Abstract
Carpal chip fractures are common causes of lameness in racehorses. Due to disadvantages in surgical management, adjuvant treatment modalities are usually necessary. Adipose-derived stem cells (ADSCs) have the potential to differentiate into other cell types including bone and cartilage cells. Adipose-derived stromal vascular fraction (SVF) is produced during ADSCs isolation from adipose tissue. The purpose of this report was to present the successful management of a grade III chip fracture in the right carpus of a 5-year-old Thoroughbred gelding by intra-articularly injected autologous SVF one month after the arthroscopic removal of the fracture. This treatment resulted in lameness improvement and short rehabilitation period to previous racing activities. High performance levels and no recurrent injuries were recorded during a twenty month follow-up period.
Key Words: Adipose-derived stromal vascular fraction, Articular cartilage, Carpal chip fracture, Horse, Osteoarthritis
Introduction
Carpal chip fractures, a common cause of poor performance in racehorses, are presented with synovial effusion and various degrees of lameness. Pain is considered to be associated with their close attachment to synovial membrane, the release of debris into the joint cavity and injuries of the apposing articular surface. The pathogenesis of chip fractures can be summarized in the following mechanisms:
i) Sclerosis of subchondral bone due to biomechanical stress during training or racing that leads to ischemic necrosis and fragmentation of the original tissue
ii) Fracturing of periarticular osteophytes formed in case of osteoarthritis (McIlWraith and Bramlage, 1996 ▶)
Chip fractures can be classified by arthroscopy into the following grades according to McIlWraith’s criteria for articular damage:
Grade Ι: Characterized by minimal articular cartilage fibrillation or fragmentation at the edge of the defect left by the fragment, extending no more than 5 mm from the fracture line
Grade ΙΙ: Characterized by articular cartilage degeneration over 5 mm from the defect and including up to 30% of the articular surface
Grade ΙIΙ: Characterized by loss of 50% of the articular cartilage
Grade ΙV: Characterized by severe bone loss associated with the fracture (McIlwraith et al., 1987 ▶)
Arhtroscopic removal of chip fractures has been indicated in order to alleviate clinical signs and prevent development of osteoarthritis (McIlwraith et al., 1991 ▶). Although arthroscopic surgical techniques have been well developed, they have limitations and thus the use of autologous biological mediators that improve tissue regeneration has recently been under investigation in both human and veterinary medicine (Monteiro et al., 2015 ▶). Adipose-derived stem cells (ADSCs), among other mesenchymal stem cells, have the potential to self-renew and differentiate in other cell types including bone and cartilage cells (Erickson et al., 2002 ▶; Zheng et al., 2006 ▶; Mehlhorn et al., 2009 ▶). Adipose-derived stromal vascular fraction (SVF) is produced during the isolation of ADSCs from adipose tissue (Gimble et al., 2007 ▶; Zuk, 2013 ▶) and has been suggested as a promising alternative for cartilage regeneration (Jurgens et al., 2009 ▶).
The purpose of this report was to present the successful clinical outcome of a grade III carpal chip fracture in a racehorse managed by intra-articular injection of autologous adipose-derived SVF that was performed one month after the arthroscopic removal of the fracture.
Case presentation
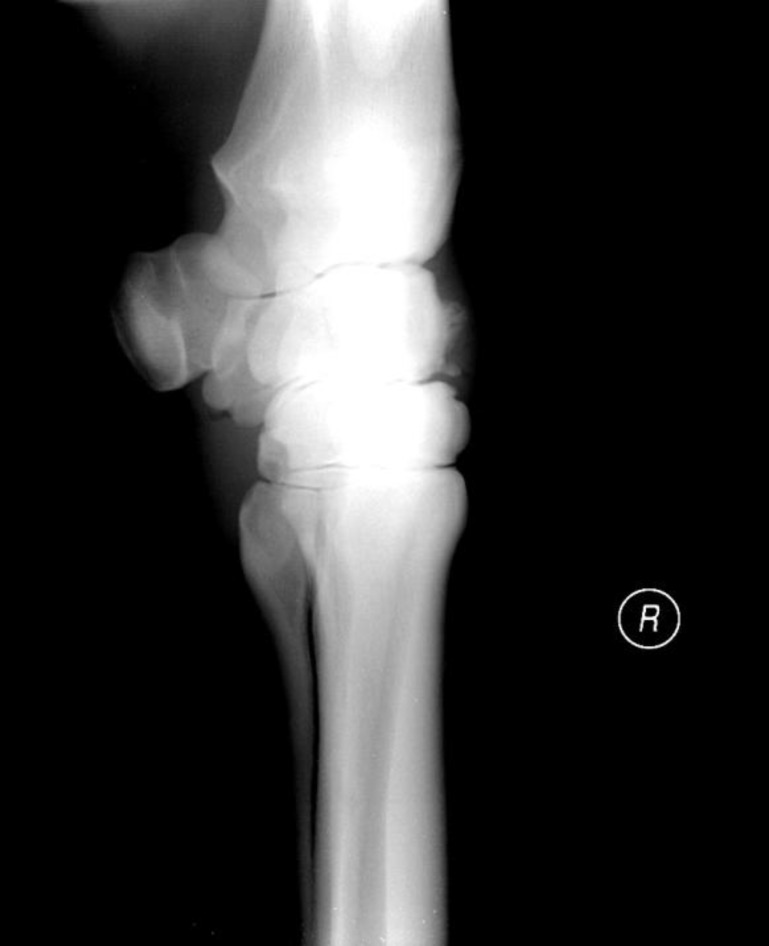
A 5-year-old Thoroughbred gelding in training was presented with severe 2-month lameness of the right forelimb, grade 3 according to the American Association of Equine Practitioners (AAEP) grading system (Anonymous, 1991 ▶). It had undergone arthroscopic removal of a chip fracture from the right intercarpal joint 6 months prior to presentation. Clinical examination revealed a slightly distended right intercarpal joint and a positive carpal flexion test. A thorough radiographic examination revealed a chip fracture in the distal ridge of the intermediate carpal bone and osteophytes formation (Fig. 1).
Fig. 1.
Lateromedial view of the right carpal joint in a racehorse. Note a chip fracture in the distal ridge of the intermediate carpal bone and osteophytes formation in the dorsal aspect of this bone
After an initial box rest and non-steroidal anti-inflammatory drugs (NSAIDs) therapy for 5 days, the horse underwent arthroscopic excision of the chip fracture in a routine manner (McIlwraith, 2005 ▶). The fracture was diagnosed during arthroscopy as grade III, whereas radiographic examination revealed its successful removal. NSAIDs and antibiotics were administered for 5 days post-operatively, the carpal region was bandaged and the horse was confined to box rest.
One month after the chip fracture removal, however, it was decided to enhance healing by injecting intra-articularly autologous adipose-derived SVF, because lameness evaluation revealed no clinical improvement. After harvesting of 20 g of adipose tissue from the region above the dorsal gluteal muscles, the isolation of adipose-derived SVF was performed based on standard techniques used in humans (Tzouvelekis et al., 2011 ▶). The adipose tissue, after mincing with a surgical blade and washing with phosphate-buffered saline solution (PBS), was treated in equal volume of PBS containing 100 U/ml type I collagenase (Biochrom, Berlin, Germany) for 1 h and under constant agitation. The digested tissue was then centrifuged at 300 g for 30 min in order to separate the SVF pellet, which was then re-suspended in PBS and washed twice. The final pellet was re-suspended in a mixture of 10% horse’s autologous serum, 10% dimethyl sulfoxide (DMSO) and 2% hydroxyethylstarch (Haes-Steril 200®) in PBS. The mean total of viable nucleated cells, counted using a Newbauer plate and trypan blue, was 20 × 106 cells. The cell suspension was placed in a cryovial, gradually cooled at a rate of 1°C per min up to -80°C and stored in liquid nitrogen until use. On the day of application, the cell suspension was rapidly defrozen at 40°C, washed with PBS and re-suspended in autologous horse serum, so as to obtain a total volume of 4 ml of SVF. The right carpal area was prepared aseptically and SVF was injected in the intercarpal joint. Thereafter, the horse continued to be confined in a box for another month, followed by increasing levels of hand-walking exercise. Lameness evaluation according to the AAEP grading system was performed every month until the horse was discharged.
Results
The horse returned to racing 4 months after the arthroscopic removal of the chip fracture, achieving excellent success rates. During the twenty month follow-up period, it sustained no injuries or recurrent lameness and maintained high performance levels.
Discussion
Current surgical strategies for treatment of articular cartilage injuries result, in most cases, in degenerative articular changes and pain, mainly because of the avascular nature of cartilage tissue and the low cell/matrix ratio, in conjunction with the poor self-repair capacity of mature chondrocytes (Cui et al., 2009 ▶). It is therefore obvious that treatment modalities adjuvant to surgery are most welcome.
In the last decades, regenerative medicine based on the use of growth factors and stem cell therapy has become increasingly popular. In most of the limited studies performed in humans or equines on articular cartilage defects and osteoarthritis, bone marrow mesenchymal stem cells were used, alone or in combination with other cellular therapy products, giving encouraging results (Monteiro et al., 2015 ▶). Adipose tissue has been considered an easily accessible source of an abundance of adult mesenchymal stem cells which can differentiate along multiple lineage pathways, especially osteogenic and chondrogenic (Erickson et al., 2002 ▶; Zheng et al., 2006 ▶; Mehlhorn et al., 2009 ▶; Sofa and Kuttapitiya, 2014 ▶). In animal models, studies evaluating the efficacy of ADSCs on cartilage regeneration have provided promising results (Dragoo et al., 2007 ▶; Cui et al., 2009 ▶; Veronesi et al., 2014 ▶). Adipose-derived SVF contains a significant number of cells that have characteristics of multipotent stem cells (Zuk, 2013 ▶). In the present case report, the use of adipose-derived SVF was chosen taking into consideration the practicability and safeness provided by the harvest technique, the easy preparation technique and the low cost.
In equine medicine, Frisbie et al. (2009) ▶ evaluated the effect of intra-articularly administered adipose-derived SVF or bone marrow-derived mesenchymal stem cells on a specific osteoarthritis (middle carpal joint) model in horses and reported that there were not significant enough findings to recommend stem cell therapy. On the contrary, Kol et al. (2012) ▶ found that adipose-derived SVF, among other examined autologous cellular therapy products, could be indicated for treating orthopaedic lesions in horses, as far as it contains mediators that have intrinsic healing function. A study on 591 horses that underwent arthroscopic removal of carpal chip fractures revealed return to racing with equal or better athletic performance post-operatively in the 71.1% of grade I, the 75.0% of grade II and the 53.2% of grade III injuries (McIlwraith et al., 1987 ▶). In our study, in which the horse had a grade III carpal chip fracture, the combination of surgical treatment and intra-articular injection of adipose-derived SVF resulted in an improved and permanent healing outcome compared to the single arthroscopic removal of the first chip fracture that relapsed six months post-surgery. The horse not only returned to its previous performance levels but also had no re-injuries during the follow-up period of 20 months. However, properly conducted clinical trials are warranted before claims implying the therapeutic efficacy of adipose-derived SVF (or ADSCs in general) can be made.
Conflict of interest
None of the authors of this paper have any financial or personal relationship with other people or organisations that might inappropriately influence or bias his/her work.
References
- Anonymous . Guide for veterinary service and judging of equestrian events: definition and classification of lameness. 4th Edn. Lexington, KY: American Association of Equine Practitioners (AAEP); 1991. 19 pp. [Google Scholar]
- Cui L, Wu Y, Cen L, Zhou H, Yin S, Liu G, Liu W, Cao Y. Repair of articular cartilage defect in non-weight bearing areas using adipose derived stem cells loaded polyglycolic acid mesh. Biomaterials. 2009;30:2683–2693. doi: 10.1016/j.biomaterials.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Dragoo JL, Carlson G, McCormick F, Khan-Farooqi H, Zhu M, Zuk PA, Benhaim P. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng. 2007;13:1615–1621. doi: 10.1089/ten.2006.0249. [DOI] [PubMed] [Google Scholar]
- Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2002;290:763–769. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J. Orthop. Res. 2009;27:1675–1680. doi: 10.1002/jor.20933. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Bunnell BA, Chiu ES, Guilak F. Adipose-derived stromal vascular fraction cells and stem cells: let’s not get lost in translation. Stem Cells. 2011;29:749–754. doi: 10.1002/stem.629. [DOI] [PubMed] [Google Scholar]
- Jurgens WJ, van Dijk A, Doulabi BZ, Niessen FB, Ritt MJ, van Milligen FJ, Helder MN. Freshly isolated stromal cells from the infrapatellar fat pad are suitable for a one-step surgical procedure to regenerate cartilage tissue. Cytotherapy. 2009;11:1052–1064. doi: 10.3109/14653240903219122. doi: 10.3109/ 14653240903219122. [DOI] [PubMed] [Google Scholar]
- Kol A, Walker NJ, Galuppo LD, Clark KC, Buerchler S, Bernanke A, Borjesson DL. Autologous point-of-care cellular therapies variably induce equine mesenchymal stem cell migration, proliferation and cytokine expression. Equine Vet. J. 2012 doi: 10.1111/j.2042-3306.2012.00600.x. doi: 10.1111/j.2042-3306.2012.00600.x. [DOI] [PubMed] [Google Scholar]
- McIlwraith CW. Diagnostic and surgical arthroscopy of the carpal joint. In: McIlWraith, CW, Nixon, AJ, Wright, IM, editors. Diagnostic and surgical arthroscopy in the horse. 3rd Edn. Edinburgh: Mosby Elsevier; 2005. pp. 347–365. [Google Scholar]
- McIlWraith CW, Bramlage LR. Surgical treatment of joint injury. In: McIlWraith, CW, Trotter, GW, editors. Joint disease in the horse. 1st Edn. Philadelphia: Saunders; 1996. pp. 292–316. [Google Scholar]
- McIlwraith CW, Foerner JJ, Davis M. Osteochondritis dissecans of the tarsocrural joint: results of treatment with arthroscopic surgery. Equine Vet. J. 1991;23:155–162. doi: 10.1111/j.2042-3306.1991.tb02746.x. [DOI] [PubMed] [Google Scholar]
- McIlwraith CW, Yovich JV, Martin GS. Arthroscopic surgery for the treatment of osteochondral chip fractures in the equine carpus. J. Am. Vet. Med. Assoc. 1987;191:531–540. [PubMed] [Google Scholar]
- Mehlhorn AT, Zwingmann J, Finkenzeller G, Niemeyer P, Danuer M, Stark B, Südkamp NP, Schmal H. Chondrogenesis of adipose-derived adult stem cells in a poly-lactide-co-glycolide scaffold. Tissue Eng.-Part A. 2009;191:1159–1167. doi: 10.1089/ten.tea.2008.0069. [DOI] [PubMed] [Google Scholar]
- Monteiro SO, Bettencourt EV, Lepage OM. Biologic strategies for intra-articular treatment and cartilage repair. J. Equine Vet. Sci. 2015;35:175–190. [Google Scholar]
- Sofa N, Kuttapitiya A. Future directions for the management of pain in osteoarthritis. Int. J. Clin. Rheumtol. 2014;9:197–276. doi: 10.2217/ijr.14.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzouvelekis A, Koliakos G, Ntolios P, Baira E, Bouros E, Oikonomou A, Zissimopoulos A, Kolios G, Kakagia D, Paspaliaris V, Kotsianidis I, Froudarakis M, Bouros D. Stem cell therapy for idiopathic pulmonary fibrosis: a protocol proposal. J. Transl. Med. 2011;9:182–190. doi: 10.1186/1479-5876-9-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronesi F, Maglio M, Tschon M, Aldini NN, Fini M. Adipose-derived mesenchymal stem cells for cartilage tissue engineering: state-of-the-art in in vivo studies (Review) J. Biomed. Mater. Res. A. 2014;102:2448–2466. doi: 10.1002/jbm.a.34896. [DOI] [PubMed] [Google Scholar]
- Zheng B, Cao B, Li G, Huard J. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chond-rogenic differentiation in vivo. Tissue Eng. 2006;12:1891–1901. doi: 10.1089/ten.2006.12.1891. [DOI] [PubMed] [Google Scholar]
- Zuk, P. Adipose-derived stem cells in tissue regeneration: a review. ISRN Stem Cells, Article ID 713959, 35 pages. 2013. http://dx.doi.org/10.1155/2013/713959.