Abstract
Quantitative histology of the descended testis of unilateral cryptorchid bucks was compared with testis of normal bucks to evaluate the reproductive potentials of the scrotal testis in unilateral cryptorchids, using light microscopy techniques. The contralateral scrotal testes of the unilateral cryptorchids and the testes of the normal bucks contained profiles of seminiferous epithelium and each showed histological evidence of normal activity. The mean heights, lengths, lumen diameter, diameter of the seminiferous tubules were significantly higher in the contralateral scrotal testes when compared to the retained testes of the unilateral cryptorchid bucks (P<0.05). Population of spermatogenic cells per testis, and ratio of germ cells to Sertoli cells were not significantly different between both groups. The percentage of the testes occupied by various germ cells did not differ between the scrotal testis of the cryptorchid bucks and those of the normal bucks. The volume occupied by the seminiferous tubules and Leydig cells in the contralateral scrotal testis of the unilateral cryptorchid bucks were significantly greater than those of the testis of normal bucks (P<0.05). From the findings, it appears that the spermatogenic efficiency of the scrotal testes of the unilateral cryptorchid bucks was significantly higher than those of the normal bucks.
Key Words: Cryptorchidism, Goat, Morphometry, Seminiferous tubules, Sperm production
Introduction
West African Dwarf (WAD) goats are short legged goats that are largely unimproved genetically, but show a certain degree of tolerance or resistance to trypano-somiasis (Chiejina and Behnke, 2011 ▶). This breed of goat is an economically important domestic ruminant in South Eastern Nigeria, where it constitutes an important source of animal protein and is utilized in socio-cultural activities (Oyeyemi and Akusu, 2011). Poor nutrition, disease and lack of genetic improvement adversely affect the productivity of these animals. These problems encouraged the local farmers to resort to unorthodox means of enhancing productivity. Among the WAD goats of Eastern Nigeria, unilateral cryptorchidism has been described as a common condition (Ezeasor, 1985 ▶; Emehelu et al., 2005 ▶). The preponderance of this condition could be attributed to the fact that the farmers prefer these cryptorchids to normal bucks for breeding as they claim that it possesses superior reproductive efficiency. Bulk of published works on unilateral cryptorchidism has been concerned with the descriptive histological changes in both the retained and con-tralateral descended testes (Dutta et al., 2013 ▶; Mechelin and Kogan, 2014 ▶; Cortes et al., 2015 ▶). Little attention has been paid to the quantitative histology of the ‘normal’ contralateral descended testes, which is probably responsible for the reproductive efficiency. Testicular morphometry has been used as indicator for assessing spermatogenic efficiency in wild boars (Costa and Silva, 2006 ▶), cats (Franca and Godinho, 2003 ▶), rodents (Segatelli et al., 2004 ▶). Testes morphometry is also essential in determination of the reproductive physiology of animals (Couto and Silva, 2006 ▶).
The paucity of information on quantitative histology of the testes of the cryptorchid bucks is worrisome as differences in testicular size and composition (Ramm and Scharer, 2014 ▶), quantitative relationships among germ cells at different developmental stages, number and efficiency of Sertoli and Leydig cells (Leel et al., 2004 ▶) and general morphometry of the seminiferous tubule (Franca and Godinho, 2003 ▶; Almeida et al., 2006 ▶) may relate to number of spermatozoa in the ejaculate as well as reproductive efficiency. Thus, comparison of spermatogenic efficiency in the scrotal testis of unilaterally cryptorchid bucks and the normal bucks using quantitative histology may provide insights for understanding the parameters controlling the re-productive capacity of the naturally unilateral cryptor-chid bucks.
Materials and Methods
Animals and management
Forty adult West African Dwarf goats consisting of 20 naturally unilateral cryptorchid bucks and 20 bucks with fully descended testes were used for the study. They were kept in two pens at the University of Nigeria, Faculty of Veterinary Medicine demonstration farm. They were fed with giant star grass, elephant grass and spent maize grain. Water was provided ad libitum. The animals were allowed to acclimatize for a period of two weeks before the onset of the experiment.
Histology method
Bucks from each group were euthanized using an overdose of pentobarbitone sodium (SagatalR). Testes samples from the retained and scrotal testes of the cryptorchid and testes of the normal bucks were dissected out and weighed. Slices of testis samples were processed to obtain semi-thin sections by fixing in Modified Karnovsky fixative composed of 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M phosphate buffer at pH = 7.4. Very thin testis samples from each buck were post fixed in osmium tetraoxide (OsO4) in Millonig’s buffer, dehydrated in increasing concentrations of ethanol, cleared in propelene oxide and embedded in epoxy resin. Semi-thin sections, 1 µm thick were cut using an ultra-microtome, stained with toluidene blue and examined using a Leica microscope (Leica Galen III).
Determination of volume density
The volume densities of the various testicular tissue components were determined using a 221 intersection grid inserted in the eye piece of a light microscope. Fifteen fields chosen randomly were scored for each animal at ×400 magnification. Points were classified as one of the following: seminiferous tubule profiles, tubular lumen, lamina propria, Sertoli cell, different spermatogenic cells, Leydig cell, blood and lymphatic vessels, connective tissue. The volume of each component of the testis was determined as the product of the volume density and testis volume (the testis volume was considered to be equivalent to testis weight since the specific gravity of testis is 1.0 (Leal et al., 2004 ▶)).
Tubular diameter and epithelial height
The tubular diameter and height of the seminiferous epithelium were measured at ×100 magnification using a motic camera (Moticam 1000, Motic China Group). For tubular diameter, 30 tubular profiles that were round or nearly round were chosen randomly and measured for each testis.
The height of the seminiferous epithelium was obtained using the same tubules that were used to determine the tubular diameter (Segatell et al., 2004 ▶).
Seminiferous tubular length
Tubular lengths per testis and tubular lengths per gram of testis were calculated using the formula:
Where,
Testis volume = length × width × height × 0.7 (Lambert, 1951)
Volume density was determined using the method of Leal et al. (2004).
Spermatogenic Cell counts and cell dimensions
The numbers of germ cells in tubular cross-sections were determined in a total of 100 cross sections of the seminiferous tubule using the formula as described by Orlu and Egbunike (2009) ▶ as follows:
Where,
Total nuclei volume = nuclei volume density × testis vol.
Nuclei volume = 4/3 πr3
Where,
r = radius of the cell nucleus
Daily sperm production (DSP) by quantitative histology technique was determined using the formula of Swierstra (1966).
The mediastenum was assumed to be composed of 1% of testis volume (Swierstra, 1968 ▶).
Volume percentages of round spermatid nuclei were 4.85 and 4.70 for the unilateral cryptorchid and normal bucks, respectively. The life span of round spermatid of 6.35 days was used (Bitto and Egbunike, 2006 ▶). Tissue shrinkage was negligible as plastic embedding method was used.
Daily sperm production per gram of testis (efficiency of sperm production) was determined by dividing the daily sperm production value by testicular parenchyma weight.
Statistical analysis
Quantitative data generated were analyzed using Student’s t-test. Significance was accepted at P<0.05.
Results
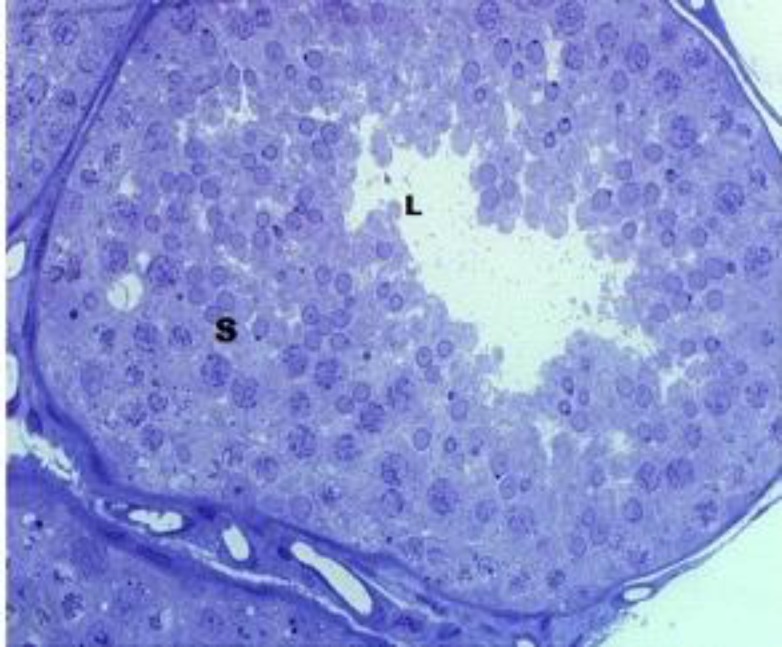
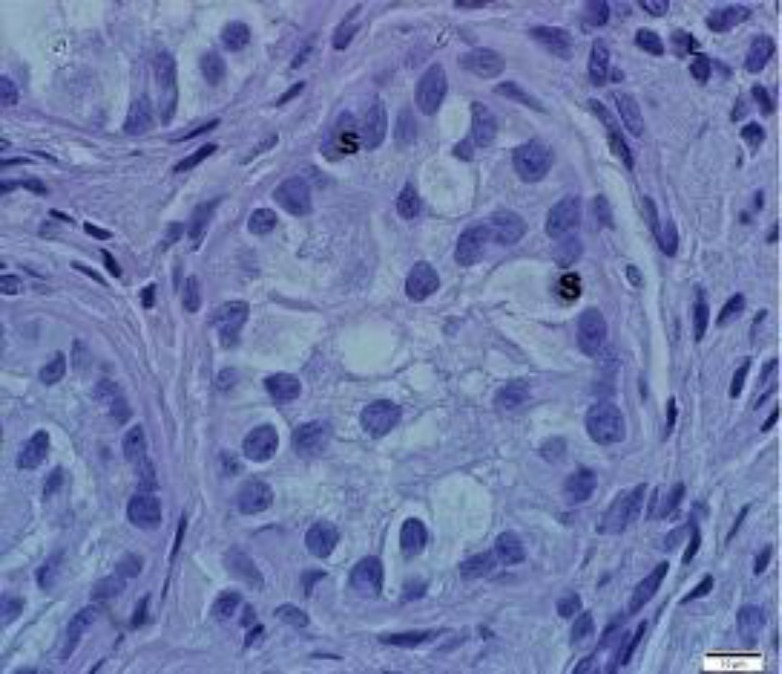
The contralateral scrotal testes of the unilateral cryptorchids and the testes of the normal bucks contained profiles of seminiferous epithelium and each showed histological evidence of normal activity (Figs. 1 and 2). However, profiles of the retained testes of the unilateral cryptorchid bucks were comprised of simple epithelium and lacked patent lumen (Fig. 3).
Fig. 1.
Seminiferous tubule profile from testis of normal bucks, showing full complement of spermatogenic cells (S) and patent lumen (L), (toluidine blue stain
Fig. 2.
Seminiferous tubule profile from contralateral scrotal testis of cryptorchid bucks, showing full complement of spermatogenic cells (S) and patent lumen (L), (toluidine blue stain, ×400
Fig. 3.
High magnification of non-canalized seminiferous tubule of retained testis in the unilateral cryptorchid buck, showing tubules with Sertoli cells only, (S) in the cytoplasm. Note the absence of patent lumen, (toluidine blue stain
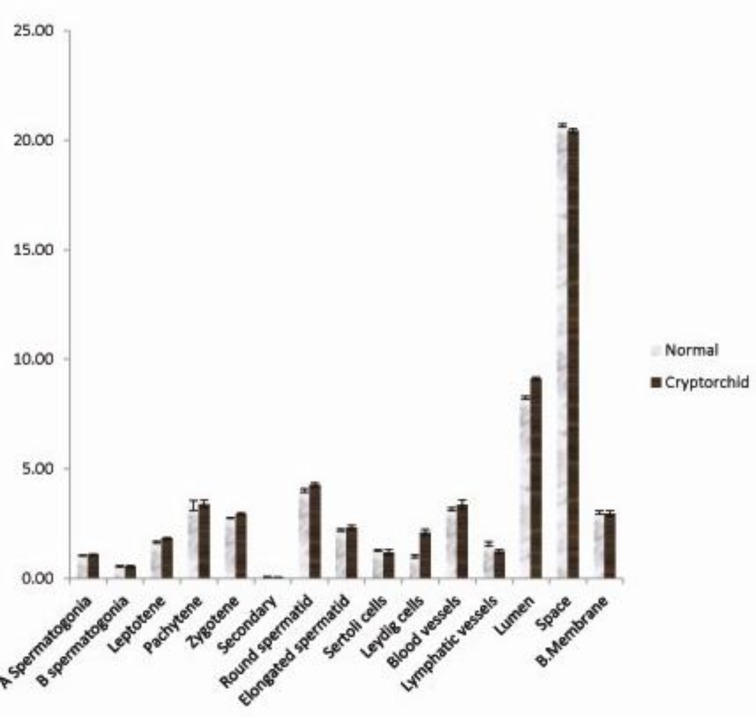
Volume densities of components of the testis
The percentage of the testis occupied by various germ cells, Sertoli cells, and several interstitial components is shown in Fig. 4. The volume densities of type A and B spermatogonia, pachytene, leptotene and zygotene primary spermatocytes, secondary spermatocytes, round and elongated spermatids, did not differ between the scrotal testis of the cryptorchid bucks and the normal bucks. However, the values obtained from the normal bucks were always lower.
Fig. 4.
Volume densities of component of the testes in normal and unilateral cryptorchid goats
The volumetric proportions of seminiferous tubule lumen were 8.25 ± 0.07 μm and 9.13 ± 0.05 μm, being the values for the normal testis and the scrotal testis of the cryptorchid bucks, respectively. The percentage volume of the lumen was greater in the scrotal testis of the cryptorchid bucks compared to the normal testis (P<0.05).
The percentage volume of the testis parenchyma occupied by the Sertoli cells was 1.19 ± 0.12 and 1.27 ± 0.04 for the normal (control) and the contralateral scrotal testis of the unilateral cryptorchid bucks, respectively. There were no significant differences in the Sertoli cell volume densities between the two groups of bucks. However, the Sertoli cells of the contralateral scrotal testis had a higher value. The interstitial space occupied by blood vessels, lymphatics and the Leydig cells was higher (P<0.05) in the testis of the normal bucks, but the volume densities of the lymphatics and blood vessels did not differ among the scrotal testis of the cryptorchid bucks and the normal testis. The volumetric proportion of the testis occupied by Leydig cells was 1.86% ± 0.37, 1.9% ± 0.89 and 1.10% ± 0.67 being values for the retained, scrotal and normal testis, respectively. The volume density of the Leydig cells from the contralateral scrotal testis of the cryptorchid bucks was significantly higher (P>0.05) than those of the normal bucks.
The percentage volume densities of the seminiferous tubules was 78.21 ± 0.15, 85.84 ± 0.73 and 80.25 ± 0.50 for the retained testis, scrotal testis and the normal (control) testis, respectively. The volume occupied by the seminiferous tubules in the contralateral scrotal testis was significantly greater than that of the normal (control) testis (P<0.05).
Height of the seminiferous epithelium
The different dimensions of the seminiferous tubules are illustrated in Table 1. The mean height of the seminiferous epithelium of the contralateral scrotal testis was significantly higher than that of the retained testes and also the control testes (P<0.05).
Table 1.
Dimensions of the seminiferous tubule of the testes of the unilaterally cryptorchid and normal goats
| Seminiferous tubule | Retained testis of cryptorchid bucks | Scrotal testis of cryptorchid bucks | Testis of normal bucks |
|---|---|---|---|
| Tubular diameter (μm) | 263.40 ± 0.41a | 285.69 ± 0.37b | 272.82 ± 0.67c |
| Epithelial height (μm) | 16.70 ± 0.45a | 98.33 ± 0.75b | 94.99 ± 0.37c |
| Tubular length per testis (m) | 346.34 ± 15.0a | 1028.67 ± 14.23b | 963.80 ± 12.43c |
| Tubular length/g of testis (m) | 12.23 ± 0.06a | 17.28 ± 0.08b | 17.96 ± 0.04b |
| Luminal diameter (μm) | 10.23 ± 0.67a | 88.94 ± 0.82b | 72.84 ± 0.52c |
| Volume density (%) | 78.21 ± 0.15a | 85.84 ± 0.73b | 80.25 ± 0.50c |
| No. of tubule/cross section | 43.50 ± 1.18a | 45.78 ± 0.97a | 43.74 ± 0.82a |
| Daily sperm production (×109) | 4.15 ± 0.35a | 3.42 ± 0.05b | |
| Daily sperm production/g of testis (×108) | 1.95 ± 0.25a | 1.02 ± 0.62b |
Means with different superscripts on the same row are significantly different (P<0.05)
Luminal diameter
The mean luminal diameter of the seminiferous tubules of the retained, contralateral scrotal testes and the normal testes is presented in Table 1. The luminal diameter of the seminiferous tubules of the contralateral scrotal testes of the cryptorchid bucks and that of the seminiferous tubules of the testes of normal (control) bucks was similar (P>0.05). However, the luminal diameter of the seminiferous tubules of the scrotal testes as well as those of the control testes were observed to be significantly greater than those of the retained testes (P<0.05).
Lengths of the seminiferous tubules
The tubular lengths of the seminiferous tubules per testis for the retained, contralataral scrotal testis and the normal (control) testis are illustrated in Table 1. The seminiferous tubules of the contralateral scrotal testis of the unilateral cryptorchid bucks were observed to be significantly (P<0.05) longer than those of the retained as well as the normal (control) testis. The tubular length per testis was significantly (P<0.05) longer in the scrotal testis compared to the normal testis. The tubular length per gram of testis was significantly (P<0.05) lower in the retained testis compared to the scrotal and normal testis. However, tubular length per gram of testis was similar comparing the tubules of the scrotal testis with those of the normal testis (P>0.05).
The diameter of the seminiferous tubules
The tubules from the contralateral scrotal testis of the cryptorchid bucks were significantly wider than those of the normal testis (P<0.05). However, the tubules from the retained testis had significantly smaller diameter compared to the normal and the contralateral scrotal testes (Table 1).
Seminiferous tubule profiles per cross section of the testes
The numbers of seminiferous tubule profiles per cross section of the testis for the retained testes, scrotal testes of the naturally unilateral cryptorchid bucks and the testes of the normal bucks are presented in Table 1.
The number of seminiferous tubule profiles in a section of the scrotal testes of the two groups was comparatively similar (P>0.05).
Dimensions of spermatogenic cells
The dimensions of the different spermatogenic cells are presented in Table 2. Comparison of the mean diameter of the spermatogonium of the normal bucks with those of the scrotal testis of the cryptorchid bucks, showed no significant difference (P>0.05). The nuclear diameter of the spermatogonia from the scrotal testis of the cryptorchid bucks was greater than those of the normal bucks (P<0.05).
Table 2.
Diameters (μm) of spermatogenic cells and their nuclei in the normal and contralateral scrotal testes of unilateral cryptorchid goats (mean±SEM
| Germ cells | Testis (normal bucks) |
Scrotal testis (cryptorchid bucks) |
|---|---|---|
| Spermatogonium | 11.60 ± 1.84a | 11.56 ± 1.66a |
| Spermatogonium nuclei | 5.30 ± 1.20a | 6.70 ± 1.80b |
| Primary spermatocyte | 17.85 ± 1.52a | 18.02 ± 1.45a |
| Primary spermatocyte nuclei | 8.70 ± 0.98a | 8.63 ± 1.76a |
| Round spermatid | 10.10 ± 1.10a | 10.22 ± 2.43a |
| Round spermatid nuclei | 7.01 ± 1.21a | 6.89 ± 1.72a |
Means with different superscripts on the same row are significantly different (P<0.05
The diameter of primary spermatocytes in the testis of normal bucks ranged from 16.20-20.34 μm. In the scrotal testis of the cryptorchid bucks, the diameter of the primary spermatocyte ranged from 16.95 to 21.22 μm. The diameter of primary spermatocytes of the cryptorchid bucks was similar to those of the normal bucks (P>0.05). The nuclear diameter of the primary spermatocytes in the testis of the normal bucks and the scrotal testis of the cryptorchid bucks were also found to be similar (P>0.05).
The diameters of the spermatids in the two groups were observed to be similar (P>0.05). The nuclear diameters of the spermatids were similar (P>0.05) comparing those of the normal testis with the scrotal testis of the cryptorchid bucks.
Population of germ cells
The total number of spermatogenic cells per testis and per cross section of the testis is presented in Table 3. There was no significant (P>0.05) difference in the number of spermatogenic cells per cross section of the testis, between the normal and the contralateral scrotal testis of the cryptorchid bucks. However, the total number of spermatogenic cells per testis was higher in the contralateral scrotal testis compared to the normal testis (P<0.05).
Table 3.
Germ cell population in the testes of the normal and unilateral cryptorchid goats (mean±SEM
| Spermatogenic cell population | Testis (normal bucks) | Scrotal testis (cryptorchid bucks) |
|---|---|---|
| No. of spermatogenic cells/cross section of testis | 257.50 ± 3.10a | 263.45 ± 2.66a |
| No. of spermatogenic cell per testis (109) | 1.12 ± 0.56a | 1.63 ± 0.75b |
Means with different superscripts on the same row are significantly different (P<0.05)
Germ cell/spermatogonia ratio
The germ cell/spermatogonia ratio is illustrated in Table 4. The ratio of primary spermatocytes to spermatogonium and round spermatid to spermato-gonium were significantly higher (P<0.05) in the scrotal testes of the cryptorchid bucks compared to the normal bucks.
Table 4.
Germ cell/spermatogonium ratio (mean±SEM) in testes of the normal and unilateral cryptorchid goats
| Germ cell | Normal bucks | Cryptorchid bucks |
|---|---|---|
| Primary spermatocytes (pachytene) | 1.92 ± 0.45a | 3.11 ± 0.51b |
| Round spermatids | 3.38 ± 0.60a | 5.78 ± 0.38b |
Means with different superscripts on the same row are significantly different (P<0.05)
Daily sperm production per testis and per gram of testis was significantly (P<0.05) higher in the scrotal testis of the unilateral cryptorchid bucks compared to the pooled means of the left and right testes of the normal bucks
Discussion
Height of the epithelium
The significantly higher epithelium of the scrotal testes of the unilateral cryptorchid bucks suggests that the scrotal testis of the unilateral cryptorchid bucks had a higher capacity for sperm production. The relative mass of seminiferous epithelium is positively correlated with sperm production. It has been accepted that species whose testes have a high proportion of seminiferous epithelium produce more sperm per unit mass (Franca and Russell, 1998 ▶; Franca et al., 2002 ▶; Hess and Fraca, 2005 ▶). It is therefore probable that the scrotal testes of the unilateral cryptorchid bucks posses superior spermatogenic efficiency compared to those of normal bucks. This superiority may have resulted not only from the epithelium density but also a combination of higher Sertoli cell support for germ cells, greater number of Sertoli cells per gram of testis, as well as higher seminiferous tubule volume density.
Luminal diameter
The findings in the present study show that the lumen of the seminiferous tubules of the scrotal testis in the unilaterally cryptorchid bucks were wider than those of the retained testes and also those of the normal bucks. This may indicate an increased production of the seminiferous tubule fluid, enhanced transmission of messages between the germinal epithelial cells and movement of spermatozoa from the lumen to the epididymis in the contralateral testis. Presence of tubular lumen is a pre-requisite for fluid formation, and the size of the lumen is determined by the volume of fluid produced. The relatively wide lumen and the probable increased volume of fluid are suggestive of an enhanced function of Sertoli cells. This assumption is based on the finding that formation of lumen in the testicular tubules is due to events occurring in the Sertoli cells, the formation of the blood-testis barrier, and fluid secretion from the Sertoli cells (Tindall et al., 1975 ▶). Bergh et al. (1978) ▶ reported an increased luminal diameter and seminiferous fluid production in congenital unilateral cryptorchidism. The increased volume of seminiferous tubule fluid promotes reproductive efficiency as it serves as a transport medium for information between cells of the germinal epithelium, and also for transportation of spermatozoa from the tubule lumen to the epididymis.
In the present study, most of the seminiferous tubules of the retained testes were not canalized, implying absence or poor fluid production by the Sertoli cells, due to the detrimental effects of the abdominal location of the testes on the Sertoli cells. Earlier studies reported decreased fluid production in cryptorchid testis, confirming impaired ability of Sertoli cells to produce fluid (Setchell, 1970 ▶; Hagenas et al., 1976 ▶). This may be a contributory factor to the non-development of germ cells observed in this study.
Length of the tubule
Seminiferous tubules were observed to be longer in the contralateral scrotal testes of the cryptorchid bucks compared to the retained testes and those of the normal bucks. The seminiferous tubule elongation observed in the contralateral scrotal testes, probably resulted from improved Sertoli cell activity, increased number of germ cells and Sertoli cell per serminiferous tubule. A highly significant correlation was reported to exist between Sertoli cell number per testis and seminiferous tubular length. Germ cell proliferation is the main factor responsible for the seminiferous tubule growth both in length and diameter (Kosco et al., 1989 ▶; Iczkowski et al., 1991 ▶). We propose a possible compensatory germ cell proliferation in the scrotal testis of the unilateral cryptorchid bucks.
Diameter of the tubule
The values obtained for the diameter of the seminiferous tubule in this study are slightly less than those reported for goats by Leal et al. (2004) ▶, but higher than those reported by Yadav and Sharma (1994) ▶ in other breeds of goats. The discrepancy probably resulted from inherent species differences and shrinkage associated with processing procedures in the earlier studies. In the present study, errors due to tissue shrinkage were minimized as we used semi-thin sections produced by plastic embedding technique.
The tubules of the contralateral scrotal testis of the cryptorchid bucks were significantly wider than those of the normal testes while the tubules of the retained testes had significantly lower diameter compared to the normal and the contralateral scrotal testes. The higher diameter of the seminiferous tubule of the contralateral scrotal testes may be a result of increased height and luminal diameter of the seminiferous tubule as reported in the present study. This may indicate high level of maturation and fluid production by the Sertoli cells of the contralateral scrotal testes, because seminiferous tubule diameter has been positively correlated with testicular maturation and spermatogenesis (Meisami et al., 1994 ▶). Also the diameter of the seminiferous tubule is an excellent parameter for determining the stage of spermatogenesis during postnatal testes development as well as the degree of Sertoli cell maturation and fluid secretion (Czykier et al., 2012 ▶).
Yield of spermatids per type A spermatogonium
The results of the present study show a high yield of spermatids per spermatogonium. This suggests less degeneration occurred in the contralateral scrotal testis of the unilateral cryptorchid bucks compared to the testis of the normal bucks. This may be attributed to the high level of FSH and testosterone recorded in the unilateral cryptorchid bucks in another study of ours. High levels of FSH and testosterone are necessary for maintenance of spermatogenesis (Walker, 2010 ▶). This may have contributed to the improved spermatogenic efficiency observed in the contralateral testis of the cryptorchid bucks. Similarly, the low index of degeneration or apoptosis in the contralateral scrotal testis contributed substantially to the speculated improvement in daily sperm production of the contralateral scrotal testes of the unilateral cryptorchid bucks.
Determination of spermatogenesis by quantitative histological technique showed that the scrotal testis of the unilateral cryptorchid bucks produced higher sperm cells per testis and per gram of testis parenchyma. This observation implies that daily sperm production of a single scrotal testis of the unilateral cryptorchid buck compared favourably with daily sperm production from two testes of the normal bucks. The only explanation for this could be that a greater percentage of the testes parenchyma is devoted to sperm production in the scrotal testis of the unilateral cryptorchid as demonstrated by the high seminiferous tubule and seminiferous epithelium relative density in the present study.
References
- Almeida FFL; Marcelo CL, Franca LR. Testis morphometry, duration of spermatogenesis and spermato-genic efficiency in the wild boar (Sus scrofa scrofa) Biol. Reprod. 2006;75:792–799. doi: 10.1095/biolreprod.106.053835. [DOI] [PubMed] [Google Scholar]
- Bergh A; Helander HF, Wahlquuist L. Studies on factors governing testicular descent in the rat-particularly the role of gubernaculums testis. Int. J. Androl. 1978;1:342–356. [Google Scholar]
- Bitto II, Egbunike GN. Seasonal variations in sperm production, gonadal and extragonadal sperm reserves in pubertal West African dwarf bucks in their native tropical environment. Livestock Res. Rural Dev., 18: Retrieved August 1, 2015. 2006. http://www.lrrd.org/lrrd18/9/bitt18134.htm.
- Chiejina SN, Behnke JM. The unique resistance and resilience of the Nigerian West African Dwarf goat to gastrointestinal nematode infections. Parasit. Vectors. 2011;4:12. doi: 10.1186/1756-3305-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes D; Laub B, Thorup J. Testicular histology in cryptorchid boys-aspects of fertility. J. Pediat. Surg. Specialt. 2015;9:1–52. [Google Scholar]
- Costa DS, Silva JFS. Wild boars (Sus scrofa scrofa) seminiferous tubules morphometry. Braz. Arch. Biol. Technol. 2006;49:739–745. [Google Scholar]
- Couto D, Talamoni SA. Reproductive condition of Akodon montensis Thomas and Bolomys lasiurus (Lund) (Rodentia, Muridae) based on histological and histometric analyses of testes and external characteristics of gonads. Acta Zool. (Stockholm) 2005;86:111–118. [Google Scholar]
- Czykier E; Bierła JB, Góral KK. Morphometric measurements of the seminiferous tubules of the testes in 2-year-old and 3-year-old European bison males with or without spermiogenesis. Polish J. Vet. Sci. 2012;15:569–570. doi: 10.2478/v10181-012-0087-2. [DOI] [PubMed] [Google Scholar]
- Dutta S; Joshi KR; Sengupta S, Bhattacharya K. Unilateral and bilateral cryptorchidism and its effect on the testicular morphology, histology, accessory sex organs, and sperm count in laboratory mice J. Human Reprod. Sci. 2013;6:106–110. doi: 10.4103/0974-1208.117172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emehelu CO; Ekwueme EC, Chah FK. Cryptorchidism in West African Dwarf goat in Nsukka agricultural zone of Enugu state, Nigeria. Sahel J. Vet. Sci. 2005;4:59–61. [Google Scholar]
- Ezeasor DN. Light and electron microscopical observations on the Leydig cells of the scrotal and abdominal testes of naturally unilateral cryptorchid West African Dwarf goats. J. Anat. 1985;141:27–40. [PMC free article] [PubMed] [Google Scholar]
- Franca LR, Godinho CL. Testis morphometry, seminiferous epithelium, cycle length and daily sperm production in domestic cats (Felis catus) Biol. Reprod. 2003;68:1554–1561. doi: 10.1095/biolreprod.102.010652. [DOI] [PubMed] [Google Scholar]
- França LR, Russell LD. The testis of domestic animals. In: Martínez, F, Regadera, J, editors. Madrid, Spain: Churchill Livingstone; 1998. pp. 197–219. [Google Scholar]
- França LR; Russell LD, Cummins JM. Is human spermatogenesis uniquely poor? Ann. Rev. Biomed. Sci. 2002;4:19–40. [Google Scholar]
- Hagenas L; Ploen L, Ritzen EM. Blood-testis barrier: maintained function of inter-sertoli cell junction in experimental cryptorchidism in the rat, as judged by a simple lanthanum-immersion technique. Andrologia. 1976;9:3–7. doi: 10.1111/j.1439-0272.1977.tb01297.x. [DOI] [PubMed] [Google Scholar]
- Hess R, França LR. History of the Sertoli cell discovery. In: Griswold, M, Skinner, M, editors. Sertoli cell biology. New York: Academic Press; 2005. pp. 3–14. [Google Scholar]
- Iczkowski KA; Sun EL, Gondos B. Morpho-metric study of the prepubertal rabbit testis: germ cell numbers and seminiferous tubule dimensions. Am. J. Anat. 1991;190:266–272. doi: 10.1002/aja.1001900306. [DOI] [PubMed] [Google Scholar]
- Kosco MS; Loseth KJ, Crabo BG. Development of the seminiferous tubules after neonatal hemicastration in the boar. J. Reprod. Fertil. 1989;87:1–11. doi: 10.1530/jrf.0.0870001. [DOI] [PubMed] [Google Scholar]
- Lambert B. The frequency of mumps and of mumps orchitis and the consiquences for sexuality and fertility. Acta Genet. Stat. Med. 1951;2:1–166. [PubMed] [Google Scholar]
- Leal MC; Becker-Silva SC; Chiarini-Garcia H, Franca LR. Sertoli cell efficiency and daily sperm production in goats (Capra hircus) Anim. Reprod. 2004;1:122–128. [Google Scholar]
- Mechelin CW, Kogan BA. What lessons can be learned from testicular histology in undescended testes. Transl. Androl. Urol. 2014;3:365–369. doi: 10.3978/j.issn.2223-4683.2014.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisami E; Azar N, Paola ST. Enhancement of seminiferous tubular growth and spermatogenesis in testes of rats recovering from early hypothyroidism: a quantitative study. Cell Tissue Res. 1994;275:503–511. doi: 10.1007/BF00318819. [DOI] [PubMed] [Google Scholar]
- Orlu EE, Egbunike GN. Daily sperm production of the domestic fowl (Gallus domesticus) as determined by quantitative testicular histology and homogenate methods. Pak. J. Biol. Sci. 2009;12:1359–1364. doi: 10.3923/pjbs.2009.1359.1364. [DOI] [PubMed] [Google Scholar]
- Ramm SA, Scharer L. The evolutionary ecology of testicular function: size isn’t everything. Biol. Rev. 2014;89:874–888. doi: 10.1111/brv.12084. [DOI] [PubMed] [Google Scholar]
- Segatell TM; Franca LR; Inheiro FP; Alemida CCD; Martinez M, Martinez FE. Spermatogenic cycle length and spermatogenic efficiency in the Gerbil (Meriones unguiculatus) J. Androl. 2004;2:13–17. doi: 10.1002/j.1939-4640.2004.tb03156.x. [DOI] [PubMed] [Google Scholar]
- Setchell BP. The secretion of fluid by the testes of rats, rams and goats with some observations on the effect of age, cryptorchidism and hypophysectomy. J. Reprod. Fertil. 1970;23:79–85. doi: 10.1530/jrf.0.0230079. [DOI] [PubMed] [Google Scholar]
- Swierstra EE. Structural composition of Shorthorn bull testes and daily spermatozoa production as determined by quantitative testicular histology. Can. J. Ani. Sci. 1966;46:107–114. [Google Scholar]
- Swierstra EE. A comparison of spermatozoa produc-tion and spermatozoa output of Yorkshire and Lacombe boars. J. Reprod. Fertil. 1968;17:459–469. doi: 10.1530/jrf.0.0170459. [DOI] [PubMed] [Google Scholar]
- Tindall DJ; Vitale R, Means AR. Androgen binding protein as a biochemical marker of formation of the blood-testis barrier. Endocrinology. 1975;97:636–648. doi: 10.1210/endo-97-3-636. [DOI] [PubMed] [Google Scholar]
- Walker WH. Non-classical actions of testosterone and spermatogenesis. Philos. Trans. R. Soc. London Bio. Sci. 2010;365:1557–1569. doi: 10.1098/rstb.2009.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav SK, Sharma AK. Seminiferous tubule length normal buffalos-bulls and bucks. Int. J. Anim. Sci. 1994;9:293–296. [Google Scholar]