Abstract
The use of liposome as an adjuvant and a vaccine carrier has been cited previously in the literature. It has also been shown to be effective in enhancing the immunogenicity of vaccine candidates. BALB/c mice immunized subcutaneously with outer membrane protein (OMP) of Brucella abortus S19 vaccine strain entrapped in a commercial cationic liposome (S19-OMP-liposome) for vaccine delivery, showed enhanced protection (P<0.05) compared to groups of mice inoculated with S19 OMP alone, S19 live B. abortus vaccine and liposome alone, when challenged intra-peritoneally with virulent B. abortus strain 544 at 30 days post-immunization (DPI). The S19-OMP-liposome preparation was found to be safer compared to the live B. abortus S19 vaccine at 15 days post challenge (DPC), as evidenced by the significant difference in spleen weight between S19-OMP-liposome, S19 OMP and S19 live as well as the liposome control groups (P<0.01). Antibody isotype response profiles of the experimental groups indicated that the immune response was Th1 cell mediated. The protective advantage conferred to mice immunized with S19-OMP entrapped in liposome over those immunized with the live B. abortus S19 version, could probably be related to the significantly different response of IgG2b at 30 DPI (P<0.01), IgG2a (P<0.01), IgG2b (P<0.01) and IgG3 (P<0.05) at the DPC stages, respectively.
Key Words: Brucella, Liposome, Mice
Introduction
Brucella abortus is the causative agent of bovine brucellosis. It causes spontaneous abortion, placentitis and infertility in pregnant cattle, hence resulting in considerable economic loss for the dairy industry (Xavier et al., 2009 ▶). It also causes infection in humans which lead to persistent undulant fever. Controlling bovine brucellosis is mainly achieved by mass immunization with live attenuated B. abortus smooth strain S19 (Graves, 1943 ▶) or the rough mutant strain RB51 (Crasta et al., 2008 ▶), but it does not confer absolute protection to cattle (Alton et al., 1984 ▶). It may revert to pathogenic form (Corner and Alton, 1981 ▶) and show residual virulence in natural hosts. The vaccine strains are also pathogenic to humans as they are excreted through milk or other secretions (Nicoletti, 1989 ▶).
Attempts have been made earlier to use purified antigens from the cell membrane of B. abortus to immunize cattle. Purified sub unit vaccines are immunogenic and safe since the possibility of reversal to virulence is eliminated. The immunogenicity of the outer membrane protein (OMP) can be improved by entrapping the OMP within liposomes that have the potential to improve the efficacy of delivery to the target antigen processing cells (APC) (Onurdag et al., 2008 ▶).
In earlier studies, the multi-lamellar molecular structure of bio-degradable cationic liposomes with an appropriate formulation of various antigens derived from F. tularensis and B. melitensis have shown versatile adjuvant property, safety, enhancement of prophylactic efficacy and the ability to elicit a strong immune response in in vitro as well in vivo models (Kulikov et al., 1985 ▶; Wong et al., 1992 ▶; Gladerio et al., 1995 ▶; Vitas et al., 1995 ▶; Vitas et al., 1996 ▶; Ireland et al., 2010 ▶). The ability of cationic liposomes to deliver liposome bound antigens in sustained and concentrated forms directly to macrophages for their further processing to APC has been cited as a reason for the better clearance of infection (Alving et al., 1986 ▶; Richard et al., 1998 ▶).
In this study, the immunogenic and protective efficacy of the outer membrane protein of B. abortus strain S19 (S19-OMP) entrapped in cationic liposome as a vaccine delivery system is evaluated by immunization and challenge experiments in a BALB/c mouse model.
Materials and Methods
Mice
Six to eight-week-old female BAB/c mice were used in this study, all provided by the Small Animal Testing (SAT) Unit, Indian Immunologicals Limited, Hyderabad. The mice were caged in biosafety level 3 (BSL 3) facilities and cared for 1 week before the start of the experiments. All experiments were approved by the Institutional Ethical Committee (IAEC) and the Committee for the Purpose of Control of Experiment of Animals (CPCSEA), Ministry of Environment, Forest and Climate Change, Government of India, and were conducted according to the standard operating procedures (SOP) and guidelines of IAEC/CPCSEA.
Bacterial culture
Bulk culture of the B. abortus S19 strain, obtained from the National Dairy Development Board, Anand, was grown in an aerated stirred-tank bioreactor using soya casein digest medium (BD, USA) and used for extraction of the outer membrane protein. Mice were immunized with 1.1 × 105 colony forming units (CFU) of B. abortus S19 vaccine (Bruvax, Indian Immunologicals Limited, Hyderabad, India). After re-constitution and dose adjustment, 0.1 ml B. abortus wild type strain 544 (ATCC, USA) was used for mice challenge experiments.
Extraction and purification of Brucella abortus S19OMP complex
Extraction of outer membrane protein complex was carried out according to the method described earlier by Verstreate et al. (1982) ▶. Downstream processing, such as cell physical disruption, purification and efficacy of extraction by electro-immunoblot transfer assay and S19-OMP complex estimation was performed as described previously by Mythili et al. (2012) ▶.
Formulation of S19-OMP-liposome
The cationic liposome 1,2, dioleoyl-3-dimethylammonium-propane (DODAP/1,2, dioleoyl-sn-glycero-3-phospho-ethanolamine (DOPE) (50:50 mol/ mol) was obtained commercially (Formumax®, USA) and used for the entrapment of the S19-OMP complex obtained as described above. The formulation was prepared fresh before immunization by manual mixing of DODAP/DOPE with S19-OMP at the recommended ratio of 1:20 (50 µL of DODAP/DOPE with 950 µL of S19-OMP) by repeated inversion (20-40 times) of the mixture in an microcentrifuge tube at room temperature, so as to obtain a concentration of 50 µg of S19-OMP/mice/dose. The process resulted in the binding of the OMP extracted from B. abortus S19 to the cationic liposome DODAP/DOPE.
Immunization of mice
Each group of six BALB/c mice was immunized sub-cutaneously with a 50 µg formulation of liposome-encapsulated B. abortus S19 OMP (S19-OMP) as described above as well as OMP alone once at day 0 of immunization. Six mice were simulatenously immunized with 1.1 × 105 CFU live attenuated B. abortus S19 once on day 0 of the immunization. Those injected with liposome alone acted as positive and negative controls on day 0 of the immunization.
Bleeding and mice challenge experiments
Serum samples from mice were collected for the antibody assay from the infra-orbital sinus using the capillary tube insertion method after proper restraining and before immunization on day 0 and days 7, 14, 21, 30 and 45 post-immunization. Mice were challenged intra-peritoneally with 2.2 × 105 CFU/mice of wild type B. abortus strain 544 irrespective of the treatment group, day 30 post-immunization.
Protective efficacy in mice
On day 15 post-challenge, mice were euthanized by CO2 asphyxiation. The spleen of each mouse from each group was collected aseptically to determine the bacterial load of wild type B. abortus challenge strain (OIE, 2009 ▶). The spleen was homogenized using mortar and pestle and re-suspended in PBS (pH = 6.4) in volumes 10 times its weight. Ten-fold serial dilutions of suspension from each spleen were plated on tryptic soya agar (TSA) to determine the load of the B. abortus strain 544. Spleens from control groups were similarly processed and samples from mice immunized with B. abortus S19 were plated on potato infusion agar (PIA). The spleen from mice immunized with liposome alone as negative control was plated on TSA to determine the bacterial load of the B. abortus 544 challenged. TSA plates were incubated at 37°C for 5-7 days with 5% CO2 to recover the wild type challenge strain. PIA plates were incubated at 37°C for the same period in air, without CO2 atmosphere. CFU enumeration from each plate was done manually. The CFU was expressed after the arithmetic values were subjected to logarithmic transformation (log y = x/log x; where x = CFU/ml). The protection expressed as log10 CFU values were compared for each group to determine the protective efficacy of each treatment.
Immunoassay
The levels of immunoglobulin antibody isotypes against anti-Brucella outer membrane protein were assayed by indirect ELISA. The well of the microtiter plate (Nunc, Denmark) was coated with 100 ng of OMP/100 µL/well in carbonate-bicarbonate buffer (pH = 9.6). The optimal concentration of antigen and serum dilution was determined by checker-board titration utilizing pre- and post-vaccinated serum. IgG antibody isotype IgG1, IgG2a, IgG2b and IgG3 against OMP was determined using a monoclonal isotype antibody kit (SIGMA, USA) with a 1:1000 dilution in 2% skimmed milk as the blocking buffer and incubated for 1 h at 37°C. Plates were washed 4-5 times with PBS-T before each subsequent step. 100 µL of recombinant protein A/G peroxidase (Thermo Scientific, USA) with a 1:20,000 dilution in skimmed milk were used as secondary conjugate after washing with PBS-T. 100 µL of Peroxidase substrate in citrate buffer (Tetra-methyl-benzidine with H2O2) were added in each well after 1 h of incubation at 37°C and washed with PBS-T. The reaction was stopped with 1.25 M H2SO4. After 10 min of color development and absorbance, optical density was measured at 450 nm (SYNERGY ST, BioTek, USA). The antibody titers were expressed as the reciprocal of 2 logarithmic transformations with the Mean ± SD of the pre-vaccinate sera with a 1:50 dilution as a cut off value for each antibody isotype.
Statistical analysis
The intensity of infection and protective efficacy of each group at 15 DPC were expressed as the mean log CFU ± SD. The values were analyzed statistically by Student’s t-tests, followed by an analysis of variance (ANOVA) with a Tukey’s Honestly Significant Difference (HSD) post-test for all groups. The specific isotype antibodies were expressed as reciprocal of log 2 end-point dilutions and analyzed by ANOVAs for statistical difference followed by Tukey’s HSD tests (Snedcor and Cochran, 1980).
Results
Antibody response
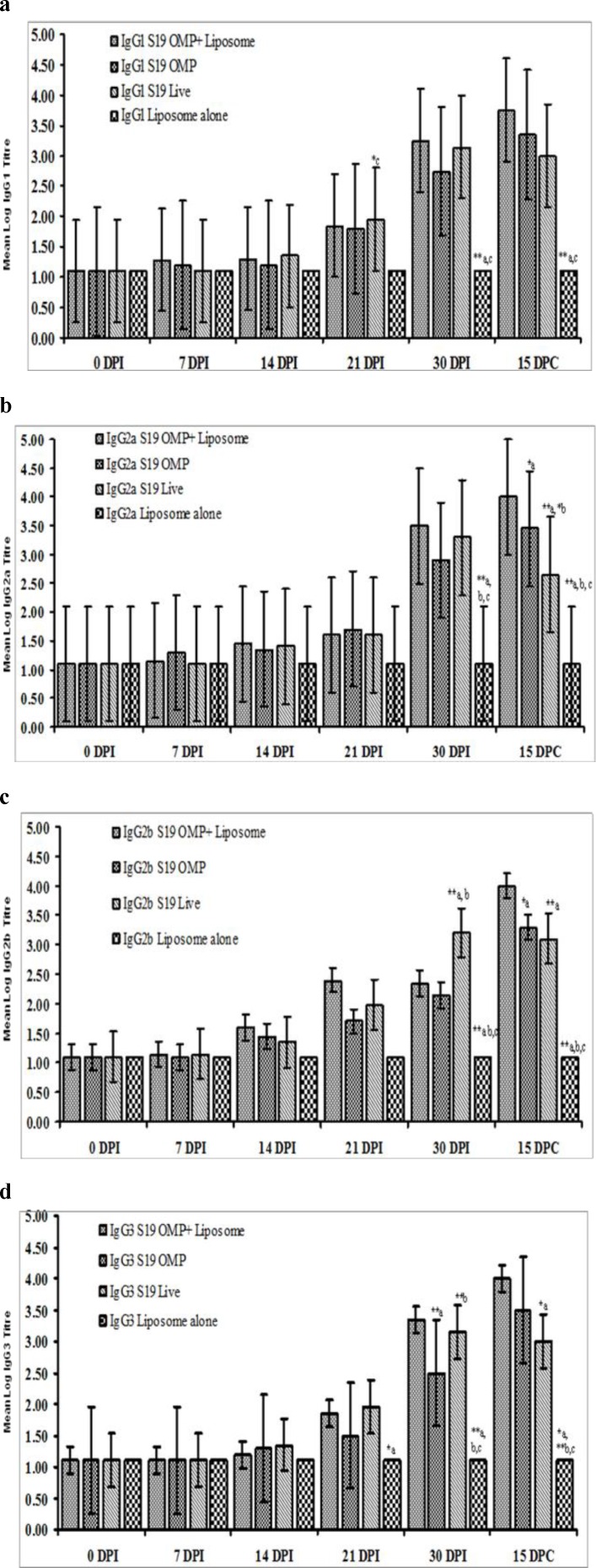
The antibody isotype response to IgG1 in mice immunized with S19-OMP-liposome and live B. abortus S19 vaccine differed significantly (P<0.01) for mice inoculated with liposome alone as a negative control group at day 30 post immunization (DPI) and day 15 post challenge (DPC) (Fig. 1a). However, the mean IgG1 titers of mice groups immunized with S19-OMP-liposome were not statistically different from those vaccinated with live B. abortus S19 vaccine (Table 1); neither the IgG1 titers of mice group immunized S19-OMP-liposome differed statistically from those immunized with S19-OMP (Table 2).
Fig. 1.
Mean log 2 isotype antibody titer of mice immunized with B. abortus S19 Liposomed – OMP (a), OMP alone (b) and S19 live vaccinated mice (c) with liposome alone (d) as negative control on DPI 0, 7, 14, 21, and 30 and DPC 15. Figs. 1a-d: Response of IgG1, IgG2a, IgG2b and IgG3 differ significantly between groups, days and between days and groups (P<0.01
Table 1.
Suggested immune response mechanism that empowered mice immunized with liposome entrapped S19-OMP protective advantage over the group immunized with B. abortus live S19 vaccine
| Vaccine formulation/antibody isotype immune response | DPI stages | 15 DPC stage |
|---|---|---|
| Antibody isotype | ||
| IgG1 | ||
| S19-OMP entrapped in liposome | NS | NS |
| Live B. abortus S19 | NS | NS |
| IgG2a | ||
| S19-OMP entrapped in liposome | NS | ** |
| Live B. abortus S19 | NS | NS |
| IgG2b | ||
| S19-OMP entrapped in liposome | (30 DPI) ** | ** |
| Live B. abortus S19 | NS | NS |
| IgG3 | ||
| S19-OMP entrapped in liposome | NS | * |
| Live B. abortus S19 | NS | NS |
Significant P<0.05, and
Significant P<0.01 by Tukey’s HSD. Protective advantage to mice conferred by immunization with S19-OMP entrapped in liposome vaccine compared to live B. abortus S19 version could probably be due to significantly different response of IgG2b at DPI 30and that of IgG2a, IgG2b and IgG3 at DPC stages. NS: Statistically not significant
Table 2.
Suggested immune response mechanism that empowered mice immunized with liposome entrapped S19-OMP protective advantage over the group immunized with S19-OMP alone
| Vaccine formulation/isotype antibody response | DPI stages | 15 DPC stage |
|---|---|---|
| Antibody isotype response | ||
| IgG1 | ||
| S19-OMP entrapped in liposome | NS | NS |
| S19-OMP alone | NS | NS |
| IgG2a | ||
| S19-OMP entrapped in liposome | NS | * |
| S19-OMP alone | NS | NS |
| IgG2b | ||
| S19-OMP entrapped in liposome | NS | * |
| S19-OMP alone | NS | NS |
| IgG3 | ||
| S19-OMP entrapped in liposome | (30 DPI) ** | NS |
| S19-OMP alone | NS | NS |
Significant P<0.05,
Significant P<0.01 Tukey’s HSD. Protective advantage to mice conferred by immunization with S19-OMP entrapped in liposome vaccine compared to S19-OMP alone could probably be due to the significantly different response of IgG3 at 30 DPI and IgG2a and IgG2b at the DPC stages. NS: Statistically not significant
The mean levels of IgG2a were significantly different (P<0.01) for mice immunized with S19-OMP-liposome, those receiving OMP alone and those vaccinated with live S19 on DPI 30 and DPC 15 compared with the liposome inoculated controls (Fig. 1b). However, at DPC 15, the mean IgG2a titers in groups immunized with S19-OMP-liposome differed significantly (P<0.01) from those immunized with S19 live vaccine (Fig. 1b, Table 1); as well as those immunized with S19-OMP alone (P<0.05) (Fig. 1b, Table 2). In addition, the mean levels in mice immunized with S19-OMP differed statistically (P<0.05) from those given the S19 live vaccine (Fig. 1b).
Similarly, the mean IgG2b response in mice immunized with S19-OMP-liposome, S19-OMP alone, and the S19 live vaccine were significantly different (P<0.01) from those inoculated with liposome controls at DPI and DPC stages (Fig. 1c). The salient response was significantly heightened IgG2b levels (P<0.01) in mice immunized with S19-OMP-liposome and S19-OMP compared to those immunized with S19 live vaccine
(P<0.01) at DPI 30. Most significantly, the mean IgG2b levels of mice immunized with the S19-OMP-liposome and those with the live B. abortus S19 vaccine were statistically different at 15 DPC (P<0.01) (Table 1).
IgG3 levels were significantly elevated (P<0.01) in mice immunized with the S19-OMP-liposome, S19-OMP, and the S19 vaccine compared to liposome inoculated control mice at the DPI 30 and DPC 15 stages (Fig. 1d). Also, at 30 DPI, the mean IgG3 levels in mice inoculated with S19-OMP-liposome were statistically different (P<0.01) from those vaccinated with S19-OMP alone (Fig. 1d). Similarly, the IgG3 levels in mice immunized with S19-OMP alone were different from those that received the live S19 vaccine (P<0.01) at DPI 30 (Fig. 1d). Importantly, the mean IgG3 titers of mice immunized with S19-OMP-liposome were significantly elevated (P<0.05) compared to those inoculated with the live S19 vaccine at the DPC stage (Fig. 1d).
Protection efficacy and safety
The level of protection, i.e. protection index, expressed as mean log10 CFU, was estimated by immunizing mice with S19-OMP-liposome, S19-OMP and S19 live vaccines, respectively, and challenged with virulent B. abortus 544 culture on day 30 post-immunization. The most striking feature was the significantly enhanced level of protection by an order of magnitude of 0.84 logs (protective index = 2.36) conferred by the immunization of mice with S19-OMP-liposome compared to those immunized with live S19 vaccine (protective index = 1.52, P<0.05) (Table 3). The efficacy of the protection enhancement (greater by 1.32 log) caused by the delivery of the antigen S19-OMP entrapped in cationic liposome formulation was observed by comparing the difference in the level of protection achieved by immunization with S19-OMP alone (P<0.05) (Table 3). However, the level of protection (1.04 log) offered to mice by immunization with the S19-OMP formulation was significantly lower (lesser by 0.48 log) compared to the group immunized with the live S19 vaccine (1.52 log) (P<0.05) (Table 3).
Table 3.
Protective indices of Brucella abortus S19 OMP with liposome (a), S19 OMP alone (b), and S19 live attenuated vaccine (c) with liposome alone (d) as control in mice
| Vaccine group |
Recovery of challenge strain B. abortus 544 |
Recovery of vaccine strain B. abortus |
Protection indices (Log 10) | Mean spleen weight (g) at 15 DPC |
|---|---|---|---|---|
| (n=6 in each group) | (Log 10 CFU) | (Log 10 CFU) | ||
| S19-OMP-liposome | 3.55 | - | 2.36* b, c | 0.165* c, d |
| S19-OMP | 4.87 | - | 1.04* a, c | 0.145* c, d |
| Live B. abortus S19 | 4.39 | 3.23 | 1.52* a, b | 0.255* a, b |
| Liposome inoculated control | 5.91 | - | - | 0.312* a, b |
Significant P<0.05, Tukey’s Honesty significant difference
Reduction in spleenomegaly, as measured by the decrease in mean spleen weight in mice groups vaccinated with live S19 strain did not differ statistically from the control group inoculated with liposome alone (Table 3). In contrast, the reduction in mean spleen weight in the group immunized with S19-OMP-lipososome and S19-OMP (P<0.05) was significantly different compared to the liposome inoculated controls (Table 3). Interestingly, the S19-OMP-liposome formulation was found safer than the S19 live vaccine since the mean spleen weight of mice immunized with S19-OMP-liposome was significantly lower compared to those immunized with the live S19 vaccine at 15 DPC (P<0.05) (Table 3).
Discussion
Citing Allison and Gregoriadis (1974) ▶, Shek and Sabiston (1981) ▶ explored the role of liposome associated protein antigens, and pointed to the initial finding regarding the immunization of animals with diphtheria toxoid entrapped in liposome which results in enhanced antibody production. With particular reference to Brucella, it has been shown that the administration of Hernandez-Caselles et al. (1989) ▶ encapsulated in cationic liposomes eliminates B. melitensis from the liver and spleen of infected mice. However, the anionic formulation cleared the infection from the spleen less effectively (Hernández-Caselles et al., 1989 ▶). The enhanced efficacy of the potentiation of humoral immune responses to lipopolysaccharide and o-polysaccharide antigens of B. abortus in mice using liposome as a vaccine carrier has also been demonstrated by Wong et al. (1992) ▶. Later, it was shown by Vitas et al. (1996) ▶ that liposomal gentamicin had protective effects against systemic acute experimental murine brucellosis due to B. abortus. The role of recombinant outer membrane proteins (rOMP) such as rOMP25, rOMP28 and rL7/L12 as sub-unit vaccine candidates has been also studied, and it has been concluded that rOMP candidates are efficacious in mouse models, if immunization and challenge experiments are employed (Mallick et al., 2007 ▶; Kaushik et al., 2010 ▶; Goel and Bhatnagar, 2012 ▶; Lim et al., 2012 ▶).
Liposome encapsulated rL7/L12 protein (Mallick et al., 2007 ▶) and rOMP25 (Goel et al., 2013 ▶) vaccine candidates have been recently tested in BALB/c mouse models. In both studies the candidates were shown to be able to elicit enhanced humoral and cell mediated immune responses compared to the live S19 vaccine and control groups. In addition, when BALB/c mice were primed by sub-cutaneous immunization with a 50 µg dose of rL7/L12 entrapped in liposomes that employed egg-phosphatidylcholine and cholesterol in a 2:1 ratio and boosted by the same route with a 30 µg dose on days 21 and 28 after priming, they induced better systemic clearance of the virulent challenge strain B. abortus 544 compared to the live B. abortus S19 vaccinated controls at DPC 15 (Mallick et al., 2007 ▶). However, BALB/c mice were able to clear the organism, B. abortus 544 challenge strain, from their spleen only when they were primed and boosted with the subcutaneous administration of 50 µg of rOMP25 entrapped in phosphatidyl-ethanlolamine (PE) and phosphatidyl-choline (PC) (in 8:2 ratio) 15 days after priming compared to groups of mice immunized with the live S19 vaccine (Goel et al., 2013 ▶). The protective efficacies resulting from the immunization of BALB/c mice with 50 µg rOM25 in PE-PC liposome without a booster were not statistically different from the S19 vaccinated group (Goel et al., 2013 ▶). In contrast to the reports cited above, we used a commercial cationic liposome, wherein the DODAP and DOPE mixture was supplied in a 50:50 (mol/mol) ratio to entrap purified total OMP extracted from B. abortus S19. Immunization of BALB/c mice with this formulation in a 50 µg subcutaneous dose regimen without a booster was able to confer significantly higher protection (log 0.84) compared to the mice immunized with the live S19 vaccine at DPC 15. The present finding indicates our formulation was much more efficacious compared to the previous report (Goel et al., 2013 ▶). Also, by adopting a single prime immunization strategy in the current study, the comparative efficacy of protection in the S19-OMP-liposome immunized BALB/c mice with respect to the S19-OMP show far better results at DPC 15 (log 2.36 versus log 1.04) compared to a previous study by Mallick et al. (2007) ▶. the comparative protection indices of mice immunized with liposome encapsulated rL7/L12 protein and rL7/L12 protein in IFA were shown as log 1.61 and log 0.066 units at DPC 30, respectively.
In the studies cited above (Mallick et al., 2007 ▶; Goel et al., 2013 ▶) liposome formulations demonstrated a predominant IgG2a antibody isotype immune response, nevertheless, cell mediated immunity indicated that the nature of the response could be influenced by the nature of the formulation. For instance, while immunization with rOMP25 in the PE-PC formulation induced both Th1 and Th2 cellular responses, the rL7/L12 in the PC-cholesterol formulation induced a Th1 response, despite the fact that most egg-based PC-cholesterol formulations are usually known to stimulate Th2 cells. It appears, therefore, that liposome formulations are distinctly unique in their ability to elicit a very specific and distinct immune response. The uniqueness of a particular liposome formulation in the elicitation of a specific response was observed in our study. Since single immunization with a 50 µg subcutaneous dose with S19-OMP entrapped in DODAP/DOPE liposomes at the DPI 30 and the DPC 15 stages was able to induce significantly elevated IgG2b responses compared to IgG1 responses, as well as significantly higher IgG2a and IgG3 response at the DPC 15, a bias is indicated towards Th1 based cellular immunity. All formulations employed in this study have shown significantly enhanced levels in the mean antibody titers of all isotypes (IgG1, IgG2a, IgG2b and IgG3) compared to the group immunized with liposome alone at the DPI and DPC stages indicating the involvement of Th1 and Th2 cells in the immune response. During the post-challenge period, the IgG1 response did not differ significantly, nevertheless, the IgG2a response was significantly elevated along with IgG2b and IgG3 in the S19-OMP-liposome immunized group in contrast to mice immunized with the live S19 vaccine, indicating the role of Th1 cells in conferring better protection. Earlier studies that used Brucella antigens such as rOMP28 as an immunizing antigen have shown strong support for the switching Th1/Th2 mechanism of Brucella immunity in mice as evidenced by a 20 fold increase in IgG2a compared to IgG1. This indicates a predominant role of the Th1 directed cellular immunity at the post-challenge stage (Lim et al., 2012 ▶). It has been shown earlier that mice infected with B. abortus S19 and 2308 strains induce intense, protracted polyclonal IgG2a and IgG3 responses compared to IgG1 and IgM isotpyes between weeks 4-8 post-infection. Furthermore, as suggested earlier, the polyclonal IgG2a and IgG3 response is mediated via IFN-γ by B. abortus primarily in a T cell independent fashion during the first week of infection, and from the T cells thereafter (Elzer et al., 1994 ▶). Current studies on the S19-OMP-liposome in mice exhibit an immune response mechanism similar to that suggested above.
Acknowledgements
The authors are grateful to the management of the National Dairy Development Board (NDDB), Anand for providing the facilities to carry out this work. The author A. Prasad expresses his gratitude to the Indian Immunologicals Limited, Hyderabad, for providing the opportunity to work on the topic for partial fulfillment of his Ph.D. thesis.
Conflict of interest
The authors do not have any conflict of interest.
References
- Allison AC, Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974 Nov 15;252(5480):252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- Alton GG, Corner LA, Plackett P. Vaccination against bovine brucellosis. Dev Biol Stand. 1984;56:643–647. [PubMed] [Google Scholar]
- Alving CR, Richard RL, Mosss J, Alving IL, Clements JD, Shiba T, Kotani S, Wirtz RA, Hockmeyer WT. Effectivness of liposome as potential carriers of vaccine: application to cholera toxin and human malaria sporozoite antigens. Vaccine. 1986;4:166–172. doi: 10.1016/0264-410x(86)90005-8. [DOI] [PubMed] [Google Scholar]
- Corner LA, Alton GG. Persistence of Brucella abortus strain 19 infection in adult cattle vaccinated with reduced doses. Res Vet Sci. 1981;31:342–344. [PubMed] [Google Scholar]
- Crasta OR, Folkerts O, Fei Z, Mane SP, Evans C, Martino-Catt S, Bricker B, Yu G, Du L, Sorbel BW. Genome sequence of Brucellla abortus vaccine strain S19 compared to virulent strains yield candidate virulence gene. PLoS One. 2008;3:e2193. doi: 10.1371/journal.pone.0002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzer PH, Jacobson RH, Nielsen KH, Douglas JT, Winter AJ. BALB/c mice infected with Brucella abortus express protracted polyclonal responses of both IgG2a and IgG3 isotypes. Immunol Lett. 1994 ;42:145–150. doi: 10.1016/0165-2478(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Galdiero F, Carratelli CR, Nuzzo I, Bentivoglio C, De Martino L, Folgore A, Galdiero M. Enhanced cellular response in mice treated with Brucella antigen-liposome mixture. FEMS Immunol Med Microbiol. 1995 ;10:235–243. doi: 10.1111/j.1574-695X.1995.tb00038.x. [DOI] [PubMed] [Google Scholar]
- Goel D, Bhatnagar R. Intradermal immunization with outer membrane protein 25 protects BALB/c mice from virulent B abortus 544. Mol Immunol. 2012 ;51:159–168. doi: 10.1016/j.molimm.2012.02.126. [DOI] [PubMed] [Google Scholar]
- Goel D, Rajendran V, Ghosh PC, Bhatnagar R. Cell mediated immune response after challenge in Omp25 liposome immunized mice contributes to protection against virulent Brucella abortus 544. Vaccine. 2013;31:1231–1237. doi: 10.1016/j.vaccine.2012.12.043. [DOI] [PubMed] [Google Scholar]
- Graves RR. The story of John M Buck’s and Matilida contribution to cattle industry. J Am Vet Med Assoc. 1943 ;102:193–195. [Google Scholar]
- Hernandez-Caselles T, Vera A, Crespo F, Villalain J, Gomez-Fernandez JC. Treatment of Brucella melitensis infection in mice by use of liposome-encapsulated gentamicin. Am J Vet Res. 1989;50:1486–1488. [PubMed] [Google Scholar]
- Ireland R, Olivares-Zavaleta N, Warawa JM, Gherardini FC, Jarrett C, Hinnebusch BJ, Belisle JT, Fairman J, Bosio CM. Effective, broad spectrum control of virulent bacterial infection using cationic DNA liposome complexes combined with bacterial antigens. PLoS Pathog. 2010;6:e1000921. doi: 10.1371/journal.ppat.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik P, Singh DK, Kumar SV, Tiwari AK, Shukla G, Dayal S, Chaudhuri P. Protection of mice against Brucella abortus 544 challenge by vaccination with recombinant OMP28 adjuvanted with CpG oligo-nucleotides. Vet Res Commun. 2010;34:119–132. doi: 10.1007/s11259-009-9337-x. [DOI] [PubMed] [Google Scholar]
- Kulikov VI, Kashkin KP, Dranovskaia EA. Incorporation of the protective antigen of Brucella abortus into liposomes and the immunogenic properties of the antigen-containing liposome. Zh Mikrobiol Epidemiol Immunobiol. 1985;12:69–72. [PubMed] [Google Scholar]
- Lim JJ, Kim DH, Lee JJ, Kim DG, Min W, Lee HJ, Rhee MH, Kim S. Protective effect of recombinat Brucella abortus Omp 28 against infection with a virulent strain of Brucella abortus 544 in mice. J Vet Sci. 2012;13:287–292. doi: 10.4142/jvs.2012.13.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick AI, Singha H, Chaudhuri P, Nadeem A, Khan SA, Dar KA, Owasis M. Liposomised recombinant ribosomal L7/L12 protein protects BALB/c mice against Brucella abortus 544 infection. Vaccine. 2007;25:3692–3704. doi: 10.1016/j.vaccine.2007.01.066. [DOI] [PubMed] [Google Scholar]
- Mythili T, Rajendra L, Srinivasan VA. A Brucella abortus glycoconjugate vaccine consisting of lipopolysacchride and outer membrane protein protects mice against challenge with Brucella abortus 544. J Adv Vet Res. 2012;2:99–106. [Google Scholar]
- Nicoletti P. Relationship between animal and human disease. In: Young EJ, Corbel MJ, editors. Brucellosis. Clinical and laboratory aspect. Inc Boca Raton: CRC Press; 1989. pp. 41–52. [Google Scholar]
- OIE Manual of Diagnostic Tests and Vaccine for Tererestrial Animals (2009). Bovine brucellosis. Chapter 2.4.3. Paris: OIE; pp. 1–35. [Google Scholar]
- Onurdag FK, Degim T, Degim Z, Kutlu I, Kaynar O, Gunes G, Abbasoglu U. The humoral immune response of mice to liposomes containing Brucella melitensis outer membrane fragments. J Ani Vet Adv. 2008;7:991–995. [Google Scholar]
- Richard RA, Wirtz RA, Hockmeyer WT, Alving CA. Liposome as drug carriers, recent trend and progress. New York: John Willey and Sons; 1998. Development of liposomes as carriers for human malaria peptide vaccine; pp. 235–241. [Google Scholar]
- Shek PN, Sabiston BH. Immune response mediated by liposome-associated protein antigens. Immunology. 1981;45:349–356. [PMC free article] [PubMed] [Google Scholar]
- Verstreate DR, Creasy MT, Caveney NT, Baldwin CL, Blab MW, Winter AJ. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982;35:979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitas AI, Diaz R, Gamozo C. Protective effect of Brucella outer membrane complex-bearing liposomes against experimental murine brucellosis. FEMS Microbiol Lett. 1995;130:231–236. doi: 10.1111/j.1574-6968.1995.tb07725.x. [DOI] [PubMed] [Google Scholar]
- Vitas AI, Diaz R, Gamozo C. Effect of composition and method of preparation of liposomes on their stability and interaction with murine monocytes infected with Brucella abortus. Antimicrob Agent Chemother. 1996;40:146–151. doi: 10.1128/aac.40.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JP, Cherewonogrodzky JW, Ninno Di VL, Stadnyk LL, Knodel MH. Liposome potentiation of humoral immune response to lipopoly-sacchride and O-polysacchride antigens of Brucella abortus. Immunology. 1992;77:123–128. [PMC free article] [PubMed] [Google Scholar]
- Xavier MN, Paixao TA, Poester FP, Lage AP, Santos RL. Pathological, immunohistochemical and bacteriological study of tissue and milk of cows and fetuses experimentally infected with Brucella abortus. J Comp Pathol. 2009;140:149–157. doi: 10.1016/j.jcpa.2008.10.004. [DOI] [PubMed] [Google Scholar]