Abstract
Chronic Hepatitis C virus (HCV) infection carries a significant clinical burden in the United States, affecting more than 4.6 million Americans. Untreated chronic HCV infection can result in cirrhosis, portal hypertension, and hepatocellular carcinoma. Previous interferon based treatment carried low rates of success and significant adverse effects. The advent of new generation oral antiviral therapy has led to major improvements in efficacy and tolerability but has also resulted in an explosion of data with increased treatment choice complexity. Treatment guidelines are constantly evolving due to emerging regimens and real world treatment data. There also still remain subpopulations for whom current treatments are lacking or unclearly defined. Thus, the race for development of HCV treatment regimens still continues. This review of the current literature will discuss the current recommended treatment strategies and briefly overview next generation agents.
Keywords: Hepatitis C, HCV infection, HCV treatment, Direct Antiviral Agents (DAA)
INTRODUCTION
Hepatitis C virus (HCV) affects more than 4.6 million people in the United States1 and is associated with more than 15,000 deaths annually.2 Chronic infection can result in cirrhosis and hepatocellular carcinoma. HCV is the leading cause of liver transplantation in the United States. Previously treatment for HCV was limited to interferon-based therapy, aimed at immunomodulation to inhibit HCV replication. These were used in conjunction with ribavirin (RBV) with limited tolerability and success. The introduction of the first generation protease inhibitors (Boceprevir and Telaperevir) improved sustained virologic response (SVR) rates in adults with HCV genotype (GT) 1 infection. Subsequently Sofosbuvir (SOF), Simeprivir (SIM) were approved by the Food and Drug Administration (FDA) in 2013. This was followed by the Ledipasvir-Sofosbuvir (LDV/SOF) combination (Harvoni), and Ombitasvir-paritaprevir-ritonavir-dasabuvir combination (Viekira Pak, 3D regimen). Following, Daclatasvir (Daklinza, DCV) and Ombitasvir-paritaprevir-ritonavir combination (Technivie, 2D regimen) were approved by the FDA for the treatment of genotype 3 and 4 respectively. Most recently, Elbasvir-Grazoprevir (EBV/GZR) was FDA approved for the treatment of genotypes 1 and 4. This article will review the current FDA approved, interferon-free, oral therapy options for chronic HCV infection with a brief discussion on future therapies.
MOLECULAR TARGETS FOR ORAL ANTIVIRAL THERAPY
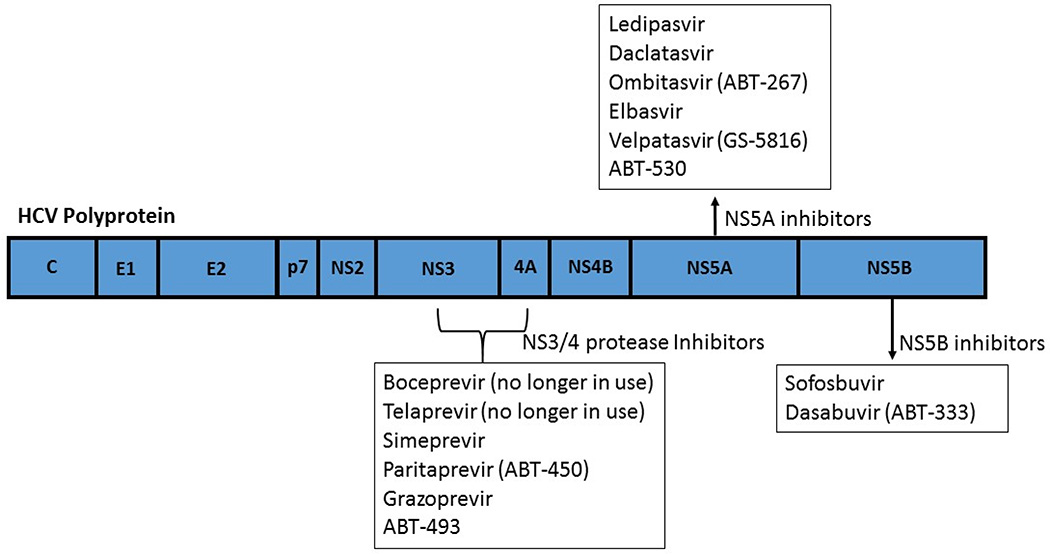
It had been observed since the 1970s that a major non-A, non B hepatitis agent was transmitted via blood products.3 HCV was discovered in 1989 when Houghton and colleagues were able to obtain a cDNA clone of the single stranded RNA virus containing up to 10,000 nucleotides.4 Since then, the structure and life cycle of the virus has been further elucidated which have been key to development of new antiviral therapies. HCV is an enveloped, single-strand, positive-sense, RNA virus that undergoes proteolytic cleavage.5 The resultant components include two structural envelope glycoproteins and the core protein. The remainder components are non-structural proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B) that are necessary viral propagation. NS2/3 and NS3/4A comprise proteases responsible for cleaving the HCV polyprotein.6 NS5B is an RNA dependent RNA polymerase required for viral replication. NS5A is involved in assembly of the cytoplasmic membrane-bound replication complex. The new direct-acting antiviral agents (DAAs) target these non-structural proteins to prevent viral replication (Figure 1).
Figure 1.
Molecular targets for HCV direct acting anti-viral therapy.
CURRENTLY US FDA APPROVED ORAL REGIMENS FOR HCV TREATMENT
The introduction of interferon-free therapies has led to marked improvement in tolerability when compared to previous IFN/RBV based regimens. Now with multiple approved regimens, the choice and duration of treatment will be dependent on several key factors including genotype, treatment experience, presence of cirrhosis, cost, potential drug interactions, and identification of special populations (end-stage renal disease, post-liver transplant, HIV co-infection).
HCV Genotype 1 Infection
GT1 accounts for about 70% of all HCV infection in the United States and currently has the most treatment options.7 SIM/SOF was the first all oral combination regimen for GT1 approved by the FDA in November of 2014. Initial experience with SIM/SOF in the COSMOS trial showed SVR12 rates of 93–94% following 12 weeks of treatment with or without RBV for GT1, treatment naïve and experienced, non-cirrhotic patients.8 COSMOS also included 41 patients with Metavir F4 score who received SIM/SOF with or without RBV for 12–24 weeks with an overall SVR rate of 93%. However, the OPTIMIST-2 study later showed that 12 weeks was suboptimal for patients with cirrhosis, resulting in SVR12 rate of only 83% (86/103).9 Analysis showed that presence of the Q80K mutation was also associated with a much lower SVR of 74% (25/34). Therefore, SIM/SOF treatment for GT1 cirrhotic patients should be extended to 24 weeks based on COSMOS and only proceed in the absence of the Q80K mutation. The main drawback to SIM/SOF regimen will be cost since SIM and SOF are produced by separate companies.
The introduction SOF/LDV (combination NS5B nucleotide inhibitor and NS5A inhibitor) was a major breakthrough which simplified treatment of GT1 infected patients to a single combination pill. In the ION 1 and ION 3 trials, SVR12 rates for GT1, treatment naïve, with or without compensated cirrhosis treated with SOF/LDV for 12 weeks were reported at 93–99%.10,11 ION 3 further showed that in a subset of treatment naïve, non-cirrhotic patients with viral load less than 6 million IU/mL, SVR12 following SOF/LDV for 8 weeks was shown non-inferior to 12 weeks (98% vs 98%). However, since these results were part of the uncontrolled post hoc analysis, the AASLD recommends caution with the 8 week treatment interval.12 Nonetheless, recent data on real world experience shows that 8 week treatment remains highly efficacious for selected patients with reported SVR rates of 93.2% (110/118) in a VA cohort, 97% (44/45) in the HCV-TARGET cohort, and 95% (251/263) in patients from the TRIO network.13,14,15 Therefore, it is recommended to use the 8 week course when the opportunity arises since it significantly reduces cost.
Treatment recommendations with LDV/SOF differ slightly for GT1 treatment experienced patients, based on the presence of cirrhosis. In the ION-2 study, patients were stratified based on GT (1a vs 1b), presence of cirrhosis, and response to previous therapy (null response, partial response, or relapse) then randomized into 4 treatment groups: LDV/SOF × 12 weeks, LDV/SOF/RBV × 12 weeks, LDV/SOF × 24 weeks, or LDV/SOF/RBV × 24 weeks. SVR 12 rates were equal in all groups (97–99%) except in subgroup analysis where patients with treatment experience and cirrhosis achieved a lower SVR of 88% (7/8) with 12 weeks LDV/SOF compared to 100% (17/17) with 24 weeks LDV/SOF. Therefore, a 24 week LDV/SOF treatment course was recommended for treatment experienced GT1 patients with cirrhosis. However additional data from the SIRIUS study showed that LDV/SOF with RBV × 12 weeks could be used with equal efficacy and tolerability compared to LDV/SOF × 24 weeks for GT1 treatment experienced cirrhotic patients16 with similar SVR rates (96% and 97% respectively).
Following SOF/LDV, Paritaprevir/ritonavir/ombitasvir plus dasabuvir (3D regimen) was approved by the FDA in December 2014 for the treatment of HCV GT1 infection. In the SAPPHIRE-1 phase 3 trial, 473 treatment naïve, non-cirrhotic, GT1 patients were treated with 12 weeks 3D regimen plus RBV resulting in overall SVR12 of 96.2%.17 Although efficacy is similar when compared to LDV/SOF, the 3D regimen’s has two main draw backs—(1) the increased pill burden with 2 separate tablets plus RBV at a twice daily dosing and (2) the increased list of potential drug-drug interactions. However, the 3D regimen also has two main strengths which are improved cost effectiveness and a niche for treatment of GT 1b subtype.18 In the PEARL-III trial, 209 HCV GT 1b infected, treatment naïve, non-cirrhotic patients were treated with 3D regimen × 12 weeks without RBV resulting in an impressive 99% SVR (207/209).19 Therefore, 3D regimen can be used for GT1b without RBV with excellent results.
The 3D regimen is also highly efficacious for GT1 treatment experienced patients. The SAPPHIRE-II phase 3 trial included 297 GT1 treatment experienced patients without cirrhosis treated with 12 weeks 3D regimen with RBV showing an overall SVR rate of 96.3% (286/297). There was no significant difference between GT1a at 96.0% (166/173) vs GT1b 96.7% (119/123). The PEARL-II trial further showed that treatment experienced GT1b patients did not require RBV.20
Unfortunately, the presence of cirrhosis in GT1a infected patients will drastically increase 3D regimen treatment duration. In the TURQUOISE II phase 3, open-label trial, patients with GT 1 infection, including both treatment experienced and naïve, who also had compensated cirrhosis (CPC A) were randomized to 12 or 24 weeks of the 3D regimen with RBV. For GT1a, SVR 12 was achieved in 91.8% of the 12 week treatment group compared to 95.9% of the 24 week treatment group.21 Given this small but significant difference, it is recommended to extend 3D regimen with RBV to 24 weeks for GT1a treatment naïve patients with compensated cirrhosis. Among GT1 treatment experienced patients in TURQUOISE II, there was also a lower SVR of 90.2% vs 96.9% in patients treated with 12 weeks compared to 24 weeks of therapy. However, out of the 12 treatment experienced patients who had treatment failure, only 1 was GT1b.
Uniquely, GT1b patients in TURQUOISE II had no significant difference comparing 12 and 24 weeks 3D regimen with SVR rates of 98.5% and 100% respectively.
Furthermore, the TURQUOISE-III evaluated the safety and efficacy of 12 week 3D regimen without RBV in GT1b patients with compensated cirrhosis.22 55% of the 60 patients enrolled in the single arm study were treatment experienced. Overall SVR was 100% (60/60) showing that the addition of RBV was not required for GT1b with cirrhosis. Despite TURQUOISE-III data, the FDA only approved use of 3D regimen with RBV for GT1b with cirrhosis. Therefore addition of RBV should still be considered.
DCV/SOF, although currently FDA approved only for GT3, can also be considered for GT1 infection. Based on ALLY-123 and ALLY-224 phase 3 studies, current AASLD guidelines recommend a 12 week course of DCV/SOF for treatment naïve non-cirrhotic GT1 patients and a 24 week course, with or without RBV, for patients with cirrhosis.12 Similarly, treatment experienced GT1 patients can also receive a 12 week course of DCV/SOF with extension to 24 weeks, with or without RBV, for patients with cirrhosis. For cirrhotic patients, data is currently limited to support an optimal treatment duration of DCV/SOF or whether RBV is required.
Elbasvir (NS5A inhibitor) and Grazeprovir (NS3/4A protease inhibitor) is a new regimen without renal limitation recently FDA approval for treatment of GT1 infection. In the C-EDGE phase 3 placebo controlled trial, EBV/GZR for 12 weeks was shown to have an overall SVR of 95% (273/288), including patients with cirrhosis.25 On subgroup analysis, GT1a patients with NS5A baseline resistance associated variants (RAVs) (M28, Q30, L31, or Y93H) had a significantly lower SVR of 70% (39/56). However, when treatment was extended to 16 weeks with the addition of RBV, SVR was 100% (6/6). Therefore, the FDA recommends baseline NS5A RAV testing for GT1a and extending treatment to 16 weeks with RBV if RAVs are present. The unique feature of EBV/GZR is its approval for use in patient with end stage renal disease. The C-SURFER phase 3 trial placebo controlled trial included both treatment naïve and experienced patients with chronic kidney disease stage 4–5, including 76% on hemodialysis, who received 12 weeks EBV/GZR with an SVR of 99% (115/116). Subgroup analysis found no difference for patients who received hemodialysis. EBV/GZR is also approved for patients who history of treatment failure with 1st generation protease inhibitors (PI) (boceprevir, simeprevir, telaprevir).26 The recommended treatment for PI experienced patents is 12 weeks with RBV.
HCV Genotype 2 Infection
Currently, the only approved oral regimen for GT 2 is SOF with RBV for 12–16 weeks. Results FISSION, POSITRON, VALENCE, and FUSION trials show a SVR rate of 93–94% for GT 2 infected patients in patients treated with SOF/RBV × 12 weeks.27,28,29 The 4 trials included both treatment naïve and treatment experienced patients as well as patients with or without compensated cirrhosis. The presence of cirrhosis was shown to be a risk factor for decreased SVR rates. Therefore, it has been proposed by the AASLD/IDSA guidelines to extend treatment to 16 weeks for patients with cirrhosis, although there is limited data at this time to support this recommendation.
DCV/SOF with or without RBV has also been studied for patients with GT2. In a study looking at DCV/SOF which included genotypes 1–3, 26 patients with GT 2 were treated with DCV/SOF either with or without RBV for 24 weeks resulting in an SVR of 92% (24/26).30
HCV Genotype 3 Infection
Currently the most difficult to treat GT is 3. Although the reasons are unclear, GT3 patients tend to have more risk factors associated with treatment resistance. GT3 is associated with a higher risk of developing cirrhosis (HR = 1.4, 95% CI, [1.32–1.5]) and hepatocellular carcinoma (HR=1.66, 95% CI, [1.48–1.85]).31 However, treatment naïve, non-cirrhotic, HCV GT3 infected patients still tend to have excellent response to DAA therapy. DCV is the first NS5A inhibitor with pan-genotypic activity that has been shown to be safe, well tolerated, and effective. It has low barrier to resistance and a low profile of drug interactions. The mechanism is unclear but thought to inhibit viral replication, assembly, and secretion. In the ALLY-3 phase 3 trial, 101 GT 3 treatment naïve patients were treated with DCV/SOF for 12 weeks, 97% of patients without cirrhosis (Metavir F0–F3) achieved SVR12.32 Alternative oral therapy with SOF/RBV × 24 weeks has also been shown to have good results with SVR 90% (65/72) in the BOSON trial.33
Currently GT3 treatment experienced cirrhotic patients remain a challenge for which optimal therapies are pending. The SVR reported in the BOSON trial for patients treated with SOF with RBV was only 76.5% even when extended to 24 weeks. Currently, addition of PEG-IFN to SOF with RBV for 12 weeks is the primary AASLD/IDSA recommended therapy for GT3 treatment experienced with cirrhosis. DCV/SOF + RBV for 24 weeks can also be considered however adequate supporting data is still lacking.
HCV Genotype 4 Infection
GT4 infection is less common in the United States and therefore data on treatment with current DAA regimens are more limited compared to GT1 however there are multiple FDA approved treatment regimens. Abbvie’s 2D regimen (paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir(25mg)) was recently approved with specific indication for GT4 treatment. In the PEARL-1 phase 2b trial, GT4 treatment naïve and experienced patients without cirrhosis achieved an SVR rate of 100% (83/83) following 12 weeks of 2D regimen with RBV.34 For treatment naïve patients who cannot tolerate RBV, 2D can be used without RBV but may have slightly lower SVR (91% vs 100%). Currently, there is no FDA indication for 2D regimen use in patients with cirrhosis. Alternate options include LDV/SOF for 12 weeks or SOF with RBV × 24 weeks. The SYNERGY trial enrolled 21 patients including both treatment naïve and experienced with and without cirrhosis resulting in a 100% SVR following 12 weeks LDV/SOF in the per-protocol analysis.35 In small studies, SOF with RBV for 24 weeks has reported SVR ranging from 84% to 100% among treatment naïve patients only.36,37,38 Most recently, EBV/GZR was FDA approved for the treatment of GT4 infection for 12 weeks in treatment naïve patients and 16 weeks with the addition of RBV in treatment experienced patients.
HCV Genotype 5 and 6 Infection
Data is limited on the efficacy of new generation DAA therapies for HCV GT 5 and 6 chronic infection however LDV/SOF for 12 weeks has received FDA approval based on small study samples for these two genotypes.39,40 Alternate AASLD recommended regimen with SOF also includes the addition of RBV and PEG-IFN for 12 weeks. New generation regimens with pan-genotypic activity pending FDA approval will be discussed later.
SPECIAL POPULATIONS
End-Stage Renal Disease (ESRD) and Hemodialysis Patients
With the introduction of EBV/GZR, GT 1 and 4 patients now have a highly effective treatment option without renal limitation although certain patients may still require the addition of RBV. SOF based regimens are not approved for patients with GFR < 30 since SOF is renally excreted. RBV use in patients with renal impairment should be approached cautiously as there is high risk for hemolysis and severe anemia. In general, use of 3D regimen with RBV for GT 1A non-cirrhotic patients with ESRD should be limited to experienced centers. Patients with ESRD and GFR < 30 currently still have no FDA approved treatment options for GT 2 and 3.
HIV/HCV Co-infection
Patients with HIV and HCV co-infection are at risk for faster progression of liver disease compared to HCV mono-infection.41 Therefore treating this special population should be considered a priority. The main consideration when choosing DAA therapy for HIV/HCV co-infected population is drug-drug interactions, particularly with HIV anti-retroviral (ARV) therapy. Interactions can result in decreased effectiveness of HCV or HIV therapy, increased risk of resistance, or toxicity. The cytochrome P450 3A4 isozyme is the most commonly affected system for these drug interactions. Key inhibitors include tenofovir and ritonavir while key inducers include efavirenz. These ARV regimens may increase or decrease DAA drug levels respectively, thus adjustment in ARV therapy should be considered prior to HCV treatment initiation. When planning treatment of HCV infection in patients with HIV/HCV co-infection, co-management with an HIV specialist and pharmacist is recommended. Otherwise, DAA therapies are equally as effective in HIV/HCV co-infected patients as HCV mono-infected patients and treatment should be a priority.
Liver Transplant Recipients
Patients with HCV who receive liver transplantation have a 100% re-infection rate of their graft. HCV re-infection of liver transplant recipients results in an accelerated disease progression with 20–30% developing cirrhosis at 5 years.42 Therefore eradication of HCV post-transplant can decrease risk of further graft damage and graft loss. The SOLAR-1 trial included a large number of liver transplant recipients (223) showing that patients with GT 1 or 4 with or without compensated cirrhosis who were treated with LDV/SOF/RBV for 12 weeks achieved a 96% SVR rate.43 Treatment can be extended to 24 weeks for patients who are RBV intolerant. DCV/SOF/RBV regimen for 12 weeks is also effective for GT 1, including those with compensated cirrhosis. Efficacy was shown in the ALLY-1 trial which included 53 transplant recipients who achieved an SVR of rate of 94%.44 In addition, the DCV/SOF/RBV regimen for 12 weeks can also be used for patients with GT 2, 3, or 4 with good efficacy.12,45 Again, 24 weeks is recommended for RBV intolerant patients. Additional alternatives for GT1 patients include SIM/SOF with or without RBV for 12 weeks and the 3D regimen with RBV for 24 weeks. SIM/SOF can be extended to patients with compensated cirrhosis of the graft however 3D regimen is limited to patients with Metavir fibrosis stage F0–F2. Furthermore, regimens that contain SIM or ritonavir will have drug-drug interaction with calcineurin inhibitors and therefore dose adjustments and/or close drug level monitoring will be needed.
FUTURE TREATMENT
Chronic hepatitis C treatment will continue to evolve with next generation regimens. The goals of future therapy will include 1) Pan-genotypic activity with high barrier to resistance 2) Simplified dosing 3) RBV free and 4) Shorter duration of treatment. Many current regimens still require RBV which can cause significant anemia and fatigue in sensitive populations. Several investigational agents have been presented with impressive pan-genotypic activity without RBV.
Sofosbuvir/Velpatasvir Regimen
In the ASTRAL-1 phase 3 trial, 624 patients with HCV Genotype 1, 2, 4, 5, and 6 were treated with Sofosbuvir and Velpatasvir (GS-5816, an NS5A inhibitor) for 12 weeks without RBV resulting in an overall SVR of 99%.46 Furthermore, 32% were treatment experienced and 19% had compensated cirrhosis. ASTRAL-2 and ASTRAL-3 phase 3 trials further extended the RBV free Sofosbuvir/Velpatasvir (SOF/VEL) regimen data in genotypes 2 and 3 showing similar SVR rates of 99% and 95% respectively.47 Limited significant adverse events were reported. SOF/VEL is an oral once daily pan-genotypic regimen pending FDA approval.
ABT-493 and ABT-530 Regimen
ABT-493 (protease inhibitor) and ABT-530 (NS5A inhibitor) is a pan-genotypic regimen with high barrier to resistance currently under investigation. The SURVEYOR I phase 2 trial included GT1 both treatment naïve and experienced patents without cirrhosis who received 8 weeks ABT-493/ABT-530 resulting in a 97% SVR (33/34).48 The SURVEYOR II phase 2 trial included GT 2 and 3 both treatment naïve and experienced patients without cirrhosis who received 12 weeks ABT 493/ABT-530 resulting in SVR 96–100% for GT 2 and 89–97% for GT 3.49,50 Limited significant adverse events were reported. Phase 3 trials are underway.
SUMMARY
Hepatitis C treatment regimens are becoming increasingly well tolerated and effective for patients. However, treatment selection has become increasingly complex due to an explosion of data on new DAA treatment regimens and the growing number of treatment options. Currently, all oral antiviral regimens are available for all genotypes (Table 1 and 2). However, patients with GT 3 who are treatment experienced with cirrhosis still remain without optimal interferon free treatment options. In the near future we are likely to see approval of new regimens which will further close gaps for difficult to treat subgroups and special populations, eventually resulting in more simplified treatment selection.
Table 1.
All oral treatment options for HCV genotype 1
| Genotype | Treatment Options |
± Ribavirin | Previous Treatment | Cirrhosis | Duration (weeks) |
SVR | Trial(s) |
|---|---|---|---|---|---|---|---|
| 1a or 1b | SIM/SOF | No RBV | Naïve or Experienced | No Cirrhosis | 12 | 93–94% | COSMOS |
| Cirrhosis | 24* | 97% | |||||
| 1a or 1b | LDV/SOF | No RBV | Naïve or Experienced | No Cirrhosis | 8–12** | 93–99% | ION-1, ION-2, ION-3, SIRIUS |
| Naïve | Cirrhosis | 12 | 97% | ||||
| Experienced | Cirrhosis | 24 | 97–100% | ||||
| + RBV | Experienced | Cirrhosis | 12 | 96% | |||
| 1a or 1b | DCV/SOF† | No RBV | Naïve or Experienced | No Cirrhosis | 12 | 96–98% | ALLY-1, ALLY-2 |
| ± RBV | Naïve or Experienced | Cirrhosis | 24 | 76% (1a), 100% (1b) |
|||
| 1a or 1b | EBV/GZR | Naïve or Experienced | ± Cirrhosis | 12 | 95% | C-EDGE TN, C-EDGE TE |
|
| + RBV | Naïve or Experienced | ± Cirrhosis | 16†† | 97–100% | |||
| 1a |
3D Regimen (Paritaprevir / ritonavir, ombitasvir, and dasabuvir) |
+ RBV | Naive or Experienced | No Cirrhosis | 12 | 96–97% | SAPPHIRE-I, SAPPHIRE-II, PEARL-II, PEARL-III, PEARL-IV, TURQUOISE-II, TURQOISE-III |
| + RBV | Naive | Cirrhosis | 24 | 96% | |||
| + RBV | Experienced | Cirrhosis | 24 | 96% | |||
| 1b | No RBV | Naïve or Experienced | No Cirrhosis | 12 | 97–100% | ||
| ± RBV | Naïve or Experienced | Cirrhosis | 12 |
Excludes patients with Q80K polymorphism
Treatment naïve patients with VL < 6 million IU can shorten therapy to 8 weeks
Not currently FDA approved for GT1
For presence of baseline NS5A RAVs (M28, Q30, L31, or Y93H) or if PegIFN/RBV/PI -experienced.
Table 2.
All oral treatment options for HCV genotypes 2, 3, 4, 5 and 6.
| Genotype | Treatment Options |
± Ribavirin | Previous Treatment | Cirrhosis | Duration (weeks) |
SVR | Trial(s) |
|---|---|---|---|---|---|---|---|
| 2 | SOF | + RBV | Naïve or Experienced | ± Cirrhosis | 12–16 | 93–94% | FISSION, POSITRON, VALENCE, FUSION |
| 3 | + RBV | Naïve | No Cirrhosis | 24 | 90–95% | BOSON, VALENCE | |
| Naïve | Cirrhosis | 24 | 92% | ||||
| Experienced | ± Cirrhosis | 24 | 62–87% | ||||
| 2 | DCV/SOF | ±RBV | Naïve | ± Cirrhosis | 24 | 92% | Sulkowski MS et al. |
| 3 | No RBV | Naïve | No Cirrhosis | 12 | 97% | ALLY-3 | |
| + RBV | Experienced | Cirrhosis | 24 | ? | ? | ||
| 4 |
2D Regimen (paritaprevir/rito navir/ombitasvir) |
+ RBV | Naïve or Experienced | No Cirrhosis | 12 | 100% | PEARL-1 |
| 4 | EBV/GZR | Naïve | ± Cirrhosis | 12 | 97% | C-SCAPE, C-EDGE TN, C-EDGE COINFECTION |
|
| + RBV | Experienced | ± Cirrhosis | 16 | 100% | C-EDGE TE | ||
| 4 | LDV/SOF | No RBV | Naïve or Experienced | ± Cirrhosis | 12 | 95% | SYNERGY |
| 5 and 6 | No RBV | Naïve | ± Cirrhosis | 12 | 95–96% | Kohler JJ et al., Abergel A et al. |
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
FINANCIAL DISCLOSURES
Dr. Zhang has nothing to disclose; Dr. Nguyen is a member of the Gilead speaker bureau; Dr. Hu is a member of Abbvie and Gilead speaker bureau.
REFERENCES
- 1.Edlin BR, Eckhardt BJ, Shu MA, et al. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62:1353–1363. doi: 10.1002/hep.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ly KN. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 3.Dienstag JL. Non-A, non-B hepatitis, I: recognition, epidemiology, and clinical features. Gastroenterology. 1983;85:439–462. [PubMed] [Google Scholar]
- 4.Choo Q-L, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 5.Ghany MG, Liang TJ. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;369(7):679–680. doi: 10.1056/NEJMc1307589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welbourn S, Pause A. HCV NS2/3 Protease. In: Tan SL, editor. Hepatitis C Viruses: Genomes and Molecular Biology. Chapter 5. Norfolk (UK): Horizon Bioscience; 2006. [PubMed] [Google Scholar]
- 7.Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60(4):691–698. doi: 10.1016/j.jhep.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384(9956):1756–1765. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 9.Lawitz E, Matusow G, DeJesus E, et al. 50th Annual Meeting of the European Association for the Study of the Liver (EASL) Vienna, Austria: 2015. A phase 3, open-label, single-arm study to evaluate the efficacy and safety of 12 weeks of simeprevir (SMV) plus sofosbuvir (SOF) in treatment-naive or -experienced patients with chronic HCV genotype 1 infection and cirrhosis: OPTIMIST-2; p. S264. [Google Scholar]
- 10.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 11.Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 12.AASLD/IDSA/IAS-USA. Recommendations for testing, managing, and treating hepatitis C. 2015 Dec; http://www.hcvguidelines.org. [Google Scholar]
- 13.Backus LI, Belperio PS, Shahoumian T, et al. Effectiveness of Ledipasvir/Sofosbuvir in Treatment Naïve Genotype 1 Patients Treated in Routine Medical Practice. AASLD Liver Meeting. 2015 Nov; Abstract ID: 93. [Google Scholar]
- 14.Terrault N. Treatment Outcomes with 8, 12 and 24 Week Regimens of Ledipasvir/Sofosbuvir for the Treatment of Hepatitis C Infection: Analysis of a Multicenter Prospective, Observational Study. AASLD Liver Meeting. 2015 Nov; Abstract ID: 94. [Google Scholar]
- 15.Curry MP. Effectiveness of 8 or 12 week LDV/SOF in treatment-naïve patients with non-cirrhotic, genotype 1 Hepatitis C: Real-world experience from the TRIO Network. AASLD Liver Meeting. 2015 Nov; Abstract ID: 1046. [Google Scholar]
- 16.Bourlière M, Bronowicki JP, de Ledinghen V, et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS) Lancet Infect Dis. 2015;15:397–404. doi: 10.1016/S1473-3099(15)70050-2. [DOI] [PubMed] [Google Scholar]
- 17.Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 18.Chidi Alexis P, PhD, MSPH, et al. Cost-Effectiveness of New Antiviral Regimens for Treatment-Naïve US Veterans with Hepatitis C. Hepatology. 2015 doi: 10.1002/hep.28327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–1992. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 20.Andreone P, Colombo MG, Enejosa JV, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147:359–365. doi: 10.1053/j.gastro.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 21.Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973–1982. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 22.Feld JJ, Moreno C, Trinh R, et al. Sustained Virologic Response of 100% in HCV Genotype 1b Patients with Cirrhosis Receiving Ombitasvir/Paritaprevir/r and Dasabuvir for 12 Weeks. J Hepatol. 2010 doi: 10.1016/j.jhep.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Poordad F, Schiff ER, Vierling JM, et al. Daclatasvir, sofosbuvir, and ribavirin combination for HCV patients with advanced cirrhosis or postransplant recurrence: phase 3 ALLY-1 study. 50th Annual Meeting of the European Association for the Study of the Liver (EASL); April 22-16, 2015; Vienna, Austria. [Abstract L08.] [Google Scholar]
- 24.Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015 doi: 10.1056/NEJMoa1503153. [DOI] [PubMed] [Google Scholar]
- 25.Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir-Elbasvir Combination Therapy for Treatment-Naive Cirrhotic and Noncirrhotic Patients with Chronic Hepatitis C Virus Genotype 1, 4, or 6 Infection: A Randomized Trial. Ann Intern Med. 2015;163(1):1–13. doi: 10.7326/M15-0785. [DOI] [PubMed] [Google Scholar]
- 26.Zepatier™ [package insert] Whitehouse Station, NJ: Merck & Co., Inc.; 2016. [Google Scholar]
- 27.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 29.Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and Ribavirin in HCV Genotype 2 and 3. N Engl J Med. 2014;370(21):1993–2001. doi: 10.1056/NEJMoa1316145. [DOI] [PubMed] [Google Scholar]
- 30.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 31.Kanwal F, Kramer JR, Ilyas J, et al. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology. 2014;60(1):98–105. doi: 10.1002/hep.27095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015 Apr;61(4):1127–1135. doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster GR, Pianko S, Brown A, et al. Efficacy of Sofosbuvir Plus Ribavirin with or Without Peginterferon-Alfa in Patients With Hepatitis C Virus Genotype 3 Infection and Treatment-Experienced Patients With Cirrhosis and Hepatitis C Virus Genotype 2 Infection. Gastroenterology. 2015;149(6):1462–1470. doi: 10.1053/j.gastro.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 34.Hézode C, Asselah T, Reddy KR, et al. Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): a randomised, open-label trial. Lancet. 2015;385(9986):2502–2509. doi: 10.1016/S0140-6736(15)60159-3. [DOI] [PubMed] [Google Scholar]
- 35.Kohli A, Kapoor R, Sims Z, et al. Ledipasvir and sofosbuvir for hepatitis C genotype 4: a proof-of-concept, single-centre, open-label phase 2a cohort study. Lancet Infect Dis. 2015;15(9):1049–1054. doi: 10.1016/S1473-3099(15)00157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruane PJ, Ain D, Stryker R, et al. Sofosbuvir plus ribavirin for the treatment of chronic genotype 4 hepatitis C virus infection in patients of Egyptian ancestry. J Hepatol. 2014 doi: 10.1016/j.jhep.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 37.Doss W, Shiha G, Hassany M, et al. Sofosbuvir plus ribavirin for treating Egyptian patients with hepatitis C genotype 4. J Hepatol. 2015 doi: 10.1016/j.jhep.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Molina JM, Orkin C, Iser DM, et al. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): a multicentre, open-label, non-randomised, phase 3 study. Lancet. 2015;385:1098–1106. doi: 10.1016/S0140-6736(14)62483-1. [DOI] [PubMed] [Google Scholar]
- 39.Kohler JJ, Nettles JH, Amblard F, et al. Approaches to hepatitis C treatment and cure using NS5A inhibitors. Infect Drug Resist. 2014;7:41–56. doi: 10.2147/IDR.S36247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abergel A, Loustaud-Ratti V, Metivier S, et al. Ledipasvir/sofosbuvir for the treatment of patients with chronic genotype 4 or 5 HCV infection. 50th Annual Meeting of the European Association for the Study of the Liver (EASL); April 22–26, 2015; Vienna, Austria. [Google Scholar]
- 41.Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 42.Fagiuoli S, Ravasio R, Lucà MG, et al. Management of hepatitis C infection before and after liver transplantation. World J Gastroenterol. 2015;21:4447–4456. doi: 10.3748/wjg.v21.i15.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charlton M, Everson GT, Flamm SL, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Poordad F, Schiff ER, Vierling JM, et al. Daclatasvir, sofosbuvir, and ribavirin combination for HCV patients with advanced cirrhosis or postransplant recurrence: phase 3 ALLY-1 study. 50th Annual Meeting of the European Association for the Study of the Liver (EASL); April 22-16, 2015; Vienna, Austria. [Abstract L08.] [Google Scholar]
- 45.Fontana RJ, Brown R, Herzer J, et al. 50th Annual Meeting of the European Association for the Study of the Liver (EASL) Vienna, Austria: 2015. Daclatasvir (DCV) combined with sofosbuvir (SOF) or simeprevir (SMV) in liver transplant (LT) recipients with severe recurrent HCV genotype 1 infection; p. S629. [Abstract P0791.] [Google Scholar]
- 46.Feld JJ, Jacobson IM, Hézode C, et al. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med. 2015 doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 47.Foster GR, Afdhal N, Roberts SK, et al. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med. 2015 doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 48.Poordad F, Felizarta F, Asatryan A, et al. 100% SVR4 in HCV Genotype 1 Non-Cirrhotic Treatment-Naïve or -Experienced Patients With the Combination of ABT-493 and ABT-530 for 8 Weeks (SURVEYOR-I) [abstract 41]. Presented at the 66th Annual Meeting of the American Association for the Study of Liver Diseases; November 13–17, 2015; San Francisco, CA. [Google Scholar]
- 49.Kwo P. SVR4 Rates with the NS3/4A Protease Inhibitor ABT-493 and NS5A Inhibitor ABT-530 in Non-Cirrhotic Treatment-Naïve and Treatment-Experienced Patients with HCV Genotype 3 Infection (SURVEYOR-2). Presented at the 66th Annual Meeting of the American Association for the Study of Liver Diseases; November 13–17, 2015; San Francisco, CA. [Oral presentation #248] [Google Scholar]
- 50.Wyles D. SVR4 Rates with the NS3/4A Protease Inhibitor ABT-493 and NS5A Inhibitor ABT-530 in Non-Cirrhotic Treatment-Naïve and Treatment-Experienced Patients with HCV Genotype 2 Infection (SURVEYOR-2). Presented at the 66th Annual Meeting of the American Association for the Study of Liver Diseases; November 13–17, 2015; San Francisco, CA. [Google Scholar]