Abstract
Objective
We aimed to summarize the pooled frequency of mediastinitis following open-heart surgery caused by Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), and Gram-negative bacteria.
Design
This study was a systematic review and a meta-analysis of prospective and retrospective cohort studies.
Materials and methods
We searched the literature, and a total of 97 cohort studies were identified. Random-effect model was used to synthesize the results. Heterogeneity between studies was examined by subgroup and meta-regression analyses, considering study and patient-level variables. Small-study effect was evaluated.
Results
Substantial heterogeneity was present. The estimated incidence of mediastinitis evaluated from 97 studies was 1.58% (95% confidence intervals [CI] 1.42, 1.75) and that of Gram-positive bacteria, Gram-negative bacteria, and MRSA bacteria evaluated from 63 studies was 0.90% (95% CI 0.81, 1.21), 0.24% (95% CI 0.18, 0.32), and 0.08% (95% CI 0.05, 0.12), respectively. A meta-regression pinpointed negative association between the frequency of mediastinitis and latitude of study place and positive association between the frequency of mediastinitis and the age of the patient at operation. Multivariate meta-regression showed that prospective cohort design and age of the patients and latitude of study place together or in combination accounted for 17% of heterogeneity for end point frequency of mediastinitis, 16.3% for Gram-positive bacteria, 14.7% for Gram-negative bacteria, and 23.3% for MRSA bacteria.
Conclusion
Evidence from this study suggests the importance of latitude of study place and advanced age as risk factors of mediastinitis. Latitude is a marker of thermally regulated bacterial virulence and other local surgical practice. There is concern of increasing risk of mediastinitis and of MRSA in elderly patients undergoing sternotomy.
Keywords: mediastinitis, sternotomy, meta-analysis
Introduction
The reported incidence of mediastinitis after coronary artery bypass grafting (CABG) is 0.4%–4%.1 Mediastinitis is associated with increased morbidity, mortality, and cost.
The etiology and pathophysiology of mediastinitis are complex and multifactorial. Also, mediastinitis carries an excess of long-term risk of mortality over 10 years of follow-up.1,2
The variability of incidence of mediastinitis in the literature is due to different study designs, population of patients, surgical cardiac procedures, and geographical area of the world with climate and latitude differences. We know that the latitude where the study took place is an important marker for variation in rainfall, humidity, temperature, and environmental mycobacteria that may alter the natural immunity and other factors. Latitude is associated with intensity and duration of sun exposure. Also, ambient temperature and latitude are strongly correlated.3 Previous studies have used latitude of study site as proxy for ambient temperature and environmental mycobacteria.4,5 We used the same method to assess the etiological role of ambient temperature and mycobacteria on the incidence and microbiology of Helicobacter pylori in a systematic review combined with meta-analysis of the literature.6 This systematic review considered worldwide studies of incidence and microbiology of mediastinitis after open-heart surgery.
Materials and methods
Literature search
A qualified medical librarian of Medical Library, Oslo University Hospital, was consulted. We searched the Cochrane Central Register of Controlled Trials (1970–2014), Medline/PubMed (1966–2014), and Embase (1980–2014) databases. We used a combination of keywords and details of the search procedure as shown in the Supplementary materials. We used similar strategies to identify previously published meta-analyses and reviews. In addition, we manually searched conference proceedings and textbooks, screened reference lists of all papers, and contacted investigators, experts, and translators. The last update for research was done on August 8, 2014. No limitation on language was considered (Figure 1).
Figure 1.
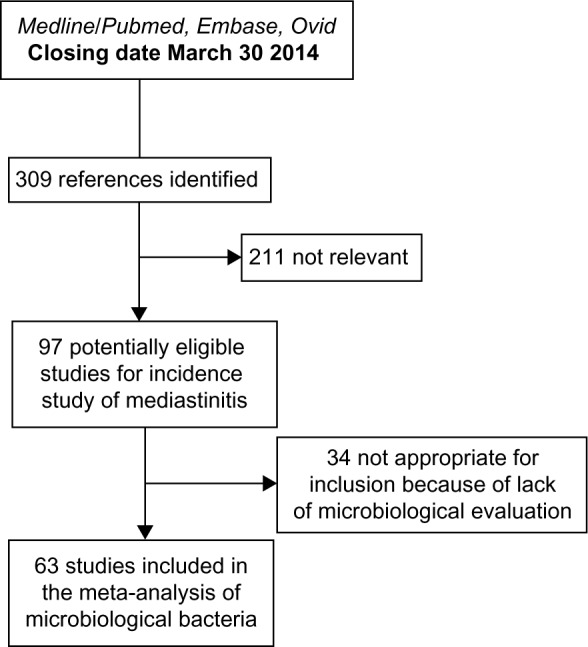
A flowchart of the literature searches for the systematic review of studies on the incidence of mediastinitis.
Study selection
We included prospective and retrospective studies on patients with open-heart surgery coronary artery bypass graft (CABG and valve replacement). Two reviewers independently evaluated reports for eligibility criteria defined as cohort of open-heart surgery patients operated in different centers of the world. Finally, 97 cohorts were considered eligible for the incidence study1,7–102 and 63 of them were considered for microbiological incidence of Gram-positive cocci and Gram-negative bacilli and fungi.
Quality assessment of the cohort studies: the component approach
Unfortunately, there is no agreed “gold standard” appraisal tool for evaluating quality in observational epidemiologic studies, and there are many tools from which to choose. For the purpose of critically appraising cohort studies, we used a checklist based on recommendations by Elwood,103 including: a) methods for selecting study participant; b) directionality of the cohort, prospective versus retrospective; and c) enough power to catch the true incidence. The poor reporting on important methodological details may be due to the fact that most papers were published before the STROBE statements.104 Sanderson et al105 evaluated a large number of tools for assessing quality of observational epidemiological studies, recommending a simple checklist, including a small number of key domains being specific to the study design under consideration rather than a scale. Scales resulting in numerical summary of quality scores might look simple to use, but such an approach can introduce a bias when assessing study quality.106
Data abstraction
Data regarding publication status, study design, patient-related characteristics, outcome methods, and results and funding were extracted in duplicate on a standardized form according to an a priori protocol. Authors were contacted for additional data and corrections. Disagreements were resolved by discussion with a third reviewer and subsequent consensus. Patient-related variables were considered in the protocol and included mean age of the cohort, frequency of female sex, and frequency of CABG surgery. The study-level variables included the nature of the cohort, prospective versus retrospective; latitude and longitude of the place of the study, year of publication, and the presence of adequate power. The adequate power was based on the total incidence of deep sternal wound infection (DSWI).
Of the 97 studies, the observed median incidence was 1.5% and the lower and higher quartile were 0.70% and 2.3%, respectively. Considering the median of 1.5% and accepting a precision of 0.8% (half the interquartile range), we will need a cohort of at least 887 patients to have a 95% probability to catch this incidence.
End points considered
The primary end point was the incidence of mediastinitis infection. The diagnosis of post sternotomy mediastinitis was based on the criteria established by the Centres for Disease Control and Prevention. We know that according to Centres for Disease Control and Prevention, surgical site infections can be classified into three categories: superficial incisional sternum superficial infections (SSIs; involving only skin and subcutaneous tissues), deep incisional SSIs (involving deep soft tissue), and organ/space SSIs (involving any part of the body other than the incision itself), which is opened or manipulated during the operative procedure. The definition of mediastinitis must meet at least one of the following criteria: 1) positive bacterial culture from the mediastinal space; 2) evidence of mediastinitis during surgery or histology; or 3) one of the following: fever (>38°C), chest pain, sternal instability, purulent discharge from the mediastinal area, organisms cultured from blood or from discharge of the mediastinal area, or mediastinal widening in radiology.
Unfortunately, in the literature, various terms for chest SSIs have been used, including sternal infection, deep sternal infection, and major infection. The inconsistent definitions could create misclassification of mediastinitis.
Secondary end points were the incidence of different microorganisms recovered in mediastinitis secondary to cardiovascular surgery. They included: 1) Gram-positive cocci (Staphylococcus aureus including methicillin- resistant S. aureus [MRSA], Staphylococcus epidermis, and Enterococcus spp); 2) Gram-negative bacilli (Esherichia coli, Enterobacter spp., Klebsiella spp., Proteus spp., and other Enterobacteri-aceae and Pseudomonas spp.); 3) fungi (Candida albicans and others); and 4) polymicrobial mediastinitis (mixed Gram-positive and Gram-negative organisms account for >10%). Other microorganisms were occasionally reported.
Quantitative data synthesis
Statistical pooling
Random-effects meta-analysis of single proportions was considered to estimate a cumulative incidence. The DerSimonian–Laird estimate107 is used in the random-effects model. To obtain summary measures, we used random-effects model due to the large heterogeneity of the results. To pool the incidence measures, logit transformation and sometimes logarithm transformation were initially performed to handle the distribution asymmetry. Sometimes, we considered the Freeman-Tukey double arcsine transformation of the proportions to calculate an overall pooled proportion and to stabilize the variance.108 These incidences were weighted by the inverse variance. When using the logit transformation, a continuity correction was applied if any studies had a zero cell count. By default, 0.5 is added to all cell frequencies of studies with a zero cell count. For individual studies, the Clopper-Pearson confidence interval was estimated.109
Sources of heterogeneity, evaluation, and quantification
Statistical heterogeneity among studies was assessed with Cochran’s Q test. The magnitude of heterogeneity was evaluated by the I2 statistics, which describes the proportion of total variation due to heterogeneity rather than chance.110 In order to investigate possible sources of heterogeneity, two different methods were used: subgroup analyses and meta-regression. We stratified our data according to the following study characteristics: nature of the cohort, prospective versus retrospective, and presence of a priori power estimation for the study.
A random-effects model was used to combine subgroups with separate estimate of τ2.110 After doing the subgroup analyses, we ran a random-effect meta-regression analysis, where the outcome variable was the transformated observed incidence from every study, indicating effect and study-level and patient-level characteristics (covariates). This random-effect regression model was done to enable exploration of the source of heterogeneity in the association between incidence of DWSI and covariates. The mixed model is an extension of the random-effect model. In this model, some heterogeneity is explained by moderators (covariates) and the rest by the random-effect term.
Publication bias or small-study effect
In order to assess potential publication bias or small-study effect, we used the funnel plot, which is a good visual evaluation of sampling bias or small-study effect.
Begg and Mazumdar rank correlation test
Begg and Mazumdar111 suggested a test statistics based on rank correlation between standardized outcome estimates and variance estimates of estimated outcome effect. Kendall’s tau is used as a correlation measure. The test statistics follows a standard normal distribution.
Egger’s test of asymmetry
Another test of asymmetry (Egger’s test)112 applied on the funnel plot was performed. The test statistics is based on a weighted linear regression of the treatment effect on its standard error. The test statistics follows a normal distribution with number of studies −2 as degrees of freedom. Nowadays, Egger’s test is mostly seen as a test of small-study effects.
Detection and correction for publication bias/small-study effect using the trim and fill method
Publication bias is a serious issue, as it may lead to biased estimates, which appears to be precise. A popular method for detecting and adjusting bias is the trim and fill method.113 When there is substantial heterogeneity, the trim and fill method is inappropriate to control for publication bias.114
Power considerations in meta-analysis and meta-regression
The test of funnel plot asymmetry and meta-regression analysis can be used only when at least ten studies are included in the meta-analysis because of power limitations. As underlined by Borenstein et al110 under the fixed model, the number of subjects in the studies and the effect size we need to pinpoint determine the power of the meta-analysis. Under the random-effect model, power is a function of not only the total number of subjects but also the number of studies, the effect size, and the variance between studies (heterogeneity).
For power consideration, we used a minimum of ten studies per covariate in a multivariate model of meta-regression. On the other hand, it is well known that aggregate variables (patient-related variables) are subject to ecological fallacy and must be considered only as hypothesis generating.
All statistical analyses were performed using the R package meta115 and Stata 13.0.116 We have followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for meta-analyses and systematic reviews of observational studies in the present report.117
Results
Trial flow
After identifying 309 references, 211 were excluded due to irrelevant content and duplicate publications, leaving 97 potentially eligible studies for incidence of mediastinitis. From these 97 eligible studies, 63 studies had microbiological evaluation. Finally, 97 studies were included in our systematic review of incidence and 63 for microbiological specific bacteria (Figure 1).
Study characteristics
The study characteristics considered were the place and latitude of the study, the year of publication, the mean age of the study population, frequency of female patients, frequency of isolated CABG, the prospective or retrospective nature of the cohort design, and the adequacy of power of the study.
End point total incidence of DSWI or mediastinitis
A total of 1,420,845 patients were included in the 97 studies, and the number of patients with DSWI or mediastinitis was 13,662. The mean age was 62.24 years (n=61 studies) with a range from 51 years to 67.9 years. The frequency of female patients was 28.2% with a range from 1% to 75% (n=68 studies). Mean frequency of CABG was 81.1% (n=61 studies) with a range from 36.6% to 100%. For the study-level characteristics, all the studies had a cohort design with 59 prospective and 48 retrospective. Of the total 97 (85.7%) studies, 84 were adequately powered.
End point bacteriology (incidence of microorganisms)
Only 63 of 97 studies have performed microbiological analysis and reported their results. Considering these studies, the number of patients included was 306,440 and the number of microorganisms (bacteria and fungi) was 3,724. The total number of Gram-positive cocci was n=2,958 (77.3%), including 343 MRSA microorganisms. Gram-negative bacilli represented 733 microorganisms, and the number of fungi infections was 33 (Table 1).
Table 1.
Incidence of microorganisms recovered in mediastinitis secondary to cardiothoracic surgery with frequency in 63 cohort studies
| Gram-positive cocci (n=2,958) |
| Staphylococcus aureus (n=1,397), including MRSA (n=343) |
| Staphylococcus epidermis (n=1057) |
| Enterococcus spp. (n=168) |
| Streptococcus spp. (n=24) |
| Gram-negative bacilli (n=733) |
| Esherichia coli (n=101) |
| Enterobacter spp. (n=142) |
| Klebsiella spp. (n=105) |
| Proteus spp. (n=19) |
| Other Enterobacteriaceae (n=52) |
| Pseudomonas spp. (n=117) |
| Fungi (n=33) |
| Other bacteria occasionally reported (n=509) |
Note: Total number of microorganisms (bacteria + fungi) is 3,724 in 306,440 patients.
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
Quantitative data synthesis
End point incidence of DSWI (mediastinitis) in 97 studies worldwide
The summarized pooled estimate incidence of DSWI in the 97 studies using the random-effect model was 0.0158 (95% CI 0.0142–0.0175) with a substantial heterogeneity (Tables 2 and 3).
Table 2.
Estimate of incidence of mediastinitis with stratification on prospective nature and power of the cohort and on latitude of the study place using the random-effect model
| Subdivision | n | Incidence (95% CI) | τ2 | I2 (%) | P-valuea |
|---|---|---|---|---|---|
| All studies | 97 | 0.0158 (0.0142–0.0175) | 0.0039 | 97.8 | |
| Prospective cohort design | |||||
| Yes | 49 | 0.02 (0.016–0.024) | 0.0075 | 97.3 | <0.0001 |
| No/Unclear | 48 | 0.012 (0.010–0.015) | 0.0019 | 95.7 | |
| Powered study | |||||
| Yes | 82 | 0.0146 (0.013–0.016) | 0.0036 | 98 | 0.021 |
| No | 15 | 0.0274 (0.0158–0.015) | 0.0225 | 93.1 | |
| Latitude over median 40.71 | |||||
| Yes | 49 | 0.0126 (0.0112–0.014) | 0.0016 | 96.4 | 0.0001 |
| No | 48 | 0.0199 (0.0162–0.024) | 0.009 | 97.7 | |
Note:
Test for subgroup differences using the random-effect model between groups with Q statistics, df =1, and P-value.
Abbreviations: CI, confidence interval; τ2, interstudy variability; df, degree of freedom.
Table 3.
Meta-regression between incidence of mediastinitis and the different study-level and patient-level variables in a univariate analysis
| Covariates | Level | β-coefficient | SE (β) | Z | P-value |
|---|---|---|---|---|---|
| Latitude | Degrees | −0.0020 | 0.0007 | −2.67 | 0.0075 |
| Age | Years | 0.0068 | 0.0029 | 2.35 | 0.018 |
| Female sex | Percent | 0.0003 | 0.0008 | 0.4367 | 0.6623 |
| CABG | Percent | 0.0006 | 0.005 | 1.20 | 0.2029 |
| Publication year | Year | 0.0010 | 0.0006 | 1.63 | 0.1023 |
Abbreviations: SE, standard error; CABG, coronary artery bypass grafting.
The funnel plot in Figure 2 visually showed evidence of bias and small-study effect. This was verified using Begg and Mazumdar rank correlation test and confirmed by the Egger’s test, which had a P-value of 0.001 accepting the null hypothesis of small-study effect. Because of substantial heterogeneity, we did not correct for selection bias considering the trim and fill simulation method.
Figure 2.
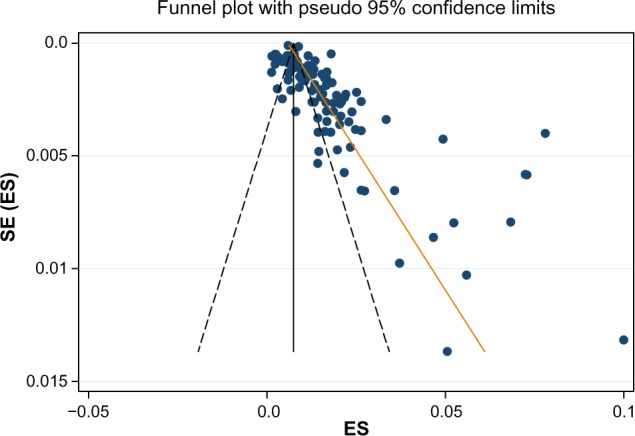
A funnel plot for the incidence of mediastinitis in 97 studies with Egger line.
Abbreviations: SE, standard error; ES, effect size.
The presence of heterogeneity led us to an investigation of its origin. A stratification analysis on the prospective versus retrospective nature of the cohorts, the adequacy of powered studies versus power-deficient studies, and the median latitude of the place of the studies showed a significant difference between subgroups. We extended the analyses with a meta-regression model taking into account moderator variables such as mean age, frequency of CABG in the total open-heart surgery, frequency of female patients, and latitude. One important study-level variable identified was latitude (Figure 3) with decreasing incidence of mediastinitis with increasing latitude. There was an increasing incidence of DSWI with the increasing age of patient at sternotomy (Figure 4).
Figure 3.
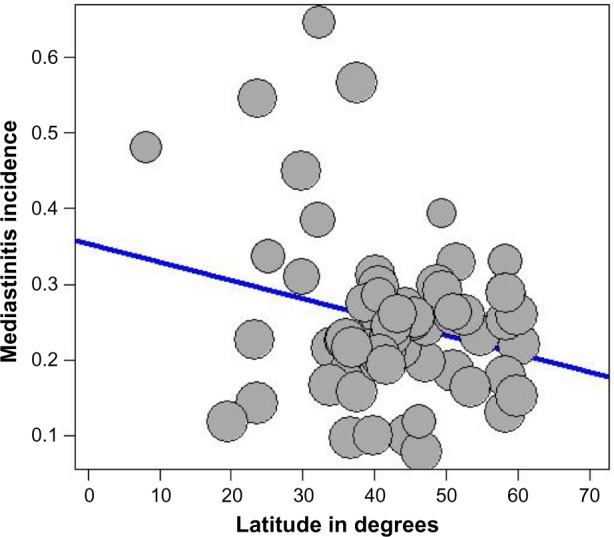
Meta-regression of incidence of mediastinitis and latitude of study place.
Note: Freeman-Tukey double arcsine transformated incidence.
Figure 4.
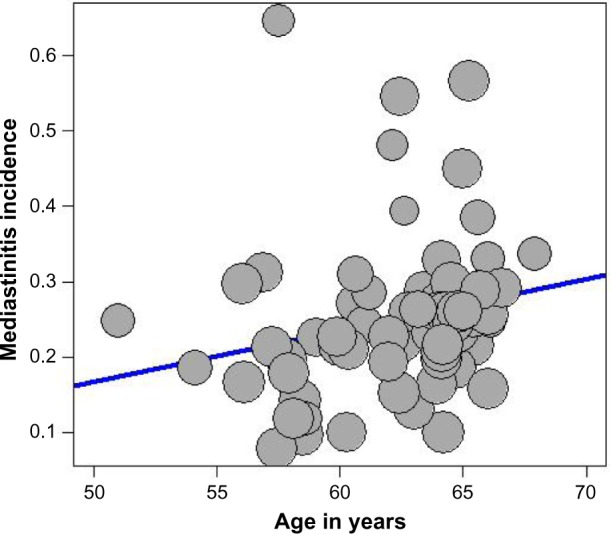
Meta-regression of incidence of mediastinitis and mean age of the patient.
Note: Freeman-Tukey double arcsine transformated incidence.
A multivariate meta-regression was performed, which considered study-level and patient-level variables together. This, using random- and mixed-effect models, identified the presence of prospective design, age of the patient, and the latitude of the study as risk factors of DSWI, with 17% of heterogeneity accounted for by these three variables together.
End points incidence of microorganisms recovered in mediastinitis
Gram-positive cocci
Tables 4 and 5 summarize the pooled estimate incidence of Gram-positive cocci in 63 studies using the random-effect model. The incidence was 0.009, 95% CI (0.0081–0.0121), with a substantial heterogeneity. The funnel plot visually showed evidence of bias and small-study effect. This was confirmed by the Begg and Mazumdar rank correlation test (P=0.018) and by the Egger’s test, which had a P-value of 0.006 accepting the null hypothesis of small-study effect or selection bias. Because of substantial heterogeneity, we did not correct for selection bias considering the trim and fill simulation method.
Table 4.
Estimate of incidence of Gram-positive cocci with stratification on prospective nature of the cohort and latitude of the study place using the random-effect model
| Subdivision | n | Incidence (95% CI) | τ2 | I2 (%) | P-valuea |
|---|---|---|---|---|---|
| All studies | 63 | 0.009 (0.0081–0.0121) | 0.6067 | 96.6 | |
| Prospective cohort design | |||||
| Yes | 33 | 0.012 (0.0095–0.0167) | 0.6219 | 96.6 | 0.0078 |
| No/Unclear | 30 | 0.007 (0.0062–0.0097) | 0.3392 | 93.4 | |
| Latitude over median 40° | |||||
| Yes | 32 | 0.009 (0.007–0.012) | 0.3894 | 95.7 | 0.8897 |
| No | 31 | 0.0101 (0.0071–0.0144) | 0.964 | 97.0 | |
Note:
Test for subgroup differences using the random-effect model between groups with Q statistics, df =1, and P-value.
Abbreviations: CI, confidence interval; τ2, interstudy variability.
Table 5.
Meta-regression model between incidence of Gram-positive cocci and the different study-level and patient-level variables in a univariate analysis using the 63 cohorts
| Covariates | Level | β-coefficient | SE (β) | Z | P-value |
|---|---|---|---|---|---|
| CABG | Percent | 0.0110 | 0.0058 | 1.89 | 0.057 |
| Age | Years | 0.0889 | 0.0429 | 2.07 | 0.038 |
| Latitude | Degrees | −0.0050 | 0.0084 | 0.596 | 0.5508 |
| Female sex | Yes/No | 0.0029 | 0.0085 | 0.342 | 0.7315 |
| Publication year | Year | 0.0012 | 0.0010 | 1.80 | 0.2378 |
Abbreviations: SE, standard error; CABG, coronary artery bypass grafting.
The presence of heterogeneity led us to an investigation of its origin. A stratification analysis on the prospective versus retrospective nature of the cohort and the median latitude of the place of the study showed a significant difference between subgroups. When we extended the analyses with a mixed-effect meta-regression model, one important patient-related variable was identified: age of the patient at sternotomy, with increasing incidence of Gram-positive cocci with increasing age. A multivariate meta-regression was performed, which considered study-level and patient-level variables together. The multivariate mixed-effect model identified age and the prospective nature of the cohort as risk factors of Gram-positive cocci with 16.3% of heterogeneity accounted for by these two variables together.
Methicillin-resistant S. aureus
Tables 6 and 7 summarize the pooled estimate incidence of MRSA in 63 studies using the random-effect model. The incidence was 0.0008, 95% CI (0.0005–0.0012), with a substantial heterogeneity. The funnel plot visually showed evidence of bias and small-study effect. This was confirmed by the Begg and Mazumdar rank correlation test with continuity correction (P=0.0007) and by the Egger’s test, which had a P-value of 0.0128 accepting the null hypothesis of small-study effect or selection bias. Because of substantial heterogeneity, we did not correct for selection bias considering the trim and fill simulation method.
Table 6.
Estimate of total incidence of MRSA with stratification on prospective nature of cohort and latitude of the study place using the random-effect model
| Subdivision | n | Incidence (95% CI) | τ2 | I2 (%) | P-valuea |
|---|---|---|---|---|---|
| All studies | 63 | 0.0008 (0.0005–0.0012) | 1.79 | 90.5 | |
| Prospective cohort design | |||||
| Yes | 33 | 0.0012 (0.0007–0.002) | 1.816 | 91.5 | 0.0417 |
| No/Unclear | 30 | 0.0005 (0.0004–0.0009) | 1.419 | 83.2 | |
| Latitude over median 40° | |||||
| Yes | 32 | 0.0006 (0.0003–0.0010) | 1.34 | 84 | 0.0853 |
| No | 31 | 0.0011 (0.0006–0.0021) | 1.90 | 84 | |
Note:
Test for subgroup differences using the random-effect model between groups with Q statistics, df =1, and P-value.
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; CI, confidence interval; τ2, interstudy variability; df, degree of freedom.
Table 7.
Meta-regression model between incidence of MRSA and the different study-level and patient-level variables in a univariate analysis using the 63 cohorts
| Covariates | Level | β-coefficient | SE (β) | Z | P-value |
|---|---|---|---|---|---|
| CABG | Percent | 0.007 | 0.005 | 1.32 | 0.1848 |
| Age | Years | 0.0079 | 0.0031 | 2.53 | 0.0112 |
| Latitude | Degrees | −0.0016 | 0.0006 | −2.53 | 0.0114 |
| Female sex | Yes/No | −0.0005 | 0.0007 | −0.78 | 0.4345 |
| Year of publication | Year | 0.0140 | 0.0227 | 0.61 | 0.5381 |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; SE, standard error; CABG, coronary artery bypass grafting.
The presence of heterogeneity led us to an investigation of its origin. A stratification analysis on the prospective versus retrospective nature of the cohort and the median latitude of the place of the study showed a significant difference between subgroups. When we extended the analyses with a mixed-effect meta-regression model, one important study-level variable was identified: the latitude of the place of the study, with decreasing incidence of MRSA with increasing latitude (Figure 5). Also, we identified increasing incidence of MRSA with increasing age of the patients (Figure 6). A multivariate meta-regression was performed, which considered study-level and patient-level variables. The multivariate using mixed-effect meta regression model identified latitude and age of the patient as independent risk factors of MRSA with 23.13% of heterogeneity accounted for by these two variables together.
Figure 5.
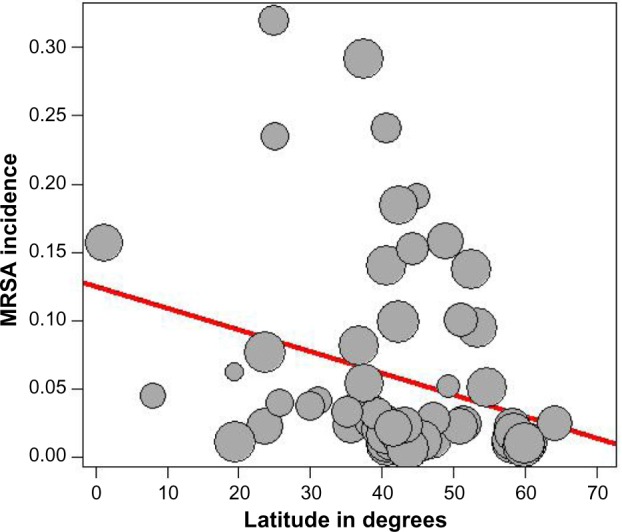
Meta-regression of incidence of MRSA and latitude of the study place.
Note: Freeman-Tukey double arcsine transformated incidence.
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
Figure 6.
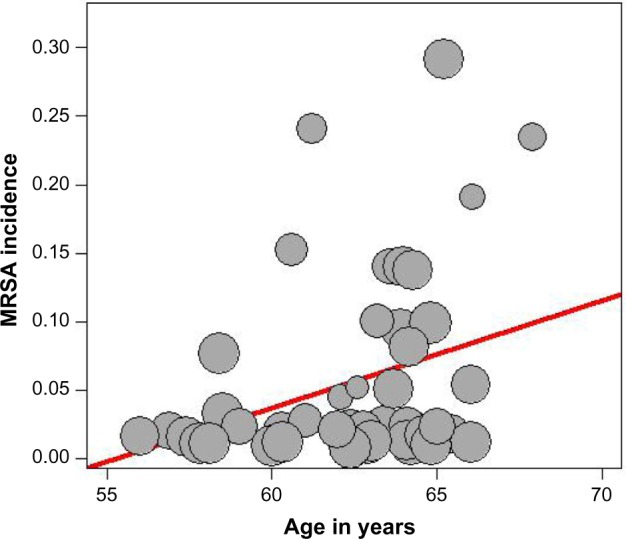
Meta-regression of incidence of MRSA and mean age of the patient.
Note: Freeman-Tukey double arcsine transformated incidence.
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
Gram-negative bacilli
Tables 8 and 9 summarize the pooled estimate incidence of Gram-negative bacilli in 63 studies using the random-effect model. The incidence was 0.0024, 95% CI (0.0018–0.032), with a substantial heterogeneity. The funnel plot visually showed evidence of bias and small-study effect. The Begg and Mazumdar rank correlation test confirmed this with continuity correction (P=0.037) and by the Egger’s test, which had a P-value of 0.00061 accepting the null hypothesis of small-study effect or selection bias. Because of substantial heterogeneity, we did not correct for selection bias considering the trim and fill simulation method.
Table 8.
Estimate of total incidence of Gram-negative bacilli with stratification on prospective nature of cohort and latitude of the study place using the random-effect model
| Subdivision | n | Incidence (95% CI) | τ2 | I2 (%) | P-valuea |
|---|---|---|---|---|---|
| All studies | 63 | 0.0024 (0.0018–0.0032) | 0.9819 | 91.8 | |
| Prospective cohort design | |||||
| Yes | 35 | 0.0058 (0.0053–0.0063) | 0.8591 | 91.9 | 0.0001 |
| No/Unclear | 30 | 0.0039 (0.0034–0.0043) | 1.2000 | 91.4 | |
| Latitude over median 40° | |||||
| Yes | 32 | 0.0018 (0.0012–0.0025) | 0.6549 | 87.4 | 0.008 |
| No | 31 | 0.0036 (0.0024–0.0054) | 1.046 | 92.4 | |
Note:
Test for subgroup differences using the random-effect model between groups with Q statistics, df =1, and P-value.
Abbreviations: CI, confidence interval; τ2, interstudy variability; df, degree of freedom.
Table 9.
Meta regression model between incidence of Gram-negative bacilli and the different study-level and patient-level variables in a univariate analysis using the 63 cohorts
| Covariates | Level | β-coefficient | SE (β) | Z | P-valuea |
|---|---|---|---|---|---|
| Latitude | Degrees | −0.0330 | 0.0108 | −3.07 | 0.002 |
| Age | Years | −0.0466 | 0.0585 | −0.79 | 0.4261 |
| Female sex | PCT | 0.0173 | 0.0126 | 1.36 | 0.1711 |
| CABG | PCT | 0.0067 | 0.0087 | 0.76 | 0.4423 |
| Publication year | Year | −0.0092 | 0.0138 | −0.66 | 0.5055 |
Note:
Test for subgroup differences using the random-effect model between groups with Q statistics, df =1, and P-value.
Abbreviations: SE, standard error; CABG, coronary artery bypass grafting; PCT, percentage; df, degree of freedom.
The presence of heterogeneity led us to an investigation of its origin. A stratification analysis on the prospective versus retrospective nature of the cohort and the median latitude of the place of the study showed a significant difference between subgroups. When we extended the analyses with a mixed-effect meta-regression model, one important study-level related variable was identified: the latitude of the place of the study, with decreasing incidence of Gram-negative bacilli with increasing latitude. A multivariate meta-regression was performed, which considered study-level variables also. The multivariate mixed-effect regression model identified latitude and the prospective nature of the cohort as risk factors of Gram-negative bacilli with 14.7% of heterogeneity accounted for by these two variables together.
Discussion
Our systematic review and meta-analysis of cohorts estimating incidence of DSWI and incidence of different microorganisms after sternotomy showed a high degree of heterogeneity among cohorts for all the outcomes. Also, publication bias or small-size effect was found to influence the outcomes. Meta-regression analyses indicated that heterogeneity could be explained by patient-related variables and study-level characteristics. For studies reporting on DSWI, the latitude of the study place and age of the patients were important determinants of incidence of this complication. Our results do not support the importance of CABG compared with valve replacement or sex of the patients as associated with incidence of DSWI. Determinants of Gram-positive cocci, including MRSA and Gram-negative bacilli microorganisms, are different. Also, latitude of the study place and age of the patients were determinants for the incidence of MRSA.
Strengths and limitations
Our review is based on a broad literature search, and it seems unlikely that we have missed relevant studies. Study selection and data extraction, including data assessment, were done by two authors to minimize bias and transcription errors.118 The major limitation of our study is the quality of cohort studies; it is clear that there is bias introduced by retrospective cohort design. The prospective cohorts showed higher incidence of mediastinitis and specific microorganisms. In the retrospective cohort studies, the accuracy of the parameters recorded and the quality of wound surveillance are therefore variable. The second problem is a misclassification of the end points DSWI, with some studies pooling DSWIs with infections at other sites, superficial sternal wound infections, or sternal and mechanical complications. Most importantly, all these studies are from different centers. It is likely that selection of patients, choice of procedures, and management of the perioperative period vary greatly among cardiac surgical units. These parameters, although difficult to record, may be important determinants of DSWI. Prospective multicentre studies would ascertain DSWI and identify high-risk patients and procedures and protect cohorts against attrition bias (dropout and loss to follow-up). Another limitation was the impossibility to control the small-study effect due to substantial heterogeneity. Finally, underpowered designed cohorts represented another limitation of our study.
Problem of heterogeneity due to patient-level and study-level variables on the incidence of mediastinitis and specific microorganisms
Latitude of the place of the study and temperature-regulated bacterial virulence
Latitude of the study site is a proxy and marker of rainfall, humidity, and environmental mycobacteria that may alter the natural immunity. Latitude and temperature are correlated.4
As it was underlined by Shapiro and Cowen119 for diverse microbial species including viruses, bacteria and fungi temperatures represent a critical environmental factor that is well established. It has been known for some time that temperature is a critical environmental trigger for many bacterial species.119
Also, temperature influences developmental transitions in the human fungal pathogen C. Albicans’s morphogenetic transition between yeast and filamentous growth and resistance to antifungal drugs.
Eber et al,120 with data from 132 US hospitals in the period 1999–2006, found that summer season and higher mean monthly outdoor temperature were associated with substantial increases of bloodstream infection. There were increases in monthly count of S. aureus including MRSA and other Gram-negative bacteria such as E. coli and Acinetobacter. This study focused on blood culture to ensure that blood culture reflects infection and not merely colonizing organisms and that the association is with monthly bacterial count and monthly mean temperature, total precipitation, and mean relative humidity. There are several potential explanations of frequency of bacterial bloodstream infections compared with outdoor temperature levels. First, relatively higher temperatures not >37°C may facilitate increased growth of bacteria in the environment, which may, in turn, increase colonization of humans.
Mechanisms of bacterial growth and optimal growth temperature vary by organism, which may explain the observed difference in association with outdoor temperature between organisms.
Investigators Mermel et al121 have observed peaks of community-associated MRSA during summer and autumn and its association with seasonal skin infections. It is well known that each infection agent has an optimal temperature for growth; most grow best at temperature close to 37°C, and too low or too high temperature will not promote growth. Sahoo et al122 in a time series analysis showed that a combination of weekly average maximum temperature above 33°C coinciding with weekly average relative humidity ranging between 55% and 78% is most favorable for occurrence of skin and soft tissue infections due to S. aureus and MRSA, and within these parameters, each unit increase in occurrence of MRSA was associated with increase in weekly average temperature of 1.7°C and weekly average relative humidity of 10%.
Another experimental work done by Madiraju et al123 challenging MRSA strains in exponential phase cultures by methicillin observed that growth and peptidoglycan synthesis were much more resistant at 30°C than 40°C.
Latitude of the study place as a marker of surgical and other local practices
The latitude of the place of the study is a marker of operating room practices in open-heart surgery. Surgical procedures as risk factors of mediastinitis were investigated extensively by Eklund et al.27 The risk of infection in the operating theater is dependent on a lot of factors. One of them the beneficial effect of a preoperative antiseptic shower for reducing the microbial colony count on the patient’s skin is still controversial on lowering the infection rate.124 Shaving the hair from the incision site, especially the night before surgery, is still controversial.72,125 A Cochrane collaboration review in 2011126 concluded that when it is necessary to remove hair, the existence evidence suggests that clippers are associated with fewer surgical site infections than razors.
In diabetic patients, during cardiopulmonary bypass, a peak core body temperature >37.9 C could increase the risk of mediastinitis postoperatively as underlined by Groom et al.127
The air contamination control in the operating room128 is important as contamination of the sternal wound was reduced by 46%, pinpointed by Verkkala et al.128
High incidence of glove perforation rates (26%–40%) was found after sternotomy.129,130
Also, an incidence of 36% of surgeons’ fingertips was contaminated by Gram-positive bacteria.131 High prevalence of skin recolonization at the end of surgery was highlighted.129
There is variability in regimens of antimicrobial prophylaxis throughout the world. Nowadays, the effectiveness of these regimens in reducing SSI is falling short of standard,132,133 and there is increasing incidence of multiresistant bacteria worldwide.
Elderly patient and increasing risk of mediastinitis and MRSA
Age of the patients was associated with increasing incidence of total mediastinitis and incidence of specifically Gram-positive bacteria, including MRSA. The elderly patients are a population of people who have a long history of using antibiotics in previous time and have acquired some resistance to these drugs. Suffering from a weakened immune system, the elderly patients are particularly susceptible to all infections, especially MRSA.
In a population study in overall Sweden,134 the incidence of MRSA per 100,000 inhabitants during the period of 2000–2003 was constant with ~5 cases/100,000 inhabitants of 1–59 years and suddenly increased to 10 cases/100,000 inhabitants between 60 and 79 years and to 20 cases/100,000 inhabitants >80 years. This study showed the same trend of increasing incidence by age as in our study on a population of open-heart operated patients.
Elderly patients have high frequency of infectious diseases such as influenza, pneumonia, and urinary tract infection with difficulties in performing early diagnosis because the usual symptoms such as fever and leukocytosis are missing and they are on a high risk of mortality. Little effectiveness of antibiotic treatment in the elderly patients has been shown. On the other hand, there is an alarming upsurge nowadays of antibiotic-resistant bacteria such as MRSA in the community and hospitals and old-people’s home institutions.
This strong association between MRSA and mortality among elderly patients is making preventive actions and politics mandatory and of paramount importance. Nowadays, MRSA is a serious threat to public health throughout the world and to the early and long-term outcome of open-heart surgery.
Conclusion
Evidence from this study suggests the importance of latitude of study place and advanced age as risk factors of mediastinitis. Latitude is a marker of thermally regulated bacterial virulence and variability of surgical and local practices in open-heart surgery in different places of the world. There is deep concern for the high incidence of mediastinitis and MRSA in the elderly patients undergoing sternotomy, and there is urgent need of preventive action.
Acknowledgments
We would like to thank our Medical Librarian, Marie Isachsen, for assisting in the literature search.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Risnes I, Abdelnoor M, Almdahl SM, Svennevig JL. Mediastinitis after coronary artery bypass grafting risk factors and long-term survival. Ann Thorac Surg. 2010;89(5):1502–1509. doi: 10.1016/j.athoracsur.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 2.Risnes I, Abdelnoor M, Veel T, Svennevig JL, Lundblad R, Rynning SE. Mediastinitis after coronary artery bypass grafting: the effect of vacuum-assisted closure versus traditional closed drainage on survival and re-infection rate. Int Wound J. 2014;11(2):177–182. doi: 10.1111/j.1742-481X.2012.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strahler AH, Strahler AN. Physical Geography: Science and Systems of the Human Environment. New York: Third Wiley; 2005. pp. 256–282. [Google Scholar]
- 4.Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271(9):698–702. [PubMed] [Google Scholar]
- 5.Cheng JY, Ko JS, Chen RY, Ng EM. Meta-regression analysis using latitude as moderator of paternal age related schizophrenia risk: high ambient temperature induced de novo mutations or is it related to the cold? Schizophr Res. 2008;99(1–3):71–76. doi: 10.1016/j.schres.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Sandven I, Abdelnoor M, Nesheim BI, Melby KK. Helicobacter pylori infection and hyperemesis gravidarum: a systematic review and meta-analysis of case control studies. Acta Obstet Gynecol Scand. 2009;88:1190–1200. doi: 10.3109/00016340903284927. [DOI] [PubMed] [Google Scholar]
- 7.Abboud CS, Wey SB, Baltar VT. Risk factors for mediastinitis after cardiac surgery. Ann Thorac Surg. 2004;77(2):676–683. doi: 10.1016/S0003-4975(03)01523-6. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed D, Cheema FH, Ahmed YI, et al. Incidence and predictors of infection in patients undergoing primary isolated coronary artery bypass grafting: a report from a tertiary care hospital in a developing country. J Cardiovasc Surg (Torino) 2011;52(1):99–104. [PubMed] [Google Scholar]
- 9.Alserius T, Anderson RE, Hammar N, Nordqvist T, Ivert T. Elevated glycosylated haemoglobin (HbA1c) is a risk marker in coronary artery bypass surgery. Scand Cardiovasc J. 2008;42(6):392–398. doi: 10.1080/14017430801942393. [DOI] [PubMed] [Google Scholar]
- 10.Antunes PE, Bernardo JE, Eugénio L, de Oliveira JF, Antunes MJ. Mediastinitis after aorto-coronary bypass surgery. Eur J Cardiothorac Surg. 1997;12(3):443–449. doi: 10.1016/s1010-7940(97)00179-6. [DOI] [PubMed] [Google Scholar]
- 11.Ariyaratnam P, Bland M, Loubani M. Risk factors and mortality associated with deep sternal wound infections following coronary bypass surgery with or without concomitant procedures in a UK population: a basis for a new risk model? Interact Cardiovasc Thorac Surg. 2010;11(5):543–546. doi: 10.1510/icvts.2010.237883. [DOI] [PubMed] [Google Scholar]
- 12.Sá MP, Figueira ES, Santos CA, et al. Validation of MagedanzSCORE as a predictor of mediastinitis after coronary artery bypass graft surgery. Rev Bras Cir Cardiovasc. 2011;26(3):386–392. doi: 10.5935/1678-9741.20110013. [DOI] [PubMed] [Google Scholar]
- 13.Baskett RJ, MacDougall CE, Ross DB. Is mediastinitis a preventable complication? A 10-year review. Ann Thorac Surg. 1999;67(2):462–465. doi: 10.1016/s0003-4975(98)01195-3. [DOI] [PubMed] [Google Scholar]
- 14.Bitkover CY, Gårdlund B. Mediastinitis after cardiovascular operations: a case-control study of risk factors. Ann Thorac Surg. 1998;65(1):36–40. doi: 10.1016/s0003-4975(97)01003-5. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard A, Hurni M, Ruchat P, Stumpe F, Fischer A, Sadeghi H. Incidence of deep and superficial sternal infection after open heart surgery. A ten years retrospective study from 1981 to 1991. Eur J Cardiothorac Surg. 1995;9(3):153–157. doi: 10.1016/s1010-7940(05)80064-8. [DOI] [PubMed] [Google Scholar]
- 16.Borger MA, Rao V, Weisel RD, et al. Deep sternal wound infection: risk factors and outcomes. Ann Thorac Surg. 1998;65(4):1050–1056. doi: 10.1016/s0003-4975(98)00063-0. [DOI] [PubMed] [Google Scholar]
- 17.Braxton JH, Marrin CA, McGrath PD, et al. 10-year follow-up of patients with and without mediastinitis. Semin Thorac Cardiovasc Surg. 2004;16(1):70–76. doi: 10.1053/j.semtcvs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Breyer RH, Mills SA, Hudspeth AS, Johnston FR, Cordell AR. A prospective study of sternal wound complications. Ann Thorac Surg. 1984;37(5):412–416. doi: 10.1016/s0003-4975(10)60767-9. [DOI] [PubMed] [Google Scholar]
- 19.Cayci C, Russo M, Cheema FH, et al. Risk analysis of deep sternal wound infections and their impact on long-term survival: a propensity analysis. Ann Plast Surg. 2008;61(3):294–301. doi: 10.1097/SAP.0b013e31815acb6a. [DOI] [PubMed] [Google Scholar]
- 20.Centofanti P, Savia F, La Torre M, et al. A prospective study of prevalence of 60-days postoperative wound infections after cardiac surgery. An updated risk factor analysis. J Cardiovasc Surg (Torino) 2007;48(5):641–646. [PubMed] [Google Scholar]
- 21.Culliford AT, Cunningham JN, Jr, Zeff RH, Isom OW, Teiko P, Spencer FC. Sternal and costochondral infections following open-heart surgery. A review of 2,594 cases. J Thorac Cardiovasc Surg. 1976;72(5):714–726. [PubMed] [Google Scholar]
- 22.Danzer D, Christenson JT, Kalangos A, Khatchatourian G, Bednarkiewicz M, Faidutti B. Impact of double internal thoracic artery grafts on long-term outcomes in coronary artery bypass grafting. Tex Heart Inst J. 2001;28(2):89–95. [PMC free article] [PubMed] [Google Scholar]
- 23.De Feo M, Renzulli A, Ismeno G, et al. Variables predicting adverse outcome in patients with deep sternal wound infection. Ann Thorac Surg. 2001;71(1):324–331. doi: 10.1016/s0003-4975(00)02137-8. [DOI] [PubMed] [Google Scholar]
- 24.De Moraes AA, Abboud CS, Chammas AZ, et al. Long term mortality of deep sternal wound infection after coronary artery bypass surgery. Rev Bras Cir Cardiovasc. 2012;27(3):377–382. doi: 10.5935/1678-9741.20120065. [DOI] [PubMed] [Google Scholar]
- 25.Demmy TL, Park SB, Liebler GA, et al. Recent experience with major sternal wound complications. Ann Thorac Surg. 1990;49(3):458–462. doi: 10.1016/0003-4975(90)90256-6. [DOI] [PubMed] [Google Scholar]
- 26.Diez C, Koch D, Kuss O, Silber RE, Friedrich I, Boergermann J. Risk factors for mediastinitis after cardiac surgery – a retrospective analysis of 1700 patients. J Cardiothorac Surg. 2007;2:23. doi: 10.1186/1749-8090-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eklund AM, Lyytikäinen O, Klemets P, et al. Mediastinitis after more than 10,000 cardiac surgical procedures. Ann Thorac Surg. 2006;82(5):1784–1789. doi: 10.1016/j.athoracsur.2006.05.097. [DOI] [PubMed] [Google Scholar]
- 28.Elenbaas TW, Soliman Hamad MA, Schönberger JP, Martens EJ, van Zundert AA, van Straten AH. Preoperative atrial fibrillation and elevated C-reactive protein levels as predictors of mediastinitis after coronary artery bypass grafting. Ann Thorac Surg. 2010;89(3):704–709. doi: 10.1016/j.athoracsur.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Engelman RM, Williams CD, Gouge TH, et al. Mediastinitis following open-heart surgery. Review of two years’ experience. Arch Surg. 1973;107(5):772–778. doi: 10.1001/archsurg.1973.01350230124022. [DOI] [PubMed] [Google Scholar]
- 30.Fakih MG, Sharma M, Khatib R, et al. Increase in the rate of sternal surgical site infection after coronary artery bypass graft: a marker of higher severity of illness. Infect Control Hosp Epidemiol. 2007;28(6):655–660. doi: 10.1086/518347. [DOI] [PubMed] [Google Scholar]
- 31.Farsky PS, Graner H, Duccini P, et al. Risk factors for sternal wound infections and application of the STS score in coronary artery bypass graft surgery. Rev Bras Cir Cardiovasc. 2011;26(4):624–629. doi: 10.5935/1678-9741.20110054. [DOI] [PubMed] [Google Scholar]
- 32.Filsoufi F, Rahmanian PB, Castillo JG, Mechanick JI, Sharma SK, Adams DH. Diabetes is not a risk factor for hospital mortality following contemporary coronary artery bypass grafting. Interact Cardiovasc Thorac Surg. 2007;6(6):753–758. doi: 10.1510/icvts.2007.158709. [DOI] [PubMed] [Google Scholar]
- 33.Fowler VG, Jr, O’Brien SM, Muhlbaier LH, Corey GR, Ferguson TB, Peterson ED. Clinical predictors of major infections after cardiac surgery. Circulation. 2005;112(9 Suppl):I358–I365. doi: 10.1161/CIRCULATIONAHA.104.525790. [DOI] [PubMed] [Google Scholar]
- 34.Garey KW, Kumar N, Dao T, Tam VH, Gentry LO. Risk factors for postoperative chest wound infections due to gram-negative bacteria in cardiac surgery patients. J Chemother. 2006;18(4):402–408. doi: 10.1179/joc.2006.18.4.402. [DOI] [PubMed] [Google Scholar]
- 35.Gaynes R, Marosok R, Mowry-Hanley J, et al. Mediastinitis following coronary artery bypass surgery: a 3-year review. J Infect Dis. 1991;163(1):117–121. doi: 10.1093/infdis/163.1.117. [DOI] [PubMed] [Google Scholar]
- 36.Ghotaslou R, Yagoubi AR, Khalili AA, Mahmodian R. Mediastinitis after cardiac surgery in Madani Heart Center, Tabriz, Iran. Jpn J Infect Dis. 2008;61(4):318–320. [PubMed] [Google Scholar]
- 37.Grmoljez PF, Barner HH, Willman VL, Kaiser GC. Major complications of median sternotomy. Am J Surg. 1975;130(6):679–681. doi: 10.1016/0002-9610(75)90419-5. [DOI] [PubMed] [Google Scholar]
- 38.Grossi EA, Culliford AT, Krieger KH, et al. A survey of 77 major infectious complications of median sternotomy: a review of 7,949 consecutive operative procedures. Ann Thorac Surg. 1985;40(3):214–223. doi: 10.1016/s0003-4975(10)60030-6. [DOI] [PubMed] [Google Scholar]
- 39.Grossi EA, Esposito R, Harris LJ, et al. Sternal wound infections and use of internal mammary artery grafts. J Thorac Cardiovasc Surg. 1991;102(3):342–346. discussion 346–347. [PubMed] [Google Scholar]
- 40.Gwely NDN, Khalaf SA, Abol Maaty RA. Deep median sternotomy wound infection after open heart surgery. Egypt J Surg. 2001;20(4):742–748. [Google Scholar]
- 41.Gårdlund B, Bitkover CY, Vaage J. Postoperative mediastinitis in cardiac surgery – microbiology and pathogenesis. Eur J Cardiothorac Surg. 2002;21(5):825–830. doi: 10.1016/s1010-7940(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 42.Harrington G, Russo P, Spelman D, et al. Surgical-site infection rates and risk factor analysis in coronary artery bypass graft surgery. Infect Control Hosp Epidemiol. 2004;25(6):472–476. doi: 10.1086/502424. [DOI] [PubMed] [Google Scholar]
- 43.Hassan M, Smith JM, Engel AM. Predictors and outcomes of sternal wound complications in patients after coronary artery bypass graft surgery. Am Surg. 2006;72(6):515–520. [PubMed] [Google Scholar]
- 44.Hazelrigg SR, Wellons HA, Jr, Schneider JA, Kolm P. Wound complications after median sternotomy. Relationship to internal mammary grafting. J Thorac Cardiovasc Surg. 1989;98(6):1096–1099. [PubMed] [Google Scholar]
- 45.Hosseinrezaei H, Rafiei H, Amiri M. Incidence and risk factors of sternal wound infection at site of incision after open-heart surgery. J Wound Care. 2012;21(8):408–411. doi: 10.12968/jowc.2012.21.8.408. [DOI] [PubMed] [Google Scholar]
- 46.Immer FF, Durrer M, Mühlemann KS, Erni D, Gahl B, Carrel TP. Deep sternal wound infection after cardiac surgery: modality of treatment and outcome. Ann Thorac Surg. 2005;80(3):957–961. doi: 10.1016/j.athoracsur.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 47.Ivert T, Lindblom D, Sahni J, Eldh J. Management of deep sternal wound infection after cardiac surgery–Hanuman syndrome. Scand J Thorac Cardiovasc Surg. 1991;25(2):111–117. doi: 10.3109/14017439109098094. [DOI] [PubMed] [Google Scholar]
- 48.Jakob HG, Borneff-Lipp M, Bach A, et al. The endogenous pathway is a major route for deep sternal wound infection. Eur J Cardiothorac Surg. 2000;17(2):154–160. doi: 10.1016/s1010-7940(00)00327-4. [DOI] [PubMed] [Google Scholar]
- 49.Jiménez-Martínez M, Argüero-Sánchez R, Pérez-Alvarez JJ, Mina-Castañeda P. Anterior mediastinitis as a complication of median sternotomy incisions: diagnostic and surgical considerations. Surgery. 1970;67(6):929–934. [PubMed] [Google Scholar]
- 50.Jonkers D, Elenbaas T, Terporten P, Nieman F, Stobberingh E. Prevalence of 90-days postoperative wound infections after cardiac surgery. Eur J Cardiothorac Surg. 2003;23(1):97–102. doi: 10.1016/s1010-7940(02)00662-0. [DOI] [PubMed] [Google Scholar]
- 51.Jurkiewicz MJ, Bostwick J, 3rd, Hester TR, Bishop JB, Craver J. Infected median sternotomy wound. Successful treatment by muscle flaps. Ann Surg. 1980;191(6):738–744. doi: 10.1097/00000658-198006000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khoo AK, Tan KC, Ong KK. Deep-seated infective mediastinitis in post-coronary artery bypass grafting patients. Ann Acad Med Singapore. 1994;23(6):832–837. [PubMed] [Google Scholar]
- 53.Kim J, Hammar N, Jakobsson K, Luepker RV, McGovern PG, Ivert T. Obesity and the risk of early and late mortality after coronary artery bypass graft surgery. Am Heart J. 2003;146(3):555–560. doi: 10.1016/S0002-8703(03)00185-6. [DOI] [PubMed] [Google Scholar]
- 54.Kohli M, Yuan L, Escobar M, et al. A risk index for sternal surgical wound infection after cardiovascular surgery. Infect Control Hosp Epidemiol. 2003;24(1):17–25. doi: 10.1086/502110. [DOI] [PubMed] [Google Scholar]
- 55.Kubota H, Miyata H, Motomura N, et al. Deep sternal wound infection after cardiac surgery. J Cardiothorac Surg. 2013;8:132. doi: 10.1186/1749-8090-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lepelletier D, Perron S, Bizouarn P, et al. Surgical-site infection after cardiac surgery: incidence, microbiology, and risk factors. Infect Control Hosp Epidemiol. 2005;26(5):466–472. doi: 10.1086/502569. [DOI] [PubMed] [Google Scholar]
- 57.Loop FD, Lytle BW, Cosgrove DM, et al. Maxwell Chamberlain memorial paper. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity, and cost of care. Ann Thorac Surg. 1990;49(2):179–186. doi: 10.1016/0003-4975(90)90136-t. discussion 186–187. [DOI] [PubMed] [Google Scholar]
- 58.Lu JC, Grayson AD, Jha P, Srinivasan AK, Fabri BM. Risk factors for sternal wound infection and mid-term survival following coronary artery bypass surgery. Eur J Cardiothorac Surg. 2003;23(6):943–949. doi: 10.1016/s1010-7940(03)00137-4. [DOI] [PubMed] [Google Scholar]
- 59.Macmanus Q, Okies JE. Mediastinal wound infection and aortocoronary graft patency. Am J Surg. 1976;132(5):558–561. doi: 10.1016/0002-9610(76)90340-8. [DOI] [PubMed] [Google Scholar]
- 60.Magedanz EH, Bodanese LC, Guaragna JC, et al. Risk score elaboration for mediastinitis after coronary artery bypass grafting. Rev Bras Cir Cardiovasc. 2010;25(2):154–159. doi: 10.1590/s0102-76382010000200005. [DOI] [PubMed] [Google Scholar]
- 61.Matros E, Aranki SF, Bayer LR, McGurk S, Neuwalder J, Orgill DP. Reduction in incidence of deep sternal wound infections: random or real? J Thorac Cardiovasc Surg. 2010;139(3):680–685. doi: 10.1016/j.jtcvs.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 62.Orita H, Shimanuki T, Fukasawa M, et al. A clinical study of postoperative infections following open-heart surgery: occurrence and microbiological findings in 782 cases. Surg Today. 1992;22(3):207–212. doi: 10.1007/BF00308824. [DOI] [PubMed] [Google Scholar]
- 63.Paul M, Raz A, Leibovici L, Madar H, Holinger R, Rubinovitch B. Sternal wound infection after coronary artery bypass graft surgery: validation of existing risk scores. J Thorac Cardiovasc Surg. 2007;133(2):397–403. doi: 10.1016/j.jtcvs.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 64.Milano CA, Kesler K, Archibald N, Sexton DJ, Jones RH. Mediastinitis after coronary artery bypass graft surgery. Risk factors and long-term survival. Circulation. 1995;92(8):2245–2251. doi: 10.1161/01.cir.92.8.2245. [DOI] [PubMed] [Google Scholar]
- 65.Muñoz P, Menasalvas A, Bernaldo de Quirós JC, Desco M, Vallejo JL, Bouza E. Postsurgical mediastinitis: a case-control study. Clin Infect Dis. 1997;25(5):1060–1064. doi: 10.1086/516068. [DOI] [PubMed] [Google Scholar]
- 66.Nagachinta T, Stephens M, Reitz B, Polk BF. Risk factors for surgical-wound infection following cardiac surgery. J Infect Dis. 1987;156(6):967–973. doi: 10.1093/infdis/156.6.967. [DOI] [PubMed] [Google Scholar]
- 67.Newman LS, Szczukowski LC, Bain RP, Perlino CA. Suppurative mediastinitis after open heart surgery. A case control study of risk factors. Chest. 1988;94(3):546–553. doi: 10.1378/chest.94.3.546. [DOI] [PubMed] [Google Scholar]
- 68.Noyez L, van Druten JA, Mulder J, Schroën AM, Skotnicki SH, Brouwer RM. Sternal wound complications after primary isolated myocardial revascularization: the importance of the post-operative variables. Eur J Cardiothorac Surg. 2001;19(4):471–476. doi: 10.1016/s1010-7940(01)00610-8. [DOI] [PubMed] [Google Scholar]
- 69.Ochsner JL, Mills NL, Woolverton WC. Disruption and infection of the median sternotomy incision. J Cardiovasc Surg (Torino) 1972;13(5):394–399. [PubMed] [Google Scholar]
- 70.Olsen MA, Lock-Buckley P, Hopkins D, Polish LB, Sundt TM, Fraser VJ. The risk factors for deep and superficial chest surgical-site infections after coronary artery bypass graft surgery are different. J Thorac Cardiovasc Surg. 2002;124(1):136–145. doi: 10.1067/mtc.2002.122306. [DOI] [PubMed] [Google Scholar]
- 71.Ottino G, De Paulis R, Pansini S, et al. Major sternal wound infection after open-heart surgery: a multivariate analysis of risk factors in 2,579 consecutive operative procedures. Ann Thorac Surg. 1987;44(2):173–179. doi: 10.1016/s0003-4975(10)62035-8. [DOI] [PubMed] [Google Scholar]
- 72.Parisian Mediastinitis Study Group Risk factors for deep sternal wound infection after sternotomy: a prospective, multicenter study. J Thorac Cardiovasc Surg. 1996;111(6):1200–1207. doi: 10.1016/s0022-5223(96)70222-2. [DOI] [PubMed] [Google Scholar]
- 73.Parissis H, Al-Alao B, Soo A, Orr D, Young V. Risk analysis and outcome of mediastinal wound and deep mediastinal wound infections with specific emphasis to omental transposition. J Cardiothorac Surg. 2011;6:111. doi: 10.1186/1749-8090-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Popovic B, Voillot D, Maureira P, et al. Bilateral internal mammary artery bypass grafting: long-term clinical benefits in a series of 1000 patients. Heart. 2013;99(12):854–859. doi: 10.1136/heartjnl-2012-303466. [DOI] [PubMed] [Google Scholar]
- 75.Prabhakar G, Haan CK, Peterson ED, Coombs LP, Cruzzavala JL, Murray GF. The risks of moderate and extreme obesity for coronary artery bypass grafting outcomes: a study from the Society of Thoracic Surgeons’ database. Ann Thorac Surg. 2002;74(4):1125–1130. doi: 10.1016/s0003-4975(02)03899-7. discussion 1130–1. [DOI] [PubMed] [Google Scholar]
- 76.Rahmanian PB, Adams DH, Castillo JG, Carpentier A, Filsoufi F. Predicting hospital mortality and analysis of long-term survival after major noncardiac complications in cardiac surgery patients. Ann Thorac Surg. 2010;90(4):1221–1229. doi: 10.1016/j.athoracsur.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 77.Careaga Reyna G, Aguirre Baca GG, Medina Concebida LE, Borrayo Sánchez G, Prado Villegas G, Argüero Sánchez R. Risk factors for mediastinitis and sternal dehiscence after cardiac surgery. Rev Esp Cardiol. 2006;59(2):130–135. [PubMed] [Google Scholar]
- 78.Ridderstolpe L, Gill H, Granfeldt H, Ahlfeldt H, Rutberg H. Superficial and deep sternal wound complications: incidence, risk factors and mortality. Eur J Cardiothorac Surg. 2001;20(6):1168–1175. doi: 10.1016/s1010-7940(01)00991-5. [DOI] [PubMed] [Google Scholar]
- 79.Russo PL, Bull A, Bennett N, et al. Infections after coronary artery bypass graft surgery in Victorian hospitals--VICNISS Hospital Acquired Infection Surveillance. Aust N Z J Public Health. 2005;29(3):244–248. doi: 10.1111/j.1467-842x.2005.tb00762.x. [DOI] [PubMed] [Google Scholar]
- 80.Sachithanandan A, Nanjaiah P, Nightingale P, et al. Deep sternal wound infection requiring revision surgery: impact on mid-term survival following cardiac surgery. Eur J Cardiothorac Surg. 2008;33(4):673–678. doi: 10.1016/j.ejcts.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 81.Sakamoto H, Fukuda I, Oosaka M, Nakata H. Risk factors and treatment of deep sternal wound infection after cardiac operation. Ann Thorac Cardiovasc Surg. 2003;9(4):226–232. [PubMed] [Google Scholar]
- 82.Salehi Omran A, Karimi A, Ahmadi SH, et al. Superficial and deep sternal wound infection after more than 9000 coronary artery bypass graft (CABG): incidence, risk factors and mortality. BMC Infect Dis. 2007;7:112. doi: 10.1186/1471-2334-7-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leung Wai Sang S, Chaturvedi R, Alam A, Samoukovic G, de Varennes B, Lachapelle K. Preoperative hospital length of stay as a modifiable risk factor for mediastinitis after cardiac surgery. J Cardio-thorac Surg. 2013;8:45. doi: 10.1186/1749-8090-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Serry C, Bleck PC, Javid H, et al. Sternal wound complications. Management and results. J Thorac Cardiovasc Surg. 1980;80(6):861–867. [PubMed] [Google Scholar]
- 85.Sharma M, Berriel-Cass D, Baran J., Jr Sternal surgical-site infection following coronary artery bypass graft: prevalence, microbiology, and complications during a 42-month period. Infect Control Hosp Epidemiol. 2004;25(6):468–471. doi: 10.1086/502423. [DOI] [PubMed] [Google Scholar]
- 86.Steingrimsson S, Gottfredsson M, Kristinsson KG, Gudbjartsson T. Deep sternal wound infections following open heart surgery in Iceland: a population-based study. Scand Cardiovasc J. 2008;42(3):208–213. doi: 10.1080/14017430801919557. [DOI] [PubMed] [Google Scholar]
- 87.Strecker T, Rösch J, Horch RE, Weyand M, Kneser U. Sternal wound infections following cardiac surgery: risk factor analysis and interdisciplinary treatment. Heart Surg Forum. 2007;10(5):E366–E371. doi: 10.1532/HSF98.20071079. [DOI] [PubMed] [Google Scholar]
- 88.Ståhle E, Tammelin A, Bergström R, Hambreus A, Nyström SO, Hansson HE. Sternal wound complications--incidence, microbiology and risk factors. Eur J Cardiothorac Surg. 1997;11(6):1146–1153. doi: 10.1016/s1010-7940(97)01210-4. [DOI] [PubMed] [Google Scholar]
- 89.Sutherland RD, Martinez HE, Guynes WA, Miller L. Postoperative chest wound infections in patients requiring coronary bypass. A controlled study evaluating prophylactic antibiotics. J Thorac Cardiovasc Surg. 1977;73(6):944–947. [PubMed] [Google Scholar]
- 90.Szabó Z, Håkanson E, Svedjeholm R. Early postoperative outcome and medium-term survival in 540 diabetic and 2239 nondiabetic patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2002;74(3):712–719. doi: 10.1016/s0003-4975(02)03778-5. [DOI] [PubMed] [Google Scholar]
- 91.Toumpoulis IK, Anagnostopoulos CE, Toumpoulis SK, De Rose JJ, Jr, Swistel DG. Risk factors for sepsis and endocarditis and long-term survival following coronary artery bypass grafting. World J Surg. 2005;29(5):621–627. doi: 10.1007/s00268-005-7756-6. discussion 627–628. [DOI] [PubMed] [Google Scholar]
- 92.Tang GH, Maganti M, Weisel RD, Borger MA. Prevention and management of deep sternal wound infection. Semin Thorac Cardiovasc Surg. 2004;16(1):62–69. doi: 10.1053/j.semtcvs.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 93.Thurer RJ, Bognolo D, Vargas A, Isch JH, Kaiser GA. The management of mediastinal infection following cardiac surgery. An experience utilizing continuous irrigation with povidone-iodine. J Thorac Cardiovasc Surg. 1974;68(6):962–968. No abstract available. [PubMed] [Google Scholar]
- 94.Tiveron MG, Fiorelli AI, Mota EM, et al. Preoperative risk factors for mediastinitis after cardiac surgery: analysis of 2768 patients. Rev Bras Cir Cardiovasc. 2012;27(2):203–210. doi: 10.5935/1678-9741.20120035. English, Portuguese. [DOI] [PubMed] [Google Scholar]
- 95.Toumpoulis IK, Anagnostopoulos CE, Derose JJ, Jr, Swistel DG. The impact of deep sternal wound infection on long-term survival after coronary artery bypass grafting. Chest. 2005;127(2):464–471. doi: 10.1378/chest.127.2.464. [DOI] [PubMed] [Google Scholar]
- 96.Trick WE, Scheckler WE, Tokars JI, et al. Modifiable risk factors associated with deep sternal site infection after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2000;119(1):108–114. doi: 10.1016/s0022-5223(00)70224-8. [DOI] [PubMed] [Google Scholar]
- 97.Upton A, Roberts SA, Milsom P, Morris AJ. Staphylococcal post-sternotomy mediastinitis: five year audit. ANZ J Surg. 2005;75(4):198–203. doi: 10.1111/j.1445-2197.2005.03371.x. [DOI] [PubMed] [Google Scholar]
- 98.Walkes JC, Earle N, Reardon MJ, et al. Outcomes in single versus bilateral internal thoracic artery grafting in coronary artery bypass surgery. Curr Opin Cardiol. 2002;17(6):598–601. doi: 10.1097/00001573-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 99.Wang FD, Chang CH. Risk factors of deep sternal wound infections in coronary artery bypass graft surgery. J Cardiovasc Surg (Torino) 2000;41(5):709–713. [PubMed] [Google Scholar]
- 100.Wilson SJ, Sexton DJ. Elevated preoperative fasting serum glucose levels increase the risk of postoperative mediastinitis in patients undergoing open heart surgery. Infect Control Hosp Epidemiol. 2003;24(10):776–778. doi: 10.1086/502133. [DOI] [PubMed] [Google Scholar]
- 101.Wouters R, Wellens F, Vanermen H, De Geest R, Degrieck I, De Meerleer F. Sternitis and mediastinitis after coronary artery bypass grafting. Analysis of risk factors. Tex Heart Inst J. 1994;21(3):183–188. [PMC free article] [PubMed] [Google Scholar]
- 102.Zacharias A, Habib RH. Factors predisposing to median sternotomy complications. Deep vs superficial infection. Chest. 1996;110(5):1173–1178. doi: 10.1378/chest.110.5.1173. [DOI] [PubMed] [Google Scholar]
- 103.Elwood M. Critical Appraisal of Epidemiological Studies and Clinical Trials. Oxford: Oxford University Press; 2007. [Google Scholar]
- 104.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 105.Sanderson S, Tatt ID, Higgins JPT. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36:666–676. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- 106.Greenland S, O’Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics. 2001;2:463–471. doi: 10.1093/biostatistics/2.4.463. [DOI] [PubMed] [Google Scholar]
- 107.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 108.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21:607–611. [Google Scholar]
- 109.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 110.Borenstein M, Hegges LV, Higgins JPT, Rothstein H. Introduction to Meta-Analysis. Third ed. Wiltshire, UK: John Wiley & Sons; 2011. [Google Scholar]
- 111.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 112.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 114.Terrin N, Schmidt C, Lau J, Olkin I. Adjusting publication bias in the presence of heterogeneity. Stat Med. 2003;22:2113–2126. doi: 10.1002/sim.1461. [DOI] [PubMed] [Google Scholar]
- 115.Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7(3):40–45. [Google Scholar]
- 116.StataCorp LP [homepage on the Internet] STATA Data Analysis and Statistical Software. College Station, TX: StataCorp LP; [Accessed March 12, 2016]. Available from: www.stata.com. [Google Scholar]
- 117.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 118.Egger M, Smith GD, Altman DG. Systematic Reviews in Health Care: Meta-Analysis in Context. Second ed. London: BMJ Publishing Group; 1995. [Google Scholar]
- 119.Shapiro RS, Cowen LE. Thermal control of microbial development and virulence: molecular mechanisms of microbial temperature sensing. MBio. 2012;2;3(5):e00238–12. doi: 10.1128/mBio.00238-12. Print 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eber MR, Shardell M, Schweizer ML, Laxminarayan R, Perencevich EN. Seasonal and temperature-associated increases in gram-negative bacterial bloodstream infections among hospitalized patients. PLoS One. 2011;6(9):e25298. doi: 10.1371/journal.pone.0025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mermel LA, Machan JT, Parenteau S. Seasonality of MRSA infections. PLoS One. 2011;6(3):e17925. doi: 10.1371/journal.pone.0017925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sahoo KC, Sahoo S, Marrone G, Pathak A, Lundborg CS, Tamhankar AJ. Climatic factors and community-associated methicillin-resistant Staphylococcus aureus skin and soft-tissue infections – a time-series analysis study. Int J Environ Res Public Health. 2014;11(9):8996–9007. doi: 10.3390/ijerph110908996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Madiraju MV, Brunner DP, Wilkinson BJ. Effects of temperature, NaCl, and methicillin on penicillin-binding proteins, growth, peptidoglycan synthesis, and autolysis in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1987;31(11):1727–1733. doi: 10.1128/aac.31.11.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ayliffe GA, Noy MF, Babb JR, Davies JG, Jackson J. A comparison of pre-operative bathing with chlorhexidine-detergent and non-medicated soap in the prevention of wound infection. J Hosp Infect. 1983;4:237–244. doi: 10.1016/0195-6701(83)90024-5. [DOI] [PubMed] [Google Scholar]
- 125.Woodhead K, Taylor EW, Bannister G, Chesworth T, Hoffman P, Humphreys H. Behaviours and rituals in the operating theatre. A report from the Hospital Infection Society Working Party on Infection Control in Operating Theatres. J Hosp Infect. 2002;51:241–255. doi: 10.1053/jhin.2002.1220. [DOI] [PubMed] [Google Scholar]
- 126.Tanner J, Norrie P, Melen K. Preoperative hair removal to reduce surgical site infection (review) Cochrane Database Syst Rev. 2011;11:CD004122. doi: 10.1002/14651858.CD004122.pub4. [DOI] [PubMed] [Google Scholar]
- 127.Groom RC, Rassias AJ, Cormack JE, et al. Northern New England Cardiovascular Disease Study Group. Highest core temperature during cardiopulmonary bypass and rate of mediastinitis. Perfusion. 2004;19:119–125. doi: 10.1191/0267659104pf731oa. [DOI] [PubMed] [Google Scholar]
- 128.Verkkala K, Makela P, Ojajarvi J, Tiittanen L, Hoborn J. Air contamination in open heart surgery with disposable coveralls, gowns and drapes. Ann Thorac Surg. 1990;50:757–760. doi: 10.1016/0003-4975(90)90678-y. [DOI] [PubMed] [Google Scholar]
- 129.Berg GA, Kirk AJ, Bain WH. Punctured surgical gloves and bacterial re-colonisation of hands during open heart surgery: implications for prosthetic valve replacement. Br J Clin Pract. 1987;41:903–906. [PubMed] [Google Scholar]
- 130.Driever R, Beie M, Schmitz E, et al. Surgical glove perforation in cardiac sugery. Thorac Cardiovasc Surg. 2001;49:328–330. doi: 10.1055/s-2001-19006. [DOI] [PubMed] [Google Scholar]
- 131.Christensen JB, Andersen BM, Thomassen SM, Johansen O, Lie M. The effects of ‘in-use’ surgical handwashing on the pre- and postoperative fingertip flora during cardiothoracic and orthopaedic surgery. J Hosp Infect. 1995;95:325–335. doi: 10.1016/0195-6701(95)90263-5. [DOI] [PubMed] [Google Scholar]
- 132.Bratzler DW, Houck PM, Richards C, et al. Use of antimicrobial prophylaxis for major surgery:baseline results from the National Surgical Infection Prevention Project. Arch Surg. 2005;38:1706–1715. doi: 10.1001/archsurg.140.2.174. [DOI] [PubMed] [Google Scholar]
- 133.Bratzler DW, Houck PM, The Surgical Infection Prevention Guidelines Writers Workgroup et al. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;38:1706–1715. doi: 10.1086/421095. [DOI] [PubMed] [Google Scholar]
- 134.Stenhem M, Ortqvist A, Ringberg H, et al. Epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) in Sweden 2000–2003, increasing incidence and regional differences. BMC Infect Dis. 2006;6:30. doi: 10.1186/1471-2334-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]


