Abstract
Objectives
With the development of effective treatments and the resulting increase in life expectancy, bone mineral density (BMD) alteration has emerged as an important comorbidity in human immunodeficiency virus type-1 (HIV-1)-infected individuals. The potential contributors to the pathogenesis of osteopenia/osteoporosis include a higher prevalence of risk factors, combined antiretroviral therapy (cART)-exposure, HIV-1 itself and chronic immune activation/inflammation. Dual-energy X-ray absorptiometry (DXA) is the “gold standard” technique for assessing bone status in HIV-1 population.
Methods
We conducted a cross-sectional study to investigate bone mineral status in a group of 158 HIV-1-infected subjects. The primary endpoint was the feasibility of calcaneal quantitative ultrasound (QUS) as a screening tool for BMD. All subjects were receiving stable cART and were virologically suppressed (HIV-RNA <37 copies/mL) from at least 12 months. Calcaneal QUS parameters were analyzed to obtain information on bone mass and microarchitecture. The results were compared with those obtained by DXA.
Results
No correlations were found between DXA/QUS parameters and demographic or HIV-1-specific characteristics, also including cART strategies. In the univariate analyses BMD, QUS indexes, and Fracture Risk Assessment Tool scores conversely showed significant associations with one or more demographic or HIV-1-related variables. Moreover, a significant relationship between calcaneal quantitative ultrasound index/stiffness and femoral/lumbar BMD values from DXA was described. The multivariate analysis showed an independent association between calcaneal quantitative ultrasound index/stiffness and body mass index, higher CD4+ T-cell numbers and low 25-OH D2/D3 vitamin D levels <10 ng/mL (P-values: 0.004, 0.016, and 0.015, respectively).
Conclusion
As an alternative and/or integrative examination to DXA, calcaneal QUS could be proposed as a useful screening in HIV-1-infected patients for assessing bone health impairment. In fact, the results obtained confirm that calcaneal QUS may be useful for monitoring bone status, being a noninvasive and inexpensive technique, especially in those subjects with the classical traditional risk factors for bone damage that were observed earlier in HIV-1 population.
Keywords: bone mineral density, fracture risk, calcaneal quantitative ultrasound, DXA
Introduction
People living with human immunodeficiency virus type-1 (HIV-1) infection have a life expectancy quite similar to that of uninfected population due to the extensive use of combined antiretroviral therapies (cARTs) that have contributed to modifying the disease prognosis.1 Aging with the virus (and on cART) is raising new challenges: “non AIDS-defining” cancers, cardiovascular diseases, bone diseases, and neurocognitive disorders are now considered a new set of comorbidities, and some of them are becoming significant causes of death among the HIV-1 population.2,3
Osteoporosis is a systemic skeletal disorder characterized by low bone mineral density (BMD) and microarchitectural deterioration of bone tissue, with a consequent raise in bone fragility and fractures. Its prevalence in HIV-1-infected subjects is approximately threefold higher than in uninfected individuals.4 This increased prevalence is likely the result of heterogeneous causes and the interplay of host, viral, and cART-related factors. A reduced BMD with increased fracture risk has been yet demonstrated in both HIV-1-infected male and female population, compared with uninfected controls.5–7
HIV-1-infected patients also have a significantly higher prevalence of vertebral, hip, wrist, and combined fractures. The fracture rate was increased both in naïve and cART-exposed subjects.8,9
Traditional osteoporosis risk factors such as low body mass index (BMI), cigarette smoking, alcohol abuse, glucocorticoid therapy, hypogonadism, growth hormone deficiency, and vitamin D insufficiency are more prevalent among HIV-1-infected individuals and likely play a causative role, but also chronic immune activation and cART-related side effects should be considered.4,10,11
Low vitamin D levels are common in HIV-1-infected patients. The potential effect of cART on 25-OH D2/D3 vitamin D levels has recently received a lot of attention, due to emerging evidences that specific drugs may affect its levels with different mechanisms.12 Also coinfection with hepatitis C virus (HCV) in HIV-1-infected patients has been demonstrated as being an independent risk factor for both fragility-induced and other kinds of fractures.13 Moreover, initiation of cART is associated with bone loss regardless of the regimen selected, as for several comorbidities linked with HIV-1 infection. Indeed, the virus itself, determines per se an increase of bone loss and fracture risk.14,15
BMD can be measured by imaging modalities, such as dual-energy X-ray absorptiometry (DXA), which helps in identifying patients at high risk of fractures.16 Routine DXA screening of all HIV-1-infected patients on cART is not recommended. It is suggested to evaluate BMD by DXA scans in: 1) men aged 40–49 years or premenopausal women aged ≥40 years, who have an intermediate- or high-risk stratification by the Fracture Risk Assessment Tool (FRAX >10%, as 10-year risk of major osteoporotic fracture); 2) all postmenopausal women; 3) all men ≥50 years of age; and 4) adults with major fragility fracture risk factors regardless of age.17,18 Repeat DXA scanning should be considered after 1–2 years for those with baseline advanced osteopenia and after 5 years for mild-to-moderate osteopenia.19,20
Calcaneal quantitative ultrasound (QUS) is an attractive prescreening investigation for osteoporosis, being an alternative and/or integrative technique to DXA scan. It is a relatively inexpensive, radiation-free, and transportable device, which has been recently proven to effectively predict fracture risk in elderly men and women. QUS uses high-frequency sound waves to evaluate bone properties. Multiple skeletal sites, such as tibia, phalanx, and radius, have been evaluated; however, the heel has been most widely studied because of several favorable characteristics, including the high content of trabecular bone and the high metabolic turnover. Although its use in clinical practice is still not well defined and depends on the type of device, it provides an appropriate tool for comparing BMD between different groups and identifying factors associated with variation in bone density, especially in settings where DXA is not available.21,22 Calcaneal QUS in a prescreen or stratification algorithm must be based on device-specific cutoff that have been validated in the populations for which they are intended to be used.23,24
In this context, we designed a cross-sectional study to measure the bone mineral quality in a group of HIV-1-in-fected subjects on cART with the aim to assess the feasibility and usefulness of calcaneal QUS as a screening investigation for the bone evaluation in comparison to DXA.
Patients and methods
Study population
We performed a cross-sectional study to investigate the bone mineral status in a group of 158 HIV-1-infected subjects followed at the Department of Public Health and Infectious Diseases, University of Rome “Sapienza”. At baseline, information regarding demographic characteristics, health-related behaviors, medical conditions, and prescribed medications were retrieved from the medical records. The following epidemiological, immunovirological, and clinical factors increasing the osteoporosis risk were included: CD4+ T-cell nadir, current CD4+ T-cell count, HIV-RNA level, years of HIV-1 infection, AIDS diagnosis, time of cART exposure, boosted protease inhibitor (PI) exposure, tenofovir difumarate (TDF) exposure, BMI, opiate, tobacco and alcohol use, hepatitis B virus (HBV) HCV coinfections, diabetes mellitus, renal function, prior or current corticosteroid use, hypogonadism, vitamin D deficiency, menopause, malabsorption, personal history of fracture, parental history of hip fracture, and rheumatoid arthritis. Moreover, nontraumatic bone fractures were clinically evaluated. Exclusion criteria were use of antiosteoporotic drugs, presence of chronic diseases known to be associated with osteoporosis, recent traumas or prolonged immobilization. Fasting blood samples were obtained from all subjects and analyzed for vitamin D and parathyroid hormone (PTH) levels, as well as for serum alkaline phosphatase, total calcium and phosphates. Serum levels of 25-OH D2/D3 vitamin D were measured by electrochemiluminescence immunoassay (ECLIA) with the Elecsys vitamin D3 (25-OH) reactive (Roche Diagnostic GmbH, Mannheim, Germany) using the Roche/Hitachi COBAS E 601 clinical chemistry analyzer (Roche Diagnostics, Indianapolis, IN, USA). Renal function and glomerular filtration rate were estimated using the Chronic Kidney Disease Epidemiology Collaboration equation.
BMI was calculated as the weight in kilograms divided by the square of the height in meters. The FRAX score and the Veterans Aging Cohort Study (VACS) index were also calculated to evaluate the 10-year probability of a major fracture (spine, forearm, proximal humerus, or hip) or hip fracture alone and to predict all-cause mortality, cause-specific mortality, and other outcomes in subjects living with HIV-1 infection. The FRAX score is based on age, sex, weight, height, femoral neck BMD and the presence of clinical risk factors that increase the fracture occurrence. The VACS index (based on age, CD4+ T-cell count, HIV-1 RNA, hemoglobin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), platelets, creatinine, and HCV status) was validated for HIV-1-infected subjects in the Women’s Interagency HIV Study.25
Ethical permission from a review board was unnecessary for this study because the QUS analysis is routinely performed, as well as the DXA analysis, to evaluate BMD, in accordance with the current osteoporosis treatment guidelines. In Italy, the calcaneal QUS is an examination tool standardized and approved by the Ministry of Health with these indications.
Written informed consent was obtained from all subjects for the use of the QUS results (appropriately anonymized) for research purposes.
BMD analysis
BMD was assessed by DXA at the lumbar spine (L1–L4) and the femoral neck. T-scores were calculated using normative data based on reference data for European Caucasian females matched for age, sex, and race. According to current international guidelines, osteopenia and osteoporosis were defined by a T-score of <−1 and <−2.5, respectively.26 The evaluation of the bone status was also investigated using the calcaneal QUS (Hologic Sahara, Hologic, Inc., Marlborough, MA, USA). QUS measures the time taken for sound waves to travel between the two transducers, referred to as speed of sound. The rate of ultrasound attenuation is defined as broadband ultrasound attenuation. Both speed of sound and broadband ultrasound attenuation are reduced in the presence of reductions in bone density and trabecular number. Hologic Sahara® also provides a combination “stiffness” index, referred to as quantitative ultrasound index (QUI).
Statistical analysis
Data were summarized as proportions and medians with interquartile ranges (IQR). Binary correlations between quantitative variables were analyzed using the Spearman’s rank correlation test. Predictive variables for the presence of low calcaneal QUI/stiffness values were identified in a multivariable logistic regression model that used as dependent variable the presence of values of calcaneal QUI/stiffness in the first decile (below the tenth centile) of the distribution, with adjusted odds ratios and 95% confidence intervals calculated. The variables entering the model (independent variables) were selected based on the best fitting in terms of model discrimination (accuracy of the model to predict cases in the first decile and area under the curve in receiver operator characteristic [ROC] curves), and amount of the variation explained by the model (Nagelkerke R square). For all tests, P-values <0.05 were considered significant. All statistical analyses were performed with the SPSS software, version 22 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
A total of 158 consecutive HIV-1-infected patients, followed at the Department of Infectious Diseases, “Sapienza” University of Rome, were included in the study between May and November 2014. All the subjects were receiving a stable and effective cART and a PI (n=52) or a nonnucleoside reverse-transcriptase inhibitor (NNRTI, n=71) or an integrase inhibitor (INI, n=35) in the scheme and were all virologically suppressed (HIV-RNA levels below <37 copies/mL) from at least 1 year. The population was characterized by a long history of HIV-1 infection (median: 17 years) and antiretroviral treatment (median: 15 years), good immunological status (median CD4: 622 cells/mm3), and relatively low values of the VACS index, suggesting overall good clinical conditions and limited risk of mortality. The general characteristics of the population are reported in detail in Table 1.
Table 1.
Population characteristics
| Parameter | Median (IQR) | N (%) |
|---|---|---|
| Age, years | 52 (46–56) | |
| BMI, kg/m2 | 22.8 (20.9–25.4) | |
| Time since HIV diagnosis, years | 18 (11–23) | |
| Prior cART exposure, years | 15 (10–20) | |
| Prior PI exposure, years | 5 (0–9) | |
| Prior TDF exposure, years | 5 (3–7) | |
| CD4+ T-cell nadir, cells/µL | 168 (108–295) | |
| Current CD4+ T-cells, cells/µL | 622 (460–860) | |
| Hgb, g/dL | 14.3 (13.6–15.3) | |
| Platelets ×1,000/µL | 208 (172–245) | |
| ALT, U/L | 23 (16–33) | |
| AST, U/L | 22 (18–31) | |
| HDL cholesterol, mg/dL | 50 (40–62) | |
| LDL cholesterol, mg/dL | 115 (95–147) | |
| Serum creatinine, mg/dL | 0.8 (0.7–1.0) | |
| e-GFR (CKD-EPI), mL/min/1.73 m2 | 89.3 (76.9–103.5) | |
| VACS index | 16 (6–23) | |
| Sex, male | 107 (67.7) | |
| HBV coinfection | 6 (3.9) | |
| HCV coinfection | 39 (25.2) | |
| Current cART: | ||
| PI-based | 52 (32.9) | |
| NNRTI-based | 71 (44.9) | |
| INI-based | 35 (22.2) | |
| NRTI in the regimen: | ||
| None | 34 (21.9) | |
| 3TC only | 3 (1.9) | |
| TDF only | 3 (1.9) | |
| ABC+3TC | 44 (28.4) | |
| TDF+FTC | 71 45.8) | |
| HIV disease stage: | ||
| CDC-A | 55 (36.2) | |
| CDC-B | 55 (36.2) | |
| CDC-C | 42 (27.6) | |
| Lumbar mineralization status: | ||
| Normal | 66 (53.7) | |
| Osteopenia | 44 (35.8) | |
| Osteoporosis | 13 (10.6) | |
| Femoral mineralization status: | ||
| Normal | 56 (45.2) | |
| Osteopenia | 57 (46.0) | |
| Osteoporosis | 11 (8.9) |
Abbreviations: ABC, abacavir; BMI, body mass index; cART, combined antiretroviral therapy; CDC, Centers for Disease Control and Prevention; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; e-GFR, estimated glomerular filtration rate; FTC, emtricitabine; HBV, hepatitis B virus; HCV, hepatitis C virus; HDL, high-density lipoproteins; Hgb, hemoglobin; HIV, human immunodeficiency virus; INI, integrase inhibitor; IQR, interquartile range; LDL, low-density lipoprotein; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleos(t)ide reverse transcriptase inhibitor; PI, protease inhibitor; TDF, tenofovir difumarate; VACS, Veterans Aging Cohort Study; 3TC, lamivudine.
Patients were analyzed for clinical and treatment variables according to the different cART used at the enrollment: no statistically significant differences were observed when age, sex, risk of HIV-1 transmission, BMI, cigarette smoking, HBV and HCV coinfections, nadir and current CD4+ T-cell counts were considered. However, subjects taking an INI-based therapy had a significantly longer extent of HIV-1 infection (20.5±6 vs 15.3±8.3 years, P<0.005) compared with those using a PI- or a NNRTI-based cART. Again, a similar result was observed when the years of prior antiretroviral therapy were considered (17.5±5.1 vs 12.9±7.2 years, P<0.004), or the VACS index was included (21.3±11.8 vs 13.1±10.9, P<0.002). Moreover, similar findings were observed in subjects belonging to the PI group when compared to those taking an NNRTI-based regimen at baseline. Years of prior TDF exposure were comparable in all groups (data not shown).
Evaluation of bone indexes
The evaluation of bone status, based on several indexes, is reported in Table 2. Blood tests revealed a frequent occurrence (45.1%) of low vitamin D levels (<30 ng/mL). Increased levels of PTH (>65 pg/mL) were found in almost 20% of cases. All subjects had serum calcium levels within the normal range, while 14/152 (9.2%) had hypophosphatemia (<2.5 mg/dL). Most of them (11/14) had one or more of the following potentially predisposing conditions: TDF-based regimens (9), increased PTH levels (1), diabetes (3), estimated glomerular filtration rate (e-GFR) <60 mL/min (1), or decreased serum vitamin D levels (5).
Table 2.
Bone metabolism and mineralization indexes of enrolled patients
| Parameter | Median (IQR) | N (%) |
|---|---|---|
| 25-OH D2/D3 vitamin D, ng/mL | 27.8 (15.3–38) | |
| PTH, pg/mL | 46.9 (31.9–58.2) | |
| Serum phosphate, mg/dL | 3.1 (2.8–3.4) | |
| Calcaneal QUI/stiffness, % | 78.1 (66.2–90.6) | |
| Calcaneal T-score | −1.5 (−0.7−2.3) | |
| Calcaneal BMD, g/cm2 | 0.42 (0.34–0.50) | |
| Lumbar spine BMD, g/cm2 | 0.97 (0.87–1.12) | |
| Femoral BMD, g/cm2 | 0.84 (0.73–0.98) | |
| FRAX score, % | 2.9 (2.1–4.8) | |
| 25-OH D2/D3 vitamin D levels <30 ng/mL | 73 (54.9) | |
| 25-OH D2/D3 vitamin D levels <10 ng/mL | 14 (10.5) | |
| PTH levels >65 pg/mL | 18 (18.4) | |
| Femoral osteoporosis or osteopenia | 68 (54.9) | |
| Femoral osteoporosis | 11 (8.9) | |
| Lumbar osteoporosis or osteopenia | 57 (46.4) | |
| Lumbar osteoporosis | 13 (10.6) | |
| Summary of clinical risk factors for | ||
| osteoporosis: | ||
| Menopausal status | 25 (16.1) | |
| Type 2 diabetes mellitus | 20 (12.9) | |
| Current or ex tobacco smoker | 101 (65.2) | |
| HBV or HCV coinfection | 45 (29.0) | |
| Hypogonadism | 2 (1.3) |
Abbreviations: BMD, bone mineral density; FRAX, Fracture Risk Assessment Tool; HBV, hepatitis B virus; HCV, hepatitis C virus; IQR, interquartile range; PTH, parathyroid hormone; QUI, quantitative ultrasound index.
The instrumental assessment of the bone compartment showed a common occurrence of reduced BMD: median BMD levels at the lumbar, femoral, and calcaneal sites were, respectively (with IQR): 0.97 (0.87–1.12), 0.84 (0.73–0.98), and 0.42 g/cm2 (0.34–0.50). Osteoporosis or osteopenia were found at the level of lumbar spine in 46.4% of subjects and at the level of femoral neck in 54.9% of subjects. Calcaneal QUI/stiffness showed a median value of 78.1 (IQR 66.2–90.6), with a median calcaneal T-score of −1.5.
The presence of clinical risk factors for osteoporosis was very common: 101 patients (65.2%) were active (or prior) tobacco smokers, 45 (29.0%) were coinfected with HBV and/or HCV, 20 had type 2 diabetes mellitus (12.6%), almost half of the women (25/51, 49%) were menopausal, and two males were affected by hypogonadism. The values of FRAX score were usually low, with only 8/152 (5.2%) showing a 10-year estimated risk of fractures higher than 10%. Posttraumatic fractures were reported by three subjects, whereas no occurrence of osteoporotic fractures was reported.
We then explored possible predictors of abnormalities in the main bone indexes evaluated. In univariate analyses, BMD levels, FRAX score, and QUIs were substantially unaffected by e-GFR, smoking, Centers for Disease Control and Prevention (CDC) HIV-1 disease stage, presence of diabetes, and current use of INIs. BMD, QUS indexes, and FRAX scores conversely showed significant associations with one or more of the following variables: sex, menopause, HCV or HBV coinfection, CD4+ T-cell levels, and vitamin D status (data not shown).
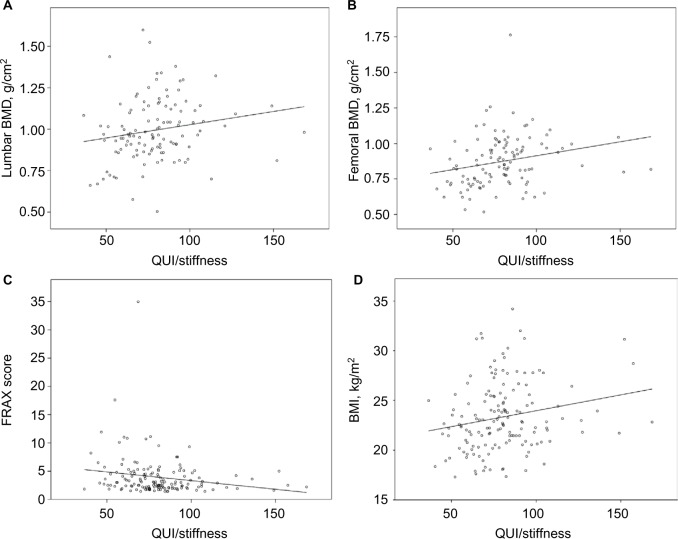
The calcaneal QUI/stiffness was highly and positively correlated with BMI values (P=0.010), with the BMD measures taken at the lumbar and femoral sites (P-values: 0.008 and <0.001, respectively) and showed an inverse significant correlation with the risk of fracture as expressed by the FRAX score (P=0.006) (Figure 1).
Figure 1.
Correlations between QUI/stiffness index and lumbar BMD (A), femoral BMD (B), FRAX score (C), and BMI (D).
Abbreviations: BMD, bone mineral density; BMI, body mass index; FRAX, Fracture Risk Assessment Tool; QUI, quantitative ultrasound index.
Assuming its potential role as a screening test, the determinants of low values of calcaneal QUI/stiffness were evaluated in a multivariate analysis. The variables in the equation of the final model, with adjusted odds ratios (with 95% confidence interval) for each variable are shown in Table 3.
Table 3.
Multivariate analysis of determinants of low calcaneal QUI/stiffness (<10° percentile)
| Parameters | AOR | AOR 95% CI
|
P-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| HCV coinfection | 0.349 | 0.018 | 6.760 | 0.487 |
| BMI (per unitary increase) | 0.699 | 0.548 | 0.891 | 0.004 |
| Duration of previous TDF exposure (per additional year) | 0.945 | 0.644 | 1.388 | 0.773 |
| Duration of previous PI exposure (per additional year) | 1.226 | 0.965 | 1.557 | 0.095 |
| Menopause | 8.787 | 0.464 | 166.350 | 0.148 |
| CDC-C stage | 9.329 | 0.781 | 111.355 | 0.078 |
| CD4+ T-cells/µL (per unitary increase) | 1.004 | 1.001 | 1.007 | 0.016 |
| Years from HIV-1 diagnosis (per unitary increase) | 0.963 | 0.826 | 1.124 | 0.635 |
| Serum 25-OH D2/D3 vitamin D levels <10 ng/mL | 35.585 | 2.003 | 632.266 | 0.015 |
| Diabetes | 2.233 | 0.135 | 36.821 | 0.574 |
| Currently on TDF-based regimens | 1.457 | 0.155 | 13.702 | 0.742 |
| Serum PTH levels (>65 pg/mL) | 1.144 | 0.059 | 22.115 | 0.929 |
Notes: Bold values indicate statistical significance (P<0.05). Fitting of the model: Nagelkerke R square: 0.853; predictive accuracy: 93.8%; area under the curve (ROC): 0.903.
Abbreviations: AOR, adjusted odds ratio; BMI, body mass index; CDC, Centers for Disease Control and Prevention; CI, confidence interval; HCV, hepatitis C virus; HIV-1, human immunodeficiency virus type-1; PI, protease inhibitor; PTH, parathyroid hormone; QUI, quantitative ultrasound index; TDF, tenofovir difumarate.
The three variables significantly and independently associated with low QUI/stiffness values were BMI (with higher BMI protective in terms of risk of low QUI/stiffness), CD4+ T-cell count (with higher CD4+ numbers associated with higher risk of low QUI/stiffness) and low (<10 ng/mL) serum levels of 25-OH D2/D3 vitamin D, which increased >30-fold the risk of low QUI/stiffness values (Table 3).
Discussion
HIV-1-infected adults are at high risk of low BMD, with up to 60% prevalence of osteopenia and up to 15% prevalence of osteoporosis. The initiation of treatment in ART-naive adults is associated with 2%–6% loss of BMD after 48–96 weeks of therapy, then followed by stabilization.27,28 The observed changes are similar, whether the regimen includes an INI or NNRTI; however, the decrease in BMD appears to be greater with TDF than with other NRTIs or when a PI is included.18 Recent findings suggest that HIV-1 infection and/or heightened inflammation could impact BMD loss in HIV-1-infected subjects, independently from cART effects.15 Several questions are still unresolved about the role of antiretroviral therapy in bone demineralization. In fact, the mechanism leading to the acute decrease in BMD after cART initiation is unclear; in addition, although a partial BMD recovery is observed after the first year of therapy or after the switch to another cART regimen, long-term clinical impact of initial BMD loss has not been clarified yet.
Some cross-sectional studies have reported a higher prevalence of low BMD, osteoporosis and fractures in HIV-1-positive adults when compared with HIV-1-negative controls.29,30 Monitoring changes in bone structure and mineral density is pivotal in the management of HIV-1 population because the viral infection and certain combinations of antiretroviral treatments elicit progressive bone loss in these patients.31
Currently, DXA is the “gold standard” technique for the assessment of the bone status in HIV-1 population, both at the time of diagnosis as well as during the first months of antiretroviral treatment. However, DXA suffers from several technical limitations, as it quantifies bone mass but does not provide information on bone quality. The DXA technique is not able to determine the strength of bone architecture, and a normal BMD could be associated with impaired bone structure and a higher risk of fractures.32 These limitations explain why HIV-1-infected patients may often present a fracture risk largely exceeding that expected from DXA evaluation.
Calcaneal QUS provides information on both bone mass and bone microarchitecture. Notwithstanding as an alternative and/or integrative technique to DXA, quantitative calcaneal ultrasound has recently been proposed as a screening method for osteopenia/osteoporosis, since it has been proven to be accurate and precise in evaluating bone mass. It is a noninvasive and low-cost technique that can be evenly used for assessing structure and bone mineralization. In seronegative patients, some studies had revealed that QUS is a cheap tool that gives an accurate and precise evaluation of bone mass and acts as an early predictive marker of bone damage. In a recent meta-analysis, one limitation emerged of QUS devices is the absence of standardized cutoffs between different manufacturers, thus limiting the comparison between results obtained from the different devices. However, the authors concluded that, in several of the available studies, the use of QUS could help save patients from undergoing DXA scans.22–24
In all HIV-1-infected men aged 40–49 years and in premenopausal women aged ≥40 years, the risk of fragility fracture should be assessed primarily using the FRAX score, without performing DXA, as it includes the use of clinical risk factors to estimate the 10-year fracture risk in individuals older than 40 years. BMD obtained by DXA should be incorporated in the algorithm, when available. Moreover, it is recommended to consider HIV-1 infection as a secondary cause of osteoporosis, including it in the FRAX calculator tool. DXA scan should be reserved for postmenopausal women, men ≥50 years old, and those showing the following: history of low impact fracture, high risk for falls, clinical hypogonadism, and oral glucocorticoid use (minimum 5 mg/daily prednisone equivalent for >3 months). Preferably, DXA should be performed in those with above the risk factors prior to cART initiation.17,18 Moreover, according to previous observations, also subjects with a poor immunological recovery after starting cART should be considered, as they are more vulnerable to develop bone fractures.6,33
In our study, we perform a bone status evaluation in HIV-1-infected individuals by means of the calcaneal QUS technique, using it as a screening tool in the assessment of bone health in this population, and comparing the results acquired with those obtained by DXA. Considering the progressive “aging” and the “frailty” condition described in HIV-1-infected patients, it would be important to have a screening instrument easy enough to perform in different clinical settings for the evaluation of bone mineral impairment. So, it would be appropriate carrying out calcaneal QUS as a screening examination of bone quality in daily clinical practice, mainly in those subjects with low BMI, smokers and persons who use alcohol, as well as in patients with diabetes mellitus, in perimenopausal women, and in male population, as these are osteoporosis risk factors or conditions frequently observed in the HIV-1 population.
Our results confirm that clinical risk factors for osteoporosis are very common in HIV-1-infected subjects and that there is a high prevalence of bone alterations in our population, as the occurrence of osteopenia/osteoporosis at the femoral neck was >50%. These findings were confirmed by the increased impairment of calcaneal QUI/stiffness, with the lower values observed when osteopenia or osteoporosis were detected by DXA. The univariate analysis confirms as predictors of BMD, QUS indexes, and FRAX scores abnormalities several variables, such as sex, menopause, HCV or HBV coinfection, CD4+ T-cell levels, and vitamin D status. The correlation observed between QUI/stiffness and HCV infection, despite unconfirmed in the multivariate analysis, support the need of making a specifically targeted screening with calcaneal QUS in the setting of HIV/HCV coinfected patients. In fact, HCV coinfection is associated with a greater risk of osteoporosis and fractures than HIV-1 monoinfection; these data advise that HIV/HCV coinfected individuals should be targeted for fracture prevention through risk factor modifications at all ages and DXA screening after 40 years.
The calcaneal QUI/stiffness was highly and positively correlated with the DXA BMD measures taken at the lumbar and femoral sites: this relationship suggests that the bone stiffness measured by calcaneal QUS may have an early predictive value in the assessment of bone status in HIV-1-infected subjects. As expected, there was a positive correlation between QUI/stiffness and BMI, as it indirectly represent an indicator of the general status of the subjects. Conversely, this QUS index was inversely correlated with the FRAX score, thus indicating its utility in predicting an increased risk of bone fractures in those subjects showing low values of QUI/stiffness.
Given its potential role as a screening test, we evaluated the determinants of low values of calcaneal QUI/stiffness in a multivariate analysis. Three variables were significantly and independently associated with low QUI/stiffness values: a higher BMI value was protective, as it indirectly represents a subject in good clinical conditions, whereas higher CD4+ T-cell counts were associated with a greater risk of low QUI/stiffness, probably reflecting a paradoxically negative effect of prolonged drug exposure and viral suppression in our population. On the contrary, the low (<10 ng/mL) serum levels of 25-OH D2/D3 vitamin D increased >30-fold the risk of low QUI/stiffness values, thus confirming the central role exerted by vitamin D in preserving a normal bone metabolism.
Accordingly, with these findings, the implementation of calcaneal QUS in the daily clinical practice may be considered an alternative to DXA in different settings for diagnosing osteopenia/osteoporosis in HIV-1-infected patients.
In some studies, a direct activity of different antiretroviral drugs on bone mineral quality and turnover has been described.14,34 Moreover, a recent study suggests a better impact of INIs vs PIs on bone mineral status.35 Our study apparently does not confirm such a result, as a direct relationship between calcaneal QUI/stiffness, BMD, and the cumulative TDF or PI exposure has not been found. The same finding has been observed when PI use was compared to NNRTI- or INI-based regimens. Moreover, no differences were found when the backbone use was compared. These different results observed could be due to the study design (cross-sectional) and/or to the relative low sample size of subjects investigated. Probably these limitations may be overwhelmed by the inclusion of additional analyses, such as the investigation of bone turnover markers and/or the evaluation of immune activation and inflammation markers, whose usefulness has been already described in HIV-related BMD alterations.
Anyway, the results obtained in our study clearly support the inclusion of calcaneal QUS as a complementary tool to DXA, which is useful for predicting the risk of bone fractures in HIV-1-infected patients, both when they start cART as well as during its follow-up.
Conclusion
Calcaneal QUS may be useful to monitor the impact of viral infection and antiretroviral drugs on bone status, being a noninvasive and inexpensive technique, easy to perform in the daily clinical practice, and requiring a short time for implementation. Using a careful monitoring of calcaneal QUS stiffness, FRAX score, and some markers of bone turnover, could be a key to reserve DXA examination only for selected patients.
Acknowledgments
The work was supported by unrestricted educational grants from ViiV Healthcare and Gileads Sciences. The authors are indebted to Dr Anna Ancona, PharmD, for her contribution to data evaluation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Battalora LA, Young B, Overton ET. Bones, fractures, antiretroviral therapy and HIV. Curr Infect Dis Rep. 2014;16(2):393. doi: 10.1007/s11908-014-0393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47(4):542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onen NF, Overton ET, Seyfried W, et al. Aging and HIV infection: a comparison between older HIV-infected persons and the general population. HIV Clin Trials. 2010;11(2):100–109. doi: 10.1310/hct1102-100. [DOI] [PubMed] [Google Scholar]
- 4.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20(17):2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 5.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93(9):3499–3504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overton ET, Mondy K, Bush TJ, et al. Factors associated with low bone mineral density (BMD) in a cohort of HIV-infected U.S. adults-baseline results from the SUN Study; 13th Conference on Retroviruses and Opportunistic Infections; February; Los Angeles, CA. 2007. Abstract 836. [Google Scholar]
- 7.McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51(8):937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen AB, Gerstoft J, Kronborg G, et al. Incidence of low- and high-energy fractures in persons with and without HIV-infection: a Danish population based cohort study. AIDS. 2012;26(3):285–293. doi: 10.1097/QAD.0b013e32834ed8a7. [DOI] [PubMed] [Google Scholar]
- 9.Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253–259. doi: 10.1016/j.bone.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Zenilman JM, Hook EW, Shepherd M, Smith P, Rompalo AM, Celentano DD. Alcohol and other substance use in STD clinic patients: relationships with STDs and prevalent HIV infection. Sex Transm Dis. 1994;21(4):220–225. doi: 10.1097/00007435-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Kwan Tat S, Padrines M, Théoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15(1):49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Pepe J, Mezzaroma I, Fantauzzi A, et al. An oral high dose of cholecalciferol restores vitamin D status in deficient postmenopausal HIV-1-infected women independently of protease inhibitors therapy: a pilot study. Endocrine. 2015 Aug 9; doi: 10.1007/s12020-015-0693-8. Epub. [DOI] [PubMed] [Google Scholar]
- 13.Dong HV, Cortés YI, Shiau S, Yin MT. Osteoporosis and fractures in HIV/hepatitis C virus coinfection: a systematic review and meta-analysis. AIDS. 2014;28(14):2119–2131. doi: 10.1097/QAD.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203(12):1791–1801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hileman CO, Labbato DE, Storer NJ, Tangpricha V, McComsey GA. Is bone loss linked to chronic inflammation in antiretroviral-naive HIV-infected adults? A 48-week matched cohort study. AIDS. 2014;28(12):1759–1767. doi: 10.1097/QAD.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker Harris V, Brown TT. Bone loss in the HIV-infected patient: evidence, clinical implications, and treatment strategies. J Infect Dis. 2012;205(Suppl 3):S391–S398. doi: 10.1093/infdis/jis199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European AIDS Clinical Society Guidelines: Prevention and Management of Non-infectious Comorbidities in HIV. 2014. [Accessed October 2015]. Available from: http://www.europeanaidsclinicalsociety.org/images/stories/EACSPdf/EacsGuidelines-v8.0-1edition.pdf.
- 18.Brown TT, Hoy J, Borderi M, et al. Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis. 2015;60(8):1242–1251. doi: 10.1093/cid/civ010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gourlay ML, Fine JP, Preisser JS, et al. Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366(3):225–233. doi: 10.1056/NEJMoa1107142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Negredo E, Bonjoch A, Gómez-Mateu M, et al. Time of progression to osteopenia/osteoporosis in chronically HIV-infected patients: screening DXA scan. PLoS One. 2012;7(10):e46031. doi: 10.1371/journal.pone.0046031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Constant D, Rosenberg L, Zhang Y, et al. Quantitative ultrasound in relation to risk factors for low bone mineral density in South African pre-menopausal women. Arch Osteoporos. 2009;4(1–2):55–65. doi: 10.1007/s11657-009-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krieg MA, Barkmann R, Gonnelli S, et al. Quantitative ultrasound in the management of osteoporosis: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11(1):163–187. doi: 10.1016/j.jocd.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Hans D, Krieg MA. Quantitative ultrasound for the detection and management of osteoporosis. Salud Publica Mex. 2009;51(Suppl 1):S25–S37. doi: 10.1590/s0036-36342009000700006. [DOI] [PubMed] [Google Scholar]
- 24.Moayyeri A, Adams JE, Adler RA, et al. Quantitative ultrasound of the heel and fracture risk assessment: an updated meta-analysis. Osteoporos Int. 2012;23(1):143–153. doi: 10.1007/s00198-011-1817-5. [DOI] [PubMed] [Google Scholar]
- 25.Cohen MH, Hotton AL, Hershow RC, et al. Gender-related risk factors improve mortality predictive ability of VACS Index among HIV-infected women. J Acquir Immune Defic Syndr. 2015;70(5):538–544. doi: 10.1097/QAI.0000000000000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis and Therapy Osteoporosis prevention, diagnosis and therapy. JAMA. 2001;285(6):785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 27.Grund B, Peng G, Gibert CL, et al. INSIGHT SMART Body Composition Substudy Group. Continuous antiretroviral therapy decreases bone mineral density. AIDS. 2009;23(12):1519–1529. doi: 10.1097/QAD.0b013e32832c1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore AL, Vashisht A, Sabin CA, et al. Reduced bone mineral density in HIV-positive individuals. AIDS. 2001;15(13):1731–1733. doi: 10.1097/00002030-200109070-00019. [DOI] [PubMed] [Google Scholar]
- 29.Dolan SE, Huang JS, Killilea KM, Sullivan MP, Aliabadi N, Grinspoon S. Reduced bone density in HIV-infected women. AIDS. 2004;18(3):475–483. doi: 10.1097/00002030-200402200-00014. [DOI] [PubMed] [Google Scholar]
- 30.Jones S, Restrepo D, Kasowitz A, et al. Risk factors for decreased bone density and effects of HIV on bone in the elderly. Osteoporos Int. 2007;19(7):913–918. doi: 10.1007/s00198-007-0524-8. [DOI] [PubMed] [Google Scholar]
- 31.Borderi M, Calza L, Colangeli V, et al. Prevalence of sub-clinical vertebral fractures in HIV-infected patients. New Microbiol. 2014;37(1):25–32. [PubMed] [Google Scholar]
- 32.Stagi S, Cavalli L, Bertini F, et al. Vitamin D levels in children, adolescents, and young adults with juvenile-onset systemic lupus erythematosus: a cross-sectional study. Lupus. 2014;23(10):1059–1065. doi: 10.1177/0961203314532564. [DOI] [PubMed] [Google Scholar]
- 33.Grant PM, Kitch D, McComsey GA, et al. Low baseline CD4+ count is associated with greater bone mineral density loss after antiretroviral therapy initiation. Clin Infect Dis. 2013;57(10):1483–1488. doi: 10.1093/cid/cit538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negredo E, Diez-Pérez A, Bonjoch A, et al. Switching from tenofovir to abacavir in HIV-1-infected patients with low bone mineral density: changes in bone turnover markers and circulating sclerostin levels. J Antimicrob Chemother. 2015;70(7):2104–2107. doi: 10.1093/jac/dkv063. [DOI] [PubMed] [Google Scholar]
- 35.Otokun I, Na LH, Landovitz RJ, et al. Comparison of the metabolic effects of ritonavir-boosted darunavir or atazanavir versus raltegravir, and the impact of ritonavir plasma exposure: ACTG 5257. Clin Infect Dis. 2015;60(12):1842–1851. doi: 10.1093/cid/civ193. [DOI] [PMC free article] [PubMed] [Google Scholar]