Abstract
A powerful combination of two-color imaging in vivo, fourier-filtered kymography, and simulations provides high-resolution view of kinesin-2 transport dynamics in cilia. This study reveals heterotrimeric kinesin-II as an obstacle course runner, homodimeric OSM-3/KIF17 as a long distance runner, and the baton handoff between these two motors on the microtubule track.
Although cellular cargos can be carried by teams of motors, the rules governing interactions of multiple motors are not well understood. What happens when different types of same-direction motors form teams to transport cargos? This question is relevant to ciliary biology because some cilia—for example, mammalian olfactory cilia, the rods and cones of the mammalian retina, and sensory neuronal cilia in C. elegans—use two different types of kinesin-2 motors for anterograde transport1, 2, but no one knows why. In a technical Tour de force, Peterman, Scholey, and colleagues provide new insights in this issue of Nature Cell Biology by studying the Intraflagellar transport (IFT) in the chemosensory phasmid cilia of C. elegans3.
Motor Interactions are broadly characterized as “coordinated” or “tug-of-war4.” For example, if kinesin and dynein are attached to a single cargo, inactivating one while the other is active provides for efficient bidirectional transport. However, simultaneous activity of both kinesin and dynein may immobilize transport4. Even in teams of identical motors, interactions can either enhance or hinder transport4.
Intraflagellar transport is a conserved process that builds and maintains virtually all eukaryotic cilia and flagella1. Early studies in the flagella of the alga Chlamydomonas reinhardti established that IFT uses heterotrimeric kinesin-II as the anterograde motor and ciliary dynein as the retrograde motor1. Over a decade ago, Scholey and colleagues used time-lapse fluorescence microscopy of GFP-tagged IFT motors and IFT particle polypeptides to show that anterograde IFT is mediated by both heterotrimeric kinesin-II (KIF3, consisting of KLP-20/KIF3A, KLP-11/KIF3B, and KAP-1/KAP) and homodimeric kinesin-2 OSM-3 (KIF17 in mammals) in C. elegans chemosesensory amphid channel cilia2. Neither ciliary transport nor ultrastructure is homogeneous, and cilia are divided into three compartments: the transition zone (TZ), characterized by microtubule (MT) doublets which are connected to the membrane by proteinacious “Y” links; the proximal segment (PS), characterized by doublets but lacking Y-links; and the distal segment (DS), characterized by MT singlets that extend to the ciliary tip5. The two kinesin-2 motors function cooperatively, moving at a rate intermediate to slow kinesin-II and fast OSM-3 in the PS. Whereas slow kinesin-II remains in the PS, OSM-3 acts as the sole IFT kinesin-2 in the DS, moving at its fast, unencumbered rate. Although slow kinesin-II and fast OSM-3 motors are redundant in building the PS, but OSM-3 alone builds the DS. Why did evolution add another anterograde motor when heterotrimeric kinesin-II seems capable of doing the job alone in algae?
Here the authors discover a heretofore unappreciated complexity in the cooperation of anterograde motors using advanced techniques such as ultrasensitive, laser-illuminated epifluorescence microscopy, simultaneous two-color time-lapse imaging, single molecule fluorescent imaging, fluorescently marked motors expressed at native levels, new automated analysis of fourier-filtered kymographs to extract instantaneous motor velocity, and simulations.
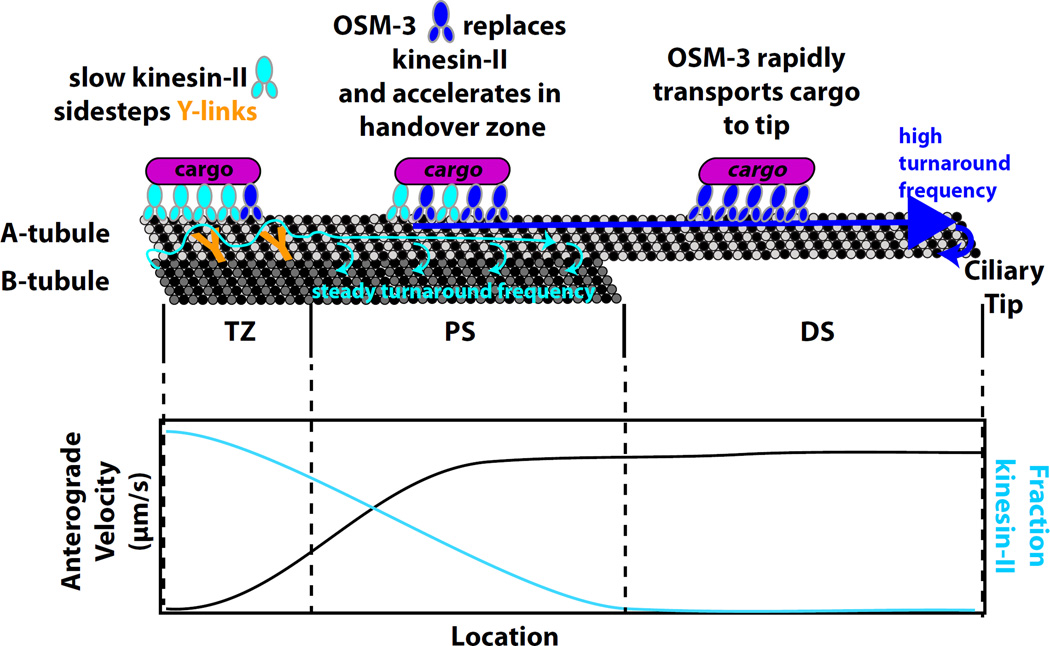
Prevo et al. find that kinesin-II acts as an import motor to bring cargos from the distal dendrite into the ciliary TZ (Figure). Because the TZ is cluttered with obstacles such as Y-link attachments6, kinesin-II, with its tendency to frequently detach from MTs, may more easily sidestep obstructions, switching to an adjacent MT protofilament, to deliver cargo to the PS. A similar model of obstacle avoidance was proposed to function in axons, in which kinesin-2 binds the same cargo as kinesin-1 to allow the complex to sidestep around Tau protein obstacles7.
Figure. A simple model of complex cooperation among anterograde motors in C. elegans phasmid cilia.
Kinesin-II imports cargos and efficiently navigates Y-links and other obstacles in the transition zone (TZ). In the proximal segment (PS) characterized by doublet microtubules (MTs) and a lack of Y-links, kinesin-II motors are steadily replaced by the homodimeric kinesin-2 OSM-3, resulting in acceleration of motor and cargo complexes. In the distal segment (DS) characterized by MT singlets, OSM-3 solely carries cargo to tip. Kinesin-II has a steady frequency of detachment and turnaround, while OSM-3 mostly turns around for retrograde transport by dynein motors (not shown) at the ciliary tip. Turnarounds ensure motors return to the right place for repeated trips.
Authors then observed gradual acceleration of IFT in the PS, accompanied by a gradual decrease in abundance of kinesin-II and concurrent increase in OSM-3/KIF17 (Figure). The acceleration resulting from changing the ratio of kinesin-II to OSM-3 did not match previous predictions from in vitro data. Instead, a biased contribution model, in which OSM-3 outcompetes kinesin-II ten-fold, fitted the acceleration observed in vivo. These results suggested a “handover zone” in the PS, in which kinesin-II gradually hands off transport duties to faster and more processive OSM-3. OSM-3 then acts as an efficient long-range motor to bring IFT complexes to the ciliary tip.
Analysis of the turnaround frequency (for example, when kinesins detach from MTs and are transported by dynein) showed that kinesin-II has a constant frequency of reversal along the ciliary PS, whereas OSM-3 reversals increase at either end.
Simulations of IFT based on motor velocity, pause durations, and turnaround probabilities as input parameters recapitulated the net motor localization concentrations observed, in which kinesin-II is more abundant at the ciliary base and TZ, whereas OSM-3 favors the DS, with only a small proportion of each kinesin-2 motor in the PS. In this minimal model, dissociated kinesin-II motors undergo rapid turnaround and recycling to the ciliary base, whereas OSM-3 is recycled mainly to the handover zone (Figure). One surprising implication of the simulation is that the MT doublet structure that defines the PS5 is coincidental, rather than causal, in kinesin-II localization. The mechanism by which kinesin-II reverse directions is not known, but phosphorylation has been implicated in kinesin-II regulation both C. elegans and Chlamydomonas8, 9.
Amongst the techniques used in this study, simultaneous two-color imaging represents an elegant approach—although not unique in the IFT field10—while the automated fourier-filtered kymographic analysis will be a boon to the field. This application, freely available on the Peterman lab website, calculates position-dependent velocities and enables analysis of velocity changes. Previous methods of kymograph analysis, which rely on manual tracing of straight lines, overlooked the handover zone, which Prevo et al. identified by curved traces.
These studies open up new lines of inquiry that include identifying OSM-3 bias contribution factors: are they intrinsic to the motors, or mediated by other molecules such as MT post-translational modifications11? What specific cargos are transported by kinesin-II and OSM-3, and how does this complex cooperativity influence cargo delivery? Is the transient interaction of kinesin-II and OSM-3 dependent on the BBS proteins, previously found to link IFT-A/IFT-B complexes12?
Another question is whether the findings of Prevo et al. can be extended diverse cilia types and to other organisms. The worm itself possesses multiple ciliated cell types. In cephalic male-specific (CEM) cilia, both heterotrimeric kinesin-II and OSM-3 traverse proximal and distal segments and act redundantly to drive ciliogenesis13. CEM cilia possess a third motor, the kinesin-3 KLP-6, which regulates the IFT kinesin-2 motors. Hence, accessory kinesins may be deployed in a cell-type specific manner to specialize a cilium, as OSM-3 acts in amphid and phasmid cilia, and KLP-6 in CEM cilia2, 13.
An emerging theme is that kinesin-II plays an essential role in ciliogenesis, whereas accessory motors are associated with specializations in ciliary form and function. KIF17 directs cyclic nucleotide gated channels to olfactory cilia in vitro14. In co-transfected mammalian cells, mCherry-KIF3A and eGFP-KIF17 and accumulate at the basal bodies and distal tips and of cilia15, respectively, reminiscent of kinesin-II and OSM-3 enrichment in the TZ and DS of C. elegans chemosensory cilia. In addition to the kinesin-2 motors, additional KIFs are found in mammalian cilia. Kinesin-8 KIF19A localizes to the tips of 9+2 motile cilia to promote MT depolymerization, while kinesin-4 KIF7/Costal-2 localizes to tips of 9+0 primary cilia to prevent new MT polymerization. Hence different kinesins and mechanisms regulate MT polymerization and ciliary length in mammalian motile and primary cilia (reviewed in16).
The work of Prevo et al. also has important implications for understanding human health; defects in cooperativity might lead to cell-type specific ciliopathies such as photoreceptor degeneration.
References
- 1.Rosenbaum JL, Witman GB. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 2.Snow JJ, et al. Nat Cell Biol. 2004;6:1109–1113. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- 3.Prevo B, et al. Nat Cell Biol. 2015 doi: 10.1038/ncb3263. [DOI] [PubMed] [Google Scholar]
- 4.Mallik R, et al. Trends Cell Biol. 2013;23:575–582. doi: 10.1016/j.tcb.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 6.Reiter JF, Blacque OE, Leroux MR. EMBO Rep. 2012;13:608–618. doi: 10.1038/embor.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoeprich GJ, et al. Biophys J. 2014;106:1691–1700. doi: 10.1016/j.bpj.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burghoorn J, et al. Proc Natl Acad Sci U S A. 2007;104:7157–7162. doi: 10.1073/pnas.0606974104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berman SA, Wilson NF, Haas NA, Lefebvre PA. Curr Biol. 2003;13:1145–1149. doi: 10.1016/s0960-9822(03)00415-9. [DOI] [PubMed] [Google Scholar]
- 10.Williams CL, et al. Nat Commun. 2014;5:5813. doi: 10.1038/ncomms6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Hagan R, et al. Curr Biol. 2011;21:1685–1694. doi: 10.1016/j.cub.2011.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ou G, et al. Nature. 2005;436:583–587. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- 13.Morsci NS, Barr MM. Curr Biol. 2011;21:1239–1244. doi: 10.1016/j.cub.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins PM, et al. Curr Biol. 2006;16:1211–1216. doi: 10.1016/j.cub.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 15.Jiang L, et al. Faseb J. 2015 [Google Scholar]
- 16.Niwa S. Anat Sci Int. 2015;90:1–6. doi: 10.1007/s12565-014-0259-5. [DOI] [PubMed] [Google Scholar]