Abstract
Low oxygen tension (hypoxia) is a hallmark of cancer that influences cancer cell function, but is also an important component of the tumour microenvironment as it alters the extracellular matrix, modulates the tumour-immune response and increases angiogenesis. Here we discuss the regulation and role of hypoxia and its key transcriptional mediators, the hypoxia inducible factor (HIF) family of transcription factors, in the tumour microenvironment and stromal compartments.
Introduction
A wealth of clinical evidence indicates the prevalence of hypoxia in solid tumours1. Hypoxia arises due to a combination of excessive oxygen consumption by cells in the tumour, and the leaky and disorganized tumour-associated vasculature, which leads to both acute fluxes in oxygen tension and diffusion-limited regions of low oxygen levels within the tumour2. Tumour hypoxia is associated with increased genetic instability, disease progression and metastasis, and can inhibit tumour response to cytotoxic and targeted therapies1. Its profound clinical implications have led to an intensive effort to characterize the cellular response to hypoxia, and to modulate it for therapeutic benefit.
Tumours are composed of a heterogenous mix of malignant and stromal cell populations, the latter including cancer associated fibroblasts (CAFs), immune cells, endothelial cells (ECs) and pericytes3. Stromal cells are intimately associated with malignant cells and participate in paracrine signaling, metabolite exchange, extracellular matrix remodeling and regulation of immune surveillance in the tumour3. Microenvironmental cues, including hypoxia, synergistically modulate the behaviour of tumour cells and associated stromal cells to potentiate tumour progression4. Central in the cellular responses of both the tumour and stromal compartments to hypoxia, is the HIF family of transcription factors. In this review, we discuss the regulation of HIF signaling and the role of the hypoxic microenvironment in shaping the tumour-stromal cell interface.
Canonical regulation of HIF signaling
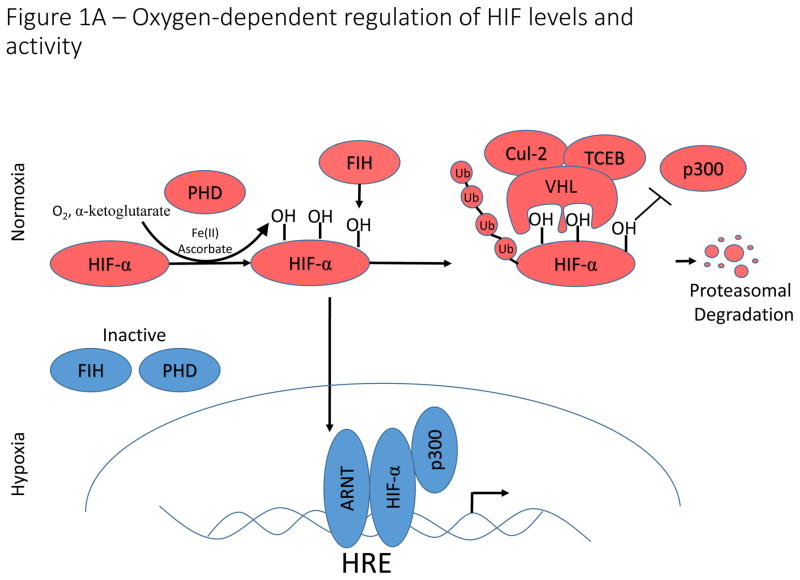
HIF-1α, HIF-2α, and the lesser studied HIF-3α constitute a family of oxygen sensitive basic helix-loop-helix transcription factors that direct the transcriptional response to hypoxia5. HIF-1α is ubiquitously expressed in human cells, whereas HIF-2α expression is restricted to specific tissues and cell types6. Transcriptional activation of HIF target genes requires assembly of a heterodimer between HIF-1α or HIF-2α and their obligate binding partner, the aryl hydrocarbon nuclear translocator (ARNT, also known as HIF-1β)7. Under normoxic conditions, α-ketoglutarate-dependent prolyl hydroxylases (PHDs) catalyze the hydroxylation of proline residues within oxygen-dependent degradation domains (ODD) of HIF-α, which are recognized by the Von Hippel-Lindau (VHL) E3 ubiquitin ligase complex, leading to HIF-α ubiquitination and subsequent degradation (Fig. 1A)8,9. Owing to its crucial role in HIF-α degradation, loss of function mutations in the VHL gene result in constitutive activation of HIF signaling and are characteristic of several cancer syndromes, including clear cell renal cell carcinoma10. In addition to regulation by prolyl hydroxylation, oxygen-dependent hydroxylation of a key asparagine residue by Factor Inhibiting HIF (FIH) disrupts binding of the p300 transcriptional co-activator to HIF, thereby inhibiting its transcriptional activation potential11. Oxygen-dependent hydroxylases provide an elegant oxygen sensing mechanism that directs the transcriptional response to hypoxia.
Figure 1. Oyxgen-dependent and -independent regulation of HIF-signaling.
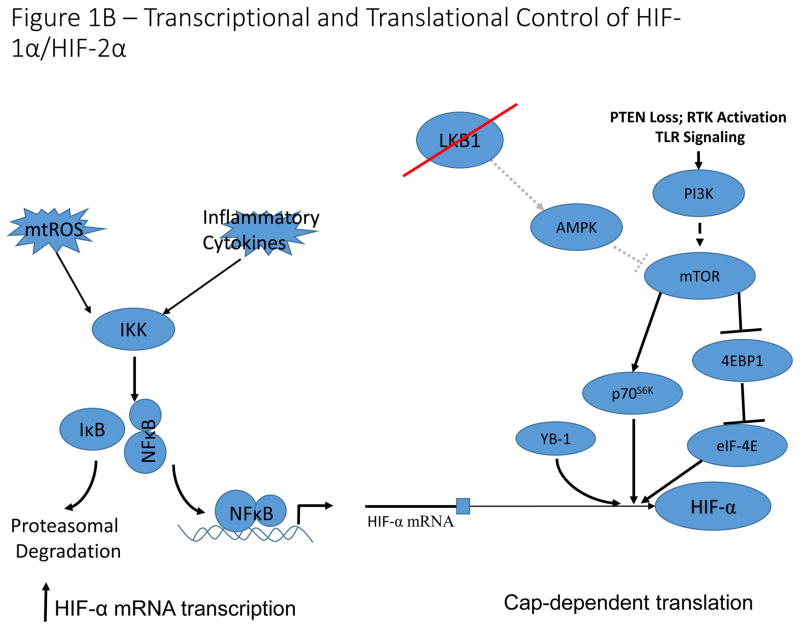
A) Oxygen-dependent regulation of HIF-1α/HIF-2α. Under conditions in which sufficient oxygen is present (normoxia), prolyl hydroxylases (PHD) catalyze the hydroxylation of two key proline residues within the oxygen dependent degradation domains of HIF-1α and HIF-2α. The hydroxylation reaction catalyzed by PHDs utilizes molecular oxygen and α-ketoglutarate (which is converted to succinate in the reaction) as co-substrates and ferrous iron (Fe(II)) and ascorbate as cofactors. These hydroxylation events form a binding site for the E3 ubiquitin ligase, VHL, which catalyzes ubiquitination and subsequent proteasomal degradation. An additional asparagine residue in the C-terminal activation domain is hydroxylated by factor inhibiting HIF (FIH). Hydroxylation of this asparagine residue disrupts binding of p300 to HIF-α, thereby inhibiting HIF-transcriptional activation potential. Under hypoxic conditions, PHD and FIH activity is inhibited and unhydroxylated HIF-1α and HIF-2α translocate to the nucleus, form a complex with ARNT and p300, and activate transcription of HIF-target genes. B). Transcriptional and translational control of HIF-1α/HIF-2α expression. Reactive oxygen species and pro-inflammatory conditions in the tumour microenvironment stimulate NF-κB-dependent transcriptional activation of HIF-1α, leading to increased expression under normoxic conditions. Activation of the mTOR signaling pathway by PI3K activation or LKB1 loss of function, results in increased cap-dependent translation of HIF-α mRNA. Additionally, the RNA/DNA binding protein, YB-1 is induced in multiple cancer types, and can bind to HIF-1α mRNA to stimulate cap-dependent translation. This leads to increased normoxic expression of HIF- α in cells with constitutively active PI3K signaling.
Non-Canonical regulation of HIF signaling
In addition to the canonical oxygen-dependent regulation of HIF-α, non-canonical regulation of HIF signaling has been demonstrated in many cell types including both malignant and stromal cells. Activation of PI3K-mTOR signaling increases cap-dependent translation of HIF-α mRNA, resulting in increased expression of HIF-α protein12–14 (Fig. 1B). In cancer cells, frequent activation of the PI3K-mTOR axis stimulates HIF-α activity and promotes tumour angiogenesis15,16. In Peutz-Jeghers syndrome, dysregulation of mTOR signaling downstream of LKB1 loss leads to metabolic reprogramming by a HIF-dependent mechanism17,18. The RNA- and DNA-binding protein YB-1, which is upregulated in sarcomas, can also regulate cap-dependent translation of HIF-1α, but not HIF-2α, and has separately been reported to indirectly regulate HIF-1α by transcriptional repression of Foxo3a19,20.
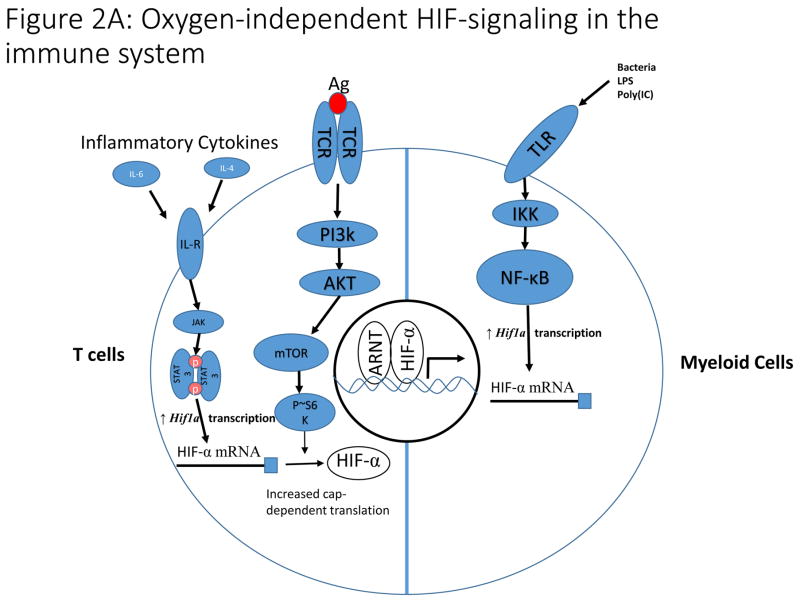
Activation of mTOR signaling, and subsequent HIF-α stabilization and activation, also occurs downstream of TCR signaling in T cells, and is imperative for their function, because it drives metabolic reprograming and prolongs survival of peripheral T cells under hypoxia21,22 (Fig. 2A). HIF-α signaling in lymphocytes is also modulated by pro-inflammatory cytokines like IL-6, which signal through the JAK/Stat3 pathway to increase transcription of HIF-α mRNA23,24. In the innate immune system, Toll-like receptor (TLR) signaling induces HIF-α expression in myeloid cells through increased NF-κB-dependent transcription of Hif1a mRNA, highlighting an important link between innate immunity and the HIF-pathway25–27 (Fig. 1B, 2A). These findings indicate that additional, oxygen-independent mechanisms in normal and malignant cells drive activation of HIF-signaling, to sustain the function of these cell types.
Figure 2. Non-canonical regulation of HIF-signaling by cell signaling and metabolism.
A). In T cells, antigen-dependent activation of the T cell Receptor (TCR) signals through the PI3K pathway to stimulate increased translation of HIF-α mRNA. Additionally, inflammatory cytokines signal through the JAK/STAT3 pathway to increase transcription of HIF-α mRNA. These pathways are essential for induction of HIF-signaling in hypoxia and during T cell activation. In myeloid cells, toll-like receptor (TLR) signaling increases NF-κB-dependent transcription of HIF-α mRNA in response to a variety of stimuli including bacterial infection leading to increased HIF-signaling.
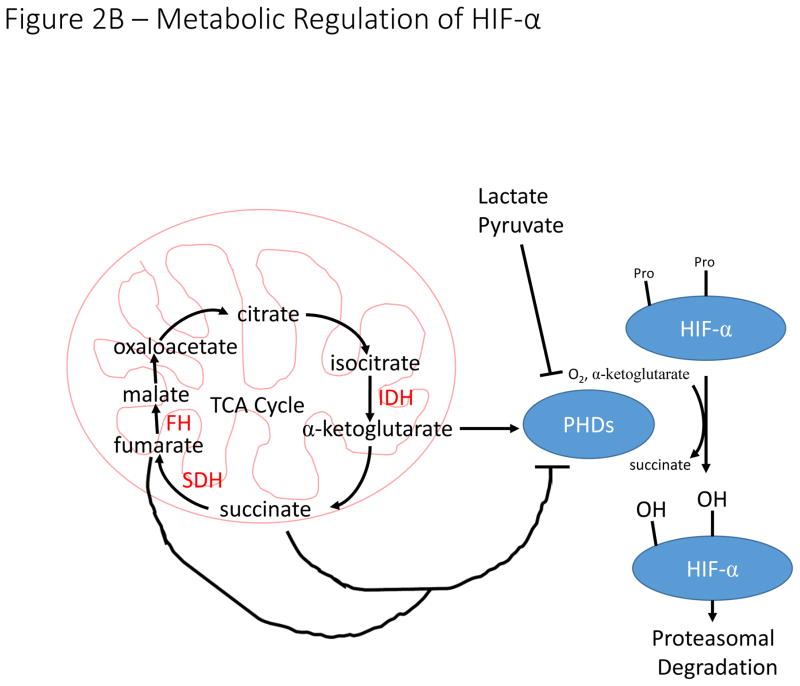
B). The hydroxylation reaction catalyzed by PHDs utilizes α-ketoglutarate as a co-substrate, producing succinate in the process. Loss of function mutations to succinate dehydrogenase (SDH) and fumarate hydratase (FH) result in accumulation of succinate, which inhibits PHD activity and stabilizes HIF-α in normoxia. The enzymes labelled in red (SDH, FH, and IDH) are frequently mutated in a variety of malignancies resulting in inhibition of PHD activity and stabilization of HIF-α subunits. Increased concentrations of lactate and pyruvate have also been demonstrated to promote stabilization of HIF-α, but the mechanism by which this stabilization occurs remains unclear.
Mitochondrial regulation of HIF activity
Reprograming of cellular metabolism towards increased glycolysis and suppressed oxidative phosphorylation is a major adaptation mechanism to hypoxia. HIF-α transcription factors are central regulators of metabolism, and several HIF target genes encode metabolic enzymes. In turn, a wide range of cellular metabolites can modulate HIF-signaling. Numerous reports indicate that mitochondria, the major site of cellular oxygen consumption, are important for HIF-α stabilization under hypoxic and non-hypoxic conditions. One hypothesis is that mitochondrial respiration regulates HIF-α stability by increasing intracellular hypoxia, thereby impairing PHD-mediated HIF-α hydroxylation. Indeed, high resolution imaging indicates that mitochondria reside in regions of lower oxygen, compared to other cellular compartments28, and mitochondrial ETC inhibitors interfere with hypoxic stabilization of HIF-α, presumably by preserving intracellular oxygen for PHD-mediated hydroxylation29. Furthermore, PGC-1α, a central regulator of mitochondrial biogenesis, stabilizes HIF-α by stimulating mitochondrial oxygen consumption and increasing intracellular hypoxia30,31. HIF was recently shown to repress PGC-1α expression in clear cell renal cell carcinoma, suggesting that a regulatory loop exists between HIF-α and PGC-1α, linking oxygen sensing to mitochondrial biogenesis32.
Aberrant metabolism and oncogene activation frequently induces reactive oxygen species (ROS) accumulation, which positively and negatively influences tumourigenesis33. In vitro models blocking mitochondrial ROS production indicate that ROS are an important mediator of HIF-stability, acting by inhibiting PHD function34–37. However, the tight coupling between ROS production and oxygen consumption in the mitochondria have made it difficult to discern whether mitochondria regulate HIF through consumption of oxygen or production of ROS. Whether ROS production in the mitochondria is increased or decreased under hypoxic conditions remains unclear and adding to the controversial role of ROS as mediators of HIF signaling, recent reports indicate that the activity of FIH, but not the PHDs, is sensitive to peroxide radicals38,39.
Influence of intracellular metabolites on HIF-α stability
Loss of function mutations to genes encoding the succinate dehydrogenase (SDH) complex and fumarate hydratase (FH) lead to accumulation of succinate or fumarate respectively, metabolites that inhibit the activity of the PHDs and result in HIF-α stabilization (Fig. 2B)40. Other intracellular metabolites, including pyruvate, lactate and oxaloacetate can also modulate HIF-signaling through PHD inhibition41. Isocitrate dehydrogenase isoforms (most commonly IDH1 and IDH2) are also frequently mutated in multiple cancer types42. These mutations result in neomorphic IDH activity, with mutant IDH1/2 producing 2-hydroxyglutarate (2-HG) instead of α-ketoglutarate, which is an essential co-substrate of the PHDs43. The impact of 2-HG on HIF-stability is hotly contested, with reports suggesting that 2-HG can either activate or inhibit PHD-mediated hydroxylation of HIF-α in an enantiomer specific manner44. Further complicating matters, IDH3α expression has been reported to repress or activate HIF-stability in CAFs and malignant cells by modulating intracellular α-ketoglutarate levels45,46. How IDH3α can increase or decrease α-ketoglutarate levels to modulate HIF signaling in different contexts remains unclear.
HIF function can also be modulated by non-catalytic functions of metabolic enzymes. The gluconeogenic enzyme fructose-1,6-bisphosphatase (FBP1) binds to the inhibitory domain on HIF, thereby preventing HIF nuclear translocation and transactivating potential, and inhibiting ccRCC tumorigenesis47. In contrast, the PKM2 isoform of pyruvate kinase can act as a HIF-1α coactivator by recruiting p30048. Dimeric PKM2 can also activate Stat3, which is known to coordinate expression of genes involved in inflammation and the hypoxic response together with HIF-α, suggesting that PKM2 may act as a metabolic sensor integrating these two gene expression programs49. Together, these studies indicate that both metabolites and metabolic enzymes can directly modulate oxygen sensing.
Hypoxia and HIF-signaling in the Tumour Microenvironment
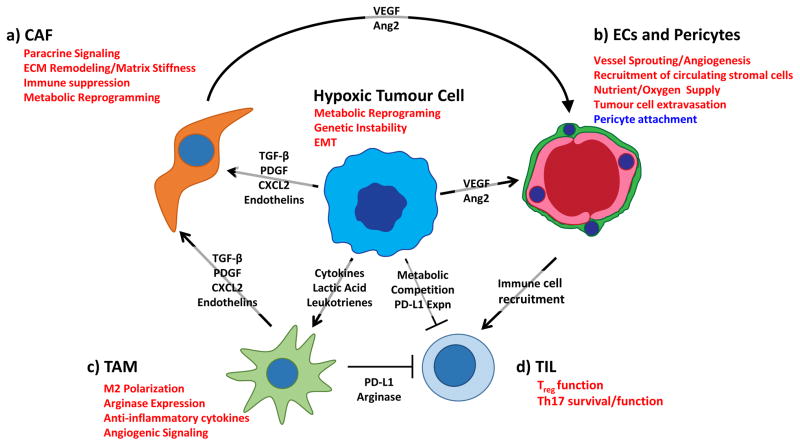
Tumour-associated stromal cells are exposed to similar, often harsh, microenvironmental cues as tumour cells. HIF signaling promotes adaptation to these microenvironmental conditions, and in doing so, induces changes in both tumour and stromal cells that potentiate tumourigenesis. Tumour hypoxia promotes the recruitment of endothelial cells and pericytes to stimulate angiogenesis and facilitates the recruitment of bone marrow derived cells. Recruited stromal cells enhance tumourigenesis through extracellular matrix remodeling, growth factor signaling and evasion of the anti-tumour immune response (Fig. 3)3.
Figure 3. Tumour hypoxia co-opts the stroma to potentiate tumourigenesis.
Hypoxic tumour and stromal cells initiate paracrine signalling to stimulate angiogenesis and recruit stromal cells from the circulation, creating an immunosuppressive microenvironment. A). Hypoxia stimulates tumour cells and TAMs to secrete paracrine factors (TGF-β, PDGF, CXCL2, Endothelin) that promote activation of cancer-associated fibroblasts. In CAFs, hypoxia stimulates extracellular matrix (ECM) remodelling, which promotes increased tumour aggressiveness through stiffening of the ECM. Hypoxic CAFs also synthesize and release factors that drive angiogenesis and immune cell recruitment to the tumour site. B). Hypoxia drives tumour and stromal secretion of vascular endothelial growth factor (VEGF) and other pro-angiogenic factors that recruit endothelial cells and pericytes from the surrounding vasculature. Hypoxia directly effects vascular barrier function by decreasing the association between pericytes and endothelial cells, thereby facilitating tumour cell extravasation and recruitment of stromal cells from the circulation. C). Hypoxia stimulates recruitment of circulating macrophages and promotes alternative (M2) activation by increasing expression of macrophage chemotractants and lactate levels. Hypoxic M2 macrophages create a functionally immunosuppressive microenvironment by increasing expression of arginase and immune checkpoint ligands. D). Hypoxia promotes a functionally immunosuppressive microenvironment by stimulating Treg cell function and increasing expression of immune checkpoint molecules such as PD-L1 and CTLA4 on tumour cells. Hypoxia also drives metabolic reprogramming in tumour cells, allowing them to out-compete T cells for key metabolites critical for T cell function. Hypoxia prolongs the survival of TH17 cells, though the role of this helper T cell subtype in tumourigenesis remains controversial. Pathways in red are induced in hypoxia, while pathways in blue are suppressed under hypoxic conditions. Ang2, angiopoietin 2; CAF, cancer associated fibroblast; CTL, cytotoxic T cell; EC, endothelial cell; EMT, epithelial to mesenchymal transition; TAM, tumour-associated macrophage.
The hypoxic tumour microenvironment spurs adaptive metabolic changes that complement the metabolic flux of normoxic tumour regions to sustain metabolic fitness in both compartments in a process termed “metabolic symbiosis.” For example, the lactate secreted by highly glycolytic hypoxic cells, is consumed by normoxic cells for ATP production via oxidative phosphorylation50 (Fig. 3). In addition to poor oxygenation, a consequence of the malformed tumour-associated vasculature is limiting nutrient supply. Byproducts of hypoxic cell metabolism may alter the function and viability of a wide range of stromal cells by increasing tissue acidity and by depriving stromal cells of access to essential nutrients51. Augmented HIF-signaling and oncogene activation in tumour cells improves metabolic fitness, allowing tumour cells to outcompete stromal cells for vital metabolites such as glucose and glutamine52. This dynamic competition for nutrients between tumour cells and their stroma has recently been implicated in the exhaustion of tumour infiltrating lymphocytes, and indicates that metabolic competition promotes immune suppression in the tumour microenvironment53,54.
Vascular Endothelial Cells and Pericytes
The contribution of angiogenesis to tumour progression is well established55. Although early in tumourigenesis the resident tissue vasculature supplies sufficient oxygen to the burgeoning tumour, larger tumours exhibit significant regions of hypoxia that limit further tumour growth. Hypoxic cancer and stromal cells secrete soluble factors, such as vascular endothelial growth factor (VEGF), that facilitate angiogenesis and thereby oxygen and nutrient delivery, to promote tumour growth under hypoxic conditions. Two major vascular cell types are the endothelial cells (ECs) that line blood vessels and pericytes which surround blood vessels, and hypoxia and HIF signaling are known to influence both.
Vascular endothelial cells
Apart from their direct impact on the tumour microenvironment by regulating oxygen and nutrient supply, ECs are a major structural component of blood vessels and consequently regulate tumour cell extravasation and the recruitment of circulating cells to the tumour56. ECs respond to VEGF secretion from cancer and stromal cells by sprouting from the existing vasculature to form new vessels during angiogenesis. Sprouting vessels culminate in specialized vascular ECs (tip cells) that direct vessel formation. As these tip cells reside far from functional vessels, they become hypoxic and must mount an adaptive response to hypoxia57. Thus, during vessel sprouting, ECs exhibit unique patterns of cellular metabolism, with high rates of glycolysis and a dependence on fatty acid oxidation for nucleic acid synthesis and proliferation58,59, despite the fact that hypoxia suppresses fatty acid oxidation in a wide range of cell types and tissues60–63. These findings indicate that ECs may exhibit unique metabolic behaviours that could be targeted therapeutically to block tumour angiogenesis.
Early evidence of the role of HIF signaling in ECs came from studies in which conditional deletion of HIF-1α in ECs impaired vascularization and tumour growth64. Surprisingly, deletion of endothelial HIF-2α enhanced tumour angiogenesis, but the resulting vasculature was highly disorganized and resulting tumours were more hypoxic65,66. Additionally, heterozygous loss of PHD2 (Egln1) resulted in increased HIF-α stabilization, normalized tumour vasculature, increased oxygenation and diminished metastasis67. These findings indicate that HIF signaling in ECs plays an essential and complex role in functional angiogenesis.
Tumour associated ECs also represent an important barrier to intravasation of invading tumour cells. HIF-1α and HIF-2α play opposing roles in regulating the barrier function of ECs: whereas loss of endothelial HIF-1α impairs tumour cell migration through endothelial layers, endothelial HIF-2α deficiency enhances tumour cell migration and metastasis68. These contradictory roles for HIF-signaling in ECs may be explained by diminished production of iNOS in HIF-1α-deficient endothelial cells, compared to enhanced iNOS production under HIF-2α deficiency, however, more work is needed to elucidate these contrasting roles of HIF-1α and HIF-2α.
Pericytes
Vascular endothelial cells are tightly associated with pericytes, which line the outside of blood vessels69. Pericytes are an important component of functional vasculature, contributing to vessel contractility and permeability to ensure efficient nutrient and oxygen delivery to healthy tissues. During angiogenesis, pericytes respond to PDGF to induce neovascularization. Whereas VEGF promotes EC proliferation and migration, it disrupts pericyte coverage of nascent blood vessels, resulting in pericytes being more weakly attached to tumour-associated vasculature70,71. Pre-clinical models of pericyte deficiency indicate that decreased pericyte vessel coverage drives increased hypoxia, tumour aggressiveness and metastasis72,73. The role of pericytes in tumour biology may fluctuate at different stages of tumour progression: whereas pericyte depletion at early stages of tumour growth can decrease growth and metastasis, their depletion at later stages enhances intratumoural hypoxia and has the converse effect72,73.
Hypoxia and anti-angiogenic therapy
A major focus of targeted cancer therapy has been the inhibition of angiogenesis through VEGF blockade74. Although these therapies often induces acute tumour shrinkage, increased hypoxia resulting from vessel pruning can promote tumour aggressiveness and metastatic dissemination69. Concomitant treatment with Sema3A during VEGF blockade can prevent invasion and metastasis by reducing hypoxia and HIF-stabilization, indicating that modulation of the hypoxic response may enhance therapeutic efficacy of anti-angiogenic agents75. Metabolic reprogramming is a key adaptation to anti-angiogenic therapies, and several lines of evidence point to increased dependency on lipid metabolism during and after treatment with VEGF inhibitors76,77. These preclinical studies indicate that understanding the dynamic response of hypoxia and HIF-signaling to anti-angiogenic therapies may contribute to enhancing their efficacy.
Although prolonged VEGF blockade enhances tumour hypoxia and promotes tumour aggressiveness, recent studies indicate a therapeutic window during which tumour vasculature appears to normalize78,79. Although vessel normalization is counterintuitive to the initial efforts of anti-angiogenic therapies, restored tumour oxygenation may enhance the delivery and efficacy of cytotoxic chemotherapies since increased interstitial pressure and hypoxia are known to decrease drug delivery and efficacy69.
Cancer-Associated Fibroblasts
CAFs directly contribute to virtually every stage of tumourigenesis80. CAFs are a heterogenous mix of myofibroblast-like cells that arise from various cell types including normal fibroblasts, endothelial cells, adipocytes, or bone marrow derived stromal cells81–83. Relative to normal fibroblasts, CAFs exhibit heightened metabolic activity and enhanced ECM remodeling, properties that promote metabolic symbiosis and facilitate metastatic dissemination. CAFs secrete a variety of growth factors, cytokines and chemokines that stimulate cancer cell proliferation and recruit bone-marrow-derived cells to the tumour site.
Hypoxic tumour cells secrete paracrine signaling factors including TGF-β, PDGF, CXCL2 and Endothelin that promote conversion of precursor cell types into CAFs, indicating the importance of hypoxia to CAF function in tumours84. Although hypoxia mediates CAF recruitment and activation through tumour cell intrinsic HIF-signaling, the direct effects of hypoxia on CAFs remain elusive. Fibroblast-specific loss of HIF-1α induces vascular normalization, decreases hypoxia and enhances tumourigenesis in a mouse model of breast cancer, indicating that HIF-signaling in CAFs may inhibit tumourigenesis85. Indeed, hypoxia or loss of PHD2 leads to a decrease in CAF-induced ECM remodeling and diminished metastasis86,87. These findings indicate the complex role of hypoxia and HIF-signaling in CAF function. Below we highlight three major mechanisms through which CAFs modulate tumourigenesis and discuss how hypoxia influences these pathways.
Extracellular matrix remodeling
The ECM is composed of fibrous proteins including collagen and proteoglycans that provide the structural fabric of tissues. During tumour growth, extensive ECM remodeling releases paracrine growth factors that stimulate tumour growth and enables tumour migration and metastasis. To facilitate ECM remodeling, CAFs express enzymes including collagen prolyl- and lysyl-hydroxylases and lysyl oxidases that catalyze crosslinking of collagens to elastin and other ECM molecules, resulting in increased matrix stiffness88. Hypoxia is an important stimulus of this process, driving increased expression of remodeling enzymes including prolyl-4-hydroxylases and lysyl oxidases leading to increased tumour stiffness and enhanced metastasis84.
Reprogrammed cellular metabolism
Compared to normal fibroblasts, CAFs exhibit increased glycolysis, which is critical for their ability to promote tumourigenesis at least in part, by activating HIF-signaling89. The metabolic byproducts of CAF metabolism can be taken up by tumour cells to feed anabolic metabolism and tumour cell proliferation, suggesting that metabolic symbiosis is a key mechanism by which CAFs support tumour growth90. Gain of function studies in fibroblasts indicate that expression of HIF-1α, but not HIF-2α, regulates aerobic metabolism and tumour promoting effects of CAFs91. In addition to exhibiting enhanced glucose uptake and glycolytic flux, cancer cells also take up significant amounts of lactate by expressing the lactate transporter MCT192,93. CAFs produce and secrete large amounts of lactate, which can be taken up by tumour cells to feed the tumour’s requirement for carbon metabolism94. Tumour cells alter ROS signaling in the microenvironment which may promote HIF-dependent and independent metabolic reprograming in CAFs, thus potentiating metabolic symbiosis between these cell types95. These results indicate a dynamic interplay between the metabolism of CAFs and tumour cells and suggest that targeting CAF metabolism could interfere with metabolic symbiosis in the tumour microenvironment and thus impair tumour growth.
Paracrine signaling
CAFs are an important source of signaling factors that act on tumour and stromal cells to potentiate paracrine signaling within the tumour microenvironment. CAFs exposed to hypoxia secrete SDF-1 (CXCL12), which promotes tumour growth through paracrine mechanisms96. Hypoxia also upregulates expression of the SDF-1 receptor, CXCR4, in numerous cell types, thus potentiating the paracrine signaling between CAFs and malignant cells97. In prostate cancer, androgen ablation therapy enhances tumour hypoxia, stimulating increased CXCL13 secretion from tumour-resident myofibroblasts, leading to disease progression98. Indeed, several factors involved in the transformation of CAFs from precursor cells, including TGF-β, PDGF-B, and bFGF are directly or indirectly regulated by HIF, indicating the importance of hypoxia in regulating paracrine signaling in CAFs99–101
Paracrine signaling by hypoxic CAFs also contributes to the immunosuppressive nature of the tumour microenvironment, by inducing secretion of arginase II (ArgII). ArgI and ArgII are important drivers of immune suppression in the tumour microenvironment because they promote conversion of L-arginine to ornithine, leading to T cell anergy and limited anti-tumour immune response102. In pancreatic cancer, CAFs localized to hypoxic tumour regions express high levels of ArgII, indicating an important role for hypoxia in CAF-mediated immunosuppression103.
Immune cells in the tumour microenvironment
Tumour infiltrating immune cells play an important role in disease progression, and anti-tumour immune therapies have been the subject of intense research for some time. Traditionally these approaches have involved augmenting intrinsic anti-tumour immunity with cytokines such as interleukin-2, or tumour-antigen vaccines. However, the tumour microenvironment is functionally immunosuppressive, and intrinsic HIF signaling drives resistance to immune-mediated tumour cell lysis, limiting the efficacy of these therapies in all but a subset of patients24,104. Consequently, a better understanding of how the microenvironment limits the effectiveness of immunotherapy could improve the efficacy of this treatment modality.
Tumour infiltrating immune cells encompass a diverse array of cell types, including tumour associated macrophages (TAM), myeloid derived suppressor cells (MDSC), T cells, neutrophils, and others3. In most tumours, immune cells are recruited from the circulation by tumour-secreted cytokines and chemokines105. Hypoxia is known to directly or indirectly modulate the function of virtually all immune cell types, thereby facilitating tumour progression106. Indeed, myeloid-specific loss of the prolyl hydroxylase PHD2 has been reported to impair tumour growth and metastasis, suggesting an important role for myeloid cell oxygen sensing in tumorigenesis107. Hypoxic regions within solid tumours offer distinct niches that fine-tune the tumour-immune system interface and enhance pro-tumorigenic phenotypes of tumour-infiltrating lymphocytes. Below, we discuss the role of hypoxia and HIF-signaling in innate and adaptive tumour immunity.
Cells of the innate immune system
Innate immune cells can potentiate the anti-tumour activity of tumour-infiltrating lymphocytes, leading to remarkable immune-mediated tumour regression in a subset of cancer patients. However, tumour microenvironmental factors, including hypoxia, limit the ability of the innate immune system to activate adaptive immunity, and instead subvert the function of the innate immune system to promote tumourigenesis.
Tumour-associated macrophages
Macrophage density correlates with poor prognosis in various cancer types, indicating an important role for macrophages in tumour progression108,109. Hypoxia in the tumour microenvironment is a potent stimulus for macrophage recruitment through the induction of chemoattractant secretion by tumour cells (Fig. 3). Semaphorin 3A (Sema3A), is such a factor, and attracts macrophages through Nrp1/Plexin signaling110. Hypoxia also induces secretion of chemokines, such as endothelial-monocyte-activating peptide II (EMAP II), endothelin-1 and endothelin-2 (ET-1 and ET-2) that recruit macrophages to the tumour111,112. In ovarian cancer, hypoxia drives macrophage recruitment by stimulating the production of pro-inflammatory leukotrienes, a process that can be reversed by pharmacologic targeting of leukotriene production113.
Macrophage activation includes the classically activated M1 phenotype, which is characterized by nitric oxide synthase 2 (iNOS) expression, pro-inflammatory cytokine production and tumour suppression. In contrast, alternatively activated (M2) macrophages express arginase 1 (Arg1), promote angiogenesis, secrete anti-inflammatory glucocorticoids and effectively dampen the anti-tumour immune response. Consequently, M1 macrophages oppose, whereas M2 macrophages promote tumour progression and metastasis114.
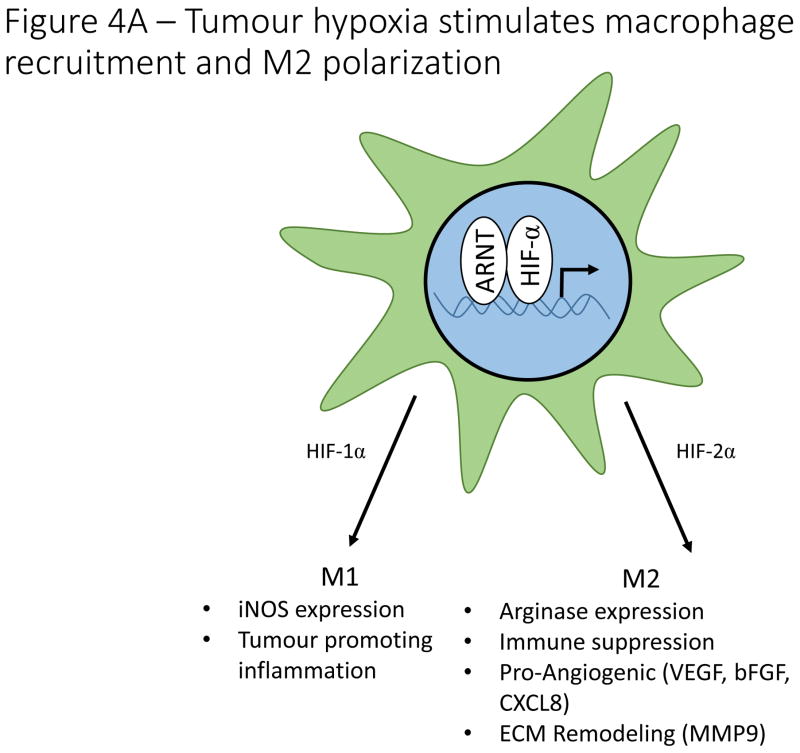
Macrophage polarization within the tumour microenvironment is heterogeneous and dynamic, and hypoxia plays an important role in this process (Fig. 4A). Blockade of Sema3A-stimulated macrophage recruitment to hypoxic tumour regions skews macrophages towards the M1 phenotype, indicating that therapeutic blockade of Sema3A may promote anti-tumour activity of TAMs110. Preclinical studies further indicate that vascular normalization after low dose irradiation decreases tumour hypoxia and promotes M1 polarization of TAMs, an effect that may be exploited to enhance anti-tumour immunity115.
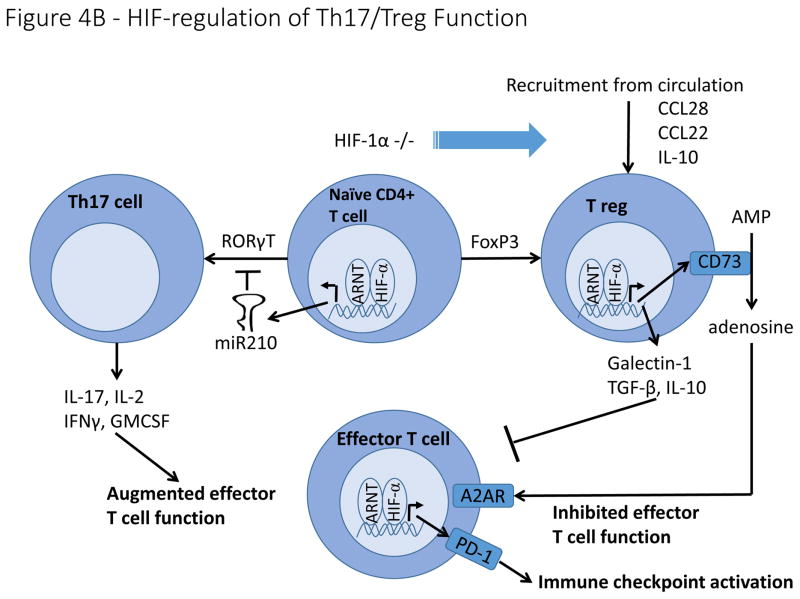
Figure 4. Hypoxia and HIF-signaling effects on immune cells in the tumour microenvironment.
A). Hypoxia regulates macrophage polarization by controlling expression of genes involved in the function of M1 and M2 macrophages. HIF-1α promotes expression of the M1 gene, iNOS, while HIF-2α promotes expression of the M2 gene, Arg1. Hypoxia induces macrophage production of genes involved in angiogenesis (VEGF, bFGF, CXCL12), ECM remodeling (MMP9), and immune suppression (Arg1). B). HIF signaling plays a controversial role in determining the differentiation of naïve CD4+ T cells into either Th17 pro-inflammatory T cells or FoxP3+ T reg cells with reports suggesting that HIF either induces or inhibits the formation of both cell types. In contrast, overwhelming evidence indicates that hypoxia stimulates the secretion of a number of cytokines and chemoattractants from cancer cells and tumour associated macrophages that recruit T regs from the circulation. On a cell intrinsic basis, hypoxia stimulates T reg production of CD73, thereby increasing adenosine levels in the tumour microenvironment, resulting in inhibition of effector T cells. Hypoxia also stimulates T reg production of other immune suppressive molecules, including Galectin-1, TGF-β and IL-10.
Hypoxia can directly impact macrophage polarization by inducing M2-like gene expression in TAMs116. HIF-1α and HIF-2 appear to play opposing roles in macrophage polarization, with HIF-2α specifically induced in response to Th2 cytokines in macrophages117. HIF-1α and HIF-2α are nevertheless essential for macrophage function, as loss of HIF-1α results in defective macrophage maturation and function118. Furthermore, genetically engineered mouse models indicate that expression of HIF-1α and HIF-2α are essential for macrophage infiltration and immune suppression within tumours and loss of either isoform in macrophages diminishes tumour growth119,120. Elevated lactate levels in tumours promotes M2 polarization in a HIF-1α dependent manner, indicating that hypoxic metabolism by tumour cells also impacts macrophage polarization121. These findings indicate that a combination of microenvironmental hypoxia and metabolic symbiosis between tumour cells and macrophages skew their polarization and shape the anti-tumour immune response122,123. In addition to mediating immunosuppression, M2 macrophages are important drivers of angiogenesis, indicating an intricate cross talk between hypoxic tumour cells, macrophages and endothelial cells that dictates oxygen availability in the tumour, cellular metabolism, and the anti-tumour immune response124. Thus, TAMs have a significant and direct impact on hypoxia in the tumour microenvironment, creating a feedback loop between oxygen availability and recruitment/polarization of TAMs.
Myeloid Derived Suppressor Cells
Myeloid derived suppressor cells (MDSCs) are bone marrow-derived cells that suppress the anti-tumour immune response. MDSCs exposed to hypoxia induce HIF-signaling and upregulate HIF targets that enhance MDSC function125. Hypoxia enhances MDSC suppressor function through a mechanism that is partially dependent on the HIF-regulated miRNA, mir-210 and increased expression of Arg1125,126. Tumour hypoxia also influences seeding of MDSCs in the pre-metastatic niche by stimulating increased secretion of lysyl oxidase127–129. This process drives ECM remodeling in the metastatic niche and suppresses natural killer anti-tumour immune responses127. The enhanced MDSC suppressor function in hypoxia indicates an additional mechanism by which the hypoxic microenvironment suppresses anti-tumour immunity.
Tumour-associated neutrophils
Tumour associated neutrophils (TANs) can have both pro- and anti-tumorigenic properties130. Hypoxia in the tumour microenvironment promotes neutrophil recruitment by changing the adherence properties of ECs to neutrophils131. In a preclinical model of uterine carcinoma, hypoxia-mediated neutrophil recruitment impeded tumour growth, indicating a tumour suppressive role for neutrophils in this cancer type132. Hypoxia prolongs neutrophil survival, and HIF-dependent increases in glycolytic metabolism are important for neutrophil function in hypoxia118,133. Similar to macrophages, neutrophil function can be classified into N1 and N2 polarization, with N2 neutrophils displaying tumour promoting properties analogous to the role of M2 macrophages in tumour progression134. TGF-β in the tumour microenvironment promotes N2 neutrophil polarization, thereby enhancing their tumour promoting function135. Despite the association between HIF-α signaling and TGF-β, whether hypoxia directly modulates neutrophil polarization remains unclear.
Cells of the adaptive immune system
Cells of the adaptive immune system, such as tumour-infiltrating lymphocytes, have the potential to effectively eliminate tumour cells from the body by recognizing tumour-associated antigens. However, the hypoxic tumour microenvironment limits their anti-tumour activity by promoting immune checkpoint activation, limiting access to key nutrients and recruiting a variety of immunosuppressive cell types to the tumour site, thereby reducing the effectiveness of the anti-tumour immune response.
Tumour-infiltrating lymphocytes
Despite frequent infiltration of T-cells into solid tumours, anti-tumour immunity is often limited by features of the tumour microenvironment, including hypoxia136. Hypoxic cancer cells and macrophages secrete chemokines and cytokines including CCL22, CCL28, and IL-10, to recruit a CD4+, CD25high, Foxp3+ subpopulation of T cells known as regulatory T cells (Tregs) from the circulation, thereby blunting the T-cell mediated anti-tumour response137,138 (Fig. 4B). Hypoxia also stimulates production of extracellular adenosine by Tregs, which inhibits effector T-cell function through activation of cAMP signaling139,140. Surprisingly, while hypoxia promotes critical Treg functions, constitutive activation of HIF-1α, achieved through Treg-specific VHL deletion, disrupts Treg function and drives interferon-γ mediated tissue inflammation, indicating that physiologically appropriate levels of HIF-signaling are important for Treg differentiation141.
Whereas Tregs are functionally immunosuppressive, Th17 cells are pro-inflammatory CD4+ T cells that play a complex and controversial role in anti-tumour immunity142. Hypoxia promotes the differentiation of naïve CD4+ T cells into Tregs or Th17 cells by modulating expression of Foxp3 and RORγt, essential transcription factors for these respective cell types23,143,144 (Fig. 4B). Induction of Th17 development is accompanied by increased glycolysis driven by mTOR-HIF-1α signaling145. In contrast, Tregs cells rely primarily on β-oxidation for their metabolic function. More work is needed to understand the complex and seemingly contradictory effects of HIF-signaling on Th17/Treg development, including whether different mechanisms of HIF-activation (hypoxia versus TCR signaling) result in different T cell fate decisions.
Promising results from clinical trials have dramatically increased interest in targeting immune checkpoints in cancer therapy. To date, most attention has focused on targeting the receptors, PD-1 and CTLA-4 and their ligands, PD-L1/PDL-2 and CD80/CD86, respectively, but the number of targets is likely to increase as knowledge of immune checkpoint regulation expands146. Recent studies revealed direct regulation of PD-L1 in MDSCs and tumour cells by HIF-1α, indicating that hypoxia transcriptionally modulates immune checkpoints147. Similarly, in vitro studies in human and canine cell lines indicate that PD-L1 is induced in a HIF-1α dependent manner upon exposure to hypoxia, and that this induction inhibits T-cell mediated lysis148.
Conclusions
Unlike genetic aberrations, which directly modulate tumour cell function, microenvironmental factors such as hypoxia influence both tumour and stromal cells. Hypoxia promotes genomic instability in cancer cells, and also recruits vascular endothelial cells and regulates pericyte function to promote angiogenesis, thereby increasing nutrient and oxygen supply and facilitating metastatic dissemination. In CAFs, hypoxia promotes symbiotic metabolism, ECM remodeling and secretion of paracrine factors that support primary tumour growth and metastasis. Hypoxia also drives recruitment of various immune cells and promotes an immunosuppressive microenvironment to limit anti-tumour immunity. On a global level, low oxygen levels trigger HIF-dependent and –independent adaptive responses in both tumour and stromal cells that increase tumour aggressiveness and metastasis.
Tumour hypoxia fluctuates during tumour growth and in response to cytotoxic and targeted therapies, with profound impacts on disease progression and therapeutic efficacy. As our understanding of the hypoxic response in stromal and tumour cells increases, developing cancer therapies against hypoxia response pathways is emerging as an attractive option. Delivery of such therapies could be timed to coincide with the increased hypoxia observed with VEGF blockade, to prevent the hypoxia-associated increased aggressiveness upon VEGF inhibition. Another potential therapeutic approach, would be to directly target HIF-transcriptional activity, for example by exploiting potential small molecule binding pockets revealed by the elucidation of the ARNT-bound HIF-1α and HIF-2α crystal structures149,150 A better understanding of the effects of hypoxia and HIF-mediated responses in CAFs and immune cells, including macrophages, T cells, and neutrophils, could also allow their therapeutic manipulation to stimulate anti-tumour immune responses. Likewise, elucidating the role of HIF signaling in controlling immune checkpoint signaling components, could be useful in cancer immunotherapy.
Given their potential to inhibit tumour promoting pathways in both malignant and stromal cells, the development of therapeutic approaches targeting HIF-signaling directly, or by inhibiting downstream effector pathways holds great promise for cancer treatment.
Acknowledgments
The authors would like to thank those whose work informed the writing of this manuscript and apologize to those authors whose elegant studies we were unable to acknowledge in this review. This work was supported by NIH grants CA-67166 and CA-197713, the Silicon Valley Foundation, the Sydney Frank Foundation and the Kimmelman Fund (AJG).
References
- 1.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 2.Nagy JA, Chang SH, Dvorak AM, Dvorak HF. Why are tumour blood vessels abnormal and why is it important to know? Br J Cancer. 2009;100:865–869. doi: 10.1038/sj.bjc.6604929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 2010;29:285–293. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- 6.Wiesener MS, et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 2003;17:271–273. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 7.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson CM, Ohh M. The multifaceted von Hippel-Lindau tumour suppressor protein. FEBS Lett. 2014;588:2704–2711. doi: 10.1016/j.febslet.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gossage L, Eisen T, Maher ER. VHL, the story of a tumour suppressor gene. Nat Rev Cancer. 2015;15:55–64. doi: 10.1038/nrc3844. [DOI] [PubMed] [Google Scholar]
- 11.Coleman ML, Ratcliffe PJ. Signalling cross talk of the HIF system: involvement of the FIH protein. Curr Pharm Des. 2009;15:3904–3907. doi: 10.2174/138161209789649448. [DOI] [PubMed] [Google Scholar]
- 12.Hudson CC, et al. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong H, et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 14.Duvel K, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG., Jr TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell. 2003;4:147–158. doi: 10.1016/s1535-6108(03)00187-9. [DOI] [PubMed] [Google Scholar]
- 16.Mayerhofer M, Valent P, Sperr WR, Griffin JD, Sillaber C. BCR/ABL induces expression of vascular endothelial growth factor and its transcriptional activator, hypoxia inducible factor-1alpha, through a pathway involving phosphoinositide 3-kinase and the mammalian target of rapamycin. Blood. 2002;100:3767–3775. doi: 10.1182/blood-2002-01-0109. [DOI] [PubMed] [Google Scholar]
- 17.Faubert B, et al. Loss of the tumor suppressor LKB1 promotes metabolic reprogramming of cancer cells via HIF-1alpha. Proc Natl Acad Sci U S A. 2014;111:2554–2559. doi: 10.1073/pnas.1312570111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shackelford DB, et al. mTOR and HIF-1alpha-mediated tumor metabolism in an LKB1 mouse model of Peutz-Jeghers syndrome. Proc Natl Acad Sci U S A. 2009;106:11137–11142. doi: 10.1073/pnas.0900465106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Naggar AM, et al. Translational Activation of HIF1alpha by YB-1 Promotes Sarcoma Metastasis. Cancer Cell. 2015;27:682–697. doi: 10.1016/j.ccell.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Chou CC, Chuang HC, Salunke SB, Kulp SK, Chen CS. A novel HIF-1alpha-integrin-linked kinase regulatory loop that facilitates hypoxia-induced HIF-1alpha expression and epithelial-mesenchymal transition in cancer cells. Oncotarget. 2015;6:8271–8285. doi: 10.18632/oncotarget.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura H, et al. TCR engagement increases hypoxia-inducible factor-1 alpha protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. J Immunol. 2005;174:7592–7599. doi: 10.4049/jimmunol.174.12.7592. [DOI] [PubMed] [Google Scholar]
- 22.Chisolm DA, Weinmann AS. TCR-Signaling Events in Cellular Metabolism and Specialization. Front Immunol. 2015;6:292. doi: 10.3389/fimmu.2015.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noman MZ, et al. The cooperative induction of hypoxia-inducible factor-1 alpha and STAT3 during hypoxia induced an impairment of tumor susceptibility to CTL-mediated cell lysis. J Immunol. 2009;182:3510–3521. doi: 10.4049/jimmunol.0800854. [DOI] [PubMed] [Google Scholar]
- 25.Rius J, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blouin CC, Page EL, Soucy GM, Richard DE. Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1alpha. Blood. 2004;103:1124–1130. doi: 10.1182/blood-2003-07-2427. [DOI] [PubMed] [Google Scholar]
- 27.Jantsch J, et al. Toll-like receptor activation and hypoxia use distinct signaling pathways to stabilize hypoxia-inducible factor 1alpha (HIF1A) and result in differential HIF1A-dependent gene expression. J Leukoc Biol. 2011;90:551–562. doi: 10.1189/jlb.1210683. [DOI] [PubMed] [Google Scholar]
- 28.Kurokawa H, et al. High resolution imaging of intracellular oxygen concentration by phosphorescence lifetime. Sci Rep. 2015;5:10657. doi: 10.1038/srep10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong Y, Agani FH. Oligomycin inhibits HIF-1alpha expression in hypoxic tumor cells. Am J Physiol Cell Physiol. 2005;288:C1023–1029. doi: 10.1152/ajpcell.00443.2004. [DOI] [PubMed] [Google Scholar]
- 30.O’Hagan KA, et al. PGC-1alpha is coupled to HIF-1alpha-dependent gene expression by increasing mitochondrial oxygen consumption in skeletal muscle cells. Proc Natl Acad Sci U S A. 2009;106:2188–2193. doi: 10.1073/pnas.0808801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan M, et al. The tumor suppressor folliculin regulates AMPK-dependent metabolic transformation. J Clin Invest. 2014;124:2640–2650. doi: 10.1172/JCI71749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaGory EL, et al. Suppression of PGC-1alpha Is Critical for Reprogramming Oxidative Metabolism in Renal Cell Carcinoma. Cell reports. 2015;12:116–127. doi: 10.1016/j.celrep.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 34.Niecknig H, et al. Role of reactive oxygen species in the regulation of HIF-1 by prolyl hydroxylase 2 under mild hypoxia. Free Radic Res. 2012;46:705–717. doi: 10.3109/10715762.2012.669041. [DOI] [PubMed] [Google Scholar]
- 35.Bell EL, Chandel NS. Mitochondrial oxygen sensing: regulation of hypoxia-inducible factor by mitochondrial generated reactive oxygen species. Essays Biochem. 2007;43:17–27. doi: 10.1042/BSE0430017. [DOI] [PubMed] [Google Scholar]
- 36.Mansfield KD, et al. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandel NS, et al. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masson N, et al. The FIH hydroxylase is a cellular peroxide sensor that modulates HIF transcriptional activity. EMBO Rep. 2012;13:251–257. doi: 10.1038/embor.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagen T. Oxygen versus Reactive Oxygen in the Regulation of HIF-1alpha: The Balance Tips. Biochem Res Int. 2012;2012:436981. doi: 10.1155/2012/436981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pollard PJ, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231–2239. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 41.Lu H, et al. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005;280:41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 42.Reitman ZJ, Yan H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J Natl Cancer Inst. 2010;102:932–941. doi: 10.1093/jnci/djq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye D, Ma S, Xiong Y, Guan KL. R-2-hydroxyglutarate as the key effector of IDH mutations promoting oncogenesis. Cancer Cell. 2013;23:274–276. doi: 10.1016/j.ccr.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong BW, Kuchnio A, Bruning U, Carmeliet P. Emerging novel functions of the oxygen-sensing prolyl hydroxylase domain enzymes. Trends Biochem Sci. 2013;38:3–11. doi: 10.1016/j.tibs.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Zhang D, et al. Metabolic reprogramming of cancer-associated fibroblasts by IDH3alpha downregulation. Cell reports. 2015;10:1335–1348. doi: 10.1016/j.celrep.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Zeng L, et al. Aberrant IDH3alpha expression promotes malignant tumor growth by inducing HIF-1-mediated metabolic reprogramming and angiogenesis. Oncogene. 2014 doi: 10.1038/onc.2014.411. [DOI] [PubMed] [Google Scholar]
- 47.Li B, et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature. 2014;513:251–255. doi: 10.1038/nature13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo W, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao X, Wang H, Yang JJ, Liu X, Liu ZR. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell. 2012;45:598–609. doi: 10.1016/j.molcel.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakajima EC, Van Houten B. Metabolic symbiosis in cancer: refocusing the Warburg lens. Mol Carcinog. 2013;52:329–337. doi: 10.1002/mc.21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peppicelli S, Bianchini F, Calorini L. Extracellular acidity, a “reappreciated” trait of tumor environment driving malignancy: perspectives in diagnosis and therapy. Cancer Metastasis Rev. 2014;33:823–832. doi: 10.1007/s10555-014-9506-4. [DOI] [PubMed] [Google Scholar]
- 52.Wang T, Liu G, Wang R. The Intercellular Metabolic Interplay between Tumor and Immune Cells. Front Immunol. 2014;5:358. doi: 10.3389/fimmu.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho PC, et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162:1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang CH, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Folkman J. Proceedings: Tumor angiogenesis factor. Cancer Res. 1974;34:2109–2113. [PubMed] [Google Scholar]
- 56.Franses JW, Baker AB, Chitalia VC, Edelman ER. Stromal endothelial cells directly influence cancer progression. Sci Transl Med. 2011;3:66ra65. doi: 10.1126/scitranslmed.3001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coulon C, et al. From vessel sprouting to normalization: role of the prolyl hydroxylase domain protein/hypoxia-inducible factor oxygen-sensing machinery. Arterioscler Thromb Vasc Biol. 2010;30:2331–2336. doi: 10.1161/ATVBAHA.110.214106. [DOI] [PubMed] [Google Scholar]
- 58.Polet F, Feron O. Endothelial cell metabolism and tumour angiogenesis: glucose and glutamine as essential fuels and lactate as the driving force. J Intern Med. 2013;273:156–165. doi: 10.1111/joim.12016. [DOI] [PubMed] [Google Scholar]
- 59.Schoors S, et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520:192–197. doi: 10.1038/nature14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang D, et al. HIF-1-mediated suppression of acyl-CoA dehydrogenases and fatty acid oxidation is critical for cancer progression. Cell reports. 2014;8:1930–1942. doi: 10.1016/j.celrep.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 61.el Azzouzi H, et al. The hypoxia-inducible microRNA cluster miR-199a approximately 214 targets myocardial PPARdelta and impairs mitochondrial fatty acid oxidation. Cell Metab. 2013;18:341–354. doi: 10.1016/j.cmet.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 62.Krishnan J, et al. Dietary obesity-associated Hif1alpha activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes Dev. 2012;26:259–270. doi: 10.1101/gad.180406.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rankin EB, et al. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol Cell Biol. 2009;29:4527–4538. doi: 10.1128/MCB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang N, et al. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 65.Skuli N, et al. Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood. 2009;114:469–477. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skuli N, et al. Endothelial HIF-2alpha regulates murine pathological angiogenesis and revascularization processes. J Clin Invest. 2012;122:1427–1443. doi: 10.1172/JCI57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mazzone M, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Branco-Price C, et al. Endothelial cell HIF-1alpha and HIF-2alpha differentially regulate metastatic success. Cancer Cell. 2012;21:52–65. doi: 10.1016/j.ccr.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 70.Morikawa S, et al. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. The American journal of pathology. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greenberg JI, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cooke VG, et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell. 2012;21:66–81. doi: 10.1016/j.ccr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keskin D, et al. Targeting vascular pericytes in hypoxic tumors increases lung metastasis via angiopoietin-2. Cell reports. 2015;10:1066–1081. doi: 10.1016/j.celrep.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17:471–494. doi: 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maione F, et al. Semaphorin 3A overcomes cancer hypoxia and metastatic dissemination induced by antiangiogenic treatment in mice. J Clin Invest. 2012;122:1832–1848. doi: 10.1172/JCI58976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sounni NE, et al. Blocking lipid synthesis overcomes tumor regrowth and metastasis after antiangiogenic therapy withdrawal. Cell Metab. 2014;20:280–294. doi: 10.1016/j.cmet.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 77.Bensaad K, et al. Fatty acid uptake and lipid storage induced by HIF-1alpha contribute to cell growth and survival after hypoxia-reoxygenation. Cell reports. 2014;9:349–365. doi: 10.1016/j.celrep.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 78.Tong RT, et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 79.Winkler F, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 80.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 81.Bochet L, et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73:5657–5668. doi: 10.1158/0008-5472.CAN-13-0530. [DOI] [PubMed] [Google Scholar]
- 82.Hinz B, et al. The myofibroblast: one function, multiple origins. The American journal of pathology. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Direkze NC, et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 84.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim JW, et al. Loss of fibroblast HIF-1alpha accelerates tumorigenesis. Cancer Res. 2012;72:3187–3195. doi: 10.1158/0008-5472.CAN-12-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Madsen CD, et al. Hypoxia and loss of PHD2 inactivate stromal fibroblasts to decrease tumour stiffness and metastasis. EMBO Rep. 2015 doi: 10.15252/embr.201540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuchnio A, et al. The Cancer Cell Oxygen Sensor PHD2 Promotes Metastasis via Activation of Cancer-Associated Fibroblasts. Cell reports. 2015 doi: 10.1016/j.celrep.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 88.Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res. 2011;1:482–497. [PMC free article] [PubMed] [Google Scholar]
- 89.Pavlides S, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 90.Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. 2014;25:47–60. doi: 10.1016/j.semcancer.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 91.Chiavarina B, et al. Metabolic reprogramming and two-compartment tumor metabolism: opposing role(s) of HIF1alpha and HIF2alpha in tumor-associated fibroblasts and human breast cancer cells. Cell Cycle. 2012;11:3280–3289. doi: 10.4161/cc.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sonveaux P, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One. 2012;7:e33418. doi: 10.1371/journal.pone.0033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sonveaux P, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fiaschi T, et al. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012;72:5130–5140. doi: 10.1158/0008-5472.CAN-12-1949. [DOI] [PubMed] [Google Scholar]
- 95.Costa A, Scholer-Dahirel A, Mechta-Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol. 2014;25:23–32. doi: 10.1016/j.semcancer.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 96.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 97.Schioppa T, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ammirante M, Shalapour S, Kang Y, Jamieson CA, Karin M. Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc Natl Acad Sci U S A. 2014;111:14776–14781. doi: 10.1073/pnas.1416498111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schito L, et al. Hypoxia-inducible factor 1-dependent expression of platelet-derived growth factor B promotes lymphatic metastasis of hypoxic breast cancer cells. Proc Natl Acad Sci U S A. 2012;109:E2707–2716. doi: 10.1073/pnas.1214019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caniggia I, et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J Clin Invest. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 102.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 103.Ino Y, et al. Arginase II expressed in cancer-associated fibroblasts indicates tissue hypoxia and predicts poor outcome in patients with pancreatic cancer. PLoS One. 2013;8:e55146. doi: 10.1371/journal.pone.0055146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baginska J, et al. Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proc Natl Acad Sci U S A. 2013;110:17450–17455. doi: 10.1073/pnas.1304790110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Du R, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 107.Mamlouk S, et al. Loss of prolyl hydroxylase-2 in myeloid cells and T-lymphocytes impairs tumor development. Int J Cancer. 2014;134:849–858. doi: 10.1002/ijc.28409. [DOI] [PubMed] [Google Scholar]
- 108.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 109.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Casazza A, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013;24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 111.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 112.Matschurat S, et al. Regulation of EMAP II by hypoxia. The American journal of pathology. 2003;162:93–103. doi: 10.1016/S0002-9440(10)63801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wen Z, et al. Increased metabolites of 5-lipoxygenase from hypoxic ovarian cancer cells promote tumor-associated macrophage infiltration. Oncogene. 2015;34:1241–1252. doi: 10.1038/onc.2014.85. [DOI] [PubMed] [Google Scholar]
- 114.Rolny C, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 115.Klug F, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 116.Laoui D, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014;74:24–30. doi: 10.1158/0008-5472.CAN-13-1196. [DOI] [PubMed] [Google Scholar]
- 117.Takeda N, et al. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24:491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cramer T, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Imtiyaz HZ, et al. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120:2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Doedens AL, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Colegio OR, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 123.Goodwin ML, et al. Modeling alveolar soft part sarcomagenesis in the mouse: a role for lactate in the tumor microenvironment. Cancer Cell. 2014;26:851–862. doi: 10.1016/j.ccell.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stockmann C, et al. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Corzo CA, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Noman MZ, et al. Tumor promoting effects of myeloid derived suppressor cells are potentiated by hypoxia-induced expression of miR-210. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-15-0405. [DOI] [PubMed] [Google Scholar]
- 127.Sceneay J, et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72:3906–3911. doi: 10.1158/0008-5472.CAN-11-3873. [DOI] [PubMed] [Google Scholar]
- 128.Cox TR, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522:106–110. doi: 10.1038/nature14492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 130.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 131.Yoshida N, et al. Anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Am J Physiol. 1992;262:H1891–1898. doi: 10.1152/ajpheart.1992.262.6.H1891. [DOI] [PubMed] [Google Scholar]
- 132.Blaisdell A, et al. Neutrophils Oppose Uterine Epithelial Carcinogenesis via Debridement of Hypoxic Tumor Cells. Cancer Cell. 2015;28:785–799. doi: 10.1016/j.ccell.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Walmsley SR, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liang W, Ferrara N. The Complex Role of Neutrophils in Tumor Angiogenesis and Metastasis. Cancer Immunol Res. 2016;4:83–91. doi: 10.1158/2326-6066.CIR-15-0313. [DOI] [PubMed] [Google Scholar]
- 135.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Le QT, et al. Galectin-1: a link between tumor hypoxia and tumor immune privilege. J Clin Oncol. 2005;23:8932–8941. doi: 10.1200/JCO.2005.02.0206. [DOI] [PubMed] [Google Scholar]
- 137.Viguier M, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 138.Facciabene A, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 139.Synnestvedt K, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ohta A, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee JH, Elly C, Park Y, Liu YC. E3 Ubiquitin Ligase VHL Regulates Hypoxia-Inducible Factor-1alpha to Maintain Regulatory T Cell Stability and Suppressive Capacity. Immunity. 2015;42:1062–1074. doi: 10.1016/j.immuni.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bailey SR, et al. Th17 cells in cancer: the ultimate identity crisis. Front Immunol. 2014;5:276. doi: 10.3389/fimmu.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1alpha. Eur J Immunol. 2008;38:2412–2418. doi: 10.1002/eji.200838318. [DOI] [PubMed] [Google Scholar]
- 144.Clambey ET, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. 2012;109:E2784–2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shi LZ, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Noman MZ, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 149.Wu D, Potluri N, Lu J, Kim Y, Rastinejad F. Structural integration in hypoxia-inducible factors. Nature. 2015 doi: 10.1038/nature14883. [DOI] [PubMed] [Google Scholar]
- 150.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]