Abstract
The aim of this study was to examine the possible morphological and morphometric changes resulting from vitrification of embryos at the cleavage stage. In this study, 30 mice early-cleavage embryos at different stages of cleavage, resulting from in vitro fertilization (IVF) techniques, were examined before and after vitrification. Digital images were taken from embryos before and after vitrification. Zona pellucida thickness, differences in zona pellucida thickness, and diameter and volume of blastomeres and embryos as morphometric parameters and current rating of appearance of embryos as morphological parameters, have been studied. According to our findings, there were significant mean differences in all morphometric parameters of the two groups except in the zona pellucid thickness (P≤0.05). With regard to the morphological parameter, the decrease in embryo quality was observed but it was not significant. According to the results, although little quantitative change observed is not necessarily synonymous with harmful intracellular damage, it seems that it is better to examine vitrification method more accurately. Because by making subtle changes in concentration and type of consumed solutions or techniques used, the changes may be minimized.
Key Words: Cleavage embryo, Mice, Morphology, Morphometry, Vitrification
Introduction
Cryopreservation of embryos in different stages of development has become a major part of all IVF laboratories (Liebermann et al., 2002 ▶). Of all methods of cryopreservation, vitrification is more common for mammalian embryos. In this physical process, by selection of high concentrations of cryoprotectants, embryos are preserved in liquid nitrogen so that no crystallization occurs (Lin et al., 2010 ▶). So, obtaining high quality embryos is an important concern in a successful vitrification protocol. Many studies have shown that morphological and morphometric parameters of the embryos can be considered as biomarkers of embryonic quality. Most of the present grading systems based on major morphological characteristics include embryonic fragmentation and blastomere uniformity (Hnide et al., 2004 ▶). The presence of thinner parts in zona pellucida can be beneficial to blastocyst expanding and hatching. Many studies have shown that ZPT and ZPTV could be beneficial supplementary characteristics in evaluation of embryos, in addition to the morphological grading system (Sun et al., 2005 ▶). Investigation of fragmentation is considered in all embryo grading systems and the existence of high amounts of fragmentation negatively relates to implantation and pregnancy rates (Ebneretal, 2001). The size of blastomeres may be considered as a biomarker of degree of fragmentation, as suggested by Hnida et al. (2004) ▶.
We could not find any research on morphological and morphometric changes occurring with embryos after vitrification. So, in this study, we aimed to examine probable effects of vitrification on early-cleavage mice embryos from morphological and morphometric points of view.
Materials and Methods
Animal selection
Female and male Balb/c mice (8-10 weeks old) were selected from animal laboratory of Shiraz University of Medical Sciences. They were kept in separate cages in the standard conditions.
Sperm preparation
Male mice were scarified by cervical dislocation and after dissection, epididymis was isolated and incubated by HamśF10 (Sigma, Germany) enriched with 5% bovine serum albumin (BSA) medium at 37°C for 1 h. Sperms released in medium were used for IVF.
Superovulation and oocyte preparation
Female mice were super-ovulated by injection of 10 IU PMSG: Pregnant Mare’s Serum Gonadotrophin (Folligon, Poland) followed 48 h later by 10 IU HCG (LG Life Sciences, Iran) intraperitoneally. Females were scarified by cervical dislocation 16 h after HCG injection. Oviducts were instantly transferred to 37°C prewarmed HamśF10 medium. Mature oocytes as cumulus-oocyte complexes in second metaphase of meiosis were separated by dissection of oviducts by two needles.
In vitro fertilization and development
Cumulus-oocyte complexes and sperms (n=106) were incubated for 6 h at 37°C and 5% CO2 concentration in HamśF10 enriched with 10% BSA drops in IVF dishes. Oocytes were observed for the existence of male and female pronuclei after 6-7 h and after 24 h post-incubation to the 2-cell stage and 48 h for 4-8-cell stages by an invert microscope (Zeiss, Germany). By using a camera (Canon, Japan) on the invert microscope, digital images were taken from these embryos (n=30) which were at different cleavage stages and considered as embryos before vitrification.
Vitrification
Mice embryos in the group of pre-vitrification were vitrified by a two-step procedure with the Dr. Alhassani protocol and using the cryoloop as carrier, as described by Lane et al. (1999). Embryos were first equilibrated in equilibration solution (ES) containing 7.5% ethylene glycol (EG) and 7.5% dimethylsulfoxide (DMSO) at room temperature for 7 min, and subsequently transferred to vitrification solution (VS) containing 15% EG, 15% DMSO and 0.5 mol/L sucrose at room temperature for 60 s. Then, any embryo with minimal VS was loaded onto the loop of the cryoloop carrier. The cryoloop was plunged vertically into liquid nitrogen vapor, sealed with protective straw-cap and then stored within liquid nitrogen tank.
Warming
After cryo-storage for 12-15 days, the embryos were warmed by using a four-step protocol with sucrose. Cryoloops consisting of the embryos were removed from the protective straw-cap and placed into warming solution (WS) consisting of 1 mol/L sucrose drops at 37°C for 1 min. Then embryos were transferred to diluent solution (DS) consisting of 0.5 mol/L sucrose drops for 3 min. In step 3, embryos were transferred into 0.25 mol/L sucrose drops for 3 min. Then washing was done with HamśF10 5 to 10 times. After warming, digital images were instantly taken from embryos and considered as the group of embryos after vitrification.
Morphological and morphometrical analysis
Images of embryos both before and after vitrification procedure were morphologically scored by using the Rezazadeh scoring system (2002) ▶ and each embryo was qualitatively classified into one of the six grades according to size of blastomeres and embryonic fragmentation. Embryos in grade I were those with equal-sized and regular blastomeres. Grade II consisted of embryos with lightly unequal-sized and irregular blastomeres. Grade III consisted of embryos with fragmentation less than 10%. Grade IV to VI consisted of embryos with more embryonic fragmentation. Grade I and II were high grade embryos and grade III to VI were low grade embryos. Zona pellucida thickness (ZPT), zona pellucida thickness variation (ZPTV), diameter and volume of blastomeres and embryonic cell mass were considered as morphometric parameters and measured by Stereo-Lite software (at Morphometry and Stereology Research Center of Shiraz University of Medical Sciences).
Measurement of ZPT and ZPTV
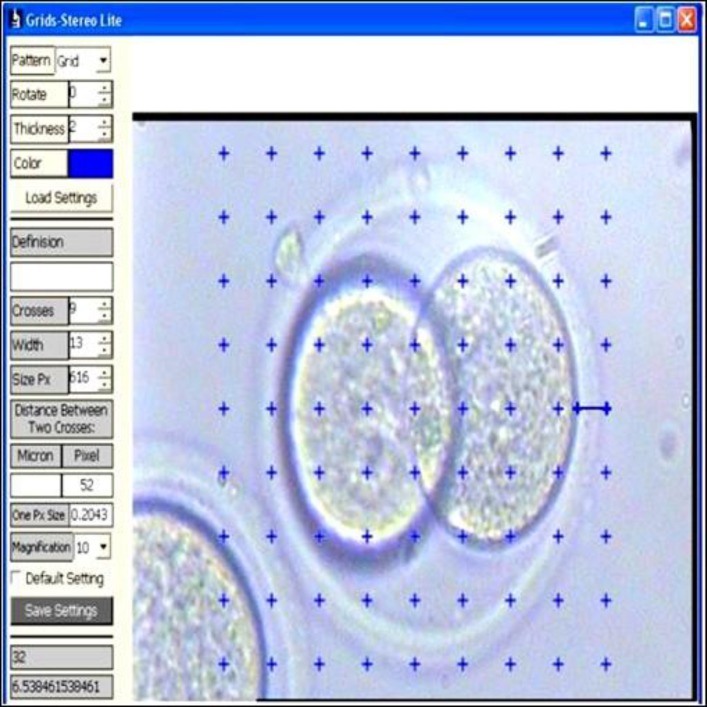
For calculating these parameters, all embryos considered as a clock-field and thickness of zona was recorded at four distinct points (Fig. 1). The maximum of the four ZPT values was considered as ZPT max. The value of mean ZPT and the value of ZPTV were calculated by means of:
Fig. 1.
Measuring zona thickness at 3:00 O’clock
ZPT mean = (ZPT1 + ZPT2 + ZPT3 + ZPT4)/4
ZPTV = [(ZPT max – ZPT mean)/ZPT mean] × 100 (Sun et al., 2005)
Measurement of diameter and volume of blastomeres and embryos
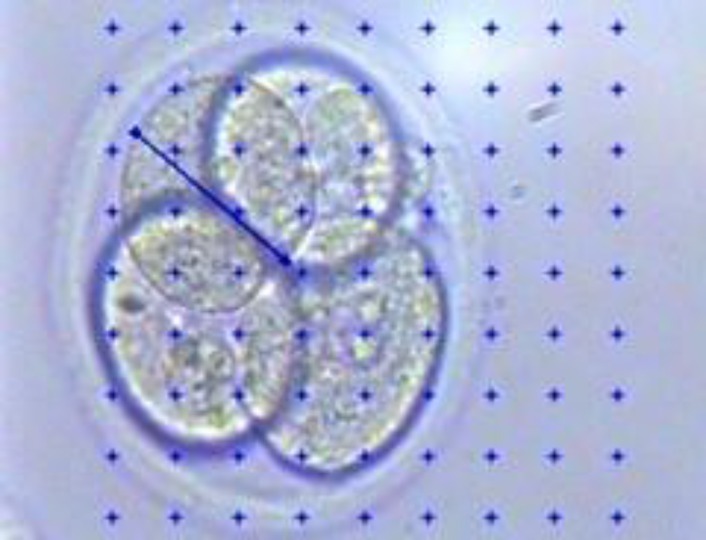
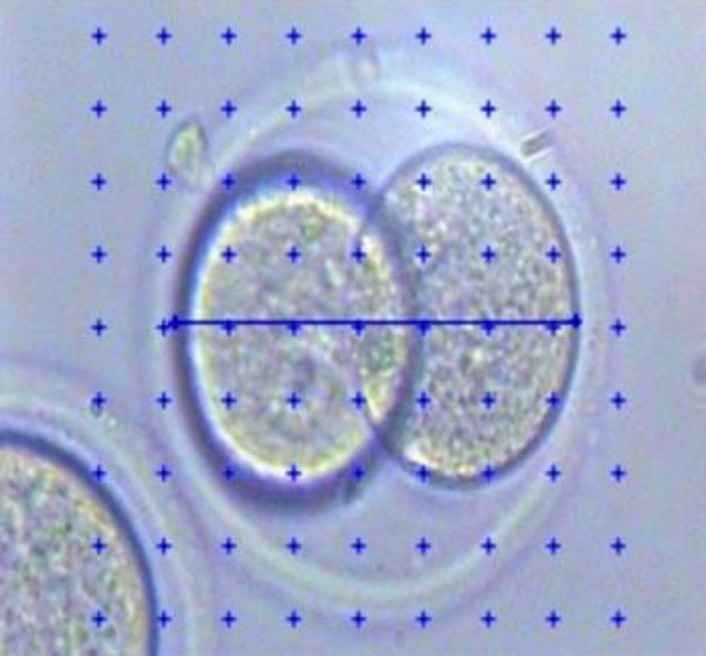
Two blastomeres were randomly considered in each embryo and four diameters of each blastomere were calculated by Stereo-Lite software (Fig. 2) and the mean diameter between two blastomeres was considered as the diameter of blastomere in each embryo. Volume of blastomeres was calculated by sphere volume formula (4/3 πr3). The biggest embryonic cell mass diameter was calculated by the mentioned software, too (Fig. 3) and the theoretical volume of the embryo was calculated by using the sphere volume formula.
Fig. 2.
Measuring diameter of blastomere
Fig. 3.
Measuring the biggest diameter of embryonic cell mass
Statistical analysis
The results were analyzed by paired sample t-test and Mann-Witney u, using the SPSS ver. 17/0 software. The means were considered significantly different at P≤0.05.
Results
Our results show that there is a significant difference between ZPT before and after vitrification as shown in Table 1 but there was not any difference in ZPTV and quality of embryos before and after vitrification (Table 1). Significant differences in diameter and volume of blastomeres and embryonic cell mass were observed before and after vitrification (Table 1).
Table 1.
Comparison of embryos parameters before and after vitrification
| Parameters of embryos | Pre-vitrification (Mean±SD) |
Post-vitrification (Mean±SD) |
|---|---|---|
| Grade | 1.86 ± 0.86 | 1.97 ± 0.80 |
| ZPT (μm) | 6.53 ± 0.46 | 6.90 ± 0.44٭ |
| ZPTV (%) | 11.43 ± 6.94 | 9.95 ± 3.75 |
| Blastomere diameter (μm) | 42.57 ± 4.28 | 36.41 ± 4.95٭ |
| Blastomere volume (μm3) | 4.60 ± 0.12 | 4.41 ± 0.18٭ |
| The biggest embryo diameter (μm) | 75.69 ± 6.21 | 71.43 ± 4.68٭ |
| Embryo volume (μm3) | 5.35 ± 0.11 | 5.27 ± 0.08٭ |
Shows statistically significant difference at (P≤0.05). Mean±SD, and n=30
Discussion
Improvement of pregnancy rates in IVF laboratories depends on selection of good quality embryos for transferring to the uterus.
Our study supports the zona change hypotheses and shows that the mean ZPT in frozen-thawed embryos increased significantly compared with that of those before cryopreservation. But the mean of ZPTV in frozen-thawed embryos compared with that of those before cryopreservation, despite reduction, did not show significant changes.
The significant increase in the mean of ZP in this study, may be due to dimethyl sulphoxide (DMSO). DMSO is used as a cryoprotectant in most cryo-preservation procedures and have been found to result in ZP hardening (Nayernia et al., 2002 ▶). Nagy et al. (2005) ▶ reported that freeze-thaw procedure result in some common cryodamages on embryo such as ZP hardening, and ZP thickening, causing failure of embryo to hatch (Gabrielsen et al., 2001 ▶).
Many reports suggested that embryo transferred with thinner zona had a higher chance for successful implantation and pregnancy than those with thicker zona (Gabrielsen et al., 2000 ▶). Cohen et al. (1988) ▶ have realized ZPTV as a reliable indicator for choosing thawed as well as fresh human embryos to transfer. Gabrielsen et al. (2000) ▶ found that embryos with a higher ZPTV had better chance for implantation compared with those with lower ZPTV.
In the present study, the mean diameter and volume of embryonic cell mass and blastomeres significantly reduced in frozen-thawed embryos compared with unfrozen embryos. Hnida et al. (2004) ▶ suggested that the degree of fragmentation can significantly affect the blastomere size. Their findings showed that the degree of fragmentation significantly increased with reduction in the mean blastomere volume. The definition of fragments is anucleate structures of embryonic origin and they may extract some major structures of cytoplasm such as cell organells, mRNA or proteins (Johansson et al., 2003). But the significant reduction of blastomere size in frozen-thawed embryos in present study may not reflect the increase of degree of fragmentation. Since fragmentation degree was considered in morphological scoring system and did not show significant change in frozen-thawed embryos compared with those before cryopreservation. So, this reduction did not reflect ultra-structural damages certainly and it may only be shrinkage due to osmotic damage followed by cryopreservation phenomenon.
In our study, the cryopreservation harmful effects on embryo morphology based on ordinary scoring system were so little and can be ignored. Cohen et al. (1988) ▶ showed that frozen-thawed cleaved embryos only implanted when they had intact and equal size blastomeres and those with abnormal morphologic features did not implant. Thus we can conclude that this vitrification protocol does not induce any damages to morphological features of embryos.
In general, vitrification method does not seem to induce severe damages to embryos and decrease good quality embryos for transferring to uterus, but more studies for establishing a protocol with minimal side effect should be conducted.
Acknowledgements
We would like to acknowledge Morphometery and Stereology Research Center and Anatomical Department of Shiraz University of Medical Sciences.
References
- Cohen J, Wiemer KE, Wright G. Prognostic value of morphologic characteristic of cryopreserved embryos: a study using videocinematography. J. Fertil. Steril. 1988;49:827–834. doi: 10.1016/s0015-0282(16)59892-6. [DOI] [PubMed] [Google Scholar]
- Gabrielsen A, Bhatnager PR, Petersen K, Lindenberg S. Influence of zona pellucid thickness of human embryos on clinical pregnancy outcome following in vitro fertilization treatment. J. Assist. Reprod. Genet. 2000;17:323–328. doi: 10.1023/A:1009453011321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen A, Lindenberg S, Petersen K. The impact of the zona pellucida thickness variation of human embryos on pregnancy outcome in relation to suboptimal embryo development A prospective randomized controlled study. J. Hum. Reprod. 2001;16:2166–2170. doi: 10.1093/humrep/16.10.2166. [DOI] [PubMed] [Google Scholar]
- Hnida C, Engenheiro E, Ziebe S. Computer-controlled, multilevel, morphometric analysis of blastomere size as biomarker of fragmentation and multinuclearity in human embryos. J. Hum. Reprod. 2004;19:288–293. doi: 10.1093/humrep/deh070. [DOI] [PubMed] [Google Scholar]
- Lane M, Schoolcraft WB, Gradner DK, Phil D. Vitrification of mouse and human blastocysts using a novel cryoloop container-less technique. J. Fertil. Steril. 1999;72:1073–1078. doi: 10.1016/s0015-0282(99)00418-5. [DOI] [PubMed] [Google Scholar]
- Liebermann J, Nawroth F, Isachenko V, Isachenco E, Rahimi G, Tucker MJ. Potential importance of vitrification in reproductive medicine. J. Biol. Reprod. 2002a;67:1671–1680. doi: 10.1095/biolreprod.102.006833. [DOI] [PubMed] [Google Scholar]
- Lin TK, Su JT, Lee FK, Lin YR, Lo HC. Cryotop vitrification as compared to conventional slow freezing for human embryos at the cleavage stage: survival and outcomes. J. Obstet. Gynecol. 2010;49:272–278. doi: 10.1016/S1028-4559(10)60060-5. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Taylor T, Elliott T, Massey J, Kort H, Shapiro D. Removal of lysed blastomeres from frozen-thawed embryos implantation and pregnancy rates in frozen embryo transfer cycles. J. Fertil. Steril. 2005;84:1606–1612. doi: 10.1016/j.fertnstert.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Nayernia K, Adham IM, Shamsadin R, Muller C, Sancken U, Engel W. Proacrosin-deficient mice and zona pellucida modifications in an experimental model of multifactorial infertility. J. Mol. Hum. Reprod. 2002;8:434–440. doi: 10.1093/molehr/8.5.434. [DOI] [PubMed] [Google Scholar]
- Rezazadeh Valojerdi M. Intracytoplasmic sperm injection. 1st Edn. Iran: Boshra; 2002. pp. 163–169. (in Persian) [Google Scholar]
- Sun YP, Xu Y, Coa T, Su YC, Guo YH. Zona pellucida thickness and clinical pregnancy outcome following in vitro fertilization. J. Obstet. Gynecol. 2005;89:258–262. doi: 10.1016/j.ijgo.2005.02.012. [DOI] [PubMed] [Google Scholar]