Abstract
Wilson's disease is an inborn error of copper metabolism characterized by inability of the liver to excrete copper into the bile, with excessive deposition of copper primarily in the liver and in the brain. The lentiform nuclei are involved, most often followed by involvement of the thalami, the pons, midbrain, superior and inferior cerebellar peduncles, and the cerebellar nuclei. Predominant involvement of the thalami and brainstem with no significant involvement of the lentiform nuclei is not common. We present a case of Wilson's disease with minimal involvement of the lentiform nuclei, with marked lesions involving the thalami, midbrain, and pons.
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging
Case report
A 32-year-old male presented with new-onset slurred speech of three weeks' duration. The patient had a known case of Wilson’s disease on znc therapy (50 mg TDS). The ceruloplasmin level was very low, at 4 mg/dl. No features of jaundice or cirrhosis were seen to suggest marked hepatic involvement. No evidence of significant abnormalities were seen on liver function tests. No features of tremors, dystonia, or dysphagia were seen. No prior CT or MRI studies were available for comparison.
An MRI study revealed marked T2 and FLAIR hyperintense lesions involving bilateral thalami, midbrain, and pons (Figure 1A, Figure 1B, Figure 1C, Fig 1D, Fig 1E). The lesions were hypointense on T1-weighted sequence and showed no evidence of restricted diffusion or postcontrast enhancement. Minimal involvement of the lentiform nuclei was seen. No significant involvement of the caudate nuclei, cerebellar white matter, centrum semiovale, or subcortical white matter was seen.
Figure 1A.
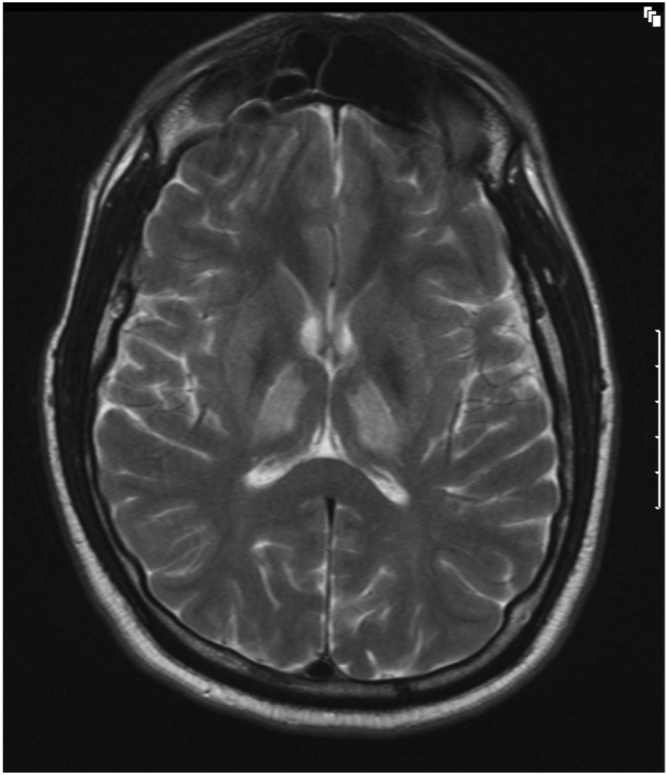
32-year-old man with history of Wilson's disease who presents with slurred speech. T2-weighed, axial MRI image shows presence of hyperintensities involving bilateral thalami. Minimal hyperintensities are seen involving bilateral lentiform nuclei.
Figure 1B.
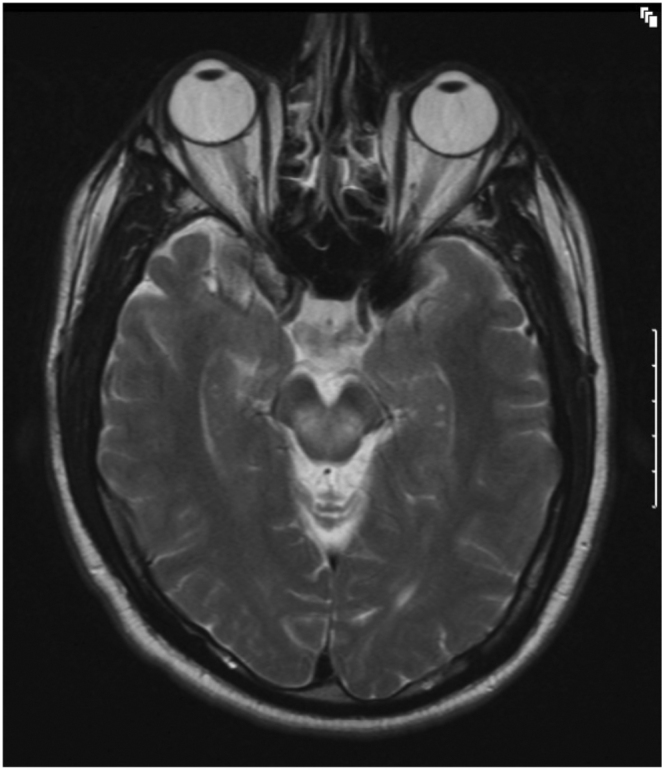
32-year-old man with history of Wilson's disease who presents with slurred speech. T2-weighed, axial MRI image shows presence of hyperintensities involving dorsal midbrain.
Figure 1C.
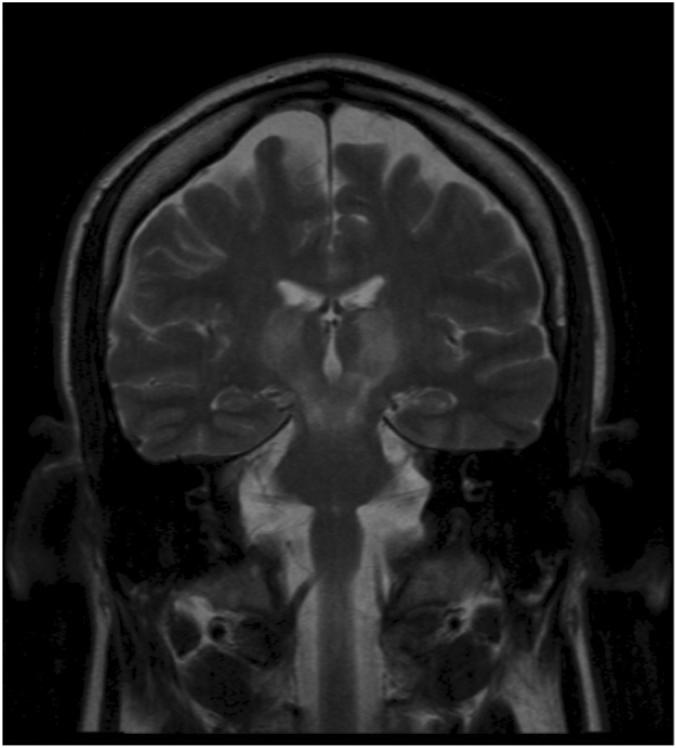
32 year old man with history of Wilson's disease who presents with slurred speech. T2-weighted, coronal MRI image shows presence of hyperintensities involving bilateral thalami, midbrain, and uppermost surface of pons.
Fig 1D.
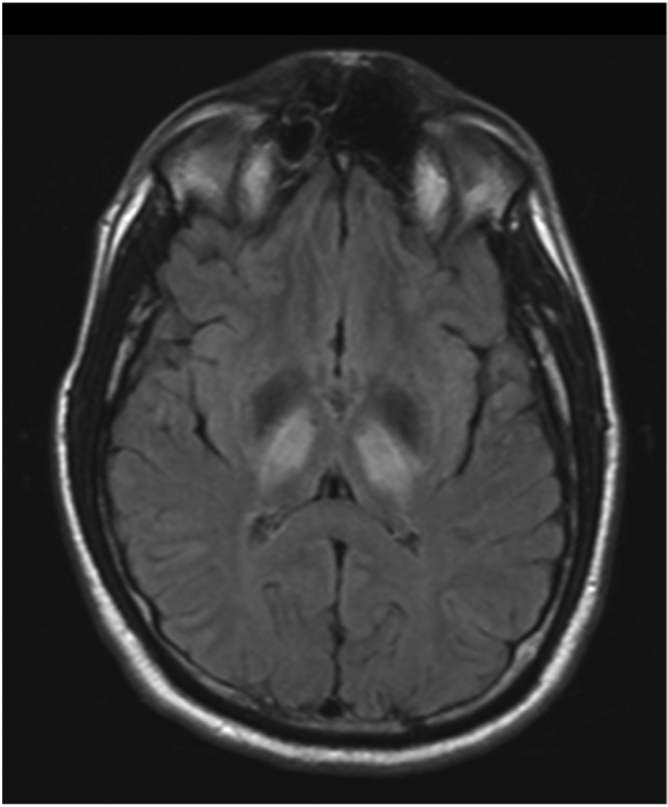
32-year-old man with history of Wilson's disease who presents with slurred speech. Fluid-attenuated inversion recovery sequence, axial MRI image shows presence of hyperintensities involving bilateral thalami. Minimal hyperintensities are seen involving bilateral nuclei.
Fig 1E.
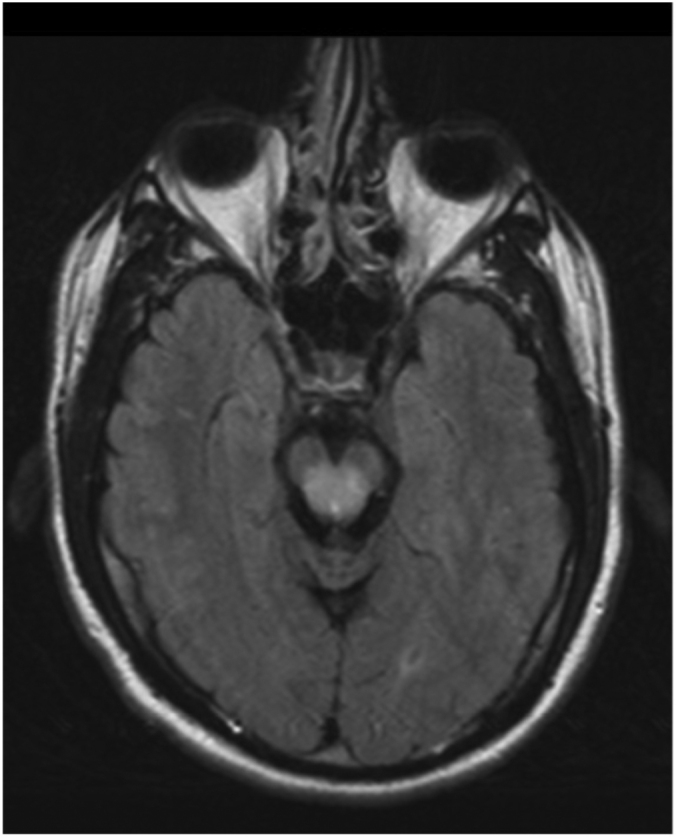
32-year-old man with history of Wilson's disease who presents with slurred speech. Fluid-attenuated inversion recovery sequence, axial MRI image shows presence of hyperintensities involving dorsal midbrain.
Discussion
Wilson’s disease is an inborn error of copper metabolism that is characterized by an inability of the liver to excrete copper into the bile (1, 2). It is named after Dr Samuel Alexander Kinnier Wilson (1878-1937), the British neurologist who first described the condition in 1912. It predominantly involves the liver and brain and is also known as hepatico-lenticular degeneration. There is abnormal accumulation of copper, primarily in the liver and in the brain. The peak age of presentation is between 8 and 16 years, though the condition can present at any age from 5 to 50 years or older (3). The definitive diagnosis is made biochemically, with low levels of serum ceruloplasmin, elevated hepatic copper levels, and increased rate of urinary copper excretion (4, 5, 6).
Most patients have abnormal liver function tests such as a raised aspartate transaminase, alanine transaminase and bilirubin level, and (when the liver damage is significant), decreased albumin, low alkaline phosphatase, and prolonged prothrombin time. Liver biopsy may reveal changes of steatosis and cirrhosis. Imaging findings on ultrasound, CT, and MRI may show multiple nodular lesions in the liver, presence of a perihepatic fat layer, and a relatively normal caudate lobe.
Normally, the human brain has high copper levels in the putamen, globus pallidus and caudate nuclei, the substantia nigra, locus caeruleus, and dentate nuclei (7, 8). In Wilson’s disease, abnormal deposition of copper is seen, especially in the putamen and globus pallidus.
Spongy softening, cavitation, and a general reduction of neurons are a few histopathological changes that are seen in Wilson’s disease involving the brain [9]. Atrophic changes are seen in longstanding cases.
The initial neurological presentation may include dysarthria and difficulty with the hands [10], with latter manifestations of personality and psychiatric problems, and neurologic abnormalities like Parkinsonian tremors, ataxia, dystonia, and chorea.
On MRI study, bilateral symmetric involvement of the putamen, caudate nuclei, thalamus, globus pallidus, dentate nucleus, pons, substantia nigra, periaqueductal gray matter, tectum, and red nucleus can be seen. Subcortical and centrum semiovale white matter involvement may also be seen. Lesions are usually hypointense on T1-weighted and hyperintense on T2-weighted images. Hypointensity on T2-weighted images may be seen sometimes, secondary to copper deposition or iron deposition (5, 11, 12). Diffusion-weighted images may show areas of restricted diffusion early in the disease process due to cytotoxic edem, or inflammation due to excessive copper deposition. However, this restricted diffusion is not seen in chronic cases. which are characterized by necrosis, spongiform degeneration, and demyelination.
In a study done in pediatric Wilson’s disease (13), three different presentation groups were found. The first group showed normal MR imaging findings. In the second group, increased signal intensity on T1-weighed images in the globus pallidus, followed by the putamen, midbrain, and caudate nucleus, was seen. The third group demonstrated increased T2-weighted signal intensity in the putamen, followed by the caudate nucleus, globus pallidus, thalamus, midbrain, and pons.
The lentiform nuclei are involved most often, followed by the thalami, pons, midbrain, superior and middle cerebellar peduncles, and cerebellar nuclei. Involvement of the thalami and brainstem structures with no significant involvement of lentiform nuclei is not common.
In our case, the lentiform nuclei show minimal involvement. However, we noted marked hyperintensity in the thalami, midbrain, and upper pons on T2-weighted and inversion recovery sequences. The differential diagnosis for these MRI findings would include vascular conditions like posterior circulation infarcts, hypoxic ischemic changes, and hypertensive encephalopathy; metabolic conditions like osmotic demyelination, Wilson’s disease, and Leigh’s disease; and inflammatory conditions like ADEM and HIV encephalopathy.
Posterior circulation infarcts, like arterial infarcts elsewhere, conform to vascular territories and bilaterally symmetric involvement of thalami, midbrain, and upper pons, and sparing of the cerebellum is not common. Hypoxic-ischemic changes generally are seen in the erinatal period and involve predominantly periventricular white matter with sequaelae of encephalomalacic changes that may have cystic changes. Global hypoxic-ischemic events generally involve the subcortical and periventricular white matter. Hypertensive encephalopathy may involve symmetric areas of brainstem, but generally the occipital lobes, cerebellum, and basal ganglia are involved. There is history of raised blood pressure, and the clinical setting and presentation are different. In osmotic demyelination, the upper and middle pons is characteristically involved, with predominant central involvement. The peripheral and ventricular surfaces of the pons are spared. Extrapontine sites of involvement may include the thalami and basal ganglia. However, the clinical presentation is different and a history of rapid correction of hyponatremia is associated.
In Leigh’s disease, bilateral symmetrical involvement of the thalami, midbrain, and pons may be seen. However the putamen, globus pallidus, caudate nuclei, cerebellar white matter, cerebral white matter, and gray matter in spinal cord may also be involved. The involvement of brainstem is characteristic. The condition generally presents in infancy or early childhood and is rapidly progressive. It is characterized clinically by respiratory failure, visual and auditory problems, ataxia, weakness, hypotonia, poor feeding, and seizures (14).
In HIV encephalopathy, symmetric involvement of brainstem and thalami may be seen. However, subcortical and periventricular white matter predilection is common, with atrophic changes involving the brain parenchyma. In ADEM, a preceding history of viral infection or vaccination is generally present. Multifocal deep white matter and subcortical lesions are seen with predilection for cerebral and cerebellar white matter. Brainstem involvement may be seen, but strict symmetry is uncommon.
Our patient. known to have Wilson’s disease, presented with symptoms of slurred speech. An MRI study showed presence of T2 and FLAIR hyperintensities involving bilateral thalami, midbrain, and upper pons. Minimal involvement of the lentiform nuclei was seen. No significant involvement of the cerebellar nuclei or superior or middle cerebellar peduncles was seen. No significant abnormalities were seen involving the cerebral cortex or white matter.
Therefore, in any case with MRI findings of T2 and FLAIR hyperintensities involving bilateral thalami and brainstem with minimal or no significant involvement of the basal ganglia, Wilson’s disease should be included in the differential diagnosis.
Footnotes
Published: August 31, 2009
References
- 1.Van Wassenaer-van Hall HN, Van den Heuvel AG, Algra A, Hoogenraad TU, Mali WPTM. Wilson disease: findings at MR imaging and CT of the brain with clinical correlation. Radiology. 1996;198:531–536. doi: 10.1148/radiology.198.2.8596862. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.King AD, Walshe JM, Kendall BE. Cranial MR imaging in Wilson's disease. AJR Am J Roentgenol. 1996;167:1584–1597. doi: 10.2214/ajr.167.6.8956601. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Walshe JM. Wilson's disease. In: Vinken PJ, Bruyn GW, Klawans HL, editors. vol. 49. Elsevier Science Publishers; Amsterdam: 1986. pp. 223–238. (Handbook of Clinical Neurology). [Google Scholar]
- 4.Starosta-Rubinstein S, Young AB, Kluin K. Clinical assessment of 31 patients with Wilson's disease. Correlations with structural changes on magnetic resonance imaging. Arch Neurol. 1987;44:365–370. doi: 10.1001/archneur.1987.00520160007005. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Yuh WT, Flickinger FW. Unusual MR findings in CNS Wilson disease (letter) AJR Am J Roentgenol. 1988;151:834. doi: 10.2214/ajr.151.4.834-a. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Lennox G, Jones R. Gaze distractibility in Wilson's disease. Ann Neurol. 1989;25:415–417. doi: 10.1002/ana.410250417. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Warren PJ, Earl CJ, Thompson RHS. The distribution of copper in human brain. Brain. 1961;83:709–717. doi: 10.1093/brain/83.4.709. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Cumings IN. Trace metals in the brain in health and in neurological disease. J Cli Path. 1968;21:1–6. [PubMed] [Google Scholar]
- 9.Greenfield JG, Hume Adams J, Duchen LW. Greenfield's neuropathology. 5th ed. Edward Arnold; London: 1992. Nutritional deficiencies and metabolic disorders; pp. 838–840. [Google Scholar]
- 10.Walshe JM, Yealland M. Wilson's disease: the problem of delayed diagnosis. J Neurol Neurosurg psychiatry. 1992;55:692–696. doi: 10.1136/jnnp.55.8.692. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hitoshi S, Iwata M, Yoshikawa K. Midbrain pathology of Wilson's disease: MRI analysis of three cases. J Neurol Neurosurg psychiatry. 1991;54:624–626. doi: 10.1136/jnnp.54.7.624. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Haan J, Grossman RI, Civitello L. High-field magnetic resonance imaging of Wilson's disease. J Comput Assist Tomogr. 1987;11:132–135. doi: 10.1016/0149-936x(87)90004-x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Kim J, Kim IO, Kim WS. MR imaging of the brain in Wilson disease of childhood: Findings before and after treatment with clinical correlation. American Journal of Neuroradiology. 2006;27:1373–1378. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 14.Barkovich AJ, Good WV, Koch TK. Mitochondrial disorders: analysis of their clinical and imaging characteristics. AJNR Am J Neuroradiol. 1993;14:1119–1137. [PubMed] [PMC free article] [PubMed] [Google Scholar]


