Abstract
The African river frog genus Amietia is found near rivers and other lentic water sources throughout central, eastern, and southern Africa. Because the genus includes multiple morphologically conservative species, taxonomic studies of river frogs have been relatively limited. We sampled 79 individuals of Amietia from multiple localities in and near the Albertine Rift (AR) of Burundi, Democratic Republic of the Congo, and Uganda. We utilized single-gene (16S) and concatenated (12S, 16S, cyt b and RAG1) gene-tree analyses and coalescent species-tree analyses to construct phylogenetic trees. Two divergence dating approaches were used in BEAST, including secondary calibration points with 12S, 16S, cyt b and RAG1, and a molecular clock with the 12S, 16S, and cyt b genes. All analyses recovered Amietia as monophyletic with strong support, and revealed several well-supported cryptic lineages, which is consistent with other recent phylogeography studies of AR amphibians. Dating estimates were similar, and Amietia diversification is coincident with global cooling and aridification events in the Miocene and Pliocene, respectively. Our results suggest additional taxonomic work is needed to describe multiple new species of AR Amietia, some of which have limited geographic distributions that are likely to be of conservation concern.
Keywords: Biodiversity, Albertine Rift, speciation, amphibians, Montane Forest
Graphical Abstract
1. Introduction
The genus Amietia currently contains 16 species of frogs (Frost, 2015) associated with permanent water sources such as rivers and lakes in central, eastern, and southern Africa. Although typically similar in morphology, Amietia vary slightly in toe webbing (Laurent, 1972; Channing, 2015), body proportions (Poynton, 1964), pattern and coloration (Channing and Baptista, 2013), and call (Visser and Channing, 1997). Amietia generally occur in habitats ranging from forests to open savannas, but they are rarely found far from water sources (Channing and Howell, 2006; Largen and Spawls, 2010). In the last decade, several species have been described, including A. lubrica (Pickersgill, 2007), A. poyntoni Channing and Baptista, 2013, A. tenuoplicata (Pickersgill, 2007), and A. viridireticulata (Pickersgill, 2007).
The current classification of the species in the genus Amietia was a result of morphological (Dubois, 2005) and genetic (van der Meijden et al., 2005) analyses that supported a monophyletic group containing both Amietia and Afrana. The species in these genera were subsequently combined into the genus Amietia in the Family Pyxicephalidae (Frost et al., 2006). Several recent studies have clarified the taxonomic status of some Amietia species (Pickersgill, 2007; Channing and Baptista, 2013; Channing, 2015), but many areas within the distribution of Amietia remain poorly sampled.
Five species of Amietia (A. amieti, A. angolensis, A. desaegeri, A. ruwenzorica, and A. wittei) are currently known from the Albertine Rift (AR) of Central Africa, where the vertebrate communities are considered to be the most species-rich in continental Africa (Plumptre et al., 2007; Greenbaum and Kusamba, 2012; Menegon et al., 2014; Greenbaum et al., 2015a; Portillo et al., 2015). Many threatened and endemic species reside within the AR (Plumptre et al., 2007; Jenkins et al., 2013; Portillo et al., 2015), making it one of the most irreplaceable and important sites for conservation in Africa (Brooks et al., 2006). Many vertebrate species in the AR are morphologically cryptic and endemic to a small number of sites, including small mammals (Kerbis Peterhans et al., 2010; Demos et al., 2014), birds (Prigogine, 1971, 1977, 1978, 1984, 1985; Bowie et al., 2006), reptiles (Greenbaum et al., 2011, 2015a; Menegon et al., 2014) and amphibians (Laurent, 1964, 1972; Evans et al., 2008, 2011; Greenbaum and Kusamba, 2012; Portillo and Greenbaum, 2014a,b; Portillo et al., 2015). Viertel et al. (2012) recently described tadpole morphology and chytrid fungal infections in a Ugandan population of A. ruwenzorica, but no previous studies have assessed AR Amietia populations with molecular data.
Herein, we utilize the General Lineage Concept of species (de Queiroz, 1998, 2007), which postulates that species are separately evolving lineages. Lineages are recognized as candidate species based on unique morphological (i.e., size, color, toe webbing), ecological, or behavioral differences in congruence with genetic differentiation (Wiens and Penkrot, 2002). We utilize concatenated gene-tree and species-tree analyses to evaluate the following questions: (1) Do populations of AR Amietia form a monophyletic group? (2) Are cryptic lineages of Amietia present in the AR? (3) Can species-tree analyses help to resolve relationships of rapidly radiating Amietia lineages? (4) When did AR Amietia populations diverge from one another? (5) Do estimated divergence dates correspond to temporal and spatial biogeographic events?
2. Materials and Methods
2.1 Taxon sampling
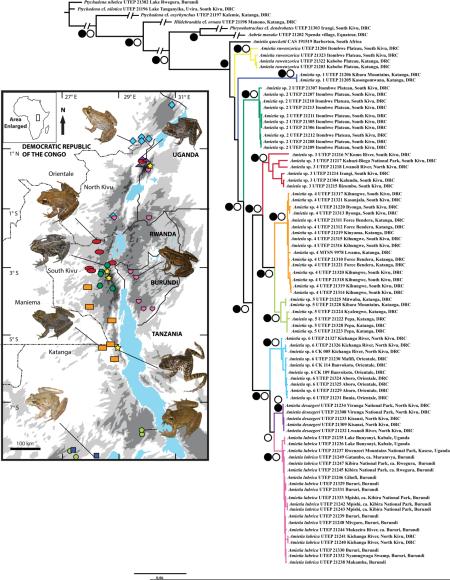
We sequenced 79 samples from the genus Amietia from locations throughout the AR in Democratic Republic of the Congo (DRC), Burundi, and Uganda (Fig. 1). Additional Amietia sequences were obtained from the studies of Dawood and Uqubay (2004), Scott (2005), van der Meijden et al. (2005, 2011), Bossuyt et al. (2006), Tarrant et al. (2008), Tolley et al. (2010), Viertel et al. (2012), Channing and Baptista (2013), and Zancolli et al. (2014), with collections as listed by Sabaj Pérez (2013). Outgroup samples included: Ptychadena nilotica, Ptychadena cf. nilotica, Ptychadena cf. oxyrhynchus, Hildebrandtia cf. ornata (Ptychadenidae), Phrynobatrachus cf. dendrobates (Phrynobatrachidae), and Aubria masako (Pyxicephalidae). Newly sequenced samples were deposited into GenBank (Table 1).
Fig. 1.
Elevation map for the Albertine Rift showing sampling localities for Amietia in this study. Colored shapes correspond to localities of clades within the phylogenies of Figs. 2–3.
Table 1.
Voucher numbers, localities, and GenBank numbers for genetic samples. DRC = Democratic Republic of the Congo.
| Species | Collection No. | Field No. | Locality | 12S | 16S | Cyt b | RAG1 |
|---|---|---|---|---|---|---|---|
| Aubria masako | UTEP 21202 | ELI 2156 | DRC: Equateur: Npenda village | KU559938 | KU560021 | KU560106 | KU560187 |
| Cacosternum boettgeri | ZFMK 66727 | — | Namibia: Hardap | AF124096 | AF215414 | — | AY571645 |
| Hildebrandtia cf. ornata | UTEP 21198 | ELI 359 | DRC: Katanga: 7 km south of Manono | KU559936 | KU560019 | KU560104 | KU560185 |
| Natalobatrachus bonebergi | ZFMK 74837 | — | South Africa: The Haven | AF215198 | AF215396 | — | DQ019502 |
| Petropedetes parkeri | No voucher | — | Cameroon: Nguti | AY341628 | AF124132 | — | DQ019505 |
| Phrynobatrachus cf. dendrobates | UTEP 21303 | EBG 1314 | DRC: South Kivu: Irangi, ca. Kahuzi-Biega National Park | KU559937 | KU560020 | KU560105 | KU560186 |
| Ptychadena cf. nilotica | UTEP 21196 | EBG 1582 | DRC: South Kivu: Lake Tanganyika, Uvira, Mulongwe | KU559934 | KU560017 | KU560102 | KU560183 |
| Ptychadena nilotica | UTEP 21302 | ELI 1243 | Burundi: Lake Rwegura | KU559933 | KU560016 | KU560101 | KU560182 |
| Ptychadena cf. oxyrhynchus | UTEP 21197 | EBG 2873 | DRC: Katanga: road south of Kalemie | KU559935 | KU560018 | KU560103 | KU560184 |
| Pyxicephalus adspersus | ZFMK 66446 | — | South Africa: KwaMbonambi | AF206091 | AF215505 | — | DQ019508 |
| Strongylopus fasciatus | ZFMK 66444 | — | South Africa: Little Brak | DQ019594 | AF215412 | — | DQ019513 |
| Tomopterna sp. | ZFMK 66403 | — | Namibia: Khorixas | DQ019595 | DQ019610 | — | DQ019514 |
| Amietia angolensis | — | AC 3080 | Angola: Humpata, Zootecnica Station | — | KC756286 | — | — |
| Amietia angolensis | — | AC 3016 | Angola: Calandula Waterfalls | — | KC756291 | — | — |
| Amietia cf. angolensis | RDS 926 | — | Tanzania: Mazumbai, West Usambara Mountains1 | — | DQ022350 | — | — |
| Amietia desaegeri | — | SL 478 | Uganda: Rwenzori Mountains | — | GQ183600 | — | — |
| Amietia desaegeri (tadpole) | UTEP 21232 | EBG 1879 | DRC: North Kivu: Lwanoli River | KU559959 | KU560042 | KU560127 | KU560208 |
| Amietia desaegeri | UTEP 21233 | EBG 1845 | DRC: North Kivu: Kisanzi | KU559957 | KU560040 | KU560125 | KU560206 |
| Amietia desaegeri | UTEP 21234 | EBG 1811 | DRC: North Kivu: Virunga National Park, Ndjuma | KU559955 | KU560038 | KU560123 | KU560204 |
| Amietia desaegeri | UTEP 21309 | EBG 1846 | DRC: North Kivu: Kisanzi | KU559958 | KU560041 | KU560126 | KU560207 |
| Amietia desaegeri | UTEP 21308 | EBG 1812 | DRC: North Kivu: Virunga National Park, Ndjuma | KU559956 | KU560039 | KU560124 | KU560205 |
| Amietia fuscigula | — | CR 1073 | Africa | — | DQ347347 | — | — |
| Amietia fuscigula | — | AC 3167 | South Africa: Western Cape: Bainskloof Pass | — | KC756306 | — | — |
| Amietia fuscigula | — | AC 2671 | South Africa: Eastern Cape: Longmore Forest Reserve | — | KC756317 | — | — |
| Amietia fuscigula | — | AC 2665 | South Africa: Western Cape: Bloukrans Pass | — | KC756321 | — | — |
| Amietia hymenopus | UFS MPU100 | — | South Africa: KwaZulu-Natal | — | FJ411435 | — | — |
| Amietia hymenopus | UFS MH1323 | — | South Africa: Free State | — | FJ411434 | — | — |
| Amietia lubrica | — | SL 538 | Uganda: Semliki | — | GQ183602 | — | — |
| Amietia lubrica | UTEP 21236 | ELI 2716 | Uganda: Kabale District: Lake Bunyonyi | KU560012 | KU560097 | KU560178 | KU560263 |
| Amietia lubrica | UTEP 21235 | ELI 2715 | Uganda: Kabale District: Lake Bunyonyi | KU560011 | KU560096 | KU560177 | KU560262 |
| Amietia lubrica | UTEP 21237 | ELI 2826 | Uganda: Kasese Disctrict: Rwenzori Mountains National Park, Ruboni Community Hotel | KU560013 | KU560098 | KU560179 | KU560264 |
| Amietia lubrica | UTEP 21238 | CFS 1586 | Burundi: western Makamba | KU560009 | KU560094 | KU560175 | KU560260 |
| Amietia lubrica | UTEP 21332 | ELI 933 | Burundi: Bururi, Nyamugwaga Swamp | KU560001 | KU560085 | KU560166 | KU560251 |
| Amietia lubrica | UTEP 21239 | ELI 867 | Burundi: Bururi | KU559998 | KU560082 | KU560163 | KU560248 |
| Amietia lubrica | UTEP 21331 | ELI 877 | Burundi: Bururi | KU560000 | KU560084 | KU560165 | KU560250 |
| Amietia lubrica | UTEP 21333 | ELI 1232 | Burundi: Mpishi, ca. Kibira National Park | — | KU560090 | KU560171 | KU560256 |
| Amietia lubrica | UTEP 21329 | ELI 865 | Burundi: Bururi | KU559997 | KU560081 | KU560162 | KU560247 |
| Amietia lubrica | UTEP 21240 | CK 051 (ELI 1562) | DRC: North Kivu: Kichanga River | KU560014 | KU560099 | KU560180 | — |
| Amietia lubrica | UTEP 21241 | CK 006 (ELI 1607) | DRC: North Kivu: Kichanga River | KU560010 | KU560095 | KU560176 | KU560261 |
| Amietia lubrica | UTEP 21242 | ELI 1152 | Burundi: Mpishi, ca. Kibira National Park | KU560004 | KU560088 | KU560169 | KU560254 |
| Amietia lubrica | UTEP 21243 | ELI 1216 | Burundi: Mpishi, Nyanutovu River, ca. Kibira National Park | KU560005 | KU560089 | KU560170 | KU560255 |
| Amietia lubrica | UTEP 21244 | CFS 1538 | Burundi: Mukazira River, ca. Bururi | KU560008 | KU560093 | KU560174 | KU560259 |
| Amietia lubrica | UTEP 21330 | ELI 873 | Burundi: Bururi | KU599999 | KU560083 | KU560164 | KU560249 |
| Amietia lubrica | UTEP 21245 | ELI 1266 | Burundi: Kibira National Park, ca. Rwegura | KU560007 | KU560092 | KU560173 | KU560258 |
| Amietia lubrica | UTEP 21246 | ELI 993 | Burundi: Gihofi | KU560003 | KU560087 | KU560168 | KU560253 |
| Amietia lubrica | UTEP 21247 | ELI 1258 | Burundi: Kibira National Park, ca. Rwegura | KU560006 | KU560091 | KU560172 | KU560257 |
| Amietia lubrica | UTEP 21248 | ELI 975 | Burundi: Bururi, Mivgaro | KU560002 | KU560086 | KU560167 | KU560252 |
| Amietia lubrica | UTEP 21249 | ELI 848 | Burundi: Gatambo, road ca. 12 km E of Muramvya | KU559996 | KU560080 | KU560161 | KU560246 |
| Amietia poyntoni | — | MH 0975 | South Africa: Harrismith | — | KC756304 | — | — |
| Amietia poyntoni | — | AC 2764 | South Africa: Rhodes | — | KC756283 | — | — |
| Amietia quecketti | — | QQ 0114 | South Africa | — | EF136555 | — | — |
| Amietia quecketti | — | KTH 406 | South Africa | — | EF136562 | — | — |
| Amietia quecketti | — | AC 2737 | South Africa: Eastern Cape | — | KC756354 | — | — |
| Amietia quecketti | CAS 191519 | — | South Africa: Barberton | DQ019576 | DQ019596 | — | DQ019493 |
| Amietia quecketti | UFS QQ00009 | — | South Africa: Free State | — | FJ411440 | — | — |
| Amietia ruwenzorica (tadpole) | UTEP 21322 | EBG 2226 | DRC: Katanga: Kabobo Plateau | KU559974 | KU560058 | KU560143 | KU560224 |
| Amietia ruwenzorica (tadpole) | UTEP 21203 | EBG 2227 | DRC: Katanga: Kabobo Plateau | KU559975 | KU560059 | KU560144 | KU560225 |
| Amietia ruwenzorica | UTEP 21204 | EBG 2094 | DRC: South Kivu: Itombwe Plateau, Mugegema | KU559976 | KU560060 | KU560145 | KU560226 |
| Amietia ruwenzorica | UTEP 21323 | EBG 1703 | DRC: South Kivu: Itombwe Plateau, Magunda | KU559977 | KU560061 | KU560146 | KU560227 |
| Amietia ruwenzorica | — | SL 437 | Uganda: Rwenzori Mountains, Mubuku River | — | JF809877 | — | — |
| Amietia ruwenzorica | — | SL 459 | Uganda: Rwenzori Mountains, Mubuku River | — | JF809880 | — | — |
| Amietia vandijki | — | MH 0107 | South Africa: Swartberg | — | HQ014418 | — | — |
| Amietia vertebralis | SAIAB DT16.1 | — | Lesotho | — | FJ411429 | — | — |
| Amietia vertebralis | — | AC 1220 | South Africa: Drakensberg: Naudes Nek | — | AY255097 | — | — |
| Amietia wittei | — | — | Tanzania: Mount Kilimanjaro | — | KJ469278 | — | — |
| Amietia wittei | — | — | Tanzania: Mount Kilimanjaro | — | KJ469273 | — | — |
| Amietia wittei | — | — | Tanzania: Mount Kilimanjaro | — | KJ469274 | — | — |
| Amietia wittei | — | — | Tanzania: Mount Kilimanjaro | — | KJ469276 | — | — |
| Amietia sp. 1 | UTEP 21205 | ELI 149 | DRC: Katanga: Kasongomwana | KU559992 | KU560076 | — | KU560242 |
| Amietia sp. 1 | UTEP 21206 | ELI 236 | DRC: Katanga: Kibara Mountains, Mayola River, ca. 3 km E of Kakunko | KU559993 | KU560077 | KU560160 | KU560243 |
| Amietia sp. 2 | UTEP 21306 | EBG 2016 | DRC: South Kivu: Itombwe Plateau, Rurambo | KU559949 | KU560032 | KU560117 | KU560198 |
| Amietia sp. 2 | UTEP 21307 | EGB 2017 | DRC: South Kivu: Itombwe Plateau, Rurambo | KU559950 | KU560033 | KU560118 | KU560199 |
| Amietia sp. 2 | UTEP 21207 | EBG 2018 | DRC: South Kivu: Itombwe Plateau, Rurambo | KU559951 | KU560034 | KU560119 | KU560200 |
| Amietia sp. 2 | UTEP 21208 | ELI 824 | DRC: South Kivu: Itombwe Plateau, Mitamba | KU559954 | KU560037 | KU560122 | KU560203 |
| Amietia sp. 2 | UTEP 21209 | ELI 818 | DRC: South Kivu: Itombwe Plateau, Mitamba | KU559953 | KU560036 | KU560121 | KU560202 |
| Amietia sp. 2 | UTEP 21210 | EBG 1588 | DRC: South Kivu: Itombwe Plateau, Miki | KU559945 | KU560028 | KU560113 | KU560194 |
| Amietia sp. 2 | UTEP 21211 | EBG 1646 | DRC: South Kivu: Itombwe Plateau, Miki | KU559946 | KU560029 | KU560114 | KU560195 |
| Amietia sp. 2 | UTEP 21212 | EBG 2058 | DRC: South Kivu: Itombwe Plateau, Komesha | KU559952 | KU560035 | KU560120 | KU560201 |
| Amietia sp. 2 | UTEP 21213 | EBG 2007 | DRC: South Kivu: Itombwe Plateau, Kasozo River | KU559947 | KU560030 | KU560115 | KU560196 |
| Amietia sp. 2 | UTEP 21305 | EBG 2008 | DRC: South Kivu: Itombwe Plateau, Kasozo River | KU559948 | KU560031 | KU560116 | KU560197 |
| Amietia sp. 3 | UTEP 21214 | EBG 1312 | DRC: South Kivu: Irangi, ca. Kahuzi-Biega National Park | KU559942 | KU560025 | KU560110 | KU560191 |
| Amietia sp. 3 | UTEP 21215 | ELI 490 | DRC: South Kivu: Bizombo | KU559944 | KU560027 | KU560112 | KU560193 |
| Amietia sp. 3 | UTEP 21304 | ELI 599 | DRC: South Kivu: Kalundu | KU559943 | KU560026 | KU560111 | KU560192 |
| Amietia sp. 3 | UTEP 21216 | EBG 1559 | DRC: South Kivu: N'Komo River near road from Uvira to Bukavu | KU559940 | KU560023 | KU560108 | KU560189 |
| Amietia sp. 3 | UTEP 21217 | EBG 1467 | DRC: South Kivu: Kahuzi-Biega National Park, Mugaba | KU559939 | KU560022 | KU560107 | KU560188 |
| Amietia sp. 3 | UTEP 21218 | EBG 1882 | DRC: North Kivu: Lwanoli River | KU559941 | KU560024 | KU560109 | KU560190 |
| Amietia sp. 4 | — | MTSN 9978 | DRC: Katanga: Lwama | KU560015 | KU560100 | KU560181 | — |
| Amietia sp. 4 | UTEP 21219 | MTSN 9837 | DRC: Katanga: Kinyama | KU559963 | KU560047 | KU560132 | KU560213 |
| Amietia sp. 4 | UTEP 21314 | ELI 1389 | DRC: South Kivu: Kihungwe | KU559970 | KU560054 | KU560139 | KU560220 |
| Amietia sp. 4 | UTEP 21319 | ELI 1405 | DRC: South Kivu: forest stream near Kihungwe | KU559968 | KU560052 | KU560137 | KU560218 |
| Amietia sp. 4 | UTEP 21320 | ELI 1426 | DRC: South Kivu: forest stream near Kihungwe | KU559969 | KU560053 | KU560138 | KU560219 |
| Amietia sp. 4 | UTEP 21318 | ELI 1404 | DRC: South Kivu: forest stream near Kihungwe | KU559967 | KU560051 | KU560136 | KU560217 |
| Amietia sp. 4 (tadpole) | UTEP 21313 | ELI 555 | DRC: South Kivu: Byonga | KU559973 | KU560057 | KU560142 | KU560223 |
| Amietia sp. 4 | UTEP 21312 | EBG 2259 | DRC: Katanga: Force Bendera | — | KU560046 | KU560131 | KU560212 |
| Amietia sp. 4 | UTEP 21315 | ELI 1400 | DRC: South Kivu: forest stream near Kihungwe | KU559964 | KU560048 | KU560133 | KU560214 |
| Amietia sp. 4 | UTEP 21310 | EBG 2200 | DRC: Katanga: Force Bendera | KU559960 | KU560043 | KU560128 | KU560209 |
| Amietia sp. 4 | UTEP 21220 | ELI 529 | DRC: South Kivu: Byonga | KU559972 | KU560056 | KU560141 | KU560222 |
| Amietia sp. 4 | UTEP 21321 | ELI 1471 | DRC: South Kivu: Kasanjala | KU559971 | KU560055 | KU560140 | KU560221 |
| Amietia sp. 4 | UTEP 21311 | EBG 2203 | DRC: Katanga: Force Bendera | KU559962 | KU560045 | KU560130 | KU560211 |
| Amietia sp. 4 | UTEP 21221 | EBG 2201 | DRC: Katanga: Force Bendera | KU559961 | KU560044 | KU560129 | KU560210 |
| Amietia sp. 4 | UTEP 21317 | ELI 1402 | DRC: South Kivu: forest stream near Kihungwe | KU559966 | KU560050 | KU560135 | KU560216 |
| Amietia sp. 4 | UTEP 21316 | ELI 1401 | DRC: South Kivu: forest stream near Kihungwe | KU559965 | KU560049 | KU560134 | KU560215 |
| Amietia sp. 5 | UTEP 21222 | EBG 2926 | DRC: Katanga: Marungu Plateau, Pepa | KU559989 | KU560073 | KU560157 | KU560239 |
| Amietia sp. 5 | UTEP 21223 | EBG 2978 | DRC: Katanga: Marungu Plateau, Pepa | KU559991 | KU560075 | KU560159 | KU560241 |
| Amietia sp. 5 | UTEP 21224 | EBG 2897 | DRC: Katanga: Marungu Plateau, Kyalengwe | KU559988 | KU560072 | KU560156 | KU560238 |
| Amietia sp. 5 (metamorph) | UTEP 21225 | ELI 186 | DRC: Katanga: Mitwaba | KU559994 | KU560078 | — | KU560244 |
| Amietia sp. 5 | UTEP 21228 | ELI 235 | DRC: Katanga: Kibara Mountains, Mayola River, ca. 3 km E of Kakunko | KU559995 | KU560079 | — | KU560245 |
| Amietia sp. 5 | UTEP 21328 | EBG 2942 | DRC: Katanga: Marungu Plateau, Pepa | KU559990 | KU560074 | KU560158 | KU560240 |
| Amietia sp. 6 | UTEP 21229 | EBG 2381 | DRC: Orientale: Lendu Plateau, Aboro | KU559980 | KU560064 | KU560149 | KU560230 |
| Amietia sp. 6 | UTEP 21230 | EBG 2309 | DRC: Orientale: Mafifi | KU559978 | KU560062 | KU560147 | KU560228 |
| Amietia sp. 6 | No Voucher | CK 114 | DRC: Orientale: Banvokoto | KU559983 | KU560067 | KU560152 | KU560233 |
| Amietia sp. 6 | UTEP 21231 | EBG 2465 | DRC: Orientale: Bunia | KU559982 | KU560066 | KU560151 | KU560232 |
| Amietia sp. 6 | No Voucher | CK 005 | DRC: North Kivu: Kichanga River | KU559986 | KU560070 | KU560155 | KU560236 |
| Amietia sp. 6 | UTEP 21326 | CK 004 (ELI 1593) | DRC: North Kivu: Kichanga River | KU559985 | KU560069 | KU560154 | KU560235 |
| Amietia sp. 6 | UTEP 21327 | CK 027 (ELI 1605) | DRC: North Kivu: Kichanga River | KU559987 | KU560071 | — | KU560237 |
| Amietia sp. 6 | No Voucher | CK 109 | DRC: Orientale: Banvokoto | KU559984 | KU560068 | KU560153 | KU560234 |
| Amietia sp. 6 | UTEP 21324 | EBG 2365 | DRC: Orientale: Lendu Plateau, Aboro | KU559979 | KU560063 | KU560148 | KU560229 |
| Amietia sp. 6 | UTEP 21325 | EBG 2368 | DRC: Orientale: Lendu Plateau, Aboro | KU559981 | KU560065 | KU560150 | KU560231 |
This locality was erroneously listed as Muzambai, Tanzania by Scott (2005).
2.2 Laboratory protocols
The Qiagen DNeasy blood and tissue kit (Qiagen Inc., Valencia, CA, USA) or IBI Scientific Genomic DNA Mini Kit (IBI Scientific, Peosta, IA, USA) were used to extract genomic DNA from alcohol-preserved muscle or liver tissue samples. We used gene-specific primers (Table 2) in 25 μl PCR reactions with an initial denaturation at 95°C for 2 min, followed by denaturation at 95°C for 35 s, annealing at 50°C for 35 s, and extension at 72°C for 95 s with 4s added to the extension per cycle for 32 (mitochondrial genes) or 34 (nuclear gene) cycles. The PCR products were visualized using a 1.5% agarose gel stained with SYBR Safe DNA gel stain (Invitrogen Corporation, Carlsbad, CA, USA). Sequencing reactions were purified with CleanSeq magnetic bead solution (Beckman Coulter, Inc., Brea, CA, USA) and then sequenced using an ABI 3130xl automated sequencer at the University of Texas at El Paso (UTEP) Border Biomedical Research Center (BBRC) Genomic Analysis Core Facility.
Table 2.
Primers used for sequencing mitochondrial and nuclear genes.
| Primer name | Primer sequence | Primer source |
|---|---|---|
| 12SA | 5’—AAACTGGGATTAGATACCCCACTAT—3’ | Kocher et al. (1989) |
| 12SB | 5’—GAGGGTGACGGGCGGTGTGT—3’ | |
| 16SA | 5’—CGCCTGTTTATCAAAAACAT—3’ | Palumbi et al. (1991) |
| 16SB | 5’—CCGGTCTGAACTCAGATCACGT—3’ | |
| cyt b-CBJ10933 | 5’—TATGTTCTACCATGAGGACAAATATC—3’ | Bossuyt and Milinkovitch (2000) |
| cyt b-C | 5’—CTACTGGTTGTCCTCCGATTCATGT—3’ | |
| RAG1MartF1 | 5’—AGCTGCAGYCARTAYCAYAARATGTA—3’ | Chiari et al. (2004); Pramuk et al. (2008) |
| RAG1AmpR1 | 5’—AACTCAGCTGCATTKCCAATRTCA—3’ |
2.3 Sequence alignment and phylogenetic analyses
Chromatograph data were interpreted with the program SeqMan Pro v. 8.0.2 (Swindell and Plasterer, 1997). Sequences were aligned using T-COFFEE (Notredame et al., 2000) with further manual adjustments in MacClade v. 4.08 (Maddison and Maddison, 2005). Phylogenetic trees were constructed using maximum likelihood (ML) and Bayesian inference (BI) criteria for both single-gene and concatenated data sets. The program RAxML v. 7.2.6 (Stamatakis, 2006) was used with the GTRGAMMA model for ML analyses, with a random starting tree and all parameters estimated. Clade support values inferred by ML analyses were estimated with the rapid bootstrap algorithm with 1000 replicates (Stamatakis et al., 2008). MrBayes v. 3.2.3 (Ronquist et al., 2012) was utilized to conduct BI analyses on the CIPRES Science Gateway v. 3.3 web portal (Miller et al., 2010). Single-gene analyses were conducted for 16S to determine genetic differences and phylogenetic relationships between all previously sequenced Amietia, because most samples from Genbank include only this gene. Concatenated analyses included GenBank samples with additional genes, but were mostly limited to newly sequenced samples for this study. Eight data partitions were used in our concatenated model: single partitions for 12S and 16S, and independent codon positions for the protein-coding genes cyt b and RAG1. The models of evolution most consistent with our data for BI analyses were determined with the Bayesian information criterion in PartitionFinder (Lanfear et al., 2012). Bayesian analyses were conducted with random starting trees, run for 20,000,000 generations, and Markov chains were sampled every 1000 generations. Convergence of multiple runs was verified through the graphical exploration using the compare tool of the MCMC program “Are We There Yet?” (AWTY) (Wilgenbusch et al., 2004; Nylander et al., 2008). A conservative estimate of 25% of trees were discarded as “burn in” once convergence was reached. Phylogenies were visualized using FigTree v. 1.4.2 (Rambaut and Drummond, 2014).
2.4 Species trees and species delimitation
Species-level phylogenetic estimates were generated using the program *BEAST v. 1.8.1 (Drummond et al., 2012) for the concatenated 12S, 16S, cyt b, and RAG1 data set. Species assignments for *BEAST were based on gene trees, collection locality, and morphology. Sequence evolution models were determined with PartitionFinder, and analyses were run with a Yule tree prior and unlinked loci and substation models. Each analysis was run for 50,000,000 iterations with Markov chains sampled every 1000 generations. Tracer v. 1.6 (http://tree.bio.ed.ac.uk/software/tracer/) was used visually to determine that convergence was reached, assess adequate sampling of all parameters, and determine “burn in.” A conservative 25% of the trees were discarded as “burn in” once convergence was reached. Trace plots were reviewed to ensure the convergence of Markov chain Monte Carlo (MCMC) runs. Gametic phases of heterozygous sites were resolved through a coalescent-based Bayesian method (PHASE v. 2.1) (Stephens et al., 2001) as executed in the software DnaSP v. 5.10.3 (Librado and Rozas, 2009). Gametic phase of alleles for polymorphic sites were inferred with probabilities ≥ 0.7.
After generating a species guide tree in *BEAST, species delimitation was tested using Bayesian Phylogenetics and Phylogeography (BPP v. 2.0; Yang and Rannala, 2010). The MCMC analyses were run for 500,000 generations, with a sampling interval of five, and a burn-in period of 10,000. Analyses were initiated with different starting seeds. Because the prior distributions on the ancestral population size and root age can affect the posterior probabilities for models, the effect of different priors was evaluated by implementing three different combinations of priors. Prior combinations were chosen from Leaché and Fujita (2010) where ϴ represents the ancestral population size and τ0 represents the divergence rates. The first combination of priors assumed small ancestral population sizes and relatively small divergence rates: ϴ = G(2,2000) and τ0 = G(2,2000), with a prior mean = 0.001 and variance = 5 × 10−7. The second combination of priors assumed large ancestral population sizes and deep divergence rates: ϴ = G(1,10) and τ0 = G(1,10), with a prior mean = 0.1 and variance = 0.01. The third combination mixes priors that assume large ancestral populations sizes ϴ = G(1,10) and relatively small divergence rates among species τ0 = G(2,2000). The third combination favors models containing fewer species and is considered to be a conservative combination of priors that would favor models with the least number of species. Each of the species delimitation models was assigned equal prior probability. Classifying of species was determined when the nodes of all three prior combination runs were > 0.95 (Smith et al., 2013).
2.5 Divergence dating
Two separate divergence dating analyses were conducted—one with secondary calibration points and another with a molecular clock for the mitochondrial genes. The program BEAST 1.8.1 (Drummond et al., 2012) was used with data sets for secondary calibrations (12S, 16S, cyt b, and RAG1) and molecular clocks (12S, 16S, and cyt b) to estimate the divergence dates for the lineages of Amietia in this study. Additional outgroups (lacking cyt b only) were sampled from the van der Meijden et al. (2005) study, including Petropedetes parkeri (Petropedetidae) and the pyxicephalids Pyxicephalus adspersus, Tomopterna sp., Natalobatrachus bonebergi, Cacosternum boettgeri, and Strongylopus fasciatus (Table 1). For the secondary calibration analyses, we applied an uncorrelated log-normal relaxed clock model with a coalescent tree prior. Analyses were run for 50,000,000 generations, sampling every 1000 generations. Based on dating estimates from Roelants et al. (2007), we used the following secondary calibrations: (1) the ancestral node of Ranoidea, which included Ptychadena, Hildebrandtia, Phrynobatrachus, Petropedetes, Pyxicephalus, Tomopterna, Natalobatrachus, Cacosternum, Strongylopus, Aubria, and Amietia, was constrained with a zero offset of 116.7 million years ago (mya), a log-normal mean of 0.01, and a log-normal standard deviation of 1.0; (2) Africanura, comprising all the previous genera with the exception of Ptychadena and Hildebrandtia, was constrained with a zero offset of 70.9 mya, a log-normal mean of 0.01, and a log-normal standard deviation of 1.0; and (3) Pyxicephalidae, comprising Pyxicephalus, Tomopterna, Natalobatrachus, Cacosternum, Strongylopus, Aubria, and Amietia, was constrained with a zero offset of 56.3 mya, a log-normal mean of 0.01, and a log-normal standard deviation of 1.0.
For molecular-clock rates, we applied a strict clock model with a coalescent tree prior to our mitochondrial data sets. Analyses were run for 50,000,000 generations, sampling every 1000 generations. We assumed a range of substitution rates from 0.60% to 1.00% per million years for cyt b and 0.20% to 0.30% per million years for both 12S and 16S, based on rates previously published for amphibians (Macey et al., 1998, 2001; Monsen and Blouin, 2003; Fouquet et al., 2009; Pröhl et al., 2010; Portillo et al., 2015).
We used the program Tracer v. 1.6 (Drummond et al., 2012) to determine the MCMC analysis stationarity, adequate effective sample sizes of the posterior probabilities, and the appropriate “burn-in” percentage. Maximum clade credibility trees were summarized using the program TreeAnnotator v. 1.8.1 (Drummond et al., 2012) and visualized in FigTree v. 1.4.2.
3. Results
3.1 Gene-tree analyses
Our concatenated data set consisted of 2499 base pairs (12S [449 bp], 16S [576 bp], cyt b [616 bp], and RAG1 [858 bp]). Five samples of Amietia failed to amplify for the gene cyt b (Table 2). Samples with missing data were still used in concatenated analyses, because branch lengths are not biased by modest amounts of missing data (Pyron et al., 2013; Jiang et al., 2014). The program PartitionFinder selected the following models of nucleotide substitution: 12S (GTR + I + G); 16S (GTR + I + G); cyt b 1st codon position (GTR + I + G), cyt b 2nd codon position (HKY + G), cyt b 3rd codon position (TrN + G); RAG1 1st and 3rd codon positions (K80 + I + G), RAG1 2nd codon position (HKY + G). When a model was not available in MrBayes, the least restrictive model (GTR) was implemented.
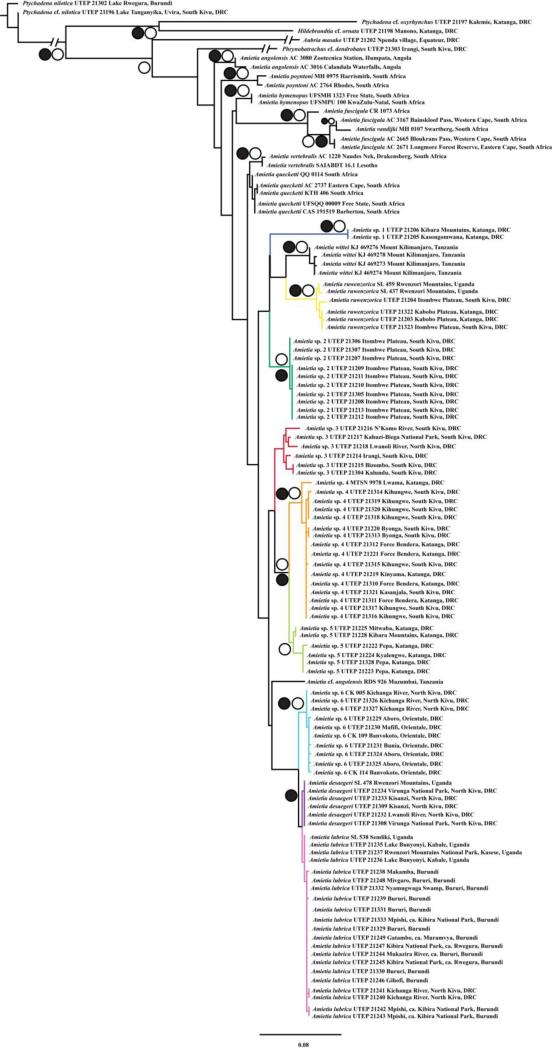
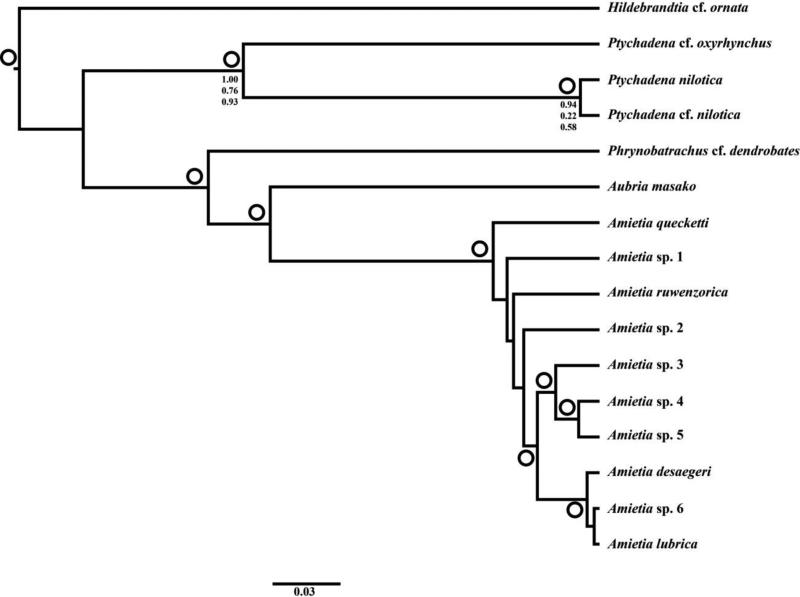
Topologies for the ML and BI analyses were similar for both the 16S and concatenated data sets (Figs. 2–3), but support for many nodes was stronger in the latter data set. The ML likelihood scores were –3497.09 for 16S and –13957.18 for the concatenated analyses, respectively. Results for 16S analyses included 15 haplotype clades of Amietia: (1) topotypic A. angolensis from Angola (GenBank); (2) A. poyntoni from South Africa (GenBank); (3) A. hymenopus from South Africa (GenBank); (4) A. fuscigula and A. vandijki from South Africa (GenBank); (5) A. vertebralis from South Africa and Lesotho (GenBank); (6) a poorly supported clade of A. quecketti from South Africa (GenBank); (7) A. sp. 1 from the Kibara Mountains and Kasongomwana, Katanga, DRC (1157–1428 m); (8) A. wittei from Mount Kilimanjaro, Tanzania above 1700 m (GenBank); (9) A. ruwenzorica from the Itombwe (ca. 2800 m) and Kabobo Plateaus (2440 m), DRC, and three topotypic GenBank samples from the Rwenzori Mountains, Uganda (above 2400 m); (10) A. sp. 2 from the Itombwe Plateau, DRC (1965–2848 m); (11) a weakly supported clade of A. sp. 3 from multiple forest localities in eastern DRC (811–2289 m); (12) A. sp. 4 from South Kivu and Katanga provinces, DRC (721–1324 m); (13) A. sp. 5 from the Marungu Plateau and proximate locations in Katanga Province, DRC (1428–2037 m), which was well supported as the sister taxon to A. sp. 4; (14) A. sp. 6 from North Kivu and Orientale Provinces, DRC (823–2088 m); and (15) A. desaegeri from Virunga National Park (DRC) and Rwenzori Mountains in DRC and Uganda (742–1543 m), and A. lubrica from multiple localities in Burundi, Uganda, and eastern DRC (1173–2303 m). Amietia vandijki was nested within the A. fuscigula clade; the former taxon is primarily distinguished from the latter by male advertisement call (Visser and Channing, 1997). Recent taxonomic changes to Amietia based on morphology (recognition of A. vertebralis and A. hymenopus) by Channing (2015) are well supported within our tree.
Fig. 2.
Maximum-likelihood phylogeny with a 16S data set of Amietia. Open circles denote clades with maximum likelihood bootstrap values ≥ 70; closed circles denote clades with Bayesian posterior probability values ≥ 0.95. Clade colors correspond to point localities in Fig. 1.
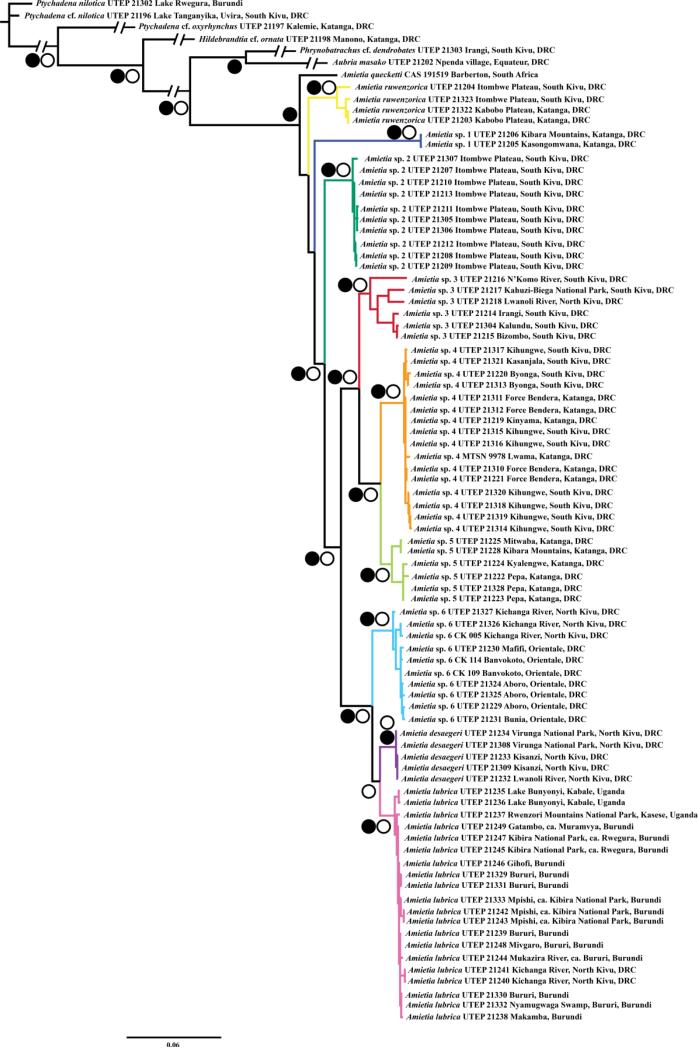
Fig. 3.
Maximum-likelihood phylogeny with combined 12S, 16S, cyt b, and RAG1 data sets of AR Amietia. Open circles denote clades with maximum likelihood bootstrap values ≥ 70; closed circles denote clades with Bayesian posterior probability values ≥ 0.95. Clade colors correspond to point localities in Fig. 1.
Our concatenated phylogeny (Fig. 3) recovered a well-supported group of Amietia that included nine strongly supported groups of recognized or candidate species from the AR: (1) A. ruwenzorica; (2) A. sp. 1; (3) A. sp. 2; (4) A. sp. 3; (5) A. sp. 4; (6) A. sp. 5; (7) A. sp. 6; (8) A. desaegeri; and (9) A. lubrica. In general, the concatenated tree showed stronger support and improved resolution compared to the single-gene analyses. There was strong support for a clade with all AR Amietia populations except for A. ruwenzorica and A. sp. 1. Another well-supported clade included A. sp. 3, A. sp. 4, A. sp. 5, A. sp. 6, A. desaegeri, and A. lubrica. The following two clades were strongly supported as sister taxa: (1) Amietia sp. 3, A. sp. 4 and A. sp. 5, and as in the single-gene tree, the latter two taxa were strongly supported as sister taxa; and (2) A. sp. 6, A. desaegeri and A. lubrica, with the latter two taxa as strongly supported sister taxa. There was weak support for the monophyly of the genus Amietia and the Amietia sp. 3 clade in the 16S analyses, but the genus was recovered as a well-supported monophyletic group in the concatenated analyses.
3.2 Species tree and species delimitation
Major clades in *BEAST analyses (Fig. 4) of AR populations of Amietia recovered the same recognized and candidate species as the concatenated gene tree. Estimates from *BEAST also recovered strong support (pp ≥ 95%) for the clade comprising A. sp. 3, A. sp. 4, A. sp. 5, A. sp. 6, A. desaegeri, and A. lubrica. Within this group, the same two major sister clades as the concatenated analyses were recovered, except that A. lubrica and A. sp. 6 were weakly supported as sister taxa. All three BPP analyses revealed nine distinct evolutionary lineages of AR Amietia (identical to those in the concatenated analyses) with the maximum possible posterior probability values (pp = 1.0). Conservative BPP models that favored fewer species did not collapse species that had relatively shallow divergences between them (e.g., A. lubrica and A.sp. 6).
Fig. 4.
Species tree generated in *BEAST for AR Amietia. Circles denote Bayesian posterior probability values ≥ 0.95. Posterior probabilities for three independent runs in BPP were 1.0 for all nodes except for those with Ptychadena, which are listed as: top run 1; middle run 2; bottom run 3 (most conservative).
3.3 Divergence dating
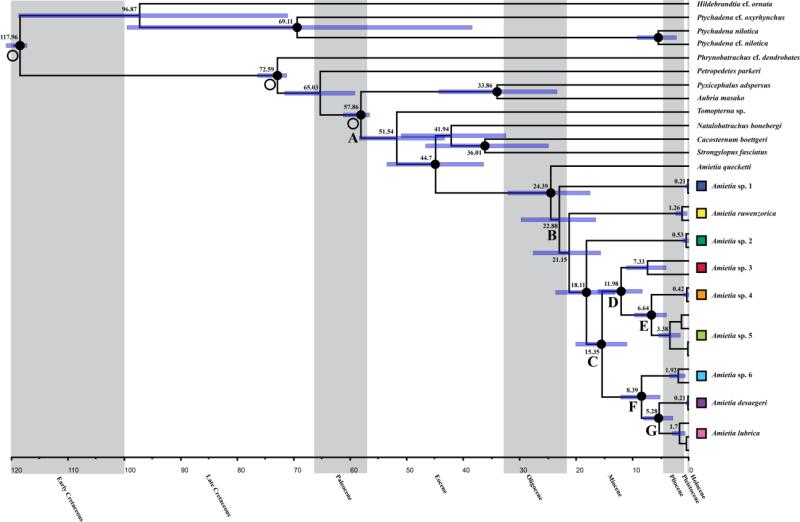
Results from the secondary calibration-based dating analysis suggest a basal divergence of Amietia in the late-Oligocene to early-Miocene 24.39 mya (17.44–31.97 mya, 95% highest posterior densities [HPD]) (Fig. 5). The common ancestor of AR Amietia diverged 22.88 mya (16.45–29.63 mya, HPD) (Table 3). Most AR Amietia lineages continued to diverge in the mid- to late Miocene. The most recent divergence between A. desaegeri and A. lubrica was 5.28 mya (2.86–8.05 mya, HPD) in the late Miocene to early Pliocene. Dating estimates from molecular-clock analyses were comparable (Table 3).
Fig. 5.
Phylogenetic tree from secondary calibration-based BEAST analyses. Open circles denote calibration points, and numbers at the base of nodes denote mean highest posterior densities (HPD). Bold circles at the nodes denote Bayesian posterior probability values ≥ 0.95. Nodes labeled with letters correspond to Table 3. Colored boxes correspond to point localities in Fig. 1.
Table 3.
Comparison of calibrated and molecular-clock time estimates for important nodes in the BEAST phylogeny of Amietia. Lettered nodes correspond to those shown in Figure 5. Data are mean highest posterior densities (in million years ago), with ranges in parentheses. Secondary calibration points are provided in the methods.
| Node | Calibrated | Molecular Clock |
|---|---|---|
| A: Split of Aubria and Amietia | 57.86 (56.33–60.98) | 55.38 (43.44–68.32) |
| B: A. sp. 1 from other Amietia | 22.88 (16.45–29.63) | 22.84 (17.79–28.39) |
| C: A. sp. 3-5 from A. sp. 6, A. desaegeri and A. lubrica | 15.35 (10.96–20.0) | 12.02 (9.4–14.87) |
| D: A. sp. 3 from A. sp. 4-5 | 11.98 (8.24–16.09) | 9.21 (7.2–11.51) |
| E: A. sp. 4 from A. sp. 5 | 6.64 (3.99–9.62) | 5.0 (3.65–6.5) |
| F: A. sp. 6 from A. desaegeri and A. lubrica | 8.39 (5.1–12.04) | 6.1 (4.53–7.81) |
| G: A. desaegeri from A. lubrica | 5.28 (2.86–8.05) | 3.95 (2.77–5.26) |
4. Discussion
4.1 Biogeography
Our results are mostly congruent with other phylogenetic studies of amphibians and reptiles from the AR (Evans et al., 2011; Greenbaum et al., 2012a,b, 2013; Portillo et al., 2015) with regard to several areas of endemism within the AR, including the Itombwe, Kabobo, Lendu, and Marungu Plateaus. Although our concatenated tree supported the monophyly of Amietia and a clade of AR Amietia (Fig. 3), the 16S analyses did not (Fig. 2), and some weakly supported clades included AR populations with A. wittei from Mt. Kilimanjaro, Tanzania and a single sample of A. cf. angolensis from Mazumbai (West Usambara Mountains), Tanzania.
Diversification of AR Amietia coincided with a global cooling trend in the late Miocene and the aridification of Africa in the Pliocene, and not the formation of the AR in the late Oligocene/early Miocene ca. 25 mya (Roberts et al., 2012). With the spread of savannas in the mid-Miocene (ca. 16 mya), Amietia associated with open, non-forested habitats may have become widespread throughout these habitats, whereas forest-specialist Amietia (i.e., A. sp. 3) were likely restricted to forest refugia. Relatively vagile and habitat generalist species of Amietia (e.g., A. lubrica) likely spread throughout large areas of the AR during relatively xeric conditions from the Miocene to Pleistocene (Fig. 5). Forest species, including snakes (Menegon et al., 2014; Greenbaum et al., 2015a), chameleons (Tolley et al., 2013), and frogs (Evans et al., 2011; Portillo et al., 2015) have shown similar patterns and dates of diversification under similar biogeographical processes. In contrast, dating analyses of AR species of birds (Fjeldså and Bowie, 2008) and mammals (Demos et al., 2014) recovered most divergence dates somewhat younger in the Pliocene, which might be explained by increased vagility of both birds and mammals, differences in molecular-clock rates, dating methods, or more recent colonization events.
The Rwenzori Mountains within the AR are unique because they likely originated relatively recently (Bauer et al., 2013) from a peneplain landcape around 2 mya (Kaufmann et al., 2015). This estimate is consistent with the relatively small genetic divergence between A. lubrica and A. desaegeri, which highlights formation of the mountain range as a potential driver of speciation for other vertebrates in the AR. However, this geological process did not influence the diversification of A. ruwenzorica, which is not restricted to the higher elevations of the Rwenzoris alone, but rather extends to several additional highlands within the AR as suggested by Frost (2015), including the Itombwe and Kabobo Plateaus.
The Itombwe Plateau harbors an unusually large number of endemic amphibians (Greenbaum and Kusamba, 2012), including recent species descriptions for Xenopus itombwensis (Evans et al., 2008) and Leptopelis anebos (Portillo and Greenbaum, 2014a). Haplotypes from Amietia sp. 2 from Itombwe formed a clade that is distinct from other AR populations, which suggests this candidate species is likely endemic to the plateau. The distinct Amietia sp. 6 lineage from the Lendu Plateau, a savanna-forest mosaic, and surrounding environs is geographically concordant with a recently described, endemic Kinyongia chameleon species (Greenbaum et al., 2012b) and a genetically distinct population of Boaedon fuliginosus (Greenbaum et al., 2015a). The historical geographic pattern relating A. sp. 6 (including the Lendu Plateau), A. desaegeri, and A. lubrica is similar to Xenopus lenduensis from the Lendu Plateau, which is closely related to X. victorianus populations from eastern DRC, Burundi, and Uganda (Furman et al., 2015).
The geological complexity of Katanga Province in DRC has led to impressive species richness, and approximately 12% of Katanga's amphibians and reptiles are endemic (Broadley and Cotterill, 2004). Our study demonstrates that the northern part of Katanga contains at least four distinct species of Amietia, which are genetically distinct from A. angolensis and all other species known from the AR. For example, A. sp. 5 is found in the Marungu Plateau and Mitwaba regions in Katanga. The Marungu Plateau is known to harbor several endemic vertebrate species, including several bird taxa, a cordylid lizard, and at least one distinct species of Hyperolius (Greenbaum et al. 2012a). Although Amietia sp. 5 seems to be the only lineage that occurs in Marungu, our study revealed that it is not endemic to the plateau.
4.2 Species limits and taxonomy
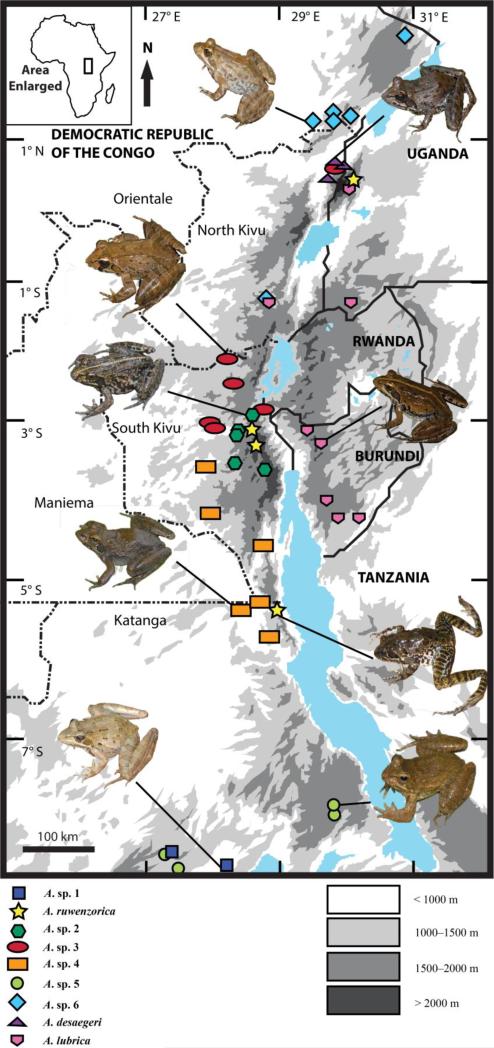
The results of our coalescent-based analyses suggest six unique genetic lineages likely warrant full species status, but additional sampling and morphological analyses are needed. Amietia sp. 1 occupies a woodland/savanna mosaic of the Kibara Mountains and nearby region from 1157–1428 m. Amietia sp. 2 was found in montane forest edges and marshes from 1965–2848 m on the Itombwe Plateau. Amietia sp. 3 seems to be the only species that is restricted to forest—it was found in transitional and montane forests from multiple localities in eastern DRC from 811–2289 m. Amietia sp. 4 occupies forest, forest edges, and woodland/savanna mosaic habitats in the regions west and south of the Itombwe Plateau in South Kivu and Katanga Provinces from 744–1324 m. Amietia sp. 5 occupies montane grassland in the Marungu Plateau and a woodland/savanna mosaic of the Kibara Mountains at elevations from 1428–2037 m. One sample of the latter clade (UTEP 21228) was found in sympatry with Amietia sp. 1 (UTEP 21206) at the Mayola River in the Kibara Mountains. Differences between A. sp. 1 and A. sp. 5 are strongly supported by both gene-tree analyses (Figs. 2–3), the species-tree analysis (Fig. 4), and by distinctive color patterns and other morphological characters (EG and TRL, unpubl. data). Amietia sp. 6 occurs in the montane woodland/savanna mosaic of the Lendu Plateau and surrounding region, marshes near the volcanoes of the southern sector of Virunga National Park (where it is sympatric with A. lubrica at the Kichanga River), and forest edges of the Ituri region from 823–2088 m. In future studies, additional morphological evidence will be combined with the genetic data presented within this study to confirm the recognition of these candidate species.
Our study revealed that Amietia angolensis does not occur in the AR, and it is likely much more limited in its distribution than currently recognized (Frost, 2015). Two distinct A. angolensis lineages were recovered in our single-gene phylogeny, and it is likely that the one from Tanzania represents a new species. Further investigation throughout the range of A. angolensis, including populations in Malawi (Conradie et al., 2011) and Tanzania (Zancolli et al., 2014), will likely reveal additional lineages in the A. angolensis complex.
Amietia lubrica is currently known only from the type locality (Lake Bunyonyi, Uganda) (Pickersgill, 2007). Our study clearly demonstrates that A. lubrica is the most widespread species within the AR, with a geographic distribution extending from western Burundi to the North Kivu Province of eastern DRC and western Uganda. These results underscore how poorly known the AR herpetofauna is in general. Amietia desaegeri is currently considered to be restricted to the Rwenzori Mountains (Frost, 2015) and southwestern Uganda (AmphibiaWeb, 2015), and although we did not detect the species in Uganda, our study shows that A. desaegeri also occurs near lowland rainforests of Virunga National Park, DRC. As noted above, A. ruwenzorica is found above 2000 m at the Itombwe Plateau, Kabobo Plateau, and Rwenzori Mountains, and the species is likely to occur in other montane regions of the AR.
According to Loveridge (1957) and Frost (2015), A. wittei should occur in eastern DRC. There is no evidence of A. wittei in eastern DRC based on our single-gene analysis, which included GenBank data from this species (Fig. 2). It is possible that the confusion over the distribution of A. wittei can be attributed to its complicated taxonomic history. Rana aberdariensis, currently a synonym of Amietia wittei, was synonymized with Rana nutti by Barbour and Loveridge (1928), then removed from this synonymy (Loveridge, 1929), and subsequently placed into the synonymy of R. (Amietia) wittei (Loveridge, 1936).
The poorly known taxon Rana chapini is currently considered to be a synonym of A. angolensis (Frost, 2015), but given the enormous disjunct distribution and ecoregion differences between the lowland Congo Basin rainforest type locality for the former taxon (Batama, Orientale Province, DRC) and the presumably topotypic A. angolensis GenBank samples from Calandula Falls and Humpata (Zootecnica Station) in Angola (Angola-Miombo Woodlands Ecoregion, sensu Burgess et al., 2004), it is likely that these taxa are not conspecific. Locality data for UTEP 21230 (Table 1) (Fig. 1) at Mafifi (near Epulu, elevation 844 m) places our most western sample of A. sp. 6 about 230 km east of Batama (528 m elevation), the only known locality for R. chapini. Noble (1924) noted that the single specimen of R. chapini was collected “in grass bordering the brook at Batama,” suggesting the habitat might have been at the edge of the rainforest and not inside it, which is consistent with A. sp. 6 at Mafifi. However, preliminary morphological data from webbing of the fourth toe of these taxa (TRL, unpubl. data) suggest A. sp. 6 is not consistent with the original description of R. chapini (Noble, 1924). Rana (Amietia) chapini is likely a distinct species, but additional sampling is needed to confirm this hypothesis.
The candidate species Amietia sp. 3 contains samples collected within 100 km of the type locality of A. amieti at Lubile (Maniema Province, DRC). The samples were collected in forests ranging from 811–2289 m. There is extensive morphological variation among the vouchers, and comparisons to type specimens are needed to clarify its taxonomic status.
4.3 Conservation concerns
Although five countries (DRC, Burundi, Rwanda, Tanzania, and Uganda) currently contain at least 13 national parks (Plumptre et al., 2007) associated with the AR, there is a need for improved habitat conservation for many of the amphibian species in the AR, including Amietia. The AR contains the most endemic vertebrate fauna in continental Africa, including four currently recognized species of Amietia, and many of these areas have high levels of endemism and are either poorly protected (e.g., Itombwe and Kabobo Plateaus) or completely unprotected (e.g., Lendu and Marungu Plateaus) (Greenbaum and Kusamba, 2012; Greenbaum et al., 2012a,b). Candidate species of Amietia that were found within protected areas of the AR include A. sp. 2 on the Itombwe Plateau (parts of which were recently made into a reserve), A. sp. 3 in the highlands of Kahuzi-Biega National Park, A. desaegeri in Virunga National Park, A. ruwenzorica in Virunga National Park and the Itombwe Plateau, and A. lubrica in Kibira National Park. The remaining candidate species of Amietia, including A. sp.1, 4, 5, and 6, have all been found outside of protected areas.
Political instability, corruption, and weak law enforcement have allowed militias to control significant parts of the region, often blocking scientific and conservation efforts (Greenbaum and Kusamba, 2012). Even where protected areas have been established, law enforcement officials are disregarded or even attacked. Emmanuel de Merode, director and chief warden of Virunga National Park, was recently shot several times, and 140 park rangers were killed for protecting the park's resources and animal fauna, which include mountain gorillas (Worrall, 2015). Political instability in the region reduces the effectiveness of law enforcement and also decreases the likelihood of significant long-term conservation goals (Omari et al. 1999; Sodhi et al., 2007). Human activities involved with the destruction of natural habitats, including deforestation, cattle ranching, and mining have been harmful to amphibian populations in the AR (Behangana et al., 2009; Greenbaum and Kusamba, 2012). Trends indicate that subsistence farming will continue to increase and place more pressure on wildlife reserves and other protected areas in many parts of Africa, including the AR (de Klerk et al., 2003; Fjeldså et al. 2004). Because many of the samples in this study were collected in relatively pristine habitats, additional conservation protection in these areas is important. A notable exception is A. lubrica, which is common in degraded and disturbed habitats (topotypic samples were collected in a hotel swimming pool adjacent to Lake Bunyonyi, Uganda).
Another major cause for concern to AR amphibian populations is the recent increase in species known to be afflicted with Batrachochytrium dendrobatidis, commonly called Bd, or chytrid fungus (Skerratt et al., 2007). The effects of Bd infection on AR amphibians are relatively unknown. However, samples of Amietia sp. 1, 2, 4, and 5 from our study tested positive for Bd (Greenbaum et al., 2014, 2015b), and samples of A. ruwenzorica from the Rwenzori Mountains (Uganda) also tested positive (Viertel et al., 2012). These studies and additional ones from Malawi (Conradie et al., 2011) and Kenya (Kielgast et al., 2010) suggest that Amietia species have relatively high rates of Bd infection compared to other African genera of frogs (Conradie et al., 2011; Greenbaum et al., 2015b). Given the high susceptibility of Amietia to chytrid infection, the limited distribution of many species within the declining pristine habitats of the AR (Fig. 1), and the suitability of the AR highlands for chytrid fungus (Seimon et al., 2015), it is likely that many additional Amietia populations are threatened by chytrid infections.
With the new understanding of Amietia diversity that is evident in this study, concomitant conservation assessments will be needed after candidate species are formally described. Given the recent discovery of other cryptic, candidate species of amphibians in the AR (e.g., Portillo et al., 2015), it is likely that many additional amphibian species await discovery in the remaining natural habitats of the region, underscoring the increasing conservation value of the AR relative to other regions of continental Africa. Because the AR has one of the highest human population densities on the continent (Barnes and Lahm, 1997), additional biodiversity studies and conservation efforts are urgently needed.
Highlights.
Fifteen different lineages of Amietia recovered from eastern to southern Africa
Nine distinct lineages of Amietia in the Albertine Rift of Central Africa
Major radiations of Amietia occurred during the Miocene
Further endemism in the Itombwe Plateau with a unique lineage of Amietia
Acknowledgments
Fieldwork by EG in DRC was funded by the Percy Sladen Memorial Fund, an IUCN/SSC Amphibian Specialist Group Seed Grant, K. Reed, M.D., research funds from the Department of Biology at Villanova University, a National Geographic Research and Exploration Grant (no. 8556-08), UTEP, and a grant from the National Science Foundation (DEB-1145459); EG thanks field companions C. Kusamba, M. M. Aristote, W. M. Muninga, M. Zigabe, A. M. Marcel, M. Luhumyo, J. and F. Akuku, F. I. Alonda, and the late A. M'Mema. We are grateful to F. B. Murutsi, former Chief Warden of the Itombwe Natural Reserve, for logistical support and permission for fieldwork in 2011; B. Bajope and M. Manunu of the Centre de Recherche en Sciences Naturelles provided project support and permits, and we thank the Institut Congolais pour la Conservation de la Nature for permits to work in protected areas in DRC. We thank the Uganda Wildlife Authority of Kampala for necessary permits to work in Uganda, and Léonidas Nzigiyimpa of the Institut National pour l'Environnement et la Conservation de la Nature (INECN) of Burundi for logistical support and permit negotiations. We thank B. Drewes, D. Blackburn and J. Vindum (CAS), P. Campbell and B. Hughes (BMNH), Alan Resetar (FMNH), and D. Meirte and G. Cael (RMCA) for access to specimens and tissues in their care, and D. Kizirian (AMNH) for photos of Rana chapini. We thank A. Plumptre, G. Mitamba and E. Muhindo, who were funded by the US Fish and Wildlife Service, for their fieldwork efforts and contribution to the UTEP collections. We thank K. Weber, M. Medina, and W. Rashid for their assistance with sequencing various samples used for the study. We acknowledge A. Betancourt of the UTEP Border Biomedical Research Center Genomic Analysis Core Facility for services and facilities provided. This work was supported by Grant G12MD007592 from the National Institutes on Minority Health and Health Disparities (NIMHD), a component of the National Institutes of Health (NIH). The authors were supported by grant DEB-1145459 from the National Science Foundation of the United States.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AmphibiaWeb . Information on amphibian biology and conservation. AmphibiaWeb; Berkeley, California, USA: 2015. [Jan 3, 2015]. http://amphibiaweb.org/. [Google Scholar]
- Barbour T, Loveridge A. A comparative study of the herpetological faunae of the Uluguru and Usumbara Mountains, Tanganyika Territory, with descriptions of new species. Mem. Mus. Comp. Zool. (Harvard) 1928;50:87–265. [Google Scholar]
- Barnes RFW, Lahm SA. An ecological perspective on human densities in the central African forests. J. Appl. Ecol. 1997;34:245–260. [Google Scholar]
- Bauer FU, Glasmacher UA, Ring U, Karl M, Schumann A, Nagudi B. Tracing the exhumation history of the Rwenzori Mountains, Albertine Rift, Uganda, using low-temperature thermochronology. Tectonophysics. 2013;599:8–28. [Google Scholar]
- Behangana M, Kasoma PMB, Luiselli L. Ecological correlates of species richness and population abundance patterns in the amphibian communities from the Albertine Rift, East Africa. Biodivers. Conserv. 2009;18:2855–2873. [Google Scholar]
- Bossuyt F, Brown RM, Hillis DM, Cannatella DC, Milinkovitch MC. Phylogeny and biogeography of a cosmopolitan frog radiation: Late Cretaceous diversification resulted in continent-scale endemism in the family Ranidae. Syst. Biol. 2006;55:579–94. doi: 10.1080/10635150600812551. [DOI] [PubMed] [Google Scholar]
- Bossuyt F, Milinkovitch MC. Convergent adaptive radiations in Madagascan and Asian ranid frogs reveal covariation between larval and adult traits. Proc. Natl. Acad. Sci. USA. 2000;97:6585–6590. doi: 10.1073/pnas.97.12.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie RCK, Fjeldså J, Hackett SJ, Bates JM, Crowe TM. Coalescent models reveal the relative roles of ancestral polymorphism, vicariance, and dispersal in shaping phylogeographical structure of an African montane forest robin. Mol. Phylogenet. Evol. 2006;38:171–188. doi: 10.1016/j.ympev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Broadley DG, Cotterill FPD. The reptiles of southeast Katanga, an overlooked ‘hot spot.’ Afr. J. Herpetol. 2004;53:35–61. [Google Scholar]
- Brooks TM, Mittermeier RA, da Fonseca GAB, Gerlach J, Hoffmann M, Lamoreux JF, Mittermeier CG, Pilgrim JD, Rodrigues ASL. Global biodiversity conservation priorities. Science. 2006;313:58–61. doi: 10.1126/science.1127609. [DOI] [PubMed] [Google Scholar]
- Burgess N, D'Amico Hales J, Underwood E, Dinerstein E, Olson D, Itoua I, Schipper J, Ricketts T, Newman K. Terrestrial Ecoregions of Africa and Madagascar: A Conservation Assessment. Island Press; Washington, DC.: 2004. [Google Scholar]
- Channing A, Howell KM. Amphibians of East Africa. Cornell University Press; Ithaca, New York: 2006. [Google Scholar]
- Channing A, Baptista N. Amietia angolensis and A. fuscigula (Anura: Pyxicephalidae) in southern Africa: a cold case reheated. Zootaxa. 2013;3640:501–520. doi: 10.11646/zootaxa.3640.4.1. [DOI] [PubMed] [Google Scholar]
- Channing A. The Maluti mystery revisited: taxonomy of African River Frogs (Pyxicephalidae, Amietia) on the Drakensberg Mountains in southern Africa. Zootaxa. 2015;3925:271–280. doi: 10.11646/zootaxa.3925.2.8. [DOI] [PubMed] [Google Scholar]
- Chiari Y, Vences M, Vieites DR, Rabemananjara F, Bora P, Ramilijaona Ravoahangimalala O, Meyer A. New evidence for parallel evolution of colour patterns in malagasy poison frogs (Mantella). Mol. Ecol. 2004;13:3763–3774. doi: 10.1111/j.1365-294X.2004.02367.x. [DOI] [PubMed] [Google Scholar]
- Conradie W, Harvey J, Kotzé A, Dalton DL, Cunningham MJ. Confirmed amphibian chytrid in Mount Mulanje area, Malawi. Herpetol. Rev. 2011;42:369–371. [Google Scholar]
- Dawood A, Uqubay SM. A molecular phylogeny of the sand frog genus Tomopterna (Amphibia: Anura: Ranidae) based on mitochondrial 12S and 16S rRNA sequences. Afr. Zool. 2004;39:145–151. [Google Scholar]
- de Klerk HM, Fjeldså J, Blyth S, Burgess ND. Gaps in the protected area network for threatened Afrotropical birds. Biol. Conserv. 2003;117:529–537. [Google Scholar]
- de Queiroz K. The general lineage concept of species, species criteria, and the process of speciation: a conceptual unification and terminological recommendations. In: Howard DJ, Berlocher SH, editors. Endless Forms: Species and Speciation. Oxford University Press; Oxford, UK: 1998. pp. 57–75. [Google Scholar]
- de Queiroz K. Species concepts and species delimitation. Syst. Biol. 2007;56:879–886. doi: 10.1080/10635150701701083. [DOI] [PubMed] [Google Scholar]
- Demos TC, Kerbis Peterhans JC, Agwanda B, Hickerson MJ. Uncovering cryptic diversity and refugial persistence among small mammal lineages across the eastern Afromontane biodiversity hotspot. Mol. Phylogenet. Evol. 2014;71:41–54. doi: 10.1016/j.ympev.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois A. Amphibia Mundi. 1.1. An ergotaxonomy of recent amphibians. Alytes. 2005;23:1–24. [Google Scholar]
- Evans BJ, Bliss SM, Mendel SA, Tinsley RC. The rift valley is a major barrier to the dispersal of African clawed frogs (Xenopus) in Ethiopia. Mol. Ecol. 2011;20:4216–4230. doi: 10.1111/j.1365-294X.2011.05262.x. [DOI] [PubMed] [Google Scholar]
- Evans BJ, Carter TF, Tobias ML, Kelley DB, Hanner R, Tinsley RC. A new species of clawed frog (genus Xenopus) from the Itombwe Massif, Democratic Republic of the Congo: implications for DNA barcodes and biodiversity conservation. Zootaxa. 2008;1780:55–68. [Google Scholar]
- Fjeldså J, Bowie RCK. New perspectives on the origin and diversification of Africa's forest avifauna. Afr. J. Ecol. 2008;46:235–247. [Google Scholar]
- Fjeldså J, Burgess ND, Blyth S, de Klerk HM. Where are the major gaps in the reserve network for Africa's mammals? Oryx. 2004;38:17–25. [Google Scholar]
- Fouquet A, Green DM, Waldman B, Bowsher JH, McBride KP, Gemmell NJ. Phylogeography of Leiopelma hochstetteri reveals strong genetic structure and suggests new conservation priorities. Conserv. Genet. 2009;11:907–919. [Google Scholar]
- Frost DR. Amphibian Species of the World: an Online Reference. Version 6.0 (April 2015) American Museum of Natural History; New York, USA: 2015. Electronic Database accessible at: http://research.amnh.org/herpetology/amphibia/index.html. [Google Scholar]
- Frost DR, Grant T, Faivovich J, Bain RH, Haas A, Haddad CFB, De Sá RO, Channing A, Wilkinson M, Donnellan SC, Raxworthy CJ, Campbell JA, Blotto BL, Moler P, Drewes RC, Nussbaum RA, Lynch JD, Green DM, Wheeler WC. The amphibian tree of life. Bull. Am. Mus. Nat. Hist. 2006;297:1–371. [Google Scholar]
- Furman BLS, Bewick AJ, Harrison TL, Greenbaum E, Gvoždík V, Kusamba C, Evans BJ. Pan-African phylogeography of a model organism, the African clawed frog ‘Xenopus laevis.'. Mol. Ecol. 2015;24:909–925. doi: 10.1111/mec.13076. [DOI] [PubMed] [Google Scholar]
- Greenbaum E, Kusamba C. Conservation implications following the rediscovery of four frog species from the Itombwe Natural Reserve, eastern Democratic Republic of the Congo. Herpetol. Rev. 2012;43:253–259. [Google Scholar]
- Greenbaum E, Meece J, Reed KD, Kusamba C. Amphibian chytrid infections in non-forested habitats of Katanga, Democratic Republic of the Congo. Herpetol. Rev. 2014;45:610–614. [Google Scholar]
- Greenbaum E, Meece J, Reed KD, Kusamba C. Extensive occurrence of the amphibian chytrid fungus in the Albertine Rift, a Central African amphibian hotspot. Herpetol. J. 2015b;25:91–100. [Google Scholar]
- Greenbaum E, Portillo F, Jackson K, Kusamba C. A phylogeny of Central African Boaedon (Serpentes: Lamprophiidae), with the description of a new cryptic species from the Albertine Rift. Afr. J. Herpetol. 2015a;64:18–38. [Google Scholar]
- Greenbaum E, Sinsch U, Lehr E, Valdez F, Kusamba C. Phylogeography of the reed frog Hyperolius castaneus (Anura: Hyperoliidae) from the Albertine Rift of Central Africa: implications for taxonomy, biogeography and conservation. Zootaxa. 2013;3731:473–494. doi: 10.11646/zootaxa.3731.4.3. [DOI] [PubMed] [Google Scholar]
- Greenbaum E, Stanley EL, Kusamba C, Moninga WM, Goldberg SR, Bursey CR. A new species of Cordylus (Squamata: Cordylidae) from the Marungu Plateau of south-eastern Democratic Republic of the Congo. Afr. J. Herpetol. 2012a;61:14–39. [Google Scholar]
- Greenbaum E, Tolley KA, Joma A, Kusamba C. A new species of chameleon (Sauria: Chamaeleonidae: Kinyongia) from the northern Albertine Rift, Central Africa. Herpetologica. 2012b;68:60–75. [Google Scholar]
- Greenbaum E, Villanueva CO, Kusamba C, Aristote MM, Branch WR. A molecular phylogeny of Equatorial African Lacertidae, with the description of a new genus and species from eastern Democratic Republic of the Congo. Zool. J. Linn. Soc. London. 2011;163:913–942. doi: 10.1111/j.1096-3642.2011.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins CN, Pimm SL, Joppa LN. Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl. Acad. Sci. USA. 2013;110:E2602–E2610. doi: 10.1073/pnas.1302251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Chen S-Y, Wang H, Li D-Z, Wiens JJ. Should genes with missing data be excluded from phylogenetic analyses? Mol. Phylogenet. Evol. 2014;80:308–318. doi: 10.1016/j.ympev.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Kaufmann G, Hinderer M, Romanov D. Shaping the Rwenzoris: balancing uplift, erosion, and glaciation. Int. J. Earth Sci. 2015 DOI: 10.1007/s00531-015-1174-2. [Google Scholar]
- Kerbis Peterhans JC, Hutterer R, Mwanga J, Ndara B, Davenport L, Karhagomba IB, Udelhoven J. African shrews endemic to the Albertine Rift: two new species of Myosorex (Mammalia: Soricidae) from Burundi and the Democratic Republic of Congo. J. East Afr. Nat. Hist. 2010;99:103–128. [Google Scholar]
- Kielgast J, Rödder D, Veith M, Lötters S. Widespread occurrence of the amphibian chytrid fungus in Kenya. Anim. Conserv. 2010;13:36–43. [Google Scholar]
- Kocher TD, Thomas WK, Meyer A, Edwards SV, Pääbo S, Villablanca FX, Wilson AC. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA. 1989;86:6196–6200. doi: 10.1073/pnas.86.16.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfear R, Calcott B, Ho SYW, Guindon S. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012;29:1695–1701. doi: 10.1093/molbev/mss020. [DOI] [PubMed] [Google Scholar]
- Largen M, Spawls S. The Amphibians and Reptiles of Ethiopia and Eritrea. Edition Chimaira; Frankfurt am Main, Germany: 2010. [Google Scholar]
- Laurent RF. Adaptive modifications in frogs of an isolated highland fauna in Central Africa. Evolution. 1964;18:458–467. [Google Scholar]
- Laurent RF. Amphibiens. Fondation pour Favoriser les Recherches Scientifiques en Afrique, Bruxelles, Exploration du Parc National des Virunga. 1972;22(2e séries):1–125. I–XI. [Google Scholar]
- Leaché AD, Fujita MK. Bayesian species delimitation in West African forest geckos (Hemidactylus fasciatus). Proc. Roy. Soc. B. Biol. Sci. 2010;277:3071–3077. doi: 10.1098/rspb.2010.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Loveridge A. East African reptiles and amphibians in the United States National Museum. Bull. U. S. Nat. Mus. 1929;151:1–135. [Google Scholar]
- Loveridge A. Scientific results of an expedition to rain forest regions in eastern Africa. VII. Amphibians. Bull. Mus. Comp. Zool. (Harvard) 1936;79:369–430. [Google Scholar]
- Loveridge A. Check list of the reptiles and amphibians of East Africa (Uganda; Kenya; Tanganyika; Zanzibar). Bull. Mus. Comp. Zool. (Harvard) 1957;117:153–362. [Google Scholar]
- Macey JR, Schulte JA, Larson A. Phylogenetic relationships of toads in the Bufo bufo species group from the eastern escarpment of the Tibetan Plateau: a case of vicariance and dispersal. Mol. Phylogenet. Evol. 1998;9:80–87. doi: 10.1006/mpev.1997.0440. [DOI] [PubMed] [Google Scholar]
- Macey JR, Strasburg JL, Brisson JA, Vredenburg VT, Jennings M, Larson A. Molecular phylogenetics of western North American frogs of the Rana boylii species group. Mol. Phylogenet. Evol. 2001;19:131–143. doi: 10.1006/mpev.2000.0908. [DOI] [PubMed] [Google Scholar]
- Maddison DR, Maddison WP. MacClade: Analysis of Phylogeny and Character Evolution. Sinauer Associates Inc.; Sunderland, Massachusetts: 2005. [Google Scholar]
- Menegon M, Loader SP, Marsden SJ, Branch WR, Davenport TRB, Ursenbacher S. The genus Atheris (Serpentes: Viperidae) in East Africa: phylogeny and the role of rifting and climate in shaping the current pattern of species diversity. Mol. Phylogenet. Evol. 2014;79:12–22. doi: 10.1016/j.ympev.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees.. Proceedings of the Gateway Computing Environments Workshop (GCE); New Orleans, LA.. 14 Nov. 2010.2010. [Google Scholar]
- Monsen KJ, Blouin MS. Genetic structure in a montane ranid frog: restricted gene flow and nuclear–mitochondrial discordance. Mol. Ecol. 2003;12:3275–3286. doi: 10.1046/j.1365-294x.2003.02001.x. [DOI] [PubMed] [Google Scholar]
- Noble GK. Contributions to the herpetology of the Belgian Congo based on the collection of the American Museum Congo Expedition, 1909–1915. Bull. Am. Mus. Nat. Hist. 1924;49:147–347. [Google Scholar]
- Notredame C, Higgins DG, Heringa J. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- Nylander JA, Wilgenbusch JC, Warren DL, Swofford DL. AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics. 2008;24:581–583. doi: 10.1093/bioinformatics/btm388. [DOI] [PubMed] [Google Scholar]
- Omari I, Hart JA, Butynski TM, Birhashirwa NR, Upoki A, M'Keyo Y, Bengana F, Bashonga M, Bagurubumwe N. The Itombwe Massif, Democratic Republic of Congo: biological surveys and conservation, with an emphasis on Grauer's gorilla and birds endemic to the Albertine Rift. Oryx. 1999;33:301–322. [Google Scholar]
- Palumbi S, Martin A, Romano S, McMillan WO, Stice L, Grabowski G. The Simple Fool's Guide to PCR. Version 2. The University of Hawaii; Honolulu, HI.: 1991. [Google Scholar]
- Pickersgill M. Frog Search: Results of Expeditions to Southern and Eastern Africa from 1993–1999. Edition Chimaira; Frankfurt am Main, Germany: 2007. [Google Scholar]
- Plumptre A, Davenport T, Behangana M, Kityo R, Eilu G, Ssegawa P, Ewango C, Meirte D, Kahindo C, Herremans M, Kerbis Peterhans J, Pilgrim JD, Wilson M, Languy M, Herremans M. The biodiversity of the Albertine Rift. Biol. Conserv. 2007;134:178–194. [Google Scholar]
- Portillo F, Greenbaum E. A new species of the Leptopelis modestus complex (Anura: Arthroleptidae) from the Albertine Rift of Central Africa. J. Herpetol. 2014a;48:394–406. [Google Scholar]
- Portillo F, Greenbaum E. At the edge of a species boundary: a new and relatively young species of Leptopelis (Anura: Arthroleptidae) from the Itombwe Plateau, Democratic Republic of the Congo. Herpetologica. 2014b;70:100–119. [Google Scholar]
- Portillo F, Greenbaum E, Menegon M, Kusamba C, Dehling JM. Phylogeography and species boundaries of Leptopelis (Anura: Arthroleptidae) from the Albertine Rift. Molec. Phylogenet. Evol. 2015;82:75–86. doi: 10.1016/j.ympev.2014.09.024. [DOI] [PubMed] [Google Scholar]
- Poynton JC. The Amphibia of southern Africa : a faunal study. Ann. Natal Mus. 1964;17:1–334. [Google Scholar]
- Pramuk JB, Robertson T, Sites JW, Jr., Noonan BP. Around the world in 10 million years: biogeography of the nearly cosmopolitan true toads (Anura: Bufonidae). Global Ecol. Biogeogr. 2008;17:72–83. [Google Scholar]
- Prigogine A. Les oiseaux de l'Itombwe et de son hinterland. Vol. 1. Musee Royal de l'Afrique Centrale. Ann. Serie 8. Sci. Zool. 1971;185:1–298. [Google Scholar]
- Prigogine A. The composition of Itombwe forest's avifauna. Bonner Zool. Beiträge. 1977;28:369–383. [Google Scholar]
- Prigogine A. Les oiseaux de l'Itombwe et de son hinterland. Vol. 2. Musee Royal de l'Afrique Centrale. Ann. Serie 8, Sci. Zool. 1978;223:1–134. figs. 15–25. [Google Scholar]
- Prigogine A. Les oiseaux de l'Itombwe et de son hinterland. Vol. 3. Musee Royal de l'Afrique Centrale. Ann. Serie 8. Sci. Zool. 1984;243:1–146. [Google Scholar]
- Prigogine A. Conservation of the avifauna of the forests of the Albertine Rift. In: Diamond AW, Lovejoy TE, editors. Conservation of Tropical Forest Birds. International Council for Bird Protection. Cambridge, UK: 1985. pp. 275–295. [Google Scholar]
- Pröhl H, Ron SR, Ryan MJ. Ecological and genetic divergence between two lineages of Middle American túngara frogs Physalaemus (=Engystomops) pustulosus. BMC Evol. Biol. 2010;10:146. doi: 10.1186/1471-2148-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyron RA, Burbrink FT, Wiens JJ. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 2013;13:93. doi: 10.1186/1471-2148-13-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A, Drummond A. FigTree v1.4.2. Institute of Evolutionary Biology. University of Edinburgh; Edinburgh, UK: 2014. http://tree.bio.ed.ac.uk/software/figtree/ [Google Scholar]
- Roberts EM, Stevens NJ, O'Connor PM, Dirks PHGM, Gottfried MD, Clyde WC, Armstrong RA, Kemp AIS, Hemming S. Initiation of the western branch of the East African Rift coeval with the eastern branch. Nat. Geosci. 2012;5:289–294. [Google Scholar]
- Roelants K, Gower DJ, Wilkinson M, Loader SP, Biju SD, Guillaume K, Moriau L, Bossuyt F. Global patterns of diversification in the history of modern amphibians. Proc. Natl. Acad. Sci. USA. 2007;104:887–892. doi: 10.1073/pnas.0608378104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larger B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabaj Pérez MH, editor. Version 5.0 (22 September 2014) American Society of Ichthyologists and Herpetologists; Washington, DC.: 2014. Standard symbolic codes for institutional resource collections in herpetology and ichthyology: an online reference. Electronically accessible at: http://www.asih.org/resources/standard-symbolic-codes-institutional-resource-collections-herpetology-ichthyology. [Google Scholar]
- Scott E. A phylogeny of ranid frogs (Anura: Ranoidea: Ranidae), based on a simultaneous analysis of morphological and molecular data. Cladistics. 2005;21:507–574. doi: 10.1111/j.1096-0031.2005.00079.x. [DOI] [PubMed] [Google Scholar]
- Seimon TA, Ayebare S, Sekisambu R, Muhindo E, Mitamba G, Greenbaum E, Menegon M, Pupin F, McAloose D, Ammazzalorso A, Meirte D, Likwago W, Behangana M, Seimon A, Plumptre AJ. Assessing the threat of amphibian chytrid fungus in the Albertine Rift: past, present and future. PLoS ONE. 2015;10:e0145841. doi: 10.1371/journal.pone.0145841. doi: 10.1371/journal.pone.0145841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, Hines HB, Kenyon N. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007;4:125–134. [Google Scholar]
- Smith BT, Ribas CC, Whitney BM, Hernandez-Banos BE, Klicka J. Identifying biases at different spatial and temporal scales of diversification: a case study in the Neotropical parrotlet genus Forpus. Mol. Ecol. 2013;22:483–494. doi: 10.1111/mec.12118. [DOI] [PubMed] [Google Scholar]
- Sodhi NS, Brook BW, Bradshaw CJA. Tropical Conservation Biology. Blackwell; Oxford, UK.: 2007. [Google Scholar]
- Stamatakis A. RAxML–VI–HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Stephens M, Smith N, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am. J. Hum. Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell SR, Plasterer TN. SEQMAN: contig assembly. Method. Mol. Biol. 1997;70:70–89. [PubMed] [Google Scholar]
- Tarrant J, Cunningham MJ, du Preez LH. Maluti mystery: a systematic review of Amietia vertebralis (Hewitt, 1927) and Strongylopus hymenopus (Boulenger, 1920) (Anura: Pyxicephalidae). Zootaxa. 2008;1962:33–48. [Google Scholar]
- Tolley KA, Braae A, Cunningham M. Phylogeography of the Clicking Stream Frog Strongylopus grayii (Anura, Pyxicephalidae) reveals cryptic divergence across climatic zones in an abundant and widespread taxon. Afr. J. Herpetol. 2010;59:17–32. [Google Scholar]
- Tolley KA, Townsend TM, Vences M. Large-scale phylogeny of chameleons suggests African origins and Eocene diversification. Proc. Roy. Soc. B, Biol. Sci. 2013;280:1–8. doi: 10.1098/rspb.2013.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meijden A, Crottini A, Tarrant J, Turner A, Vences M. Multi-locus phylogeny and evolution of reproductive modes in the Pyxicephalidae, an African endemic clade of frogs. Afr. J. Herpetol. 2011;60:1–12. [Google Scholar]
- van der Meijden A, Vences M, Hoegg S, Meyer A. A previously unrecognized radiation of ranid frogs in southern Africa revealed by nuclear and mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2005;37:674–685. doi: 10.1016/j.ympev.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Viertel B, Veith M, Schick S, Channing A, Kigoolo S, Baeza-Urrea O, Sinsch U, Lötters S. The stream-dwelling larva of the Ruwenzori River Frog, Amietia ruwenzorica, its buccal cavity and pathology of chytridiomycosis. Zootaxa. 2012;57:43–57. [Google Scholar]
- Visser J, Channing A. A new species of river frog from the Swartberg, South Africa (Ranidae: Afrana). J. Afr. Zool. 1997;111:191–198. [Google Scholar]
- Wiens JJ, Penkrot TA. Delimiting species using DNA and morphological variation and discordant species limits in spiny lizards (Sceloporus). Syst. Biol. 2002;51:69–91. doi: 10.1080/106351502753475880. [DOI] [PubMed] [Google Scholar]
- Wilgenbusch JC, Warren DL, Swofford DL. AWTY: a system for graphical exploration of MCMC convergence in Bayesian phylogenetic inference. 2004 doi: 10.1093/bioinformatics/btm388. Accessed at: http://ceb.csit.fsu.edu/awty. [DOI] [PubMed]
- Worrall S. A prince battles to save gorillas amid brutal conflict. [December 10, 2015];National Geographic. 2015 http://news.nationalgeographic.com/2015/06/150611-virunga-national-park-emmanuel-de-merode-africa-world/
- Yang Z, Rannala B. Bayesian species delimitation using multilocus sequence data. Proc. Natl. Acad. Sci. USA. 2010;107:9264–9269. doi: 10.1073/pnas.0913022107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zancolli G, Rödel M, Steffan-Dewenter I, Storfer A. Comparative landscape genetics of two river frog species occurring at different elevations on Mount Kilimanjaro. Mol. Ecol. 2014;23:4989–5002. doi: 10.1111/mec.12921. [DOI] [PubMed] [Google Scholar]