Summary
Background
Cancer-induced muscle wasting begins early in the course of a patient's malignant disease, resulting in declining physical function and other detrimental clinical consequences. This randomised, double-blind, placebo-controlled phase 2 trial assessed the efficacy and safety of enobosarm, a selective androgen receptor modulator, in patients with cancer.
Methods
We enrolled male (>45 years) and female (postmenopausal) patients with cancer who were not obese and who had at least 2% weight loss in the previous 6 months. Participants were randomly assigned (1:1:1 ratio, by computer generated list, block size three, stratified by cancer type) to receive once-daily oral enobosarm 1 mg, 3 mg, or placebo for up to 113 days at US and Argentinian oncology clinics. The sponsor, study personnel, and participants were masked to assignment. The primary endpoint was change in total lean body mass from baseline, assessed by dual-energy x-ray absorptiometry. Efficacy analyses were done only in patients who had a baseline and an on-treatment assessment in the protocol-specified window of within 10 days before baseline or first study drug, and within 10 days of day 113 or end of study (evaluable efficacy population). Adverse events and other safety measurements were assessed in the intention-to-treat (safety) population. This trial is registered with ClinicalTrials.gov, number NCT00467844.
Findings
Enrolment started on July 3, 2007, and the last patient completed the trial on Aug 1, 2008. 159 patients were analysed for safety (placebo, n=52; enobosarm 1 mg, n=53; enobosarm 3 mg, n=54). The evaluable efficacy population included 100 participants (placebo, n=34; enobosarm 1 mg, n=32; enobosarm 3 mg, n=34). Compared with baseline, significant increases in total lean body mass by day 113 or end of study were noted in both enobosarm groups (enobosarm 1 mg median 1·5 kg, range −2·1 to 12·6, p=0·0012; enodosarm 3 mg 1·0 kg, −4·8 to 11·5, p=0·046). Change in total lean body mass within the placebo group (median 0·02 kg, range –5·8 to 6·7) was not significant (p=0·88). The most common serious adverse events were malignant neoplasm progression (eight of 52 [15%] with placebo vs five of 53 [9%] with enobosarm 1 mg vs seven of 54 [13%] with enobosarm 3 mg), pneumonia (two [4%] vs two [4%] vs three [6%]), and febrile neutropenia (three [6% vs one [2%] vs none). None of these events were deemed related to study drug.
Interpretation
Cancer cachexia is an unmet medical need and our data suggest that use of enobosarm might lead to improvements in lean body mass, without the toxic effects associated with androgens and progestational agents.
Funding
GTx.
Introduction
Cancer cachexia, also known as muscle wasting, is a multifactorial syndrome characterised by continuing loss of skeletal muscle mass (with or without loss of fat mass) that leads to progressive functional impairment.1–3 Cachexia often develops progressively through a continuum of stages (precachexia, cachexia, and refractory cachexia) that are associated with increased proinflammatory cytokines and defined by worsening metabolic and clinical signs, including anorexia, impaired glucose tolerance, muscle wasting, involuntary weight loss, and decreased life expectancy.1 Between 15% and 30% of patients with cancer are estimated to be affected by cachexia.2,3 However, these estimates do not account for precachexia, or muscle loss with no change in body-mass index (BMI), and probably underestimate the actual prevalence of this disorder. Muscle wasting was shown to be a prominent clinical sign in patients with non-small-cell lung cancer (NSCLC), with nearly 50% of patients exhibiting severe muscle depletion at time of referral, irrespective of BMI.4
Cancer-induced muscle wasting begins early in the disease process, resulting in decreased physical function and other detrimental consequences. Patients with muscle wasting commonly present with fatigue, weight loss, and reduced physical function, which can contribute to disability, reduced quality of life, and shorter overall survival compared with patients without muscle loss.5,6 Muscle wasting is also associated with worse treatment outcomes and reduced tolerance and response to chemotherapy.7–9 Loss of muscle mass, irrespective of BMI, has detrimental clinical consequences early in the course of a patient's malignant disease, underscoring the importance of diagnosis and treatment of cachexia at an early stage.1 Although anorexia or reduced food intake is also a common clinical sign, nutritional supplementation is unable to reverse the underlying catabolic cause of wasting or restore muscle mass.10,11 As far as we are aware, no products are approved for prevention or treatment of muscle wasting in patients with cancer.
Agents that directly address the accelerated loss of skeletal muscle mass are being assessed for prevention and treatment of muscle wasting. Anabolic androgenic steroids, such as testosterone, have been shown to increase lean body mass.5 However, the absence of tissue specificity and associated side-effects of these agents have limited their use.12 Selective androgen receptor modulators are a new class of non-steroidal, tissue-specific, anabolic agents that have potential to increase muscle mass and improve physical function without the unwanted effects on the prostate, skin, or hair that are commonly associated with testosterone or other non-selective, synthetic anabolic steroids.13–20 Enobosarm (GTx-024; GTx, Memphis, TN, USA) is a selective androgen receptor modulator that induces conformational changes in the androgen receptor upon binding, which selectively alters the interaction of the receptor with coactivator and corepressor proteins that exist in different tissues and changes the receptor's ability to regulate gene expression.7,21
Improvements in lean body mass and physical function were shown in a phase 2, double-blind, placebo-controlled study of enobosarm in healthy postmenopausal women and elderly men.8 As far as we are aware, the present study is the first randomised, double-blind, placebo-controlled, multicentre phase 2 trial to assess efficacy and safety of a selective androgen receptor modulator for the treatment of muscle wasting in patients with cancer.
Methods
Study design and participants
This randomised, double-blind, placebo-controlled study was done at 27 US sites and 12 Argentinian sites. Eligible patients had a BMI of 35 kg/m2 or less and were men older than 45 years and postmenopausal women. Additional inclusion criteria included at least 2% weight loss in the 6 months before randomisation, life expectancy of more than 6 months, and an Eastern Cooperative Oncology Group (ECOG) score of 1 or less. Patients must have been diagnosed with NSCLC (stage II, III, or IV), colorectal cancer (stage II, III, or IV), non-Hodgkin lymphoma, chronic lymphocytic leukaemia, or breast cancer (stage III, or IV) and had not yet begun chemotherapy or were between chemotherapy cycles.
Two amendments to the protocol affected eligibility. The protocol was amended on Sept 27, 2007, to allow patients with stage 4 cancer to be enrolled in the study because patients with stage 4 cancer and an ECOG of less than 1 are likely to have a life expectancy of more than 6 months. The next amendment on March 12, 2008, opened up the study to patients with stage III and IV breast cancer. We included patients with breast cancer because this population was identified as having a substantial problem with cachexia by several sites. Key exclusion criteria were a history of active or uncontrolled congestive heart failure, hypertension, chronic hepatitis, hepatic cirrhosis, or infection with HIV or hepatitis A, B, or C. Patients with aspartate aminotransferase or alanine aminotransferase concentrations more than three times the upper limit of normal or total bilirubin concentrations of more than 34 μmol/L were excluded. Patients taking testosterone, oxandrolone, testosterone-like agents, megestrol acetate, dronabinol, or any prescription drug intended to increase appetite or treat weight loss within the past 30 days (or 6 months if long-term depository) were also excluded. We did not prescribe a specific diet or exercise programme for study participants.
The protocol was approved by the institutional review board at each investigative site and we obtained written informed consent from each patient before study initiation. The study was done in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines.
Randomisation and masking
Treatment assignments were randomly generated by a program created by the study contract research organisation with a block size of three. Treatment was stratified by cancer type and was assigned 1:1:1 to enobosarm 1 mg, enobosarm 3 mg, or placebo. The sponsor, study personnel, sites, and patients were masked to treatment assignments.
Procedures
On the basis of several phase 1 studies and a phase 2 study in healthy, elderly men and postmenopausal women, 1 mg and 3 mg were selected as the most efficacious doses with acceptable safety profiles.8 Participants received enobosarm 1 mg or 3 mg or matching placebo orally once daily for up to 113 days. No dose modifications or reductions were allowed per protocol.
The primary objective was to assess the effects of enobosarm on total lean body mass. Secondary objectives were assessment of the effects of enobosarm on total bodyweight, physical function (stair climb, 6 m walk, grip test), bone turnover markers, total body fat mass, hair growth, prostate-specific antigen, haemoglobin, appetite, quality of life (assessed by Functional Assessment of Anorexia/Cachexia Therapy score [FAACT] and Functional Assessment of Chronic Illness Therapy-Fatigue [FACIT-F]), and inflammation markers. Study visits occurred at screening, randomisation, day 1, day 29, day 57, day 85, before day 113 (between days 104 and 113), and day 113 or end of study (ie, the patient's last recorded assesment on study drug). Lean body mass and body fat mass were assessed by dual-energy x-ray absorptiometry (DXA) at baseline and on day 113 or end of study. Patients who stopped treatment because of malignant progression or adverse events did not have a final scan. Scans were read locally and, as a post-hoc analysis, at a central radiology facility (SYNARC, San Francisco, CA, USA). Changes in bodyweight were measured with scale weight on day 1 and day 113 or end of study. Physical function was assessed at baseline and before day 113 or end of study. Stair climb time was recorded with electronic step switch pads placed on steps 1, 4, 8, and 12. Study site personnel were trained on how to use the equipment via hands-on training, a computer presentation, and a training manual. Stair climb power (watts) was calculated with the formula: P (watts) = (m × g × h)/t, where m is patient's scale weight in kilograms, g is acceleration due to gravity (9·8 m/s2), h is stair height in metres, and t is stair climb time in seconds. Grip strength was measured using the Jamar Hydraulic Hand Dynamometer–5030J1 (Lafayette Instruments Company, Lafayette, IN, USA) in both the left and right hands.
The occurrence, severity, duration, and possible relation of adverse events to enobosarm were recorded from the first dose through the treatment period. Severity of adverse events was graded according to our own criteria: 0=no adverse event or within normal limits; 1=mild adverse event (minor; no specific medical intervention; asymptomatic laboratory findings only, radiographic findings only; marginal clinical relevance); 2=moderate adverse event (minimal intervention; local intervention; non-invasive intervention [packing, cautery]); 3=severe and undesirable adverse event (significant symptoms requiring admission to hospital or invasive inter vention; transfusion; elective interventional radiological procedure; therapeutic endoscopy or operation); 4=life-threatening or disabling adverse event (complicated by acute, life-threatening metabolic or cardiovascular complications, such as circulatory failure, haemorrhage, sepsis. Life-threatening physiological consequences; need for intensive care or emergent invasive procedure; emergent interventional radiological procedure, therapeutic endoscopy or operation); and 5=fatal adverse event. Clinical laboratory results, vital signs, and physical examination findings were also assessed.
Statistical analyses
We calculated our sample size on the basis of data from the phase 2 study in healthy volunteers, with 80% power and an α of 0·05.8 Estimated effect size was based on a difference of 1·4 kg lean body mass (SD 1·73 kg), and the sample size was increased to accommodate a 50% drop out in this population with advanced cancer.
The intention-to-treat (ITT) and safety populations were identical and included all randomised patients who received at least one dose of enobosarm or placebo post-baseline. Efficacy analyses were done only in patients who had a baseline and an on-treatment assessment within the protocol-specified window of within 10 days before baseline or first study drug and within 10 days of day 113 or end of study (evaluable efficacy population), with no adjustments for missing data. Because of the anticipated life expectancy of this population of patients and the fact that the primary endpoint was an on-study assessment, all statistical analyses were done with the efficacy evaluable population except those related to safety and survival, which were done with the ITT or safety population, as previously described.9
The primary efficacy endpoint was change in total lean body mass from baseline to day 113 or end of study in the evaluable efficacy population. Changes from baseline to day 113 or end of study were tested for significance with the exact Wilcoxon signed rank test (StatXact 7 Procs for SAS Users, version 8). We used the exact Wilcoxon rank sum test, stratified by cancer type, to test for significance between groups. Secondary outcomes were summarised with descriptive statistics in a manner much the same as that for the primary endpoint. For changes from baseline in FAACT and FACIT-F score, significance was established with a paired t test. As a post-hoc analysis, we used the Cox proportional hazard model to estimate the hazard ratio (HR) for death and its associated 95% CI. All participants enrolled were included in the survival analysis. Survival time was the time from start of drug until date of death or date of last contact for those who remained alive (censored). No adjustment was made for multiplicity.
We did sample size computations and statistical analyses with SAS/STAT (version 9.1) of the SAS System. This trial is registered with ClinicalTrials.gov, number NCT00467844.
Role of the funding source
GTx sponsored designing of the study and medical writing support, which was was provided by Complete Publication Solutions (Horsham, PA, USA). All authors (those from GTx and those from other institutions) were involved in writing and statistical support, and data interpretation and analysis. All authors had full data access and made the final decision to submit the manuscript for publication.
Results
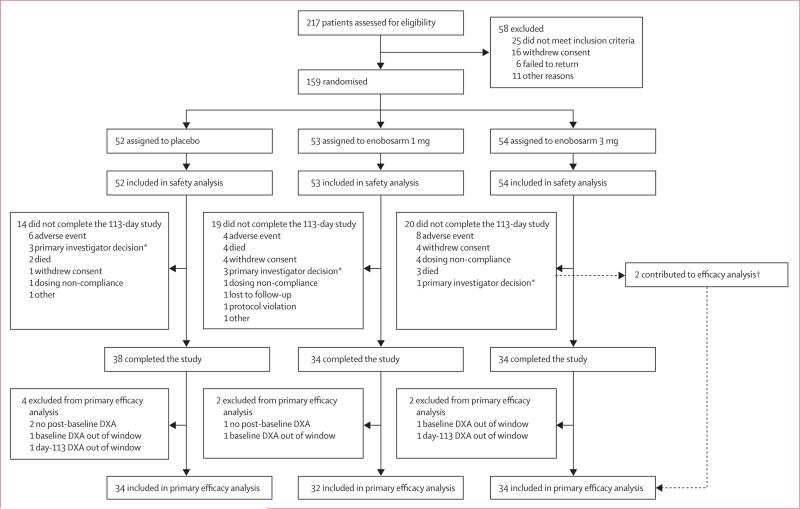
Enrolment started on July 3, 2007, and the last patient completed the trial on Aug 1, 2008. 159 patients were randomised and received at least one dose of study drug (placebo, n=52; enobosarm 1 mg, n=53; or enobosarm 3 mg, n=54). Most (112 of 159; 70%) patients were enrolled at US sites, with the remainder (47 of 159; 30%) enrolled in Argentina. In the ITT (safety) population (n=159), 53 patients discontinued treatment (figure 1). The most common reason for discontinuation was the occurrence of an adverse event (18 of 159; 11%). We identified no between-group differences in distributions of reasons for discontinuation.
Figure 1. Trial profile.
Because of the prevalence of end-stage cancer in this study, 27 deaths occurred; nine discontinued because of death, 16 died after study discontinuation, and two died after study completion. *Removals were done before unmasking of primary investigators. †Two participants in the enobosarm 3 mg group were included in the efficacy analysis but did not complete the trial (one withdrew consent, one because of non-compliance).
Baseline demographic (age, sex, race, and BMI) and clinical characteristics (cancer type and cancer stage) were much the same across treatment groups (table 1). Among patients in the ITT (safety) population, mean age was 65·9 years (SD 9·7) and most patients were male (103 of 159; 65%) and white (143 of 159; 90%). In this population median time since diagnosis of cancer was 11·9 months (range 0–217) and median time since last treatment with chemotherapy was 0·9 months (0–97), which is consistent with a population actively receiving chemotherapy. At baseline, mean percentage weight loss in the 6 months before screening was substantial in all treatment groups (8·8%; SD 5·2%). Substantial comorbidities common among elderly participants included hypertension, diabetes, depression, and hypercholesterolaemia (data not shown).
Table 1.
Demographics and clinical characteristics of patients
| Placebo |
Enobosarm 1 mg |
Enobosarm 3 mg |
||||
|---|---|---|---|---|---|---|
| Safety (n=52) | Efficacy (n=34) | Safety (n=53) | Efficacy (n=32) | Safety (n=54) | Efficacy (n=34) | |
| Mean age, years (range) | 66 (41 to 83) | 65 (41 to 81) | 66 (43 to 87) | 63 (43 to 87) | 66 (39 to 82) | 71 (39 to 81) |
| Sex | ||||||
| Men | 35 (67%) | 21 (62%) | 34 (64%) | 16 (50%) | 34 (63%) | 21 (62%) |
| Women | 17 (33%) | 13 (38%) | 19 (36%) | 16 (50%) | 20 (37%) | 13 (38%) |
| Race | ||||||
| White | 47 (90%) | 32 (94%) | 45 (85%) | 26 (81%) | 51 (94%) | 32 (94%) |
| Other* | 5 (10%) | 2 (6%) | 8 (15%) | 6 (19%) | 3 (6%) | 2 (6%) |
| Cancer type | ||||||
| NSCLC | 21 (40%) | 10 (29%) | 21 (40%) | 8 (25%) | 19 (35%) | 13 (38%) |
| Stage II; III; IV | 1; 5; 15 | 1; 3; 6 | 0; 9; 12 | 0; 5; 3 | 3; 7; 9 | 2; 6; 5 |
| Colorectal | 21 (40%) | 15 (44%) | 21 (40%) | 17 (53%) | 20 (37%) | 12 (35%) |
| Stage II; III; IV | 3; 5; 13 | 3; 4; 8 | 3; 6; 12 | 3; 4; 10 | 1; 7; 12 | 0; 3; 9 |
| Other† | 10 (19%) | 9 (26%) | 11 (21%) | 7 (22%) | 15 (28%) | 9 (26%) |
| Chemotherapy with CRF | ||||||
| n | 39 | 26 | 36 | 24 | 44 | 31 |
| Chemotherapy on study | 31 (79%) | 24 (92%) | 31 (86%) | 24 (100%) | 36 (82%) | 26 (84%) |
| Weight change‡ | ||||||
| kg | –7·4 (5·3) | –6·5 (4·9) | –7·2 (4·0) | –7·4 (4·4) | –5·6 (3·2) | –5·8 (3·6) |
| % | –9·4 (6·0) | –7·9 (4·8) | –9·7 (5·1) | –10·2 (5·6) | –7·4 (3·9) | –7·5 (4·3) |
| BMI (kg/m2) | 24·1 (4·6) | 24·8 (4·9) | 23·5 (4·8) | 23·6 (4·6) | 24·0 (3·9) | 24·1 (4·1) |
| Lean body mass (kg) | 47·2 (27·7 to 72·1) | 46·6 (27·7 to 72·1) | 44·9 (27·0 to 63·6) | 41·6 (27·0 to 63·6) | 44·8 (28·4 to 68·4) | 46·3 (30·6 to 68·4) |
| Stair climb power§ | ||||||
| n | 52 | 36 | 53 | 31 | 53 | 28 |
| Stairs 1–12 (watts) | 137·6 (48·8–442·3) | 150·2 (72·8–442·3) | 127·9 (25·3–375·5) | 116·4 (43·9–375·5) | 154·4 (16·6–413·3) | 154·5 (16·6–286·9) |
| Grip strength | ||||||
| n | 52 | 34 | 53 | 30 | 54 | 33 |
| kg | 27·0 (6·4–56·3) | 26·5 (6·4–56·3) | 25·5 (2·3–85·0) | 24·1 (2·3–85·0) | 26·0 (8·0–95·6) | 26·0 (8·0–95·6) |
Data are n (%), mean (SD), or median (range) unless otherwise specified. Efficacy=patients for whom efficacy data was available, no adjustment for missing data. NSCLC=non-small-cell lung cancer. CRF=cancer-related fatigue. BMI=body-mass index.
Hispanic, black, and Native American or Alaskan.
Non-Hodgkin lymphoma, chronic lymphocytic leukaemia, and breast cancer.
Weight change in the previous 6 months.
Not all patients had a baseline value.
We identified a statistically significant increase from baseline to day 113 or end of study in total lean body mass in patients who received enobosarm 1 mg (p=0·0012) and enobosarm 3 mg (p=0·046), but not in patients assigned placebo (table 2). Results of centrally read DXA scans were consistent with those of the primary local read (data not shown).
Table 2.
Change in total lean body mass at day 113 or end of study compared with baseline
| Placebo | Enobosarm 1 mg | Enobosarm 3 mg | |
|---|---|---|---|
| N | 34 | 32 | 34 |
| Mean (SD), kg | 0·1 (2·7) | 1·5 (2·7) | 1·3 (3·5) |
| Median (range), kg | 0·02 (–5·8 to 6·7) | 1·5 (–2·1 to 12·6) | 1·0 (–4·8 to 11·5) |
| p value* | 0·88 | 0·0012 | 0·046 |
These analyses were done for patients for whom efficacy data were evaluable; no adjustments have been made for missing data. p value for enobosarm 1 mg vs placebo is 0·066; for enobosarm 3 mg vs placebo is 0·041.
p values are from an exact Wilcoxon signed rank test.
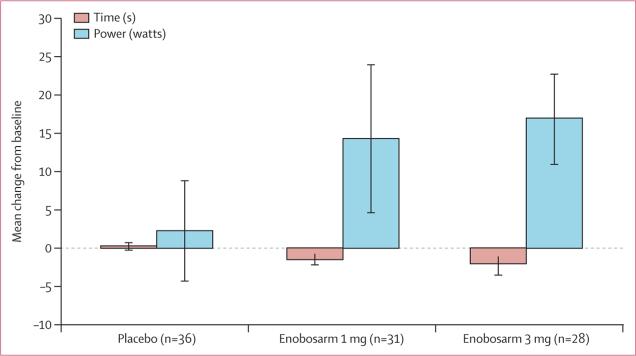
Median time required to climb 12 stairs changed little from baseline to day 113 or end of study in the placebo group, but was significantly decreased in the enobosarm 1 mg group and enobosarm 3 mg group (table 3, figure 2). We recorded a significant increase from baseline to day 113 or end of study in mean stair climb power (stairs 1–12) among patients who received enobosarm 1 mg and enobosarm 3 mg, but not in patients assigned placebo (table 3, figure 2). Absolute changes in stair climb power represented a mean of 18·0% (SD 31·1) improvement compared with baseline for enobosarm 1 mg and 21·7% (65·7) for enobosarm 3 mg (vs placebo, 4·8% [SD 23·2]).
Table 3.
Change in stair climb test at day 113 or end of study compared with baseline
| Placebo | Enobosarm 1 mg | Enobosarm 3 mg | |
|---|---|---|---|
|
Stair climb time (s)
| |||
| N | 36 | 32 | 28 |
| Mean (SD) | 0·20 (2·98) | –1·63 (3·39) | –2·22 (7·05) |
| Median (range) | –0·14 (–4·61 to 14·54) | –0·84 (–12·67 to 5·56) | –0·46 (–31·01 to 5·06) |
| p value* | 0·26 | 0·0019 | 0·0065 |
|
Stair climb power (watts)† | |||
| N | 36 | 31 | 28 |
| Mean (SD) | 2·21 (39·30) | 14·26 (53·77) | 16·81 (31·08) |
| Median (range) | 11·34 (–156·36 to 56·37) | 19·93 (–235·34 to 110·14) | 12·84 (–77·74 to 93·15) |
| p value* | 0·11 | 0·0008 | 0·0006 |
These analyses were done for patients for whom efficacy data were evaluable; no adjustments have been made for missing data.
p values are from an exact Wilcoxon signed rank test.
Power=(9·8 [m/s2]× stair height [m] × weight [kg])/stair climb time (s).
Figure 2. Mean absolute change from baseline to day 113 or end of study in stair climb time and power.
These analyses were done for patients for whom efficacy data were evaluable; no adjustments have been made for missing data. Error bars represent 1 SE.
We identified no significant differences in the secondary endpoints of absolute change or percentage change in hand grip strength (average of both hands) from baseline to day 113 or end of study (table 4).
Table 4.
Change in secondary endpoints at day 113 or end of study compared with baseline
| Placebo | Enobosarm 1 mg | Enobosarm 3 mg | |
|---|---|---|---|
|
Total bodyweight (scale weight)
| |||
| n | 36 | 34 | 35 |
| Mean (SD) | 0·93 (4·13) | 1·00 (4·27) | 1·12 (4·02) |
| Median (range) | 0·90 (–9·6 to 12·0) | 0·80 (–7·3 to 12·3) | 0·40 (–7·7 to 9·9) |
| p value | 0·091 | 0·205 | 0·169 |
|
Total bodyweight (DXA weight) | |||
| n | 34 | 32 | 34 |
| Mean (SD) | 0·52 (3·79) | 0·85 (4·29) | 0·51 (4·20) |
| Median (range) | 0·68 (–8·55 to 10·45) | 0·30 (–6·53 to 9·71) | 0·21 (–9·97 to 9·39) |
| p value | 0·270 | 0·400 | 0·586 |
|
6 m walk (gait speed) | |||
| n | 36 | 33 | 34 |
| Mean (SD) | 0·13 (1·61) | –0·73 (2·72) | 0·36 (2·90) |
| Median (range) | –0·04 (–3·18 to 5·87) | –0·40 (–10·15 to 7·27) | 0·00 (–3·39 to 13·63) |
| p value | 0·871 | 0·068 | 0·987 |
|
Grip test (both hands) | |||
| n | 36 | 32 | 34 |
| Mean (SD) | 6·42 (–0·90) | 8·57 (0·52) | 10·80 (–0·45) |
| Median (range) | –0·06 (–30·00 to 16·25) | 2·00 (–37·50 to 14·50) | 0·00 (–50·13 to 16·25) |
| p value | 0·439 | 0·222 | 0·747 |
|
Bone turnover markers (type I collagen C-telopeptide, ng/mL) | |||
| n | 35 | 23 | 26 |
| Mean (SD) | 0·00 (0·23) | –0·05 (0·16) | 0·05 (0·33) |
| Median (range) | –0·02 (–0·35 to 0·64) | –0·06 (–0·32 to 0·30) | 0·01 (–0·49 to 1·00) |
| p value | 0·913 | 0·145 | 0·447 |
|
Bone turnover markers (N-telopeptide, nmol/L BCE) | |||
| n | 35 | 23 | 26 |
| Mean (SD) | 0·76 (4·81) | –0·09 (3·93) | 3·27 (6·05) |
| Median (range) | 0·70 (–7·6 to 17·3) | 0·40 (–7·4 to 8·4) | 2·05 (–8·6 to 15·5) |
| p value | 0·354 | 0·912 | 0·011 |
|
Bone turnover markers (osteocalcin, ng/mL) | |||
| n | 35 | 28 | 30 |
| Mean (SD) | 0·29 (3·41) | 1·25 (3·98) | –0·39 (4·35) |
| Median (range) | –0·10 (–7·7 to 9·8) | 0·60 (–5·5 to 10·5) | –0·80 (–8·3 to 11·2) |
| p value | 0·613 | 0·109 | 0·624 |
|
Bone turnover markers (bone-specific alkaline phosphatase, U/L) | |||
| n | 35 | 28 | 30 |
| Mean (SD) | –1·47 (9·51) | 1·99 (10·32) | –0·37 (13·02) |
| Median (range) | 0·00 (–21·9 to 17·1) | –1·10 (–11·8 to 28·2) | –4·55 (–19·0 to 34·0) |
| p value | 0·367 | 0·317 | 0·877 |
|
Total body fat mass | |||
| n | 34 | 32 | 34 |
| Mean (SD) | 411·72 (3562·55) | –641·83 (3571·48) | –764·40 (3617·23) |
| Median (range) | 479·80 (–8792·3 to 9723·0) | –962·00 (–6341·0 to 8605·0) | –759·50 (12747·0 to 8691·0) |
| p value | 0·272 | 0·210 | 0·328 |
|
Hair growth (women only) | |||
| n | 13 | 14 | 15 |
| Mean (SD) | 0·0 (2·48) | –0·4 (1·95) | 0·4 (1·40) |
| Median (range) | 0·0 (–6 to 6) | 0·0 (–7 to 1) | 0·0 (–1 to 4) |
| p value | >0·999 | 0·504 | 0·288 |
|
Prostate-specific antigen | |||
| n | 23 | 17 | 20 |
| Mean (SD) | –0·08 (0·45) | –0·42 (0·79) | –0·09 (0·61) |
| Median (range) | 0·00 (–1·4 to 0·8) | –0·10 (–2·5 to 0·3) | –0·05 (–0·9 to 1·7) |
| p value | 0·415 | 0·041 | 0·518 |
|
Haemoglobin A1c | |||
| n | 36 | 32 | 34 |
| Mean (SD) | –0·14 (0·45) | –0·15 (0·62) | –0·06 (0·68) |
| Median (range) | –0·15 (–1·0 to 0·7) | –0·10 (–1·9 to 1·3) | –0·05 (–1·5 to 1·7) |
| p value | 0·064 | 0·193 | 0·633 |
|
Anorexia cachexia subscale | |||
| n | 36 | 32 | 34 |
| Mean (SD) | 2·31 (9·64) | 6·95 (4·63) | 3·12 (8·98) |
| Median (range) | 2·0 (–20 to 23) | 2·0 (–6 to 20) | 3·5 (–14 to 24) |
| p value | 0·160 | 0·001 | 0·051 |
|
FAACT | |||
| n | 36 | 32 | 34 |
| Mean (SD) | 2·30 (16·54) | 9·48 (18·52) | 4·14 (16·22) |
| Median (range) | 2·40 (–31·83 to 48·00) | 7·50 (–19·00 to 49·83) | 4·08 (–23·83 to 42·50) |
| p value | 0·411 | 0·007 | 0·146 |
|
FACIT-F | |||
| n | 36 | 32 | 34 |
| Mean (SD) | 1·60 (17·70) | 9·41 (23·38) | 0·99 (20·36) |
| Median (range) | –0·30 (–42·00 to 44·00) | 2·92 (–39·00 to 60·00) | 2·12 (–33·83 to 46·50) |
| p value | 0·591 | 0·030 | 0·779 |
|
Inflammation markers (C-reactive protein, mg/dL) | |||
| n | 35 | 32 | 29 |
| Mean (SD) | 3·69 (0·31) | 2·46 (0·59) | 6·54 (1·96) |
| Median (range) | 0·09 (–14·01 to 10·57) | 0·12 (–3·57 to 7·96) | –0·01 (–6·37 to 28·28) |
| p value | 0·624 | 0·186 | 0·118 |
|
Inflammation markers (interleukin 6, pg/mL) | |||
| n | 33 | 25 | 29 |
| Mean (SD) | 40·43 (–4·01) | 13·41 (2·09) | 114·76 (17·07) |
| Median (range) | 0·45 (–210·07 to 65·89) | –0·12 (–13·19 to 59·71) | –0·04 (–174·70 to 580·61) |
| p value | 0·573 | 0·444 | 0·430 |
|
Inflammation markers (TNFα, pg/mL) | |||
| n | 35 | 25 | 30 |
| Mean (SD) | 1·43 (0·37) | 1·09 (0·78) | 6·58 (1·97) |
| Median (range) | 0·59 (–6·38 to 2·18) | 0·66 (–1·09 to 3·10) | 0·75 (–1·99 to 35·74) |
| p value | 0·132 | 0·001 | 0·111 |
These analyses were done for patients for whom efficacy data were evaluable; no adjustments have been made for missing data. DXA=x-ray absorptiometry. BCE=bone collagen equivalent. FAACT=Functional Assessment of Anorexia/Cachexia Therapy. FACIT-F=Functional Assessment of Chronic Illness Therapy: Fatigue. TNFα=tumour necrosis factor α.
We identified no significant differences in ECOG or frailty status between patients in the enobosarm or placebo groups (tables 5 and 6).
Table 5.
Change in ECOG status at day 133 or end of study compared with baseline
| Placebo (n=38) | Enobosarm 1 mg (n=34) | Enobosarm 3 mg (n=35) | |
|---|---|---|---|
| Worsened | 6 (15·8%) | 5 (14·7%) | 8 (22·9%) |
| No change | 27 (71·1%) | 22 (64·7%) | 22 (62·9%) |
| Improved | 5 (13·2%) | 7 (20·6%) | 5 (14·3%) |
Data are n (%). These analyses were done for patients for whom efficacy data were evaluable; no adjustments have been made for missing data. ECOG=Eastern Cooperative Oncology Group.
Table 6.
Change in frailty status at day 113 or end of study compared with baseline
| Placebo (n=38) | Enobosarm 1 mg (n=35) | Enobosarm 3 mg (n=41) | ||||
|---|---|---|---|---|---|---|
| Frail at baseline | Not frail at baseline | Frail at baseline | Not frail at baseline | Frail at baseline | Not frail at baseline | |
|
Weight
| ||||||
| Frail at day 113 | 6 (17%) | 3 (8%) | 11 (32%) | 6 (18%) | 6 (17%) | 3 (9%) |
| Not frail at day 113 | 3 (8%) | 24 (67%) | 3 (9%) | 14 (41%) | 4 (11%) | 22 (63%) |
|
Exhaustion | ||||||
| Frail at day 113 | 10 (29%) | 5 (15%) | 5 (18%) | 6 (21%) | 7 (23%) | 6 (19%) |
| Not frail at day 113 | 7 (21%) | 12 (35%) | 5 (18%) | 12 (43%) | 3 (10%) | 15 (48%) |
|
Physical activity | ||||||
| Frail at day 113 | 11 (31%) | 7 (20%) | 10 (33%) | 3 (10%) | 10 (30%) | 8 (24%) |
| Not frail at day 113 | 3 (9%) | 14 (40%) | 2 (7%) | 15 (50%) | 7 (21%) | 8 (24%) |
|
Walk time | ||||||
| Frail at day 113 | 2 (6%) | 0 | 4 (12%) | 5 (15%) | 2 (6%) | 1 (3%) |
| Not frail at day 113 | 3 (9%) | 30 (86%) | 0 | 24 (73%) | 1 (3%) | 29 (88%) |
|
Grip strength | ||||||
| Frail at day 113 | 9 (26%) | 1 (3%) | 8 (26%) | 4 (13%) | 7 (21%) | 5 (15%) |
| Not frail at day 113 | 4 (11%) | 21 (60%) | 1 (3%) | 18 (58%) | 5 (15%) | 16 (48%) |
Data are n (%). These analyses were done for patients for whom efficacy data were evaluable; no adjustments have been made for missing data.
Notably, there were no significant differences within the enobosarm and placebo groups in change in hair growth in women (modified Ferriman-Gallwey score22,23) or serum prostate-specific antigen concentrations in men (table 4).
The total number of adverse events was much the same between treatment groups, with 46 (88%) of 52 patients in the placebo group, 47 (89%) of 53 patients in the enobosarm 1 mg group, and 49 (91%) of 54 patients in the enobosarm 3 mg group having an adverse event. The most common grade 1–2 adverse events in the placebo group were fatigue (11 of 52, 21%) and nausea (seven, 13%); in the enobosarm 1 mg group were nausea (12 of 53, 23%) and anaemia and constipation (both nine, 17%); and in the enobosarm 3 mg group were fatigue (11 of 54, 20%), and diarrhoea and cough (both nine, 17%). These events are consistent with patients with cancer who are undergoing chemotherapy (table 7). Although treatment-related adverse events were more common in the enobosarm 3 mg group (15 of 54, 28%) than in the enobosarm 1 mg group (seven of 53, 13%) or placebo group (eight of 52, 15%; appendix), the frequency of specific adverse events was generally much the same across treatment groups and we identified no notable group differences in the number of patients discontinuing because of adverse events or in the type of adverse events that led to study discontinuation (placebo, six of 52 [12%] discontinued: idiosyncratic drug reaction 1, malignant neoplasm progression 3, NSCLC progression 1, disorientation 1. Enobosarm 1 mg, four of 53 [8%] discontinued: therapeutic agent toxicity 1, NSCLC progression 1, brain stem infarction 1, dizziness 1. Enobosarm 3 mg, eight of 54 [15%] discontinued: asthenia 1, fatigue 2, cellulitis 1, malignant neoplasm progression 2, headache 1, depression 1).
Table 7.
Summary of adverse events (safety population)
| Placebo (n=52) |
Enobosarm 1 mg (n=53) |
Enobosarm 3 mg (n=54) |
||||
|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | |
| Abdominal pain | 3 (6%) | 0 | 2 (4%) | 0 | 8 (15%) | 0 |
| Anaemia | 6 (12%) | 2 (4%) | 9 (17%) | 4 (8%) | 5 (9%) | 2 (4%) |
| Anorexia | 2 (4%) | 0 | 4 (8%) | 0 | 6 (11%) | 0 |
| Asthenia | 4 (8%) | 0 | 1 (2%) | 0 | 7 (13%) | 0 |
| Back pain | 3 (6%) | 0 | 5 (9%) | 0 | 1 (2%) | 0 |
| Constipation | 2 (4%) | 0 | 9 (17%) | 0 | 6 (11%) | 0 |
| Cough | 6 (12%) | 0 | 2 (4%) | 0 | 9 (17%) | 0 |
| Dehydration | 3 (6%) | 3 (6%) | 1 (2%) | 0 | 7 (13%) | 0 |
| Diarrhoea | 4 (8%) | 0 | 8 (15%) | 0 | 9 (17%) | 0 |
| Dyspnoea | 5 (10%) | 0 | 6 (11%) | 0 | 3 (6%) | 0 |
| Fatigue | 11 (21%) | 0 | 8 (15%) | 0 | 11 (20%) | 3 (6%) |
| Febrile neutropenia | 0 | 3 (6%) | 0 | 0 | 0 | 0 |
| Headache | 1 (2%) | 0 | 3 (6%) | 0 | 3 (6%) | 0 |
| Malignant neoplasm progression | 0 | 8 (15%) | 0 | 5 (9%) | 0 | 7 (13%) |
| Nausea | 7 (13%) | 0 | 12 (23%) | 0 | 6 (11%) | 0 |
| Neutropenia | 2 (4%) | 0 | 1 (2%) | 0 | 4 (7%) | 0 |
| Pneumonia | 1 (2%) | 0 | 1 (2%) | 0 | 4 (7%) | 0 |
| Pyrexia | 2 (4%) | 0 | 5 (9%) | 0 | 8 (15%) | 0 |
| Thrombocytopenia | 0 | 3 (6%) | 3 (6%) | 1 (2%) | 4 (7%) | 1 (2%) |
| Vomiting | 6 (12%) | 0 | 8 (15%) | 0 | 4 (7%) | 0 |
| Weight decreased | 5 (10%) | 0 | 3 (6%) | 0 | 8 (15%) | 0 |
Data are n (%). Adverse event occurring in >5% of patients in any treatment group. Severity criteria: 0=no adverse event or within normal limits; 1=mild adverse event (minor; no specific medical intervention; asymptomatic laboratory findings only, radiographic findings only; marginal clinical relevance); 2=moderate adverse event (minimal intervention; local intervention; non-invasive intervention [packing, cautery]); 3=severe and undesirable adverse event (significant symptoms requiring admission to hospital or invasive intervention; transfusion; elective interventional radiological procedure; therapeutic endoscopy or operation); 4=life-threatening or disabling adverse event (complicated by acute, life-threatening metabolic or cardiovascular complications, such as circulatory failure, haemorrhage, sepsis; life-threatening physiologic consequences; need for intensive care or emergent invasive procedure; emergent interventional radiological procedure, therapeutic endoscopy or operation); and 5=fatal adverse event.
The occurrence of serious adverse events (those with significant symptoms requiring admission to hospital or invasive intervention) was 14 of 53 (26%) in the enobosarm 1 mg group, 15 of 54 (28%) in the enobosarm 3 mg group, and 17 of 52 (33%) the placebo group. The most common serious adverse event (those occurring in more than 3% of the patients in any treatment group) was malignant neoplasm progression in eight (15%) patients in the placebo group, five (9%) patients in the enobosarm 1 mg group, and seven (13%) patients in the enobosarm 3 mg group. Two patients (4%) in the placebo group also had pneumonia and three (6%) had febrile neutropenia. Three patients (6%) in enobosarm 3 mg group had pneumonia. Two patients (4%) in the enobosarm 1 mg group had anaemia, two (4%) had therapeutic agent toxicity, two (4%) had pneumonia, and one (2%) had febrile neutropenia.
Life-threatening adverse events (those complicated by acute, life-threatening metabolic or cardiovascular complications) were reported by six of 53 (11%) patients in the enobosarm 1 mg group, four of 54 (7%) patients in the enobosarm 3 mg group, and eight of 52 (15%) patients in the placebo group. No serious or life-threatening adverse events were deemed to be related to study drug.
We noted no difference in occurrence of tumour progression for the treated versus placebo groups (eight of 52 [15·4%] with placebo vs five of 53 [9·4%] with enobosarm 1 mg vs seven of 54 [13·0%] with enobosarm 3 mg). 27 deaths occurred (placebo, n=11; enobosarm 1 mg, n=8; enobosarm 3 mg, n=8), a rate consistent with the large percentage of patients with late-stage cancer in the trial. No deaths were attributed to study drug. The trial was not powered to assess survival; however, in a post-hoc analysis, the survival HR for patients assigned enobosarm 1 mg versus placebo was 0·80 (95% CI 0·31– 2·02) and for those assigned enobosarm 3 mg versus placebo was 0·70 (0·28–1·74).
Although variability was noted in laboratory values in this population of patients receiving treatment for their malignancy, none of these values raised safety concerns. Most patients maintained normal alanine amino-transferase concentrations (data not shown). Three patients in the enobosarm 3 mg treatment group had transient (returning to normal while still on drug) alanine aminotransferase increases that were two to three times the upper limit of normal; one patient each in the enobosarm 3 mg and placebo groups had a transient alanine aminotransferase increase to more than three times the upper limit of normal. No patient had an alanine aminotransferase increase more than four times the upper limit of normal or discontinued because of increased alanine aminotransferase. The number of patients with clinically significant changes in total bilirubin was much the same across treatment groups (data not shown).
Discussion
Both 1 mg and 3 mg of enobosarm resulted in increases in lean body mass in patients with advanced cancer, compared with baseline measurements. No significant changes from baseline were noted in patients assigned placebo. Adverse events were generally much the same between groups and were consistent with those for patients undergoing chemotherapy.
As far as we are aware, this study is the first clinical trial to examine safety and efficacy of a selective androgen receptor modulator for cancer-induced muscle wasting. Enobosarm was generally well tolerated, with the occurrence of serious adverse events and overall pattern of adverse events being much the same among placebo and treatment groups. Increases in alanine aminotransferase concentrations were transient and no patient discontinued treatment because of increased alanine aminotransferase concentrations. The population of patients enrolled in this study was heterogeneous in terms of cancer type and included a high proportion of patients with advanced stage disease. Patients entering the study had a mean weight loss of about 9% within the previous 6 months, which is substantially higher than the commonly defined 5% weight loss in 6 months for a clinical diagnosis of cachexia and far above the 2% weight loss specified in the inclusion criteria.1,3 Although these patients had greater than the 2% weight loss for study entry, the amount of weight loss at baseline was not judged to be atypical for a population of patients with advanced cancer. Previous studies have shown that about 50% of patients with cancer have significant weight and muscle loss at the time of cancer diagnosis and that this weight or muscle loss continues throughout the course of malignant disease, affecting about 80% of patients at the time of death.4 The population studied in this trial ranged from patients who were newly diagnosed with cancer to patients who had already received several previous treatments for their malignancy. Therefore we would expect that some of these patients had refractory cachexia with substantial weight loss, consistent the average weight loss at baseline in this population.1 Overall, despite the heterogeneity and advanced stage of disease, treatment with enobosarm resulted in clinical benefit as shown by statistically significant improvements in lean body mass and stair climb power.
This study is one of the first to report stair climb power as an assessment of physical function in patients with cancer. The stair climb test is a common measure of physical function in elderly populations. Studies in older adults have shown that reduced lower leg function and low leg power predict subsequent disability, morbidity, and mortality.24–27 The stair climb test is a simple and reliable measure of leg power and, importantly, provides a direct measure of a clinically meaningful activity that takes into consideration several muscle-related attributes (eg, balance, strength, mobility, and endurance) of a major muscle group used for locomotion.10,11 Although Schroeder and colleagues13 showed that changes in lean body mass do not always temporally coincide with changes in physical function, we identified a modest correlation (Spearman's rank correlation coefficient 0·21, p=0·052) between changes in total lean body mass and stair climb power in this study. Hand grip strength is also commonly used to assess physical function but might not be as reliable because of arthritis, atrophy, oedema, or compensatory use of the hands and arms by people who are sick or elderly as they become physically impaired.14,15 Elderly and sick people commonly rely on their arms and hands to assist them as they stand or do other activities of daily living, which could delay or lessen muscle wasting in the upper arms and mask the effects of a pharmacological agent on hand grip strength in these smaller muscle groups.16,17 Physical function tests of gait speed (6 m walk) and hand grip strength were done during this study as exploratory endpoints but no significant differences were noted (tables 4–6).
Irrespective of the physical function test used, thresholds of clinically meaningful change in performance have been established. A minimally clinically meaningful change in physical function is generally defined as a 5% increase from baseline, whereas a substantial clinically meaningful change is a 10% increase from baseline. Published work in healthy elderly and mobility-limited participants has correlated measures of physical function with clinically meaningful changes as established in the Short Physical Performance Battery. In a large randomised trial with adults aged 70–89 years (n=424), Kwon and colleagues26 used 400 m walk and gait speed and showed that a 4–4·5% improvement in physical function translates into “minimally meaningful” change, whereas an improvement of 10% is a “substantial meaningful” change. Perera and colleages27 showed much the same results with gait speed, 6 min walk distance, and self-reported mobility in older adults with mobility disabilities (n=492), concluding that an improvement in performance of 6–8% represents a “small meaningful change” and 11–17% a “substantial meaningful change.” These studies define thresholds for minimally meaningful and substantial meaningful clinical change that can be applied irrespective of the physical function test used. Mean improvement in stair climb power from baseline was 5% in the placebo group, 18% in the enobosarm 1 mg group, and 22% in the enobosarm 3 mg group, providing evidence that the increases in lean body mass were accompanied by substantial clinically meaningful changes in physical function at both doses of enobosarm. The percentage of participants with substantial clinically meaningful increases in physical function (≥10% increase in stair climb power) was 39% (14 of 36) with placebo, 61% (19 of 31) with enobosarm 1 mg, and 61% (17 of 28) with enobosarm 3 mg.
Although as far as we are aware this study is the first to show that a selective androgen receptor modulator increased lean body mass and improved physical function in patients with cancer with existing cachexia (muscle loss), the small sample size and heterogeneity of cancer types studied could be deemed potential limitations. Additionally, since this was not a cancer treatment trial, we did not collect information regarding previous therapy for the underlying cancer, including surgery, radiation, and type of previous chemotherapy and number of previous chemotherapy cycles. Although data for concurrent chemotherapy were collected during the course of the trial (appendix), we cannot be sure whether aggressive previous treatment or absence thereof had an effect on the results of the trial. However, baseline characteristics were much the same for all subgroups of patients, all patients must have been cachectic at study entry and we thus enrolled participants who should be representative of most of those with advanced cancer. Data for nutritional therapy and concurrent supportive care were also not collected during the study and the effect of these interventions is unknown. Nutritional therapy alone has no effect on the underlying catabolic process of cachexia, but it would be interesting to know the potentially synergistic effect that could accrue from nutritional therapy in conjunction with enobosarm.19 With regard to supportive care, Temel and colleagues20 showed that supportive care leads to improved treatment outcomes. Although this association might be true for survival and overall quality of life, we are not aware of data suggesting that supportive care alone leads to improvements in lean body mass and physical function. We believe that a multimodality approach to treatment with supportive care, diet, and nutritional support along with a pharmacological intervention designed to correct the underlying catabolic process of cachexia will ultimately lead to improved outcomes for patients with cancer (panel).
Another limitation is the number of missing patients in the efficacy analysis. Because of the anticipated short (ie, <12 months) life expectancy of a cancer population with cachexia, the evaluable efficacy population (ie, only patients for whom efficacy data were available, with no adjustment for missing data) was chosen for this phase 2 study to understand the therapeutic benefit of a selective androgen receptor modulator. We recognised that missing data would be a limitation of the study. Fewer male patients (68 of 103, 66%) than female patients (46 of 56, 82%) were in the evaluable efficacy population; however, this discrepancy might have arisen because fewer patients with NSCLC (36 of 61, 59%), most of whom were men (46 of 61, 75%), were eligible for inclusion in the efficacy evaluable population compared with those with other cancers. Also, 60% (47 of 78) of participants with stage IV disease were included in the evaluable efficacy population compared with 83% (67 of 81) of participants with cancers of other stages. Age, BMI, and lean body mass at baseline did not differ between those in the evaluable efficacy population and those not (p>0·70 for all comparisons); however, participants assigned placebo in the evaluable efficacy population population had a tendency toward higher baseline physical function (p=0·094) with median power of 77 watts versus 66 watts, possibly suggesting that those patients were more fit at baseline and were more likely to complete the study.
Cancer-induced muscle wasting is a highly prevalent and potentially severe disorder that can adversely affect treatment outcomes, quality of life, and mortality.4,5,7,9 Cancer-induced muscle wasting occurs early in the course of a patient's malignant disease,28 before overt weight loss, resulting in a decline in physical function and other detrimental clinical consequences.4,22,26 Loss of lean body mass and physical function has also been noted during prolonged bed rest.23,24 Thus, effective new therapies and management strategies are needed for prevention and treatment of muscle wasting in patients with cancer. Enobosarm resulted in significant increases in lean body mass and improvements in physical function as assessed by stair climb power at both doses. However, response differences between doses were identified in lean body mass and physical function in different cancer types. In each of the cancer types (NSCLC, colorectal cancer, and others cancers), we identified an increase in median lean body mass and an increase in stair climb power (physical function) for both enobosarm 1 mg and 3 mg. We noted a greater increase in lean body mass in patients with colorectal cancer whereas we recorded the greatest increases in stair climb power in patients with NSCLC.
Enobosarm might offer a promising option for the prevention and treatment of muscle wasting due to cancer by increasing muscle mass without the unwanted virilising effects associated with non-selective anabolic androgenic steroids or the oedema associated with growth hormone or growth hormone-releasing hormone therapies.29,30 Importantly, this study provides evidence of substantial clinically meaningful improvements in physical function with enobosarm treatment at both doses (1 mg and 3 mg). Additionally, a previous clinical study8 showed that the 3 mg dose of enobosarm resulted in larger improvements in lean body mass and physical function in healthy older men and postmenopausal women as compared with the 1 mg dose. The 3 mg dose of enobosarm was chosen for the phase 3 studies because of its similar safety profile to the 1 mg dose and the ability of the 3 mg dose to improve lean body mass and physical function in several populations, ranging from healthy older men and women to older patients with cancer. The 3 mg dose of enobosarm is now being assessed for its ability to prevent and treat muscle wasting in patients with NSCLC in two phase 3 clinical trials (NCT01355484, NCT01355497). In each of these placebo-controlled, double-blind clinical trials, about 300 patients with stage III or IV NSCLC have been randomised to oral daily doses of placebo or enobosarm 3 mg at the time they begin first-line standard platinum doublet chemotherapy. The studies are assessing as coprimary endpoints at 3 months of treatment the responder rates of enobosarm versus placebo on maintenance or improvement of total lean body mass (muscle) assessed by DXA and improvement in physical function measured by the stair climb test (power). These two trials will establish the ability of enobosarm to prevent and treat muscle wasting in patients with NSCLC and might establish a new role for selective anabolic therapies as supportive care for men and women with cancer.8
Supplementary Material
Panel: Research in context.
Systematic review
We searched PubMed with the terms “cancer” and “cachexia” for research reported between January, 2001, and January, 2006; results were filtered for “clinical trial”. We also searched ClinicalTrials.gov for trials reported between January, 1990, and January, 2006, with the terms “cancer” and “cachexia”. Because of the scarcity of information and few studies of cancer cachexia at this time, a very low number of results were returned in both of these searches. Therefore, the final examination and selection of articles and studies was done by individual review. Studies that were not published in English, observational studies without any interventions, and those that did not contain a treatment group with patients with active cancer were excluded from this analysis. Data were compiled for the remaining studies on type of cancer, mode of intervention, and endpoints.
During the design of the present study no agents or interventions were approved for the treatment of cancer-associated muscle wasting. The interventions described in the identified studies included drugs, biological agents, nutritional supplements, and behavioural modifications. Many variable responses to these interventions were reported along with several potentially limiting side-effects. However, the sum of this information suggested that patients with cancer do have the capability to respond to and benefit from treatments targeting muscle wasting. The desired profile of an agent ultimately used to treat cancer-induced muscle wasting would include a strong anabolic effect on skeletal muscle, lean body mass, and metabolic processes, improve the functional capacity of the patient, and exhibit a safe and tolerable side-effect profile. The data available at the time for the interventions uncovered in our search did not suggest that any exhibited a complement of these qualities.
Interpretation
Our results provide evidence that a targeted anabolic agent can increase lean body mass in conjunction with improved physical function and quality of life throughout various stages of a broad range of cancers. Furthermore, the favourable side-effect profile reported in this population suggests that this could be a treatment option well suited for use in conjunction with a broad spectrum of the cancer treatment options that are currently available. The response to enobosarm described in our investigation warrants further investigation and suggests that patients might benefit from a treatment affecting multiple aspects of metabolism, physical function, and quality of life.
Acknowledgments
We thank Jamie L Kistler (Complete Publication Solutions, Horsham, PA, USA) for medical writing and editorial assistance; this work was funded by GTx.
Footnotes
Contributors
ASD assisted with the study design, recruited patients, and contributed to data analysis and writing of the report. RVB contributed to patient accrual and data collection and analysis. CCC recruited patients to the trial and was involved in analysis of data. NYG contributed to accrual and recruitment of patients and data collection. JTD contributed to the design of the clinical protocol; analysis and interpretation of data; and writing and revision of the report text, tables, and figures. MLH did the statistical analysis and assisted with data interpretation. MAJ contributed to the scientific literature review; data review, analysis and interpretation; and writing of the report. MSS contributed to the study design; scientific literature search; data collection, analysis, and interpretation; and writing of the report.
The G200502 investigators
James Beck, Donald Berdeaux, Cesar Blajman, Janet Bull, Pablo Casella, Terrence Cescon, Christopher Chay, Lucio Gordan, Michael Greenhawt, Adrian Hannois, Carlos Ivulich, Gustavo Jankilevich, Haresh Jhnajiani, Peter Jiang, Jesus Larravide, Leslie Laufman, Cynthia Lewis, An Nguyen, Miguel Pavlosky, Shannon Penland, Farid Qazi, Edgard P Quintana, Evangeline Reyes, Monica Rondinon, Marc Rovito, Kert Sabbath, Fred Schreiber, Brad Somer, Jerome Spunberg, Douglas Testori, Nadagopal Vrindavanam, David Young, James Young.
Conflicts of interest
ASD, RVB, CCC, NYG, and EPQ received research funds from GTx for this study. MLH, MAJ, JTD, and MSS are employees of and have stock and stock options with GTx.
References
- 1.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 2.Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006;83:735–43. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 3.Fox KM, Brooks JM, Gandra SR, Markus R, Chiou CF. Estimation of cachexia among cancer patients based on four definitions. J Oncol. 2009;2009:693458. doi: 10.1155/2009/693458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;91:S1133–37. doi: 10.3945/ajcn.2010.28608C. [DOI] [PubMed] [Google Scholar]
- 5.Basaria S, Wahlstrom JT, Dobs AS. Clinical review 138: anabolic-androgenic steroid therapy in the treatment of chronic diseases. J Clin Endocrinol Metab. 2001;86:5108–17. doi: 10.1210/jcem.86.11.7983. [DOI] [PubMed] [Google Scholar]
- 6.Prado CMM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 7.Narayanan R, Coss CC, Yepuru M, Kearbey JD, Miller DD, Dalton JT. Steroidal androgens and nonsteroidal, tissue-selective androgen receptor modulator, S-22, regulate androgen receptor function through distinct genomic and nongenomic signaling pathways. Mol Endocrinol. 2008;22:2448–65. doi: 10.1210/me.2008-0160. [DOI] [PubMed] [Google Scholar]
- 8.Dalton JT, Barnette KG, Bohl CE, et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2:153–61. doi: 10.1007/s13539-011-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moyle GJ, Daar ES, Gertner JM, et al. Growth hormone improves lean body mass, physical performance, and quality of life in subjects with HIV-associated weight loss or wasting on highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2004;35:367–75. doi: 10.1097/00126334-200404010-00006. [DOI] [PubMed] [Google Scholar]
- 10.Bean JF, Kiely DK, LaRose S, Alian J, Frontera WR. Is stair climb power a clinically relevant measure of leg power impairments in at-risk older adults? Arch Phys Med Rehabil. 2007;88:604–09. doi: 10.1016/j.apmr.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 11.LeBrasseur NK, Bhasin S, Miciek R, Storer TW. Tests of muscle strength and physical function: reliability and discrimination of performance in younger and older men and older men with mobility limitations. J Am Geriatr Soc. 2008;56:2118–23. doi: 10.1111/j.1532-5415.2008.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhasin S, Calof OM, Storer TW, et al. Drug insight: testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab. 2006;2:146–59. doi: 10.1038/ncpendmet0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroeder ET, He J, Yarasheski KE, et al. Value of measuring muscle performance to assess changes in lean mass with testosterone and growth hormone supplementation. Eur J Appl Physiol. 2012;112:1123–31. doi: 10.1007/s00421-011-2077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Oliveira DG, Nunes PM, Aruin AS, Dos Santos MJ. Grip force control in individuals with hand osteoarthritis. J Hand Ther. 2011;24:345–54. doi: 10.1016/j.jht.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Bagis S, Sahin G, Yapici Y, Cimen OB, Erdogan C. The effect of hand osteoarthritis on grip and pinch strength and hand function in postmenopausal women. Clin Rheumatol. 2003;22:420–24. doi: 10.1007/s10067-003-0792-4. [DOI] [PubMed] [Google Scholar]
- 16.Janssen WG, Bussmann HB, Stam HJ. Determinants of the sit-to-stand movement: a review. Phys Ther. 2002;82:866–79. [PubMed] [Google Scholar]
- 17.Mazzà C, Benvenuti F, Bimbi C, Stanhope SJ. Association between subject functional status, seat height, and movement strategy in sit-to-stand performance. J Am Geriatr Soc. 2004;52:1750–54. doi: 10.1111/j.1532-5415.2004.52472.x. [DOI] [PubMed] [Google Scholar]
- 18.Klein S, Kinney J, Jeejeebhoy K, et al. Nutrition support in clinical practice: review of published data and recommendations for future research directions. Summary of a conference sponsored by the National Institutes of Health, American Society for Parenteral and Enteral Nutrition, and American Society for Clinical Nutrition. Am J Clin Nutr. 1997;66:683–706. doi: 10.1093/ajcn/66.3.683. [DOI] [PubMed] [Google Scholar]
- 19.Grimble RF. Nutritional therapy for cancer cachexia. Gut. 2003;52:1391–92. doi: 10.1136/gut.52.10.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–42. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 21.Bohl CE, Miller DD, Chen J, Bell CE, Dalton JT. Structural basis for accommodation of nonsteroidal ligands in the androgen receptor. J Biol Chem. 2005;280:37747–54. doi: 10.1074/jbc.M507464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moncada E. Familial study of hirsutism. J Clin Endocrinol Metab. 1970;31:556–64. doi: 10.1210/jcem-31-5-556. [DOI] [PubMed] [Google Scholar]
- 23.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–47. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 24.Wilcock A. Anorexia: A taste of things to come? Palliat Med. 2006;20:43–45. doi: 10.1191/0269216306pm1089xx. [DOI] [PubMed] [Google Scholar]
- 25.Lieffers J, Bathe O, Fassbender K, Winget M, Baracos V. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–36. doi: 10.1038/bjc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297:1772–74. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 25.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatric Soc. 2003;51:314–22. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 26.Kwon S, Perera S, Pahor M, et al. What is meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging. 2009;13:538–44. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:746–49. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 28.Bruera E. ABC of palliative care. Anorexia, cachexia, and nutrition. BMJ. 1997;315:1219–22. doi: 10.1136/bmj.315.7117.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hazem A, Elamin MB, Bancos I, et al. Body composition and quality of life in older adults treated with GH therapy: a systemic review and meta-analysis. Eur J Endocrinol. 2012;166:13–20. doi: 10.1530/EJE-11-0558. [DOI] [PubMed] [Google Scholar]
- 30.Dhillon S. Tesamorelin: a review of its use in the management of HIV-associated lipodystrophy. Drugs. 2011;71:1071–91. doi: 10.2165/11202240-000000000-00000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.