysis. Glomerular injury score (GIS) was measured in Periodic Acid Schiff–stained kidney sections by an experienced pathologist (H.F.) purposely blinded to the different experimental conditions and using a 0+ to 4+ scale as previously described.21 All glomeruli available in each slide (n=32–263) were analyzed. Renal fibrosis was evaluated blindly by the same pathologist in trichrome-stained kidney sections and expressed as percent of fibrosis in the interstitium.
Proteinuria
Proteinuria was measured by the Lowry method and adjusted for urine volume in the 24-hour collection (Cayman, Ann Arbor, MI). Urinary albumin concentrations were determined using a rat albumin ELISA quantitation kit from Bethyl (Montgomery, TX) and adjusted for urine volume obtained during the 24-hour collection period.
Urinary TGF-β1 and Angiotensinogen
Active TGF-β1 was measured in urine by ELISA (MB100B, R&D Systems, Minneapolis, MN) following the manufacturer’s instructions and adjusted for 24 hours urine volume. Urinary angiotensinogen was measured by ELISA as described previously and adjusted for 24 hours urine volume.22
Renal Cortical Ang II
The cortical concentration of Ang II was measured by radioimmunoassay as previously described and expressed as fmol/g of total protein.23
Statistical Analysis
Results were expressed as means±SEM. The data were evaluated by 1-way or 2-way ANOVA. When the overall F test result of ANOVA was significant, a multiple-comparison Dunnett test was applied. Student t test was used in 2 mean comparisons. Differences were reported as significant when p value was < 0.05.
Results
Blood Pressure
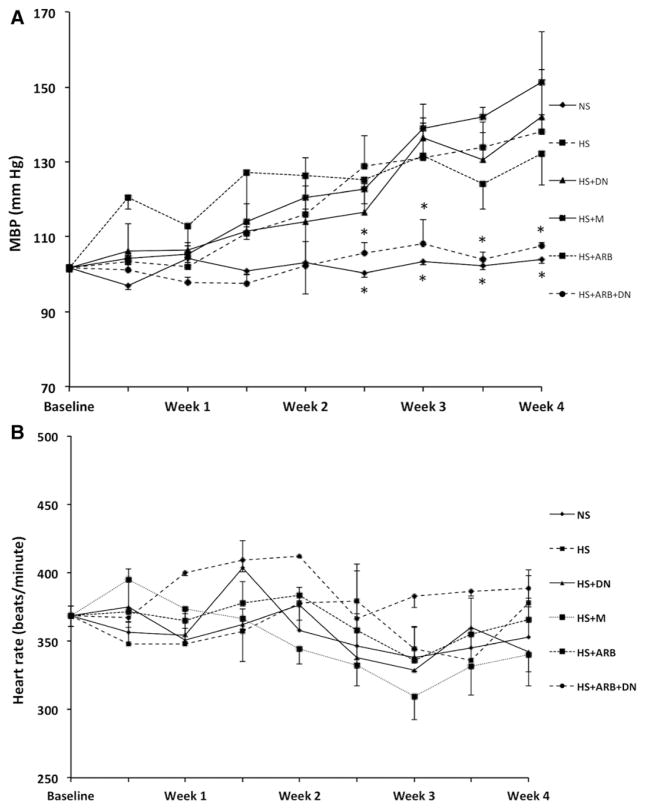
DS rats fed a high-salt diet showed a progressive increase in blood pressure as assessed by radiotelemetry. As shown in Table, treatment with either DN or ARB resulted in modest albeit significant reductions in blood pressure. DS rats receiving concomitant treatment with ARB and DN did not increase blood pressure while on a high-salt diet and had blood pressures similar to those observed in rats receiving a normal-salt diet (Figure 1A). Neither treatment had a significant effect on heart rat as shown in Figure 1B.
Table.
Effects of ETS-1 and Renin–Angiotensin System Blockade on Proteinuria, TGF-β, Angiotensinogen Urinary Excretion, and Cortical Angiotensin II
| NS | HS | HS/DN | HS/MU | HS/ARB | HS/DN/ARB | |
|---|---|---|---|---|---|---|
| Proteinuria, mg/24 h | 44.8±3.9 | 91.3±6.1* | 65.3±6.6*† | 82.8±16.3* | 51.9±3.2† | 42.5±3.0†‡ |
| Albuminuria, mg/24 h | 3.7±1.1 | 56.4±17.2* | 25.4±2.7*† | 36.0±10.5* | 11.8±6.4† | 11.6±1.8† |
| Urinary TGF-β, pg/24 h | 13.0±8.8 | 417±87.6* | 142.2±64.9*† | 498.3±138.3* | 604.1±94.9* | 23.0±9.3†‡ |
| Angiotensinogen, ng/24 h | 382.8±153 | 1105±148* | 538.4±114† | 812.2±372.9* | 446±81.2† | 330.2±116† |
| Renal cortical angiotensin II, fmol/g | 1237±212 | 3197±675* | 1008.6±127 | 1525.6±298† | 927.2±204† | 1352±397† |
Data are presented as mean±SEM. ARB indicates angiotensin II receptor I blocker; DN, dominant negative peptide; ETS-1, transcription factor avian virus E26 oncogen homolog-1; HS, high-salt diet; MU, mutant peptide; NS, normal-salt diet; and TGF-β, transforming growth factor-β.
P<0.05 vs NS.
P<0.05 vs HS.
P<0.05 vs HS/DN and HS/ARB.
Figure 1.
Effects of transcription factor avian erythroblastosis virus E26 oncogen homolog-1 (ETS-1) blockade and renin–angiotensin system blockade on blood pressure and heart rate. A, Blood pressure increased progressively in high-salt diet (HS), HS/mutant peptide (MU), and HS/ dominant-negative peptide (DN). Rats on HS/angiotensin II receptor I blocker (ARB) had slightly lower blood pressures that however were not significantly lower compared with normal-salt diet (NS) rats. HS/ARB/DN did not have a significant increase in blood pressure compared with the other groups on HS and their blood pressure was no different when compared with the HS rats (*P<0.05 vs HS rats). B, There were no significant differences in heart rate in all the groups studied throughout the duration of the studies (P=not significant). MBP indicates mean blood pressure.
ETS-1 Expression Is Increased in Hypertensive DS Rats
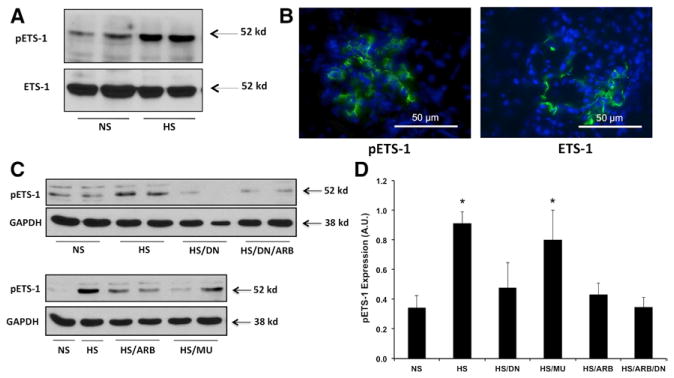
Hypertensive DS rats had increased glomerular expression of the phosphorylated form of ETS-1 compared with normotensive DS rats as assessed by Western blot (Figure 2A). No significant changes in the expression of total ETS-1 were observed in hypertensive DS rats compared with their normotensive counterparts. The expression of ETS-1 and phospho-ETS-1 was exclusively glomerular as assessed by immunofluorescence (Figure 2B). To determine the role of RAS activation on ETS-1 expression in hypertensive Dahl rats, we determined the effects of RAS blockade on ETS-1 expression. As shown in Figure 2C and 2D, the administration of the ARB candesartan significantly reduced the expression of the phosphorylated form of ETS-1 as assessed by western blot. In addition, the administration of the DN as well as the combination of the DN and ARB resulted in significant reductions in ETS-1 phosphorylation.
Figure 2.
Transcription factor avian erythroblastosis virus E26 oncogen homolog-1 (ETS-1) expression in hypertensive Dahl salt-sensitive (DS) rats. A, Hypertensive DS rats have increased cortical expression of the phosphorylated form of ETS-1 (pETS-1) but not of total ETS-1. B, Expression of total and pETS-1 is exclusively glomerular (×40 magnification). C, Renin–angiotensin system (RAS) blockade with the angiotensin II receptor I blocker (ARB) candesartan and dominant-negative peptide (DN) reduces the expression of pETS-1 in the renal cortex of hypertensive DS rats. D, Densitometry analysis for pETS-1 showing significant increases in pETS-1 protein expression in hypertensive DS rats that is prevented by RAS blockade with candesartan and DN. Data are expressed as mean±SEM and are normalized to GAPDH. (*P<0.05 vs normal-salt diet [NS]; n=6). HS indicates high-salt diet; and MU, mutant peptide.
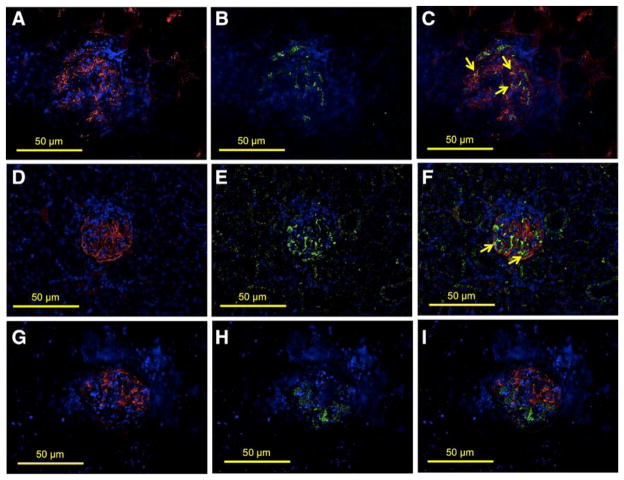
We performed colocalization studies to characterize the glomerular cells that express ETS-1 in hypertensive DS rats, using specific markers for vascular endothelium (CD31), mesangial cells (desmin), and podocytes (synaptopodin). As shown in Figure 3, in hypertensive DS rats, the expression of total ETS-1 partially colocalized with CD31 (Figure 3A–3C) indicating expression of ETS-1 in the glomerular endothelium. Following a similar strategy, we performed colocalization studies using synaptopodin as a podocyte marker, and we observed strong colocalization of ETS-1 with synaptopodin-expressing cells, indicating that podocytes express ETS-1 (Figure 3D–3F). We also evaluated the expression of ETS-1 in the glomerular mesangium using desmin as a marker for mesangial expression. As shown in Figure 4, there was no clear colocalization of ETS-1 and desmin, suggesting that in vivo there is no significant expression of ETS-1 in the glomerular mesangium.
Figure 3.
Glomerular expression of transcription factor avian erythroblastosis virus E26 oncogen homolog-1 (ETS-1) in hypertensive Dahl salt-sensitive (DS) rats. Colocalization studies were performed to characterize the expression of ETS-1 in the glomerular endothelium (CD31), podocytes (synaptopodin), or mesangium (desmin). A–C show positive stain for CD31 (A, red), ETS-1 (B, green), and some areas in which there is colocalization of CD31 and ETS-1 indicating endothelial expression of ETS-1 (C, arrows). D–F show positive stain for synaptopodin (D, red), ETS-1 (E, green), and areas in which there is colocalization of ETS-1 and synaptopodin indicating expression of ETS-1 in podocytes (F, arrows). G–I show positive stain for desmin (G, red), ETS-1 (H, green); no clear evidence of colocalization of ETS-1 and desmin was observed (I), suggesting lack of expression of ETS-1 in mesangial areas (×40 magnification).
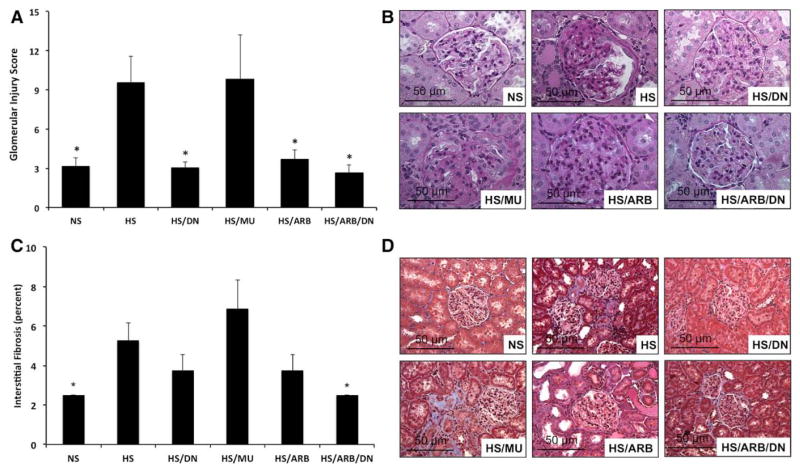
Figure 4.
Effects of transcription factor avian erythroblastosis virus E26 oncogen homolog-1 (ETS-1) and renin–angiotensin system (RAS) blockade on glomerular injury score (GIS) and interstitial fibrosis. A, GIS is increased in hypertensive Dahl salt-sensitive (DS) rats and improved by ETS-1 blockade, RAS blockade, or combination treatment (*P<0.05 vs high-salt diet [HS] and HS/mutant peptide [MU], n=8). B, Representative photomicrographs for the different experimental groups (Periodic Acid Schiff stain) used to assess GIS. C, Interstitial fibrosis is increased in hypertensive DS rats and improved by combination treatment of ETS-1 and RAS blockade. (*P<0.05 vs HS and HS/MU, n=8). D, Representative photomicrographs for the different experimental groups (trichrome) used to assess interstitial fibrosis (×40 magnification). ARB indicates angiotensin II receptor I blocker; DN, dominant-negative peptide; and NS, normal-salt diet.
ETS-1 Blockade Reduces Proteinuria in Hypertensive DS Rats
As others and we have shown, hypertensive DS rats had a significant increase in the urinary excretion of protein2 and albumin compared with normotensive DS rats (Table). Treatment with DN peptide but not MU resulted in significant reductions in both proteinuria and albuminuria. Treatment with ARB also resulted in significant reductions in protein excretion and albuminuria, while the combination of ARB and DN completely normalized the urinary excretion of protein in these rats but had no additional effect on albuminuria (Table).
ETS-1 Blockade Improves GIS and Interstitial Fibrosis
To assess the effect of ETS-1 blockade alone or in combination with RAS blockade on renal injury, we measured GIS in Periodic Acid Schiff–stained kidney sections and interstitial fibrosis in trichrome-stained sections. As expected, the GIS was significantly increased in hypertensive DS rats compared with normotensive DS rats (Figure 4). Treatment with DN, ARB, or DN/ ARB, but not with MU, resulted in significant improvements in GIS (Figure 4). Similarly, we observed significant increases in interstitial fibrosis in hypertensive DS rats compared with normotensive DS rats (Figure 4). Treatment with DN or ARB alone resulted in a small and nonsignificant improvement in the severity of interstitial fibrosis. However, the combination of DN and ARB reduced interstitial fibrosis to levels similar to those seen in the control rats. Altogether, these findings suggest that RAS blockade and ETS-1 blockade alone improve GIS in hypertensive DS rats; however, only concomitant RAS and ETS-1 blockade had a significant effect on interstitial fibrosis.
ETS-1 Blockade Reduces Urinary TGF-β and Cortical Fibronectin Expression
To better assess the role of ETS-1 blockade alone or in combination with RAS blockade on renal injury, we determined their effects on cortical fibronectin expression and on urinary excretion of TGF-β. As shown in Figure 5, hypertensive DS rats had a significant increase in the cortical expression of fibronectin compared with normotensive DS rats. Treatment with DN, but not with MU, peptide normalized fibronectin expression as assessed by Western blot. Treatment with ARB, however, had no significant effect on fibronectin, whereas concomitant RAS and ETS-1 blockade had similar effects to ETS-1 blockade alone. To determine whether these changes in fibronectin expression were linked to changes in TGF-β production, a critical growth factor implicated in the pathogenesis of renal fibrosis, we measured the urinary excretion of TGF-β as we have and others have previously described.24 As shown in Table, hypertensive DS rats had significantly higher urinary excretion of TGF-β compared with normotensive DS rats. The administration of DN peptide resulted in significant reductions in the urinary excretion of TGF-β while treatment with the MU peptide had no effect. Treatment with ARB alone had no effect, whereas the combination of DN/ARB completely normalized the urinary excretion of TGF-β in hypertensive DS rats.
Figure 5.
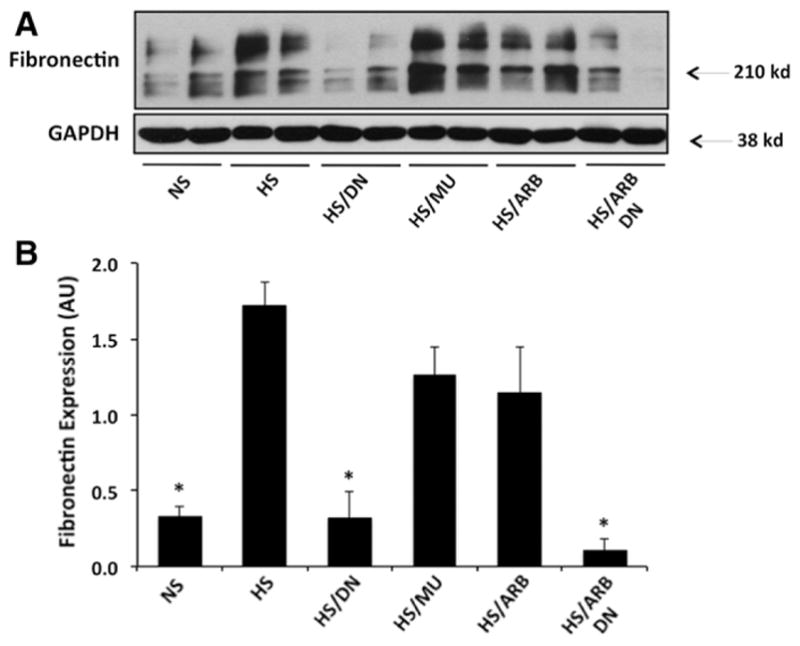
Effects of transcription factor avian erythroblastosis virus E26 oncogen homolog-1 (ETS-1) and renin–angiotensin system (RAS) blockade on fibronectin expression in hypertensive Dahl salt-sensitive (DS) rats. A, Representative Western blot for fibronectin and actin that was used as a loading control. B, Densitometry analysis for cortical fibronectin and actin. The cortical expression of fibronectin is increased in hypertensive DS rats (high-salt diet [HS]) and reduced by ETS-1 blockade alone (HS/dominant-negative peptide [DN]) or in combination with RAS blockade (HS/ DN/angiotensin II receptor I blocker [ARB]). Treatment with inactive peptide (HS/mutant peptide [MU]) or RAS blockade alone (HS/ARB) had no significant effect on fibronectin expression (*P<0.05 vs HS, HS/MU, and HS/ARB; n=6). NS indicates normal-salt diet.
ETS-1 Blockade Reduces Macrophage Infiltration in Hypertensive DS rats
Given the well-known role of inflammation as mediator of the effects of renal injury in hypertensive DS rats,25,26 we determined the effects of ETS-1 blockade alone or in combination with RAS blockade on macrophage infiltration. As shown in Figure 6, we observed a significant increase in macrophage infiltration in hypertensive DS rats that was more evident in periglomerular areas and the cortical interstitium. Treatment with DN normalized the number of macrophages infiltrating the cortex of hypertensive DS rats while the MU peptide had no effect. Treatment with ARB had a modest albeit significant effect on macrophage infiltration while dual ETS-1 and RAS blockade reduced macrophage infiltration to levels similar to those of control rats on low-salt diet.
Figure 6.
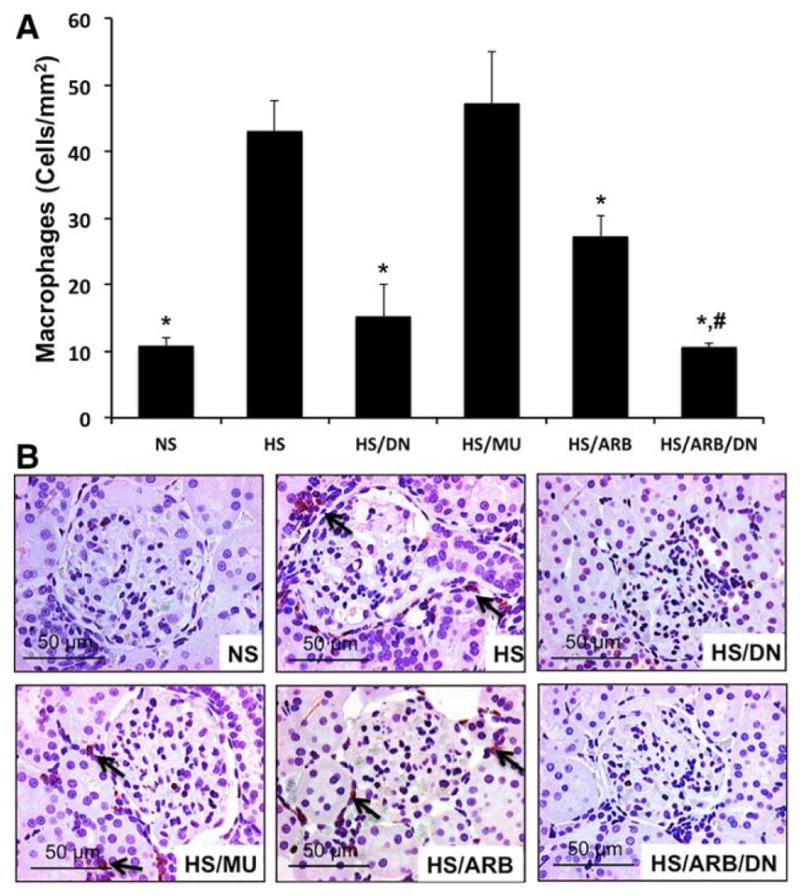
Effects of transcription factor avian erythroblastosis virus E26 oncogen homolog-1 (ETS-1) and renin–angiotensin system (RAS) blockade on macrophage infiltration in hypertensive Dahl salt-sensitive (DS) rats. A, Hypertensive DS rats had a significant increase in the number of infiltrating macrophages (arrows) that was significantly improved by ETS-1 blockade (high-salt diet [HS]/dominant-negative peptide [DN]) or RAS blockade (HS/angiotensin II receptor I blocker [ARB]). Combination treatment (HS/DN/ARB) completely normalized macrophage infiltration (*P<0.05 vs HS and HS/mutant peptide [MU], #P<0.05 vs HS/ARB, n=6). B, Representative photomicrograph showing the presence of macrophages predominantly in the interstitium (×40 magnification). NS indicates normal-salt diet.
Effects of ETS-1 Blockade on Components of the RAS
To determine the effects of ETS 1 on specific components of the RAS, we determined the effects of ETS 1 blockade on the cortical concentrations of Ang II and the urinary excretion of angiotensinogen. As shown in the Table, hypertensive DS rats on a high-salt diet had a significant increase in the urinary excretion of angiotensinogen that was significantly reduced by ETS 1 blockade and ARB and normalized by the combination of ETS-1 and RAS blockade. Hypertensive DS rats had increased cortical expression of Ang II that was partially reduced by DN, MU, ARB, and concomitant ETS-1 and RAS blockade.
Discussion
ETS-1 is a member of the ETS family of transcription factors that share a highly conserved DNA-binding domain (ETS domain)6 that originates from the sequence described in the E26 avian erythroblastosis virus (E26 Transformation-specific Sequence).27 Studies in mutant mice have demonstrated that ETS-1 knockout animals have a lower number of glomeruli, and among the existing glomeruli, a higher number are immature,28 highlighting the important role of ETS-1 in the regulation of normal kidney development.29–31 The transcriptional activity of ETS-1 is modulated through post-translational modifications. Phosphorylation of threonine-38 increases the transcriptional activity of ETS-132 while calmodulin-dependent kinase II inhibits DNA binding through serine phosphorylation of ETS-1 inhibitory domains.33 ETS-1 is also regulated through nuclear transport via specific nuclear localization sequences that facilitate the movement of ETS-1 from the cytoplasm into the nucleus.34
The renal expression of ETS-1 is increased in a variety of models of renal injury. The anti-Thy1 model of glomerulonephritis is associated with a 4-fold increase in ETS-1 expression predominantly in the glomerular mesangium and at a lesser degree in podocytes and the glomerular endothelium.35 In rats with antiglomerular basement–induced glomerulonephritis, there is also increased upregulation of ETS-1 in the glomeruli and in the interstitium,36 and in an ischemic model of acute renal failure, the tubular expression of ETS-1 is increased and associated with augmented expression of cyclin D, suggesting a role for ETS-1 in the control of tubular regeneration in acute kidney injury.37 In previous studies, we also demonstrated that Ang II increases the cortical expression of ETS-1 in Sprague-Dawley rats and that knockdown of ETS-1 reduces Ang II-stimulated fibronectin production in rat mesangial cells.38 In other studies, we demonstrated that Ang II increases the glomerular expression of ETS-1 in mice and that blockade of ETS-1 using a specific DN reduces proteinuria, inflammation, and fibrosis induced by Ang II.13 Importantly, in these studies, ETS-1 blockade did not reduce blood pressure, suggesting that hemodynamic effects did not mediate the beneficial actions of ETS-1 blockade.
In the present studies, we have demonstrated increased glomerular expression of ETS-1 in hypertensive Dahl/Rapp salt-sensitive rats, a paradigm of salt-sensitive hypertension in humans. We demonstrated that hypertensive Dahl/Rapp salt-sensitive rats have a significant increase in the expression of the phosphorylated (T38) form of ETS-1 without significant changes in the expression of total ETS-1. Although we did not observe changes in the expression of total ETS-1, we cannot rule out that changes in the expression of total ETS-1 may occur at other time points. Using colocalization methods, we demonstrated that the expression of ETS-1 is mostly glomerular and predominantly expressed in the glomerular epithelium and to a lesser degree in the glomerular endothelium.
Several studies have demonstrated that DS rats when fed a high-salt diet and made hypertensive have increased local activation of the RAS, characterized by sustained levels of Ang II, increased levels of angiotensinogen, and increased expression of the AT1 receptor.14 In addition, as others and we have shown, salt-sensitive hypertension is associated with reduced nitric oxide bioavailability and increased reactive oxygen species production.2,39,40 In support of the role for increased RAS activation in salt-sensitive hypertension, AT1 receptor blockade ameliorates cardiac or renal dysfunction in these rats, suggesting an important role for RAS in the development of end-organ injury in salt-sensitive hypertension.15,16 In the current studies, we observed increased expression of the phosphorylated form of ETS-1 in hypertensive DS rats that was significantly reduced by RAS blockade with ARB, suggesting that increased RAS activation mediates increased ETS-1 phosphorylation and activation in hypertensive DS rats. To determine the role of ETS-1 in the pathogenesis of renal injury in salt-sensitive hypertension, we used a DN ETS-1 peptide that competes for DNA binding with ETS-1 but does not initiate gene transcription. In our studies, we observed that ETS-1 blockade reduced ETS-1 phosphorylation at T38; these findings are consistent with a positive feedback of ETS-1 on its own activation as has been previously described.
ETS-1 blockade resulted in significant reductions in GIS, fibronectin expression, proteinuria, and macrophage infiltration but had no significant effect on interstitial fibrosis. RAS blockade also reduced GIS, proteinuria, and macrophage infiltration and had a no significant effect on fibronectin or fibrosis. By contrast, concomitant ETS-1 and RAS blockade had additive effects on all parameters examined. In addition, we observed that ETS-1 blockade resulted in a significant reduction in the urinary excretion of TGF-β, suggesting that ETS-1 may be a direct regulator of TGF-β in hypertensive DS rats. By contrast, RAS blockade did not modify the urinary excretion of TGF-β, indicating that other pathways independent of RAS participate in the production of TGF-β in hypertensive DS rats. Both ETS-1 blockade and RAS blockade had small effects on blood pressure as measured by radiotelemetry; however, rats with ETS-1 and RAS blockade had blood pressures that were similar to those from rats on a normal-salt diet, indicating that ETS-1 may also be playing a role in blood pressure regulation either directly or indirectly. In addition, these findings suggest that the additional beneficial effects of concomitant ETS-1 and RAS blockade are in large part due to their effects on blood pressure.
To better understand the interaction between RAS and ETS-1, we measured the expression of some of main components of RAS in the different experimental groups. As previously shown by us and others, hypertensive DS rats had significant increases in the urinary excretion of angiotensinogen and intrarenal concentration of Ang II indicative of increased RAS activation. Both ETS-1 and RAS blockade induced significant reductions in the urinary excretion of angiotensinogen and tissue levels of Ang II. In addition, concomitant ETS-1 and RAS blockade further reduced the urinary excretion of angiotensinogen. These findings suggest that ETS-1 also plays a role in the regulation of the RAS, the mechanisms by which ETS-1 could be modulating RAS activity are unclear and are the subject of active investigation in our laboratory.
Perspective
In these studies, we have unveiled the role of the transcription factor ETS-1 as a mediator of renal injury in salt-sensitive hypertension. In addition, we determined that the activation of RAS mediates ETS-1 phosphorylation in hypertensive salt-sensitive rats and that concomitant RAS and ETS-1 blockade have beneficial additive effects on renal injury, suggesting that concomitant blockade of RAS and ETS-1 could be a novel therapeutic strategy for the treatment and prevention of end-organ injury in hypertension.
Novelty and Significance.
What Is New?
These studies demonstrate increased expression of the transcription factor avian erythroblastosis virus E26 oncogen homolog-1 (ETS-1) in salt-sensitive hypertension.
Blockade of ETS-1 reduces renal injury in salt-sensitive hypertension.
What Is Relevant?
These studies unveil a potential target for the treatment and prevention of end-organ injury in hypertension.
Summary
Expression of the phosphorylated form of ETS-1 is increased in glomeruli from hypertensive Dahl salt-sensitive rats.
ETS-1 and renin–angiotensin system blockade have additive beneficial effects in salt-sensitive hypertension by reducing proteinuria, glomerular injury score, interstitial fibrosis, fibronectin, macrophage infiltration, and transforming growth factor-β excretion.
Acknowledgments
We are grateful for the support of Core Facilities at the O’Brien Kidney Center at the University of Alabama at Birmingham.
Sources of Funding
A Merit Review Award (E.A. Jaimes), a Research Grant (1 IP1 BX001595; P.W. Sanders and E.A. Jaimes) from the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, a National Institute of Diabetes and Digestive and Kidney Diseases George M. O’Brien Kidney and Urological Research Centers Program Grant P30-DK-079337, and a Byrne Fund Grant (E.A. Jaimes) from Memorial Sloan Kettering Cancer Center funded these studies.
Footnotes
Disclosures
None.
References
- 1.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8(6 Part 2):II127–II134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 2.Zhou MS, Adam AG, Jaimes EA, Raij L. In salt-sensitive hypertension, increased superoxide production is linked to functional upregulation of angiotensin II. Hypertension. 2003;42:945–951. doi: 10.1161/01.HYP.0000094220.06020.C8. [DOI] [PubMed] [Google Scholar]
- 3.Jaimes EA, Galceran JM, Raij L. Angiotensin II induces superoxide anion production by mesangial cells. Kidney Int. 1998;54:775–784. doi: 10.1046/j.1523-1755.1998.00068.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhou MS, Jaimes EA, Raij L. Inhibition of oxidative stress and improvement of endothelial function by amlodipine in angiotensin II-infused rats. Am J Hypertens. 2004;17:167–171. doi: 10.1016/j.amjhyper.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Liao TD, Yang XP, Liu YH, Shesely EG, Cavasin MA, Kuziel WA, Pagano PJ, Carretero OA. Role of inflammation in the development of renal damage and dysfunction in angiotensin II-induced hypertension. Hypertension. 2008;52:256–263. doi: 10.1161/HYPERTENSIONAHA.108.112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oettgen P. Regulation of vascular inflammation and remodeling by ETS factors. Circ Res. 2006;99:1159–1166. doi: 10.1161/01.RES.0000251056.85990.db. [DOI] [PubMed] [Google Scholar]
- 7.Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, Oettgen P. Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest. 2005;115:2508–2516. doi: 10.1172/JCI24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naito S, Shimizu S, Maeda S, Wang J, Paul R, Fagin JA. Ets-1 is an early response gene activated by ET-1 and PDGF-BB in vascular smooth muscle cells. Am J Physiol. 1998;274(2 Part 1):C472–C480. doi: 10.1152/ajpcell.1998.274.2.C472. [DOI] [PubMed] [Google Scholar]
- 9.Hultgårdh-Nilsson A, Cercek B, Wang JW, Naito S, Lövdahl C, Sharifi B, Forrester JS, Fagin JA. Regulated expression of the ets-1 transcription factor in vascular smooth muscle cells in vivo and in vitro. Circ Res. 1996;78:589–595. doi: 10.1161/01.res.78.4.589. [DOI] [PubMed] [Google Scholar]
- 10.Redlich K, Kiener HP, Schett G, Tohidast-Akrad M, Selzer E, Radda I, Stummvoll GH, Steiner CW, Gröger M, Bitzan P, Zenz P, Smolen JS, Steiner G. Overexpression of transcription factor Ets-1 in rheumatoid arthritis synovial membrane: regulation of expression and activation by interleukin-1 and tumor necrosis factor alpha. Arthritis Rheum. 2001;44:266–274. doi: 10.1002/1529-0131(200102)44:2<266::AID-ANR43>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 11.Goetze S, Kintscher U, Kaneshiro K, Meehan WP, Collins A, Fleck E, Hsueh WA, Law RE. TNFalpha induces expression of transcription factors c-fos, Egr-1, and Ets-1 in vascular lesions through extracellular signal-regulated kinases ½. Atherosclerosis. 2001;159:93–101. doi: 10.1016/s0021-9150(01)00497-x. [DOI] [PubMed] [Google Scholar]
- 12.Pearse DD, Tian RX, Nigro J, Iorgulescu JB, Puzis L, Jaimes EA. Angiotensin II increases the expression of the transcription factor ETS-1 in mesangial cells. Am J Physiol Renal Physiol. 2008;294:F1094–F1100. doi: 10.1152/ajprenal.00458.2007. [DOI] [PubMed] [Google Scholar]
- 13.Feng W, Chumley P, Hua P, Rezonzew G, Jaimes D, Duckworth MW, Xing D, Jaimes EA. Role of the transcription factor erythroblastosis virus E26 oncogen homolog-1 (ETS-1) as mediator of the renal proinflammatory and profibrotic effects of angiotensin II. Hypertension. 2012;60:1226–1233. doi: 10.1161/HYPERTENSIONAHA.112.197871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishiyama A, Yoshizumi M, Rahman M, Kobori H, Seth DM, Miyatake A, Zhang GX, Yao L, Hitomi H, Shokoji T, Kiyomoto H, Kimura S, Tamaki T, Kohno M, Abe Y. Effects of AT1 receptor blockade on renal injury and mitogen-activated protein activity in Dahl salt-sensitive rats. Kidney Int. 2004;65:972–981. doi: 10.1111/j.1523-1755.2004.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onozato ML, Tojo A, Kobayashi N, Goto A, Matsuoka H, Fujita T. Dual blockade of aldosterone and angiotensin II additively suppresses TGF-beta and NADPH oxidase in the hypertensive kidney. Nephrol Dial Transplant. 2007;22:1314–1322. doi: 10.1093/ndt/gfl780. [DOI] [PubMed] [Google Scholar]
- 17.Ni W, Zhan Y, He H, Maynard E, Balschi JA, Oettgen P. Ets-1 is a critical transcriptional regulator of reactive oxygen species and p47(phox) gene expression in response to angiotensin II. Circ Res. 2007;101:985–994. doi: 10.1161/CIRCRESAHA.107.152439. [DOI] [PubMed] [Google Scholar]
- 18.Feng W, Xing D, Hua P, Zhang Y, Chen YF, Oparil S, Jaimes EA. The transcription factor ETS-1 mediates proinflammatory responses and neointima formation in carotid artery endoluminal vascular injury. Hypertension. 2010;55:1381–1388. doi: 10.1161/HYPERTENSIONAHA.110.150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaimes EA, Zhou MS, Pearse DD, Puzis L, Raij L. Upregulation of cortical COX-2 in salt-sensitive hypertension: role of angiotensin II and reactive oxygen species. Am J Physiol Renal Physiol. 2008;294:F385–F392. doi: 10.1152/ajprenal.00302.2007. [DOI] [PubMed] [Google Scholar]
- 20.Feng W, Xing D, Hua P, Zhang Y, Chen YF, Oparil S, Jaimes EA. The transcription factor ETS-1 mediates proinflammatory responses and neointima formation in carotid artery endoluminal vascular injury. Hypertension. 2010;55:1381–1388. doi: 10.1161/HYPERTENSIONAHA.110.150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raij L, Chiou XC, Owens R, Wrigley B. Therapeutic implications of hypertension-induced glomerular injury. Comparison of enalapril and a combination of hydralazine, reserpine, and hydrochlorothiazide in an experimental model. Am J Med. 1985;79(3C):37–41. doi: 10.1016/0002-9343(85)90078-6. [DOI] [PubMed] [Google Scholar]
- 22.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navar LG, Mitchell KD, Harrison-Bernard LM, Kobori H, Nishiyama A. Intrarenal angiotensin II levels in normal and hypertensive states. J Renin Angiotensin Aldosterone Syst. 2001;2:S176–S184. doi: 10.1177/14703203010020013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rezonzew G, Chumley P, Feng W, Hua P, Siegal GP, Jaimes EA. Nicotine exposure and the progression of chronic kidney disease: role of the α7-nicotinic acetylcholine receptor. Am J Physiol Renal Physiol. 2012;303:F304–F312. doi: 10.1152/ajprenal.00661.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou MS, Schulman IH, Raij L. Vascular inflammation, insulin resistance, and endothelial dysfunction in salt-sensitive hypertension: role of nuclear factor kappa B activation. J Hypertens. 2010;28:527–535. doi: 10.1097/HJH.0b013e3283340da8. [DOI] [PubMed] [Google Scholar]
- 26.Lin L, Phillips WE, Manning RD. Intrarenal angiotensin ii is associated with inflammation, renal damage and dysfunction in dahl salt-sensitive hypertension. J Am Soc Hypertens. 2009;3:306–314. doi: 10.1016/j.jash.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nunn MF, Seeburg PH, Moscovici C, Duesberg PH. Tripartite structure of the avian erythroblastosis virus E26 transforming gene. Nature. 1983;306:391–395. doi: 10.1038/306391a0. [DOI] [PubMed] [Google Scholar]
- 28.Gomez RA, Norwood VF. Recent advances in renal development. Curr Opin Pediatr. 1999;11:135–140. doi: 10.1097/00008480-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Chernavsky D, Fernandez L, Barton K, Muthusamy N, Leiden J, Gomez R. Expression and function of ETS-1 in the developing kidney. J Am Soc Nephrol. 1998;9:360. [Google Scholar]
- 30.Cederberg A, Hulander M, Carlsson P, Enerbäck S. The kidney-expressed winged helix transcription factor FREAC-4 is regulated by Ets-1. A possible role in kidney development. J Biol Chem. 1999;274:165–169. doi: 10.1074/jbc.274.1.165. [DOI] [PubMed] [Google Scholar]
- 31.Gomez RA, Norwood VF. Recent advances in renal development. Curr Opin Pediatr. 1999;11:135–140. doi: 10.1097/00008480-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Wasylyk B, Hagman J, Gutierrez-Hartmann A. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci. 1998;23:213–216. doi: 10.1016/s0968-0004(98)01211-0. [DOI] [PubMed] [Google Scholar]
- 33.Cowley DO, Graves BJ. Phosphorylation represses Ets-1 DNA binding by reinforcing autoinhibition. Genes Dev. 2000;14:366–376. [PMC free article] [PubMed] [Google Scholar]
- 34.Boulukos KE, Pognonec P, Rabault B, Begue A, Ghysdael J. Definition of an Ets1 protein domain required for nuclear localization in cells and DNA-binding activity in vitro. Mol Cell Biol. 1989;9:5718–5721. doi: 10.1128/mcb.9.12.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raffetseder U, Wernert N, Ostendorf T, van Roeyen C, Rauen T, Behrens P, Floege J, Mertens PR. Mesangial cell expression of proto-oncogene Ets-1 during progression of mesangioproliferative glomerulonephritis. Kidney Int. 2004;66:622–632. doi: 10.1111/j.1523-1755.2004.00782.x. [DOI] [PubMed] [Google Scholar]
- 36.Naito T, Razzaque MS, Nazneen A, Liu D, Nihei H, Koji T, Taguchi T. Renal expression of the Ets-1 proto-oncogene during progression of rat crescentic glomerulonephritis. J Am Soc Nephrol. 2000;11:2243–2255. doi: 10.1681/ASN.V11122243. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka H, Terada Y, Kobayashi T, Okado T, Inoshita S, Kuwahara M, Seth A, Sato Y, Sasaki S. Expression and function of Ets-1 during experimental acute renal failure in rats. J Am Soc Nephrol. 2004;15:3083–3092. doi: 10.1097/01.ASN.0000145459.54236.D3. [DOI] [PubMed] [Google Scholar]
- 38.Pearse DD, Tian RX, Nigro J, Iorgulescu JB, Puzis L, Jaimes EA. Angiotensin II increases the expression of the transcription factor ETS-1 in mesangial cells. Am J Physiol Renal Physiol. 2008;294:F1094–F1100. doi: 10.1152/ajprenal.00458.2007. [DOI] [PubMed] [Google Scholar]
- 39.Zhou MS, Hernandez Schulman I, Pagano PJ, Jaimes EA, Raij L. Reduced NAD(P)H oxidase in low renin hypertension: link among angiotensin II, atherogenesis, and blood pressure. Hypertension. 2006;47:81–86. doi: 10.1161/01.HYP.0000197182.65554.c7. [DOI] [PubMed] [Google Scholar]
- 40.Elbashir SM, Harborth J, Weber K, Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26:199–213. doi: 10.1016/S1046-2023(02)00023-3. [DOI] [PubMed] [Google Scholar]