Abstract
Foot-and-mouth disease is an important viral disease of cloven-hoofed animals. Inactivated whole particle virus vaccines are still widely used in prophylactic vaccination campaigns. The choice of adjuvant is a very important factor in enhancing immune responses and the efficacy of inactivated vaccines. Montanide ISA 61 VG is a new ready-to-use mineral oil-based adjuvant developed by SEPPIC Inc. (SEPPIC, France) with high-potential immune responses needed for clinical protection against FMD infection. In this study, we compared the efficacy of two FMD vaccines either formulated with the new oil-based adjuvant ISA 61 VG and saponin, or with aluminum hydroxide gel and saponin. Both vaccines contained the same antigen payloads of O2010/IR. Two groups of 15 naive cattle received a single vaccination with different doses (full dose, 1/3 dose and 1/9 dose) to calculate their PD50 (50% protective dose) after being challenged with the homologous virulent virus. The mean neutralizing antibody titer was determined at 0, 7, 14 and 21 days after vaccination, measured by a micro neutralization test. The new vaccine improved humoral immune responses by 19%, while inducing a higher geometric mean. The titer for neutralizing antibodies was 2.91 log10 compared to the alum-gel based adjuvant vaccine which was 2.44 log10 (P-value=0.1782). The new vaccine showed a PD50 value of 10.05 as compared to a PD50 value of 4.171, respectively. According to the results, the FMD vaccine formulated with the new oil adjuvant, ISA 61 VG, shows potential as an alternative vaccine for routine and emergency vaccinations in the FMD enzootic region.
Key Words: Cattle, Foot-and-mouth disease, Montanide ISA 61 VG, Oil adjuvant, Vaccine potency
Introduction
Foot-and-mouth disease (FMD) is an acute and highly contagious viral disease of cloven-hoofed animals, especially ruminants and pigs. FMD is an important economical disease with low mortality (except for newborn and young animals) and high morbidity in susceptible animals. The causative agent of this disease belongs to the genus Aphtovirus of the Picornaviridae family which includes seven distinct serological serotypes A, O, Asia1, C, and South African Territories (SAT) types 1, 2, 3 and many (sub)lineages (Grubman et al., 2004 ▶; Jamal et al., 2013 ▶). In Iran, FMD is an enzootic disease first reported by the World Reference Laboratory (WRL) in 1956 when it appeared as a serotype O virus. Serotypes of Asia1 and A were also isolated in 1957 and 1960, respectively (WRLFMD, 1956-1960 Iran). One of the most important strategies for controlling and eradicating FMD is vaccination with high quality vaccines, especially in enzootic areas (Brückner et al., 2010 ▶).
The goal of vaccination is to generate immune responses to the administered antigen which should provide long-term protection against infection. Despite advances in the field of vaccinology, FMD inactivated whole-virus vaccines are still commonly used to combat the disease. One of the most important factors that enhance the immunogenicity of these vaccines is the nature of the adjuvant. The induction of strong and long lasting immune responses with inactivated as opposed to live attenuated viral vaccines often requires the addition of a potent and safe adjuvant (Petrovsky et al., 2004 ▶). Many types of adjuvants are employed in veterinary vaccines, however, alum-based and mineral oil-based adjuvants with or without saponin are most frequently used for inactivated FMD vaccines (Park et al., 2014 ▶).
Vaccines containing aluminum hydroxide and saponin as adjuvants have several deficiencies such as the induction of short-lived antibody responses which require relatively frequent revaccinations at intervals of 6 or even 4 months. In contrast, oil-based adjuvant FMD vaccines appear to have several advantages such as the induction of high titers and long-lived antibody responses, resulting in more effective protection (Aucouturier et al., 2001 ▶; Cloete et al., 2008 ▶). Unlike alum-based adjuvant vaccines, oil-based adjuvant vaccines can overcome interference by maternal antibodies in neonates and can consequently be applied earlier in life (Iyer et al., 2000 ▶).
Montanide ISA 61 VG is a new mineral oil-based adjuvant developed by SEPPIC. According to the manufacturer, Montanide ISA 61 VG water-in-oil (W/O) emulsion is robust, stable, easy to inject, induces strong and long lasting protection and is especially suitable for antigens with a relatively low immunogenicity (SEPPIC, 2010 ▶).
In the case of FMD, the serum neutralization test (SNT) is serotype-specific and is considered highly sensitive to antibodies against the FMD virus (Selim et al., 2010 ▶; OIE, 2012 ▶).
Potency testing is another reliable method of estimating vaccine efficacy by determining the protection of cattle vaccinated with different doses of the vaccine after being challenged with a homologous virus (OIE, 2012 ▶).
In the present study, we evaluated a monovalent O2010/IR oil-based FMD vaccine formulated with the new Montanide ISA 61 VG W/O emulsion adjuvant, and compared it with a conventional vaccine formulated with an alum-based adjuvant and saponin with the same vaccine virus strain and antigen payload. We determined which formulation would elicit the highest neutralization antibody titers and protection against the homologous virulent virus challenge PD50 (50% protective dose).
Materials and Methods
Animals
Naive Holstein calves used in this study were between 6 to 9 months old and weighted between 300 and 400 kg. These calves were monitored and controlled from birth until the beginning of the experiment, and had never been infected or vaccinated against the FMD virus as evidenced by the absence of neutralizing antibodies and NSP (nonstructural proteins) antibodies prior to vaccination in all groups and before the challenge in the non-vaccinated control group.
Preparation of vaccines
The virus was propagated on a monolayer and suspension BHK21 cell culture. It was then harvested and centrifuged to remove cell debris and concentrated by 8% polyethylene glycol 6000. The virus concentration titer was measured by the TCID50 method and inactivated by 4 mM w/v Binary Ethyleneimine (BEI) for 30 h at 30°C. To neutralize and remove residues of BEI, 2 mM sodium thiosulfate was added. Finally, the formulation was carried out as follows:
2.5 × 107 TCID50 inactivated FMD virus type O2010/IR was used as the antigen payload per dose. Three mg/doses saponin was added and maintained for the aqueous phase of the vaccine bulk. Vaccines were then prepared for experiments 1 and 2 as follows:
Experiment 1: A stable and efficient vaccine was obtained by mixing the aqueous phase bulk into the Montanide ISA 61 VG (W/O) at lower than 20 +/-2°C using a high shear mixer, which was prepared based on 2 ml per dose.
Experiment 2: 2.5% aluminum hydroxide gel was prepared as an alum-based adjuvant and a 30% per dose was added to the aqueous phase of the vaccine bulk, mixed for 1 h in a low speed agitating mixer at 25°C. The vaccines were then prepared and employed in 5 ml-volumes per dose for subcutaneous injection.
Vaccine and challenge experiment
Initially, 32 naive calves were assigned to three groups; group A for experiment 1 with 15 calves, group B for experiment 2 with 15 calves and group C with 2 calves as the control group (OIE, 2012 ▶). Groups A and B were then divided into three subgroups (A1, A2, A3 and B1, B2, B3) each including 5 calves. Animals were kept in a controlled area and housed in separated boxes. Vaccination was finally performed as follows:
Group A: A1, 2 ml (full dose); A2, 0.67 ml (1:3 dose) and A3, 0.22 ml (1:9 dose) vaccination.
Group B: B1, 5 ml (full dose); B2, 1.67 ml (1:3 dose) and B3, 0.55 ml (1:9 dose) vaccination.
Group C: Unvaccinated control animals.
All animals were monitored daily for 21 days post vaccination and before the challenge with 10000 BID50 virulent virus.
Sample collection
Clotted blood samples were collected in 10 ml venoject tubes from all cattle on days 0, 7, 14 and 21 after vaccination. Sera were harvested after incubating the clotted blood samples at 4-8°C for 4-5 h and centrifuged at 2,500 rpm at 4°C for 20 min. The sera were then stored at -20°C until used (Rweyemamu et al., 1978 ▶; OIE, 2012 ▶).
Antibody assays
The titer of neutralizing antibodies against FMD virus type O2010/IR was measured by micro neutralization tests, and expressed as the reciprocal of the dilution that neutralized 50% of the virus in BHK-21 cells. Average SNT titers were calculated as log10 geometric mean titers (Patil et al., 2002 ▶).
Potency test
Potency tests of the two vaccines (experiment 1 and 2) were carried out in compliance with the OIE terrestrial manual and European Pharmacopeia (SEPPIC). Briefly, the challenge was carried out at day 21 post vaccination for all vaccinated (A and B) and unvaccinated calves (C) by intradermolingual injection of 0.2 ml of 10,000 bovine infectious dose 50% (BID50), homologous to the virulent virus, onto the two sides of the tongue (0.1 ml in each side). All animals were monitored for 8 days and their body temperature was measured and recorded daily. Animals were tranquillized using Xylazine. Their hooves were then washed carefully to determine protected and unprotected animals by investigating lesions at sites other than the tongue. The PD50 was estimated for both experiments using Karber’s method (Karber, 1931 ▶).
Statistical analysis
Raw data of all experiments were used for statistical analysis. The collected data were analyzed using Graph Pad Prism 6 software. Analyses were carried out at a 95% confidence level and p-values of less than 0.05 were considered as significant.
Results
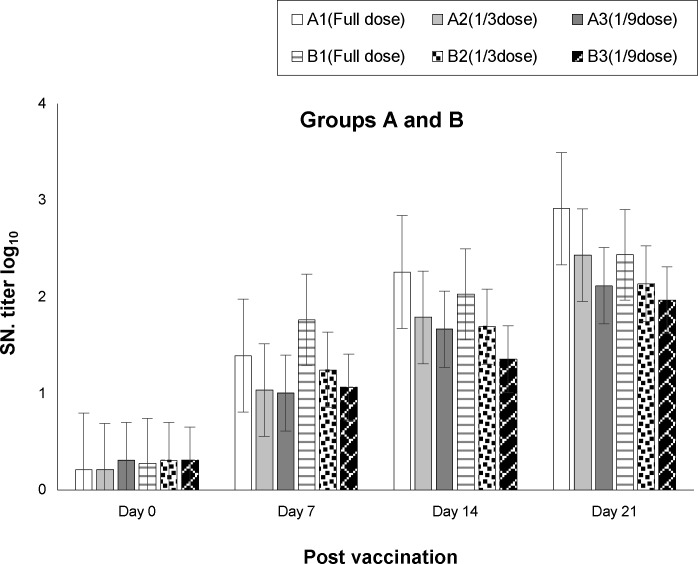
The geometric mean SNT titer at day 7 post vaccination for experiment 1 and experiment 2 indicated a more rapid antibody induction for the full dose of alum-based adjuvant vaccine, reaching to 1.764 log10 and 1.36 log10 for Montanide ISA 61 VG. Antibody titers increased to maximum levels at day 21 after vaccination. Details of full and reduced doses (1:3 and 1:9) of both vaccines 1 and 2, are shown in Table 1 and Fig. 1. Geometric mean antibody titers for full dose vaccination with the new oil-based adjuvant ISA 61 VG and alum-based adjuvant vaccine at day 21 post vaccination were 2.91 and 2.44 log10, respectively, which were not significantly different (P-value=0.1782). Nevertheless, it is clear that the new ISA 61 VG oil-based adjuvant vaccine improved at least 19% of the humoral response compared to the alum-based adjuvant vaccine.
Table 1.
Summary of mean antibody titers obtained by SNT (log10
| Vaccine | Experiment 1 (ISA 61 VG) group A |
Experiment 2 (alum-gel) group B |
||||
|---|---|---|---|---|---|---|
| Sub groups |
A1 full dose |
A2 1:3 dose |
A3 1:9 dose |
B1 Full dose |
B2 1:3 dose |
B3 1:9 dose |
| Days post vaccination | Mean SNT titer log10 | Mean SNT titer log10 | ||||
| D0 | 0.214 | 0.214 | 0.308 | 0.274 | 0.308 | 0.308 |
| D7 | 1.39 | 1.036 | 1.004 | 1.764a | 1.244 | 1.062 |
| D14 | 2.256 | 1.788 | 1.666 | 2.028 | 1.692 | 1.354 |
| D21 | 2.914 | 2.432 | 2.056 | 2.44 | 2.136 | 1.968 |
Higher antibody titer induced by the vaccine formulated in experiment 2 than experiment 1 at day 7
Fig. 1.
Trend of induction of means of the neutralizing antibody titers (humoral response) in each period of sampling from day zero to day 21 (the day of challenge) for the two groups of vaccinated cattle. Group A (A1, A2 and A3) was vaccinated with ISA 61 VG oil-based adjuvant and group B (B1, B2 and B3) with alum-based adjuvant
The ability of the two vaccines with different adjuvants (experiments 1 and 2) to protect cattle against the generalization of the disease after being challenged with virulent FMD virus type O2010/IR is presented in Tables 2 and 3. As expected, body temperature in the two control group animals increased 24 h after challenge and both calves showed typical clinical signs of FMD with vesicle formation in all four feet at the 2nd-day post challenge. For experiment 1, one calf vaccinated with a 1:3 dose, subgroup A2, and one calf vaccinated with a 1:9 dose, subgroup A3, showed clinical lesions in two or more feet. Thirteen cattle were clinically protected and this vaccine passed with a PD50 value of 10.05. In experiment 2, two vaccinated cattle from subgroup B2 which received 1:3 dose, and four cattle from subgroup B3, which received 1:9 dose, showed clinical lesions on their feet. Nine cattle were protected and this vaccine passed with a PD50 value of 4.171 as calculated by Spaerman and Karber (1931). The PD50 value for the oil-based adjuvant ISA 61 VG (W/O) emulsified FMD vaccine (experiment 1) appeared to be higher than the PD50 value of the alum-based adjuvant (experiment 2).
Table 2.
FMD experiment vaccine 1 (ISA 61 VG new oil-based adjuvant) inspection at necropsy on day 8 post challenge
| Details | Sub groups |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | |||||||||||||
| Animal ID | 090 | 181 | 194 | 146 | 222 | 091 | 111 | 147 | 208 | 209 | 139 | 186 | 202 | 210 | 232 |
| Vaccine dose/ml | 2 | 2 | 2 | 2 | 2 | 0.67 | 0.67 | 0.67 | 0.67 | 0.67 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 |
| LFL lesion | 0a | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1b | 0 |
| RFL lesion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| LHL lesion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| RHL lesion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Tongue | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total score | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 5 | 1 |
0: There is no FMD virus lesion, and 1: There is at least one FMD virus lesion. LFL: Left front leg, RFL: Right front leg, LHL: Left hind leg, and RHL: Right hind leg
Table 3.
FMD experiment vaccine 2 (alum-based adjuvant) inspection at necropsy on day 8 post challenge
| Details | Sub groups |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | B3 | |||||||||||||
| Animal ID | 120 | 177 | 143 | 231 | 233 | 093 | 125 | 199 | 219 | 234 | 162 | 197 | 198 | 203 | 229 |
| Vaccine dose/ml | 5 | 5 | 5 | 5 | 5 | 1.67 | 1.67 | 1.67 | 1.67 | 1.67 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| LFL lesion | 0a | 0 | 0 | 0 | 0 | 0 | 1b | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 |
| RFL lesion | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 |
| LHL lesion | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| RHL lesion | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| Tongue | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total score | 1 | 1 | 1 | 1 | 1 | 1 | 5 | 1 | 1 | 5 | 5 | 5 | 3 | 5 | 1 |
0: There is no FMD virus lesions, and 1: There is at least one FMD virus lesion. LFL: Left front leg, RFL: Right front leg, LHL: Left hind leg, and RHL: Right hind leg
Discussion
Vaccination with high potency vaccines may prevent virus replication in the pharyngeal region, reduce clinical symptoms and finally decrease the incidence of FMD infections (Selim et al., 2010 ▶). Since we could not find any report of Montanide ISA 61 VG adjuvanted FMD vaccines, we hypothesized that this new oil-based adjuvant formulation might induce a better immune response and protection in cattle as compared to a conventional alum-based adjuvant vaccine. In this study, the following mean neutralizing antibody titers were detected in all vaccinated cattle in groups A and B. These data were in agreement with earlier reports (Rweyemamu et al., 1978 ▶; Bahnemann et al., 1987 ▶; Barteling et al., 1991 ▶; Cloete et al., 2008 ▶; Dar et al., 2013 ▶). On the other hand, it was shown that the immune response to a full dose of the experiment 2 vaccines induced an earlier immune response than the experiment 1 vaccine. However, the results showed that after 14 days post-vaccination, antibody titers were higher in all groups vaccinated with different volumes of the experiment 1 vaccine as compared to the experiment 2 vaccine, which was similar to the results reported by the previous studies (Bahnemann et al., 1987 ▶; De Diego et al., 1997 ▶; Cloete et al., 2008 ▶; Cox et al., 2010 ▶; Selim et al., 2010 ▶). These data were also consistent with previous reports regarding oil-based adjuvant vaccines and the better immune responses they caused at all-time points (except at day 7) as compared to an alum-based adjuvant vaccine, suggesting that a vaccine containing ISA 61 VG may be a good candidate for replacing conventional the alum-based vaccine (Selim et al., 2010 ▶). Three different doses of each vaccine (full, 1:3 and 1:9 doses) were used in two potency tests. The relationship between dose and clinical protection obtained in this study was similar to the findings of other researchers (McCullough et al., 1992 ▶; Patil et al., 2002 ▶; Cox et al., 2010 ▶; Selim et al., 2010 ▶; Oh et al., 2012 ▶; Li et al., 2013 ▶). According to the statistical analysis, there was no significant difference (P≤0.05) in the SNT titer log10 between experiments 1 and 2. However, the PD50 value of the ISA 61 VG formulated vaccine was higher than that of the alum-based adjuvant vaccine. The potency of FMD vaccines can be negatively influenced by the disruption of the 146S antigen during adsorption processes to the aluminum hydroxide gel (Doel et al., 1990 ▶; Li et al., 2013 ▶). We found in both potency tests that all cattle with neutralizing antibody titers higher than 2.1 log10 were fully protected, whereas cattle with neutralizing antibody titers of 1.5 log10 (group A) and 1.8-1.98 log10 (group B) were not. However, one case in group A was protected with an antibody titer of 1.8 log10. Since the amount of antigen used in both vaccines was equal, this efficacy in protection was presumably related to the type of adjuvant used in the vaccine formulation and the capacity of its humoral and cellular response stimulation (Petrovsky et al., 2004 ▶; Meeusen et al., 2007 ▶; SEPPIC, 2010 ▶; Park, 2013 ▶). Although humoral immunity is the most important factor in protection against FMD, this protection can also be determined by reticuloendothelial system cells (phagocytosis) that play a crucial role in the immune defense against the FMD virus (McCullough et al., 1992 ▶; Oh et al., 2012 ▶; Park, 2013 ▶) and are strongly influenced by the quality (avidity and affinities) of the antibodies produced following vaccination (McCullough et al., 1992 ▶; Summerfield et al., 2009 ▶). The new oil FMD vaccine seems to have induced antibodies with high avidity and affinity. In the new oil FMD vaccine, there is a case indicating a lower antibody of 1.8 log10, that protected the animals against the challenge, while the two cases in the aqueous vaccine demonstrating antibodies of 1.8 and 1.9 log10, did not.
According to OIE manual and EU pharmacopeia, the PD50 value obtained against the homologues virulent virus challenge is a standard and acceptable method for improving FMD vaccines potency (OIE, 2012 ▶). Therefore, the new oil FMD vaccine showed 10.05 PD50 whereas the aqueous vaccine showed 4.17 PD50. It can be inferred that at least 1/10 of the dose of the new oil FMD vaccine (0.2 ml) is required to protect 50% of the cattle while at least 1/4 dose of aqueous vaccine (1.25 ml) protects 50% of the animals. Thus, the new oil FMD vaccine is shown to have better efficiency and potency to protect the cattle against the FMD virus.
In conclusion, the results of this study showed that a single administration of ISA 61 VG oil-based adjuvant vaccine induced high mean neutralizing antibody titers at any given period of the study (except day 7 post vaccination) and provided superior clinical protection against a homologous challenge (PD50≥10.05) as compared to an alum-based adjuvant vaccine with the same antigen payload (PD50≥4.171). These observations suggest that the vaccine formulated with new ISA 61 VG W/O emulsion oil-based adjuvant can be a good alternative to alum-based adjuvant vaccines in enzootic countries such as the IRI.
Acknowledgements
This study was conducted and supported as a virology Ph.D. thesis by the Faculty of Veterinary Medicine of Tehran University (No. 28088/6/10) and funded by a grant from Razi Institute. The authors gratefully appreciate the assistance of Dr. H. Izadi for cell culture, Mr. M. Ranji and M. Sotoodeh for preparing viruses and antigens, Mr. F. Jeirani for IPQC, and Dr. V. Khaze and Mr. M. Kameli for the analysis of the data.
Conflict of interest
The authors declare no conflict of interest.
References
- Aucouturier J, Dupuis L, Ganne V. Adjuvants designed for veterinary and human vaccines. Vaccine. 2001;19:2666–2672. doi: 10.1016/s0264-410x(00)00498-9. [DOI] [PubMed] [Google Scholar]
- Bahnemann HG, Mesquita JA. Oil-adjuvant vaccine against foot-and-mouth disease. Bol Centr Panam Fiebre Aftosa. 1987;53:25–30. [Google Scholar]
- Barteling S, Vreeswijk J. Developments in foot-and-mouth disease vaccines. Vaccine. 1991;9:75–88. doi: 10.1016/0264-410x(91)90261-4. [DOI] [PubMed] [Google Scholar]
- Brückner G, Saraiva-Vieira V. OIE strategy for the control and eradication of foot and mouth disease at regional and global levels. Compendium of technical items presented to the OIE World Assembly of Delegates and to OIE Regional Commissions. 2010. pp. 187–211. [Google Scholar]
- Cloete M, Dungu B, Van Staden L, Ismail-Cassim N, Vosloo W. Evaluation of different adjuvants for foot-and-mouth disease vaccine containing all the SAT serotypes. Onderstepoort J Vet Res. 2008;75:17–31. doi: 10.4102/ojvr.v75i1.84. [DOI] [PubMed] [Google Scholar]
- Cox SJ, Carr BV, Parida S, Hamblin PA, Prentice H, Charleston B. Longevity of protection in cattle following immunisation with emergency FMD A22 serotype vaccine from the UK strategic reserve. Vaccine. 28:2318–2322. doi: 10.1016/j.vaccine.2009.12.065. [DOI] [PubMed] [Google Scholar]
- Dar P, Kalaivanan R, Sied N, Mamo B, Kishore S, Suryanarayana V. Montanide ISA™ 201 adjuvanted FMD vaccine induces improved immune responses and protection in cattle. Vaccine. 2013;31:3327–3332. doi: 10.1016/j.vaccine.2013.05.078. [DOI] [PubMed] [Google Scholar]
- De Diego M, Brocchi E, Mackay D, De Simone F. The non-structural polyprotein 3ABC of foot-and-mouth disease virus as a diagnostic antigen in ELISA to differentiate infected from vaccinated cattle. Arch Virol. 1997;142:2021–2033. doi: 10.1007/s007050050219. [DOI] [PubMed] [Google Scholar]
- Doel T, Pullen L. International bank for foot-and-mouth disease vaccine: stability studies with virus con-centrates and vaccines prepared from them. Vaccine. 1990;8:473–478. doi: 10.1016/0264-410x(90)90249-l. [DOI] [PubMed] [Google Scholar]
- Grubman MJ, Baxt B. Foot-and-mouth disease. Clin Microbiol Rev. 2004;17:465–493. doi: 10.1128/CMR.17.2.465-493.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer A, Ghosh S, Singh S, Deshmukh R. Evaluation of three ‘ready to formulate’ oil adjuvants for foot-and-mouth disease vaccine production. Vaccine. 2000;19:1097–1105. doi: 10.1016/s0264-410x(00)00337-6. [DOI] [PubMed] [Google Scholar]
- Jamal SM, Belsham GJ. Foot-and-mouth disease: past, present and future. Vet Res. 2013;44:1–14. doi: 10.1186/1297-9716-44-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karber G. 50% end-point calculation. Arch Exp Pathol Pharmak. 1931;162:480–483. [Google Scholar]
- Li D, Zhou C, She D, Li P, Sun P, Bai X, Chen Y, Xie B, Liu Z. The comparison of the efficacy of swine FMD vaccine emulsified with oil adjuvant of ISA 201 VG or ISA 206 VG. J Biosci Med. 2013;1:22–25. [Google Scholar]
- McCullough K, De Simone F, Brocchi E, Capucci L, Crowther J, Kihm U. Protective immune response against foot-and-mouth disease. J Virol. 1992;66:1835–1840. doi: 10.1128/jvi.66.4.1835-1840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen EN, Walker J, Peters A, Pastoret PP, Jungersen G. Current status of veterinary vaccines. Clin Microbiol Rev. 2007;20:489–510. doi: 10.1128/CMR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y, Fleming L, Statham B, Hamblin P, Barnett P, Paton DJ. Interferon-γ induced by in vitro re-stimulation of CD4+ T-cells correlates with in vivo FMD vaccine induced protection of cattle against disease and persistent infection. PloS one. 2012;7:e44365. doi: 10.1371/journal.pone.0044365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE. Manual of diagnostic tests and vaccines for terrestrial animals. OIE, Paris, France: 2012. Foot and mouth disease. Chapter 2.1.5. http://www.oie.int/fileadmin/Home/eng/Health_ standards/tahm/2.01.05_FMD.pdf. [Google Scholar]
- Park JH. Requirements for improved vaccines against foot-and-mouth disease epidemics. Clin Exp Vaccine Res. 2013;2:8–18. doi: 10.7774/cevr.2013.2.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park ME, Lee SY, Kim RH, Ko MK, Lee KN, Kim SM. Enhanced immune responses of foot-and-mouth disease vaccine using new oil/gel adjuvant mixtures in pigs and goats. Vaccine. 2014;32:5221–5227. doi: 10.1016/j.vaccine.2014.07.040. [DOI] [PubMed] [Google Scholar]
- Patil P, Bayry J, Ramakrishna C, Hugar B, Misra L, Natarajan C. Immune responses of goats against foot-and-mouth disease quadrivalent vaccine: comparison of double oil emulsion and aluminium hydroxide gel vaccines in eliciting immunity. Vaccine. 2002;20:2781–2789. doi: 10.1016/s0264-410x(02)00184-6. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82:488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- Rweyemamu M, Booth J, Head M, Pay T. Microneutralization tests for serological typing and subtyping of foot-and-mouth disease virus strains. J Hyg. 1978;81:107–123. doi: 10.1017/s002217240005381x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim A, Abouzeid N, Aggour A, Sobhy N. Comparative study for immune efficacy of two different adjuvants bivalent FMD vaccines in sheep. J Am Sci. 2010;6:1292–1298. [Google Scholar]
- S. Inc, editor. SEPPIC. Montanide ISA 61 VG. abbreviated Montanide ISA 61 VG. 2010. http://www.seppic.com/file/galleryelement/pj/7d/7b/48/55/4524-technical-bulletin-mtd-isa-61-vg3096961497520821468.pdf.
- Summerfield A, Guzylack-Piriou L, Harwood L, McCullough KC. Innate immune responses against foot-and-mouth disease virus: current understanding and future directions. Vet Immunol Immunopathol. 2009;128:205–210. doi: 10.1016/j.vetimm.2008.10.296. [DOI] [PubMed] [Google Scholar]
- WRLFMD. FAO World Reference Laboratory for Foot-and-Mouth Disease. 1956-1960 Iran . http://www.wrlfmd.org/fmd_genotyping/me/irn.htm.