Abstract
In recent times increasing occurrence of dermatophytosis, especially among the school children in eastern India was evidenced along with increased tendency of keeping companion animals such as dogs and cats. This study was undertaken to detect the occurrence of dermatophytes with antifungal susceptibility among the companion animals. A total of 1501 healthy companion animals comprising 1209 dogs and 292 cats belonged to individual owners in and around Kolkata (West Bengal, India) were examined for the evidence of dermatophytosis during 2011-2013. The collected samples were subjected to direct examination by standard KOH mount technique. The samples were inoculated into both Sabouraud dextrose agar (SDA) with 0.05% chloramphenicol and 0.5% cycloheximide and dermatophyte test medium (DTM). Each of the fungal isolate was identified based upon its colony characteristics and hyphal and conidial cells it produced. Antifungal susceptibility of the isolates was tested by broth micro dilution assay using fluconazole, ketoconazole, itraconazole, miconazole, griseofulvin and amphotericin-B antifungals. Among the 1209 samples from dogs and 292 samples from cats, 253 (20.93%) and 109 (37.33%) samples were positive for dermatophytes by direct examination. Three identified species of dermatophytes with predominant occurrence were Microsporum canis, Microsporum gypseum and Trichophyton mentagrophytes. Ketoconazole (0.06-0.5 µgm/ml), itraconazole (0.03-0.5 µgm/ml) and amphotericin-B (0.03-0.5 µgm/ml) showed lowest MIC values against M. canis, T. mentagrophytes and M. gypseum, respectively. This is the first systemic report of dermatophytes in healthy companion animals with large numbers of samples in India.
Key Words: Cat, Dermatophytes, Dog, Microsporum, Trichophyton
Introduction
Dermatophytosis is a superficial fungal infection of hair and keratinized layers of the epidermis and is caused by keratinophilic and keratinolytic genera such as Microsporum, Trichophyton and Epidermophyton. It is an endemic infection in many countries throughout the world affecting companion animals (dogs, cats), domestic animals (calves), and laboratory animals (rabbits) as well as humans. High animal density in a farm and close contact between the companion animals and human facilitates transmission (Lund et al., 2014 ▶; Samanta, 2015 ▶).
The companion animals (dogs and cats) can act as carriers of dermatophyte (Microsporum) spores which cannot invade the healthy skin of the animals. This carrier stage may progress to infection based on certain predisposing factors such as young age, immuno-suppression, nutritional deficiency, high environmental temperature with high humidity and skin trauma. After penetration through the damaged skin the spores germinate in the stratum corneum and the fungal metabolites induce inflammatory reaction at the site of infection (Weitzman and Summerbell, 1995 ▶).
The ‘gold standard’ diagnostic techniques for identification of dermatophytosis involve direct microscopic examination of clinical specimens followed by in vitro isolation and identification (Nardoni et al., 2010 ▶). The antifungals commonly used in systemic treatment of dermatophytosis in dogs and cats include itraconazole, terbinafine and griseofulvin (Gupta and Del Rosso, 2000 ▶). Currently emergence of antifungal resistant clinical isolates leads to failure in the treatment of mycosis (Alcazar-Fuoli and Mellado, 2014 ▶). Therefore, in vitro antifungal susceptibility test could help to optimize the therapy and select an effective antifungal agent against the clinical isolates (Araújo et al., 2009 ▶).
Indian human population mostly suffers from tinea corporis type of dermatophytosis which is associated with age, occupation, prior exposure and personal hygiene (Mahapatra, 1989 ▶; Das et al., 2009 ▶). However, in recent times increasing occurrence of tinea capitis (scalp infection) especially among the school children in the study area (West Bengal, India) was evidenced which was not associated with personal hygiene (Kundu et al., 2012 ▶). An increased tendency of keeping companion animals such as dogs and cats was observed in the study area and the pets are very closely associated with the daily life of their owners, especially the children. Historically very few reports regarding the occurrence of dermatophytes in pet animals in the study area were noted (Chakrabarty et al., 1954 ▶; Chatterjee et al., 1980 ▶). Furthermore, the studies were performed with limited number of samples and it could not provide the current trend of infection and their antifungal susceptibility.
Thus the present study reported the occurrence of dermatophytes carrier state and their antifungal suscep-tibility in healthy companion animals (dogs and cats) which are considered as the most potent carriers in India.
Materials and Methods
Study population
A total 1501 companion animals comprising of 1209 dogs and 292 cats belonging to individual owners in and around Kolkata (West Bengal, India) were examined for the evidence of dermatophytosis during 2011-2013. The skin of the animals was examined by veterinarian for any lesion and only the healthy animals without lesions were selected. The animals were both male and female. The dogs belonged to different breeds (German Shepherd, Spitz, Labrador, Golden Retriever) whereas the cats were indigenous. The age of the animals was divided into two groups i.e., group 1 (0-6 months) and group 2 (>6 months). The samples were collected during four seasons i.e., spring (March-May), summer (June-August), autumn (September-November) and winter (December-February). The dogs were kept indoors and they often shared the common floor, bed, sofa with their owners specifically, the children. However the cats preferred to roam outside the house during the daytime and were dirty in appearance.
Sampling
The sampling was done either at pet clinics of veterinarians or at owner’s house. The hairs and unused toothbrush or hair brush after brushing the animal skin over the back, shoulders, sides, hindquarters and legs for 5-7 min were collected. Both the hair and the brushes were wrapped in a coloured paper and were kept in a tight container preferably without moisture for transport to the laboratory.
Direct examination
The collected samples were subjected to direct examination by standard KOH mount technique (Rippons, 1983 ▶).
Isolation and identification
The clinical samples were inoculated into both Sabouraud dextrose agar (SDA) with 0.05% chloramphenicol and 0.5% cycloheximide and dermato-phyte test medium (DTM). The SDA plates were incubated at 28°C for four weeks and were observed periodically for appearance of fungal growth. The DTM tubes were incubated at 28°C for three weeks to detect any change in colour (Robert and Pihet, 2008 ▶).
Each of the fungal isolate was identified based upon its colony characteristics and hyphal and conidial cells it produced. The conidia were identified after lactophenol cotton blue staining on the basis of their size, shape, presence of septa, thickness of conidial wall and arrangement of conidial cells around the hyphae (Pang et al., 2008 ▶).
Antifungal susceptibility test
Antifungal susceptibility of the isolates was tested by broth micro dilution assay using fluconazole, ketoconazole, itraconazole, miconazole, griseofulvin and amphotericin-B antifungals (CLSI, 2002 ▶).
Statistical analysis
Differences in occurrence rates of dermatophytosis were compared according to age, sex and season using Chi-square test (SPSS Inc., Chicago, IL).
Results
Occurrence of dermatophytes in cats and dogs
Out of 1209 samples from dogs, 253 (20.93%) samples were positive for dermatophyte spores by direct examination. Out of 253 samples, deramtophytes were isolated from 248 (248/253, 98.02%) samples. Three identified species of dermatophytes were M. canis (43.55%), M. gypseum (36.69%) and T. mentagrophytes (19.79%) (Figs. 1-3; Table 1). Younger pups (<6 months) showed statistically highly significant (P<0.0001) levels of occurrence of the disease than the adults (>6 months). Higher rate of carriage in dogs was observed during summer and autumn than the winter and spring (P=0.0091; Table 2). Male animals showed a relatively higher occurrence of dermatophytes than the females, however, this was not statistically significant (P=0.571; Table 2).
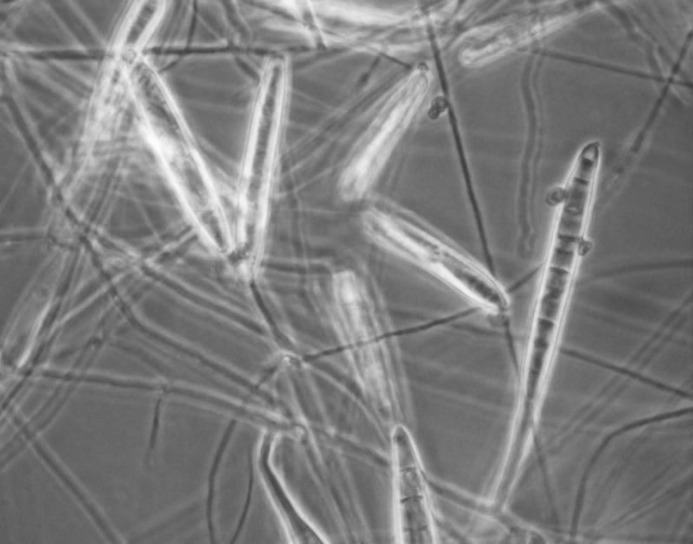
Fig. 1.
Macroconidia of M. canis (lactophenol cotton blue) detected from healthy dogs in India
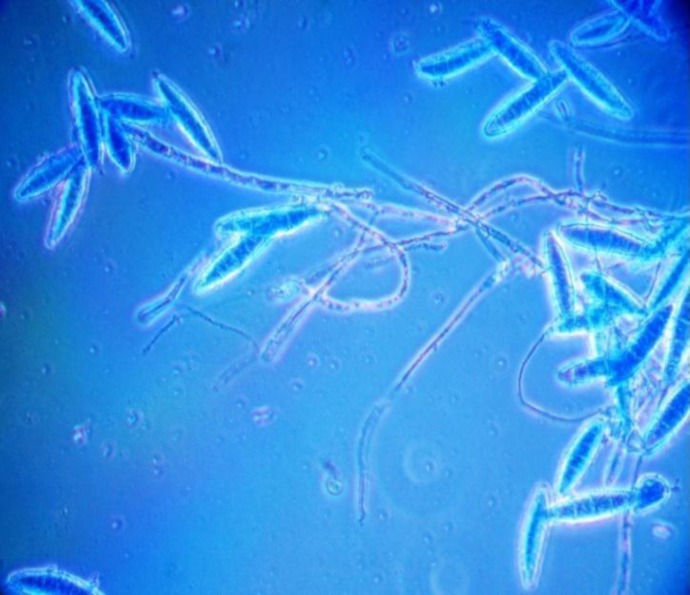
Fig. 3.
Microconidia of T. mentagrophytes (lactophenol cotton blue) detected from healthy dogs in India
Table 1.
Occurrence of dermatophyte species in dogs and cats in West Bengal, India
| Dermatophytes | Dogs |
Cats |
||
|---|---|---|---|---|
| n | Percentage | n | Percentage | |
| M. canis | 108 | 43.55 | 57 | 55.34 |
| M. gypseum | 91 | 36.69 | 32 | 31.07 |
| T. mentagrophytes | 49 | 19.76 | 14 | 13.59 |
| Total | 248 | 100.00 | 103 | 100.00 |
Table 2.
Variables of age, sex and season on dog and cat dermatophytosis in West Bengal, India
| Variables | No. of positive/No. of animals examined (percentage) |
|
|---|---|---|
| Dog | Cat | |
| Age | ||
| 0-6 | 172/484 (35.54) | 68/178 (38.20) |
| >6 | 76/725 (10.48) | 35/114 (30.70) |
| P<0.0001 | P=0.2106 | |
| Sex | ||
| Male | 133/629 (21.14) | 47/127 (37.00) |
| Female | 115/580 (19.83) | 56/165 (33.93) |
| P=0.571 | P=0.5864 | |
| Season | ||
| Spring | 14/119 (11.76) | 09/53 (16.98) |
| Summer | 103/454 (22.69) | 43/94 45.74) |
| Autumn | 117/528 (22.16) | 38/87 (43.68) |
| Winter | 14/108 (12.96) | 13/58 (22.41) |
| P=0.0091 | P=0.0039 | |
Among the 292 samples from cats, 109 (37.33%) samples were positive for dermatophyte spores by direct examination. Further, out of 109 samples, deramtophytes were isolated from 103 (103/109, 94.49%) samples (Table 1). Three identified species of dermatophytes were M. canis (55.34%), M. gypseum (31.07%) and T. mentagrophytes (13.59%) (Table 2). The occurrence of dermatophytes in cats was significantly higher in summer and autumn (P=0.0039) than in spring and winter (Table 2). Younger (<6 months) and male cats showed a relatively higher occurrence of dermatophytes than the adult (>6 months) and female cats, however, this was not significantly different (P=0.2106 and P=0.5864; Table 2).
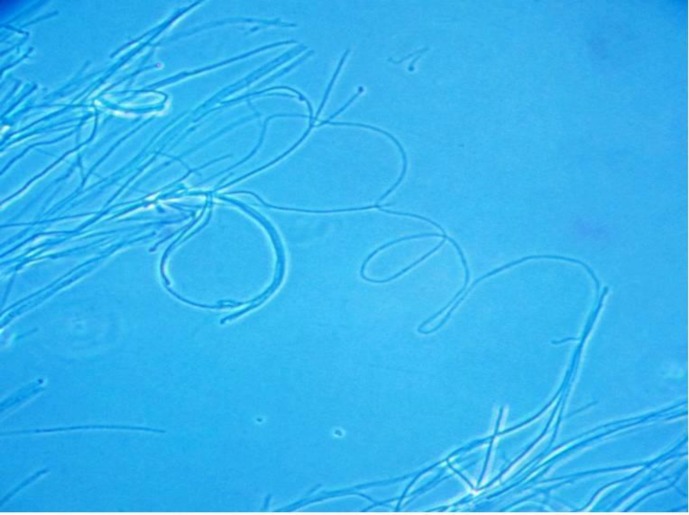
Fig. 2.
Macroconidia of M. gypseum (lactophenol cotton blue) detected from healthy dogs in India
Antifungal susceptibility test
Antifungal susceptibility pattern of isolated dermato-phytes by broth micro dilution method revealed minimal inhibitory concentration (MIC) of 6 antifungal agents for 351 (cat-103, dog-248) dermatophyte isolates. Ketoconazole (0.06-0.5 µgm/ml), itraconazole (0.03-0.5 µgm/ml) and amphotericin-B (0.03-0.5 µgm/ml) showed lowest MIC values against M. canis, T. mentagrophytes and M. gypseum, respectively. Further, 90% of M. canis, T. mentagrophytes and M. gypseum isolates were inhibited by 0.125 µgm/ml of ketoconazole (MIC90), 0.25 µgm/ml of itraconazole (MIC90) and 0.125 µgm/ml of amphotericin-B (MIC90), respectively. Fluconazole and miconazole showed highest MIC value against the isolates indicating antifungal resistance (Table 3).
Table 3.
In vitro antifungal susceptibility of dermatophyte isolates from cats and dogs in West Bengal, India
| Species (No. of isolates) | Antifungal agents | MIC (µg/ml) |
||
|---|---|---|---|---|
| Range | MIC50 | MIC90 | ||
| T. mentagrophytes (63) | Fluconazole | 4-64 | 16 | 64 |
| Ketoconazole | 0.06-2 | 0.125 | 0.25 | |
| Itraconazole | 0.03-0.5 | 0.125 | 0.25 | |
| Miconazole | 0.03-1 | 0.06 | 0.125 | |
| Griseofulvin | 0.6-1 | 0.125 | 0.25 | |
| Amphotericin-B | 0.03-1 | 0.03 | 0.125 | |
| M. canis (165) | Fluconazole | 4-64 | 16 | 32 |
| Ketoconazole | 0.06-0.5 | 0.06 | 0.125 | |
| Itraconazole | 0.03-1 | 0.06 | 0.125 | |
| Miconazole | 0.03-0.5 | 0.06 | 0.25 | |
| Griseofulvin | 0.06-4 | 0.125 | 0.25 | |
| Amphotericin-B | 0.03-1 | 0.06 | 0.125 | |
| M. gypseum (123) | Fluconazole | 8-64 | 16 | 32 |
| Ketoconazole | 0.03-1 | 0.06 | 0.125 | |
| Itraconazole | 0.03-2 | 0.25 | 0.5 | |
| Miconazole | 0.03-0.25 | 0.06 | 0.25 | |
| Griseofulvin | 0.06-2 | 0.125 | 0.5 | |
| Amphotericin-B | 0.03-0.5 | 0.06 | 0.125 | |
Discussion
The present study detected moderate occurrence (21%) of dermatophytes in the companion dog population in eastern India which is consistent with earlier findings throughout the world (Faggi et al., 1987 ▶; Seker et al., 2011 ▶). In corroboration with our findings, earlier reports are also available from India and abroad regarding isolation of M. canis as the predominant species of dermatophyte in dogs (Seker et al., 2011 ▶; da Costa et al., 2013 ▶; Beigh et al., 2014 ▶).
The present study detected moderately higher occurrence of dermatophytes (37.33%) in healthy cats without any skin lesions in eastern India. Similar prevalence of dermatophytosis (25-35%) in stray or companion cats was detected earlier in other countries such as New Zealand, Iran and Portugal (Carman et al., 1979 ▶; Khosravi, 1996 ▶; Duarte et al., 2010 ▶). Although lower prevalence (5%) of dermatophytosis in cats was observed in the UK and Italy (Patel et al., 2005 ▶; Proverbio et al., 2014 ▶). The unhygienic condition in which the companion cats were kept as detected in the present study is the probable reason of higher occurrence of dermatophytes. Microsporum canis was the most frequently isolated dermatophyte from the companion cats which is in agreement with earlier findings in India and throughout the world (Chatterjee et al., 1980 ▶; Duarte et al., 2010 ▶; Lopez et al., 2012).
Further, comparative occurrence rate of dermato-phytes between the studied cats and dogs revealed higher occurrence in cats than the dogs which is also consistent with earlier findings (Guzman-Chavez et al., 2000 ▶). Probably the unhygienic conditions in which the cats were kept is responsible for more dermatophyte carriage than the dogs.
Group-1 pups (<6 months of age) showed a significantly higher occurrence (P<0.05) of dermato-phytes than the group-2 (>6 months age). While, relatively higher occurrence of dermatophytes in group-1 cats (<6 month age) was detected than the group-2 cats (>6 months age), which was not significantly different. Higher susceptibility of young companion animals to dermatophytes was also reported earlier (Marchisio and Gallo, 1995 ▶; Seker et al., 2011 ▶). It might be due to their poorly developed immune system and deficiency of fungistatic linoleic acid (Al-Ali et al., 1997 ▶).
Further, male cats showed significantly higher occurrence of dermatophytes than the female cats. The samples were not collected equally from male and female cats which could explain the higher occurrence in male cats. Whereas, there was no significant difference of occurrence between male and female dogs. Similarly previous studies did not find the sex as a contributing factor in occurrence of dermatophytosis in cats and dogs (Guzman-Chavez et al., 2000 ▶; Seker et al., 2011 ▶; Lopez et al., 2012). The occurrence of dermatophytes in cats and dogs was significantly higher in summer and autumn than in spring and winter season. During summer the owners preferred to keep their dogs indoor only due to harsh external weather which could explain the higher occurrence as observed in temperate countries during winter (English, 1972 ▶).
Ketoconazole, itraconazole and amphotericin-B showed lowest MIC values against M. canis, T. mentagrophytes and M. gypseum, respectively. Whereas fluconazole and miconazole showed highest MIC value against the isolates, indicating the antifungal resistance. Similarly, itraconazole with lowest MIC activity (0.03-0.5 µgm/ml) and fluconazole with highest MIC activity (0-24 µgm/ml) was detected against human dermato-phyte isolates (Santos et al., 2006 ▶; Araújo et al., 2009 ▶; Aktas et al., 2014 ▶).
Thus the present study identified companion animals like dogs and cats of West Bengal (India) as potential carriers of dermatophytes in their hair or skin without showing any clinical symptom. The infection may be transmitted to the owners, especially among the children who came in close contact and mingled with them.
Acknowledgements
The authors provide sincere thanks to Honorable Vice Chancellor, West Bengal University of Animal and Fishery Sciences (WBUAFS) for providing infra-structure. This work was financially supported by Indian Council of Agricultural Research (ICAR) under outreach programme.
References
- Aktas AE; Yigit N; Aktas A, Gozubuyuk SG. Investigation of in vitro activity of five antifungal drugs against Dermatophytes species isolated from clinical samples using the E-test method. Eurasian J. Med. 2014;46:26–31. doi: 10.5152/eajm.2014.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ali G; Natour RM, Al-Bitar W. Distribution and prevalence of superficial fungal infections in Amman and other Jordan cities. Med. Biol. Sci. 1997;24:219–227. [Google Scholar]
- Alcazar-Fuoli L, Mellado E. Current status of antifungal resistance and its impact on clinical practice. Br. J. Haematol. 2014;166:471–484. doi: 10.1111/bjh.12896. [DOI] [PubMed] [Google Scholar]
- Araújo CR; Miranda KC; Fernandes OFL; Soares AJ, Silva MRR. In vitro susceptibility testing of dermatophytes isolated in Goiania, Brazil, against five antifungal agents by broth microdilution method. Rev. Inst. Med. Trop. S. Paulo. 2009;51:9–12. doi: 10.1590/s0036-46652009000100002. [DOI] [PubMed] [Google Scholar]
- Beigh SA; Soodan JS; Singh R; Khan AM, Dar MA. Evaluation of trace elements, oxidant/antioxidant status, vitamin C and β-carotene in dogs with dermato-phytosis. Mycoses. 2014;57:358–365. doi: 10.1111/myc.12163. [DOI] [PubMed] [Google Scholar]
- Carman MG; Rush-Munro FM, Carter ME. Dermatophytes isolated from domestic and feral animals. New Zealand Vet. J. 1979;27:136–144. doi: 10.1080/00480169.1979.34628. [DOI] [PubMed] [Google Scholar]
- Chakrabarty AN; Ghosh S, Blank F. Isolation of Trichophyton rubrum (Castollani) Sab., 1911 from animals. Can. J. Comp. Med. 1954;18:436–438. [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Sengupta DN, Chattopadhyay D, Bhattacharya D, Dutta AK. Some epidemiological aspects of zoophilic dermatophytosis. Int. J. Zoonoses. 1980;7:19–33. [PubMed] [Google Scholar]
- CLSI. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Wayne: CLSI; 2002. [Google Scholar]
- da Costa FV, Farias MR, Bier D, de Andrade CP, de Castro LA, da Silva SC, Ferreiro L. Genetic variability in Microsporum canis isolated from cats, dogs and humans in Brazil. Mycoses. 2013;56:582–588. doi: 10.1111/myc.12078. [DOI] [PubMed] [Google Scholar]
- Das K, Basak S, Ray S. A study on superficial fungal infection from West Bengal: a brief report. J. Life Sci. 2009;1:51–55. [Google Scholar]
- Duarte A, Castro I, Pereira da Fonseca IM, Almeida V, Madeira De Carvalho LM, Meireles J, Fazendeiro MI, Tavares L, Vaz Y. Survey of infectious and parasitic diseases in stray cats at the Lisbon Metropolitan area, Portugal. J. Feline Med. Sur. 2010;12:441–446. doi: 10.1016/j.jfms.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English MP. The epidemiology of animal ringworm in man. Bri. J. Dermatol. 1972;86(Suppl. ):78–87. [Google Scholar]
- Faggi E, Saponetto N, Sagone M. Dermatophyte isolated from domestic carnivores in Florence (Italy): epidemiological investigation. Bull. Soc. Fr. Mycol. Med. 1987;16:297–301. [Google Scholar]
- Gupta AK, Del Rosso JQ. An evaluation of intermittent therapies used to treat onychomycosis and other dermatomycoses with the oral antifungal agents. Int. J. Derm. 2000;39:401–411. doi: 10.1046/j.1365-4362.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- Guzman-Chavez RE, Segundo-Zaragoza C, Arnulfo R, Olivares C, Tapia-Perez G. Presence of keratinophilic fungi with special reference to Dermatophytes on the haircoat of dogs and cats in México and Nezahualcoyotl cities. Rev. Latinoam. Microbiol. 2000;42:41–44. [PubMed] [Google Scholar]
- Khosravi AR. Fungal flora of the hair coat of stray cats in Iran. Mycoses. 1996;39:241–243. doi: 10.1111/j.1439-0507.1996.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Kundu D, Mandal L, Sen G. Prevalence of Tinea capitisin school going children in Kolkata, West Bengal. J. Nat. Sci. Biol. Med. 2012;3:152–155. doi: 10.4103/0976-9668.101894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund A, Bratberg AM, Nass B, Gudding R. Control of bovine ringworm by vaccination in Norway. Vet. Immunol. Immunopathol. 2014;158:37–45. doi: 10.1016/j.vetimm.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Mahapatra LN. Study of medical mycology in India - an overview. Indian J. Med. Res. 1989;89:351–353. [PubMed] [Google Scholar]
- Marchisio VF, Gallo MG. Dermatophytes from cases of skin disease in cats and dogs in Turin, Italy. Mycoses. 1995;38:239–244. doi: 10.1111/j.1439-0507.1995.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Nardoni S, Papini R, Verin R, Mancianti F. Survey on the role of brown hares (Lepus europaeus, Pallas 1778) as carriers of zoonotic dermatophytes. Italian J. Anim. Sci. 2010;9:126–128. [Google Scholar]
- Pang SH, Ren P, Wu XJ. Diagnosis and treatment of rabbit skin mildew. Anim. Sci. Vet. Med. 2008;4:70. [Google Scholar]
- Patel A, Lloyd DH, Lamport AI. Survey of dermato-phytes on clinically normal cats in the southeast of England. J. Small Anim. Pract. 2005;46:436–439. doi: 10.1111/j.1748-5827.2005.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Proverbio D, Perego R, Spada E, Bagnagatti de Giorgi G, Pepa AD, Ferro E. Survey of Dermatophytes in stray cats with and without skin lesions in Northern Italy. Vet. Med. Int. 2014. http://dx.doi.org/10.1155/2014/565470. [DOI] [PMC free article] [PubMed]
- Rippons JW. Dermatophytosis and dermatomycosis. In: Rippons, JW, editor. Medical mycology, the pathogenic fungi and the pathogenic Actinomycetes. 2nd Edn. Philadelphia: W. B. Saunders; 1983. 213 pp. [Google Scholar]
- Robert R, Pihet M. Conventional methods for the diagnosis of dermatophytosis. Mycopathologia. 2008;166:295–306. doi: 10.1007/s11046-008-9106-3. [DOI] [PubMed] [Google Scholar]
- Samanta I. Veterinary mycology. 1st Edn. India: Springer. P; 2015. 16 pp. [Google Scholar]
- Santos DA, Barros MES, Hamdan JS. Establishing a method of inoculum preparation for suscep-tibility testing of Trichophyton rubrum and Trichophyton mentagrophytes. J. Clin. Microbiol. 2006;44:98–101. doi: 10.1128/JCM.44.1.98-101.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seker E, Dogan N. Isolation of dermatophytes from dogs and cats with suspected dermatophytosis in Western Turkey. Prev. Vet. Med. 2011;98:46–51. doi: 10.1016/j.prevetmed.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Weitzman I, Summerbell RC. The dermato-phytes. Clin. Microbiol. Rev. 1995;8:240–259. doi: 10.1128/cmr.8.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]