Combined central and peripheral demyelination (CCPD) encompasses a wide array of disorders ranging from myeloradiculoneuritis and encephalomyeloradiculoneuritis, which often occur after infection or vaccination, to co-occurrence of multiple sclerosis (MS) and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).1,2
Antibodies directed to neurofascin-155 (NF155), a protein expressed in both CNS and peripheral nervous system myelin and involved in axo-glial coupling at the paranodal regions that flank the node of Ranvier, have been identified with high frequency in Japanese patients with CCPD.3,4 However, these data await independent confirmation in different case series. Moreover, antibodies to NF155 have been reported consistently, although with low frequency, in patients with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP),5–7 thus questioning their specificity and pathogenicity in CCDP. Finally, there is no current consensus on the gold standard technique for anti-NF155 antibody testing, and previous studies used rat or human NF155-based ELISA, cell-based assay (CBA), and immunohistochemistry (IHC) on mouse teased fibers.
The aims of this study were to confirm the association between anti-NF155 antibodies and CCPD in a Caucasian population and to assess the diagnostic accuracy of the currently available techniques for anti-NF155 antibody testing.
Research question and classification of evidence.
Our primary research question was as follows: Do anti-NF155 antibodies within Caucasian patients distinguish those with CCPD from those with other neurologic conditions? This study provides Class IV evidence that serum anti-NF155 antibodies within Caucasian patients do not distinguish those with CCPD from those with other neurologic conditions.
Methods.
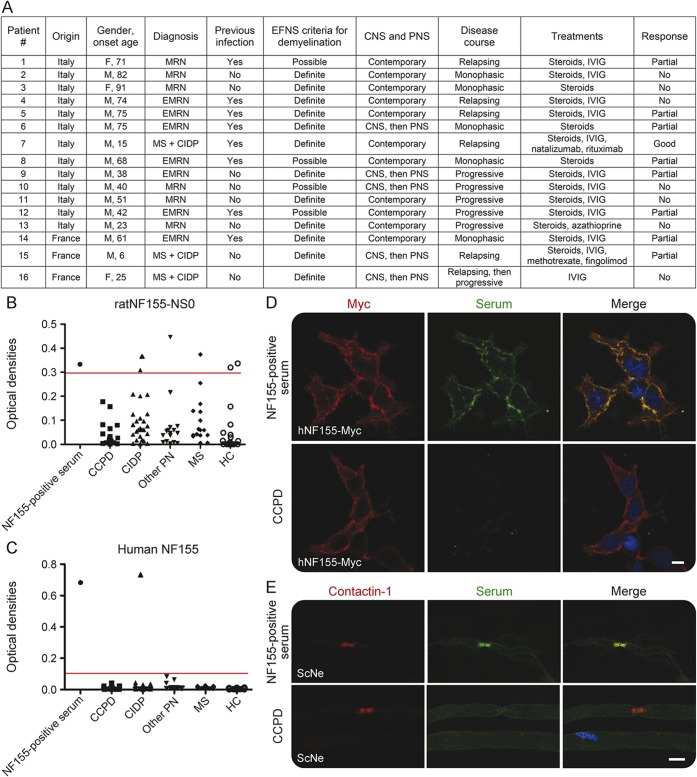
We examined sera from 16 patients with CCPD (7 myeloradiculoneuritis, 6 encephalomyeloradiculoneuritis, 3 MS with CIDP). CSF was available for testing in 8 of them. Their clinical features are summarized in figure 1A. All patients underwent a thorough screening to exclude different metabolic, infectious, rheumatologic, and inherited causes of combined inflammatory disorders of CNS and peripheral nervous system, as detailed previously.2 As controls, 26 patients with CIDP, 15 patients with MS, 15 patients with other peripheral neuropathy (PN), and 20 healthy controls (HC) were used. All the sera were collected for diagnostic purposes and stored at −80°C. A previously identified patient with CIDP with antibody to NF155 was used as positive control. Anti-NF155 antibodies were tested by ELISA and CBA in sera from all patients and controls and in CSF from patients with CCPD. In addition, reactivity against the node of Ranvier was assessed by IHC on teased fibers from mouse sciatic nerve. Sera samples were tested in duplicate by 2 independent laboratories, which were blinded to patients' diagnoses. Briefly, for ELISA, polystyrene microwells were coated with 4 μg/mL rat NF155-NS0 (R&D Systems, Minneapolis, MN), or 1 μg/mL human NF155 (OriGene, Rockville, MD), blocked with 5% nonfat milk in phosphate-buffered saline (PBS), and, after washing, incubated with sera that were previously diluted at 1:200 (rat ELISA) or 1:100 (human ELISA) with 2% nonfat milk in PBS. Detection system was based on horseradish peroxidase/tetramethyl benzidine reaction. CBA and IHC were carried out as previously reported.7 Undiluted CSF samples were tested by CBA. To determine immunoglobulin G (IgG) subclass, peroxidase-conjugated (Thermo Fisher Scientific, Waltham, MA) or FITC-conjugated (Abcam, Cambridge, UK) anti-human IgG1, IgG2, IgG3, and IgG4 secondary antibodies were used in both CBA and ELISA.
Figure 1. Absence of anti–neurofascin-155 (NF155) antibodies in combined central and peripheral demyelination (CCPD).
(A) Clinical features of the patients with CCPD. EMRN = encephalomyeloradiculoneuritis; IVIG = IV immunoglobulin; MRN = myeloradiculoneuritis; PNS = peripheral nervous system. (B–E) Serum samples from patients with CCPD (n = 16), patients with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) (n = 26), patients with other peripheral neuropathy (PN) (n = 15), patients with multiple sclerosis (MS) (n = 15), and healthy controls (HC) (n = 20) were tested for autoantibodies to rat NF155 (B) and human NF155 (C) by ELISA. Optical densities (OD) to NF155 are shown after subtraction of baseline OD reading to bovine serum albumin. The cutoff (red line) represents the mean of HC and other PN groups plus 3 standard deviations. (D) Serum from NF155-positive patients with CIDP, but not sera of patients with CCPD, reacted against myc-tagged human NF155 in HEK293 cells. (E) NF155-positive CIDP serum, but not sera of patients with CCPD, colocalized with contactin-1, an axonal protein that interacts with glial NF155 at paranodes, in mouse sciatic nerve fibers. Scale bars (D, E): 10 μm.
Results.
On rat NF155-based ELISA, anti-NF155 antibodies were found in 2/26 (7.6%) patients with CIDP, 1/15 (6.6%) patients with MS, 1/15 (6.6%) patients with other PN, and 2/20 (10%) HC, but in none of the 16 patients with CCPD (figure 1B). On human NF155-based ELISA, anti-NF155 antibodies were found in 1/26 (3.8%) CIDP serum, but not in CCPD or healthy and disease controls sera (figure 1C) or CSF samples.
Such isolated antibody reactivity, along with the positive control serum, was confirmed by CBA (figure 1D) and showed paranodal staining on teased nerve fibers (figure 1E). No CCPD sera stained the nodes or paranodes. Anti-NF155 antibodies in the seropositive patient with CIDP were mainly of IgG4 isotype (figure e-1 at Neurology.org/nn).
Discussion.
None of our patients with CCPD had serum antibody reactivity to NF155, differently from previous studies reporting their high prevalence (45.5%–86%).3,4 Differences in ethnicity (Caucasian vs Japanese) and in clinical features (our patients had later onset, more heterogeneous clinical presentations, and unsatisfactory response to IV immunoglobulin [IVIg]) can at least partially account for the discrepancies. Therefore, we cannot exclude that anti-NF155 antibodies may be present in particular subgroups of patients with CCPD, such as those with clear response to immunotherapies or of Japanese ethnicity.
We found anti-NF155 IgG4 antibodies in 1/26 patients with CIDP. The patient had the classical clinical features of NF155-positive CIDP, including distally predominant weakness, ataxia, tremor, and IVIg resistance, but no central demyelination. In a recent study, one of us also found that anti-NF155 antibodies were specifically associated with CIDP in a Japanese cohort.7 A minority of those patients showed CNS demyelinating lesions (3/35), which suggested that the presence of antibodies to NF155 may increase the susceptibility to develop CNS disorders.
Regarding anti-NF155 antibody testing, ELISA using rat NF155 showed low analytical specificity, possibly due to nonspecific reactivity to NS0 myeloma cell line–derived rat NF155, which is abnormally glycosylated and potentially immunogenic.5 By contrast, human NF155-based ELISA and CBA yielded concordant results, and allowed a clear distinction between anti-NF155 seropositive and seronegative cases. For this reason, both human NF155-based ELISAs and CBAs, followed by determination of IgG subclass in positive cases, should be preferred for anti-NF155 antibody testing.
Supplementary Material
Acknowledgments
Acknowledgment: The authors thank Dr. Isabel Illa (Hospital de la Santa Creu I Sant Pau de Barcelona, Universitat Autònoma de Barcelona, Spain) for providing the control NF155-positive CIDP serum; Dr. Fabio Blandini (C. Mondino National Neurological Institute, IRCCS, Pavia, Italy) for providing the rat sciatic nerves; and Dr. Emilio Ciusani (IRCCS Foundation, C. Besta Neurological Institute, Milan, Italy) for contributing with CCPD sera collection.
Footnotes
Supplemental data at Neurology.org/nn
Author contributions: Andrea Cortese: development of the study concept and design, study supervision, acquisition of clinical and laboratory data, analysis and interpretation, writing, reviewing, and revising of manuscript. Jérôme J. Devaux: study supervision, acquisition of laboratory data, analysis and interpretation, reviewing, and revising of manuscript. Elisabetta Zardini: acquisition of laboratory data, analysis and interpretation, reviewing, and revising of manuscript. Constance Manso: acquisition of laboratory data, analysis and interpretation, reviewing, and revising of manuscript. Guillaume Taieb: acquisition of clinical data, analysis and interpretation, reviewing, and revising of manuscript. Clarisse Carra Dallière: acquisition of clinical data, analysis and interpretation, reviewing, and revising of manuscript. Philippe Merle: acquisition of clinical data, analysis and interpretation, reviewing, and revising of manuscript. Silvia Romagnolo: acquisition of laboratory data, analysis and interpretation, reviewing, and revising of manuscript. Cecilia Osera: acquisition of laboratory data, analysis and interpretation, reviewing, and revising of manuscript. Nicolò Visigalli: acquisition of laboratory data, analysis and interpretation, reviewing, and revising of manuscript. Giuseppe Piscosquito: acquisition of clinical data, analysis and interpretation, reviewing, and revising of manuscript. Ettore Salsano: acquisition of clinical data, analysis and interpretation, reviewing, and revising of manuscript. Enrico Alfonsi: development of the study concept and design, study supervision, acquisition of clinical data, analysis and interpretation, reviewing, and revising of manuscript. Arrigo Moglia: development of the study concept and design, study supervision, acquisition of clinical data, analysis and interpretation, reviewing, and revising of manuscript. Davide Pareyson: development of the study concept and design, study supervision, acquisition of clinical data, analysis and interpretation, reviewing, and revising of manuscript. Enrico Marchioni: development of the study concept and design, study supervision, acquisition of clinical data, analysis and interpretation, reviewing, and revising of manuscript. Diego Franciotta: development of the study concept and design, study supervision, acquisition of laboratory data, analysis and interpretation, reviewing, and revising of manuscript.
Study funding: Supported by the Italian Ministry of Health (RF-2011-02347955; E.M.), the Agence Nationale pour la Recherche (ACAMIN; J.D.), under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases, and the Association Française contre les Myopathies (MNM1 2012-14580; J.D.).
Disclosure: A. Cortese reports no disclosures. J.J. Devaux received research support from Agence Nationale pur la Recherche, The Association Française contre les Myopathies. E. Zardini, C. Manso, and G. Taieb report no disclosures. C.C. Dalliere received travel funding from Merck Serono, TEVA, and Novartis. P. Merle, C. Osera, S. Romagnolo, N. Visigalli, and G. Piscosquito report no disclosures. E. Salsano served on a scientific advisory board for and received travel expenses from Actelion Pharmaceuticals Ltd. E. Alfonsi and A. Moglia report no disclosures. D. Pareyson is on the editorial board for Journal of Peripheral Nervous System, Neurological Sciences, and Journal of Neuromuscular Disorders; and received research support from Pfizer Italia srs, Kedrion SpA, Regione Lombardia, and Telethon-UILDM Italy. E. Marchioni received research support from Fondazione Istituto Neurologicao Casimiro Mondino, Ministero della Sanità D. Franciotta reports no disclosures. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Kamm C, Zettl UK. Autoimmune disorders affecting both the central and peripheral nervous system. Autoimmun Rev 2012;11:196–202. [DOI] [PubMed] [Google Scholar]
- 2.Marchioni E, Ravaglia S, Montomoli C, et al. Postinfectious neurologic syndromes: a prospective cohort study. Neurology 2013;80:882–889. [DOI] [PubMed] [Google Scholar]
- 3.Kawamura N, Yamasaki R, Yonekawa T, et al. Anti-neurofascin antibody in patients with combined central and peripheral demyelination. Neurology 2013;81:714–722. [DOI] [PubMed] [Google Scholar]
- 4.Ogata H, Matsuse D, Yamasaki R, et al. A nationwide survey of combined central and peripheral demyelination in Japan. J Neurol Neurosurg Psychiatry 2016;87:29–36. [DOI] [PubMed] [Google Scholar]
- 5.Ng JK, Malotka J, Kawakami N, et al. Neurofascin as a target for autoantibodies in peripheral neuropathies. Neurology 2012;79:2241–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Querol L, Nogales-Gadea G, Rojas-Garcia R, et al. Neurofascin IgG4 antibodies in CIDP associate with disabling tremor and poor response to IVIg. Neurology 2014;82:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devaux JJ, Miura Y, Fukami Y, et al. Neurofascin-155 IgG4 in chronic inflammatory demyelinating polyneuropathy. Neurology 2016;86:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.