Summary
TSH receptor antibodies (TRAbs) are the pathological hallmark of Graves’ disease, present in nearly all patients with the disease. Euthyroid Graves’ ophthalmopathy (EGO) is a well-recognized clinical entity, but its occurrence in patients with negative TRAbs is a potential source of diagnostic confusion. A 66-year-old female presented to our endocrinology clinic with right eye pain and diplopia in the absence of thyroid dysfunction. TRAbs were negative, as measured with a highly sensitive third-generation thyrotropin-binding inhibitory immunoglobulin (TBII) ELISA assay. CT and MRI scans of the orbit showed asymmetrical thickening of the inferior rectus muscles but no other inflammatory or malignant orbital pathology. Graves’ ophthalmopathy (GO) was diagnosed on the basis of the clinical and radiological features, and she underwent surgical recession of the inferior rectus muscle with complete resolution of the diplopia and orbital pain. She remained euthyroid over the course of follow-up but ultimately developed overt clinical and biochemical hyperthyroidism, 24 months after the initial presentation. By this time, she had developed positive TRAb as well as thyroid peroxidase antibodies. She responded to treatment with thionamides and remains euthyroid. This case highlights the potential for negative thyroid-specific autoantibodies in the presentation of EGO and underscores the variable temporal relationship between the clinical expression of thyroid dysfunction and orbital disease in the natural evolution of Graves’ disease.
Learning points
Euthyroid Graves’ ophthalmopathy can present initially with negative thyroid-specific autoantibodies.
Patients with suggestive symptoms of ophthalmopathy should be carefully evaluated for GO with imaging studies even when thyroid function and autoantibodies are normal.
Patients with EGO can develop thyroid dysfunction within 4 years of follow-up underpinning the need for long-term follow-up and continued patient and physician vigilance in patients who have been treated for EGO.
Background
Graves’ ophthalmopathy (GO) is a chronic inflammatory disease of the orbits typically affecting women in their productive years of life (1, 2). Affected patients suffer distressing and disfiguring eye disease with a small risk of sight loss in severe cases (1, 2). GO classically occurs in patients with Graves’ hyperthyroidism, but 5–10% of patients have hypothyroidism or normal thyroid function (3). Individuals with GO and normal thyroid status are said to have euthyroid Graves’ ophthalmopathy (EGO), the diagnosis of which is supported by the presence of one or more thyroid-specific antibodies, namely antibodies to thyroid peroxidase (TPOAb) and the TSH receptor (TRAbs). TRAbs, the pathological hallmark of Graves’ disease, are present in virtually every patient with the disease (2), and thus, the occurrence of GO in the absence of thyroid dysfunction and thyroid antibodies is a cause of diagnostic uncertainty and has been rarely reported (4). We report a case of GO without thyroid dysfunction or thyroid antibodies at presentation who subsequently developed hyperthyroidism 24 months after the initial presentation.
Case presentation
A 66-year-old female presented with a 4-month history of double vision, excessive tearing, sticky feeling in the eyes, and orbital pain in all gaze directions. She had no symptoms of thyroid dysfunction, did not smoke, and denied any personal or family history of thyroid disease. She was clinically euthyroid and had no palpable goiter. Her visual acuity was 5/6 in both the eyes. She had fullness of her eyelids on the right side with erythema below the right inferior orbital rim. She had right eye proptosis and diplopia on vertical gaze but with no lid lag or retraction. Her intraocular pressures were normal and the optic discs were normal on fundoscopy. At this point, a differential diagnosis of right inferior rectus mass and thyroid eye disease was considered.
Investigation
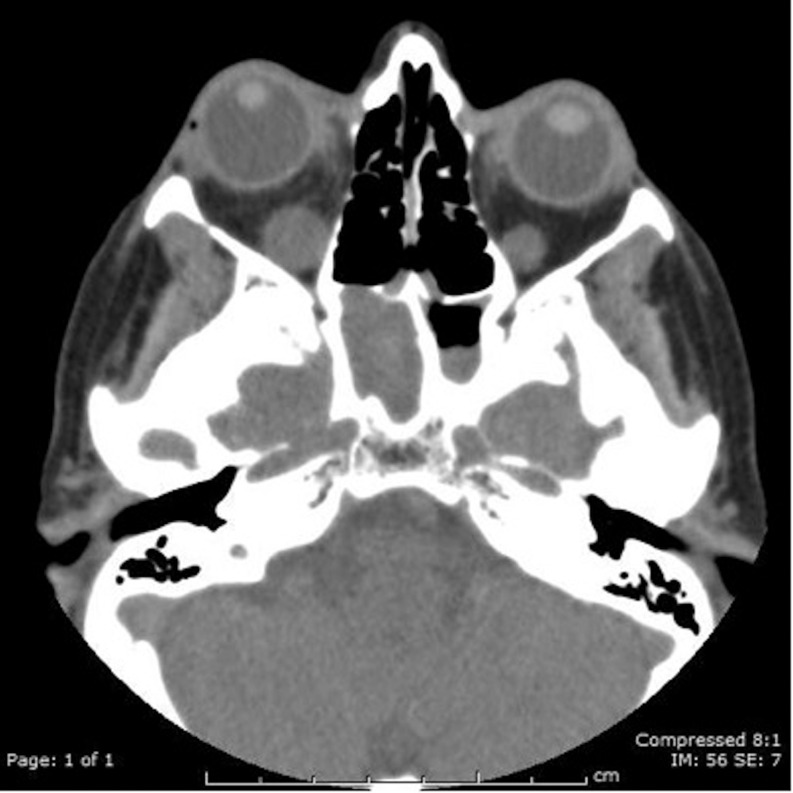
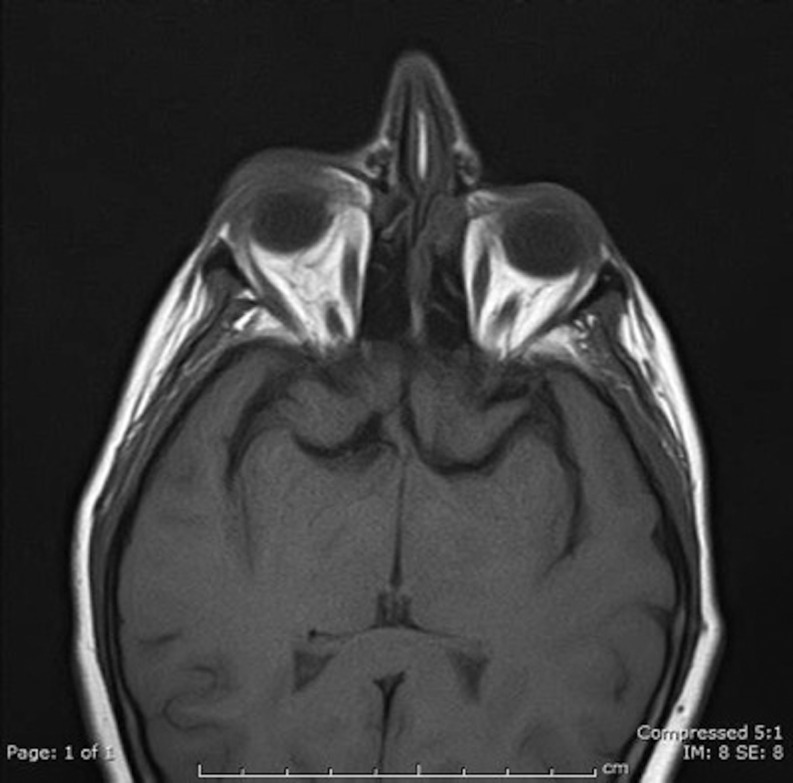
Thyroid function test was normal: TSH 2.25 U/L (reference range 0.4–4.5), FT4 11.6pmol/L (reference range 11.0–24.0), and FT3 4.3pmol/L (reference range 2.67–7.03) (Table 1). TRAbs and TPOAbs were negative and thyroid ultrasound scan showed no evidence of thyroid disease. TRAb measurement was performed using a commercial third-generation ELISA kit that detects both thyroid-stimulating (TSAbs) and -blocking antibodies (TBAbs) with manufacturer specificity and sensitivity of 100 and 95%, respectively, and positive cut-off of >0.4 U/L (RSR Laboratories, Cardiff, UK) (5). In the ELISA, serum TRAbs inhibit the binding of human biotin-labeled monoclonal antibody to immobilized recombinant TSH receptor on the ELISA plate. The amount of M22-biotin bound to the plate is then determined by the addition of streptavidin peroxidase and tetramethylbenzidine and the absorbance of the mixture is read at 450 nm using a plate reader (5). A CT scan of the orbit showed bilateral asymmetrical enlargement of the inferior rectus, more marked on the right than on the left, and was highly suggestive of GO inflammation (Fig. 1). An MRI orbit (Fig. 2) also confirmed inflammation in keeping with GO (Fig. 2). There was no evidence of structural or other inflammatory lesions on imaging. Serum calcium and angiotensin-converting enzyme (ACE) levels were normal.
Table 1.
Evolution of thyroid function and autoimmunity in patient.
| Date | Clinical status | FT4 (pmol/L) | FT3 (pmol/L) | TSH (U/L) | TRAbs (U/L) |
|---|---|---|---|---|---|
| 06/07/2010 | Onset of ophthalmopathy | 11.6 | 5.43 | 1.49 | 0.2 |
| 24/09/2012 | Onset of hyperthyroidism | 16.6 | 9.56 | 0.04 | 7.6 |
| 11/05/2013 | During thionamide treatment | 10.9 | 4.8 | 3.33 | 0.3 |
| 14/07/2014 | After thionamide treatment | 11 | 5.4 | 2.96 | 0.1 |
Reference ranges: FT4, 11.0–23.0 pmol/L; FT3, 2.67–7.03 pmol/L; TSH, 0.4–4.5 U/L; TRAbs <0.1 U/L. The patient had normal thyroid function and negative TRAbs at the onset of ophthalmopathy, but developed thyroid dysfunction with TRAb positivity 24 months after the initial presentation. TRAb levels returned to normal following treatment with thionamides.
Figure 1.
CT orbit showing bilateral asymmetrical enlargement of the inferior rectus.
Figure 2.
MRI orbit showing bilateral enlargement of inferior rectus and mild right-sided proptosis.
Treatment
She was treated symptomatically in the regional multidisciplinary Thyroid Eye Disease clinic with topical lubricants and fitted with a corrective prism. She later underwent a surgical recession of 4mm of the inferior rectus muscle with complete resolution of diplopia and headaches. A biopsy of the inferior rectus muscle taken at surgery showed a low-grade infiltrate with B and T lymphocytes. She had no further eye complaints and continued to be followed up with annual thyroid function tests.
Outcome and follow-up
Twenty-four months after initial presentation, she developed symptoms of anxiety, sweating, tremor, and palpitations. She had no goiter or eye signs. Her thyroid hormone levels were consistent with T3 toxicosis: TSH 0.04U/L, FT3 9.56mol/L, and FT4 15.5pmol/L. At this point, repeat TRAb and TPOAb measurements were positive: 7.6IU/L and 67.9IU/mL, respectively (Table 1). She was commenced on carbimazole initially and subsequently treated with a ‘block and replace’ regimen of carbimazole and levothyroxine. She responded satisfactorily with clinical and biochemical resolution and treatment was discontinued after 12 months. Her vision has since remained intact with no further disturbance of thyroid function.
Discussion
The advent of sensitive second- and third-generation TRAb assays has simplified the clinical recognition of Graves’ disease, especially in patients with atypical presentations such as euthyroid Graves’ ophthalmopathy (EGO) (6). This condition occurs in approximately 5% of the cases of GO and refers to the occurrence of ophthalmopathy in the absence of current or past history of hyperthyroidism (3). Patients with EGO typically have relatively mild disease, characterized by unilateral or asymmetrical disease and lesser degrees of soft tissue inflammation and muscle involvement than patients exposed to hyperthyroidism (7). However, regardless of the degree of thyroid dysfunction or orbital involvement, it is unusual for GO to occur in the absence of thyroid autoantibodies. For patients with established hyperthyroidism, TRAb estimation is not essential for the recognition or management of GO (8). However, a patient with orbital symptoms in the absence of thyroid disease or autoantibodies presents a diagnostic dilemma, and the clinician may be tempted to attribute such cases to nonthyroid causes. Our report illustrates the importance of a thorough clinical and radiological evaluation and close monitoring of long-term thyroid status in such individuals.
As we can see from the case, CT or MRI imaging of the orbit is essential in excluding other causes of orbital disease including meningiomas, lymphomas, cavernous carotid fistula, orbital cellulitis, Cushing’s disease, sarcoidosis, pseudotumor cerebri, and primary and metastatic tumors (9). Imaging will also confirm the features of GO such as extraocular muscle enlargement, adipose tissue expansion, or the sight-threatening complication of dysthyroid optic neuropathy (DON), which may require urgent intervention to prevent sight loss. CT or MRI will also detect bilateral disease that may not be clinically apparent, as some patients with asymmetric bilateral disease may present with unilateral symptoms and signs. Biopsy of the orbital tissue may be useful to differentiate EGO from other inflammatory or infiltrating diseases, but the diagnosis of EGO can often be made on clinical and radiological grounds alone. 18-Fluorodeoxyglucose positron emission tomography scans have been used in some series in the diagnosis of cases where clinical doubt exists (10).
The pathological explanation for negative TRAbs in our patient is unclear. Serum TRAbs are a central pathogenetic event in the initiation of GO, and it is widely accepted that TRAbs cross-react with TSH receptors on orbital fibroblasts and preadipocytes, leading to activation and production of hydrophilic glycosaminoglycans (GAG) which in turn cause the orbital expansion seen in GO (1, 2). It is possible that the TRAb assay used in this case lacked adequate sensitivity to detect low-level antibody activity at the time of presentation. TRAb levels are reported to be very low in patients with EGO and decrease over time after the onset of ophthalmopathy (11). Our patient presented to our clinic 4 months after the start of her symptoms, and it is thus possible that our assay lacked the necessary sensitivity to detect very low levels of TRAbs by the time of presentation. The third-generation ELISA TBII assay used in our case has 95% diagnostic sensitivity, meaning that 5% of patients with Graves’ disease will test negative (5). However, a retrospective study in a U.K. clinic, which included patients at varying stages of treatment, reported a lower sensitivity of 85% using the same third-generation assay at a positive cut-off of >0.4 U/L (12). Thus, the sensitivity of the assay may vary depending on the stage of disease, which was uncertain in our patient. Bioassays which measure TSH receptor-induced cyclic AMP (cAMP) activity in cultured cell preparations could potentially improve sensitivity but are not routinely available in practice. In some instances and depending on the assays used, a specific screen for TRAb IgG subclasses may need to be undertaken, as studies have shown that patients with Graves’ disease may express thyrotropin-binding inhibitory immunoglobulins of varied subclass distribution (13).
A further consideration is that our patient had relatively mild disease, which may not have been severe enough to manifest with circulating TRAbs. TRAb levels correlate with the severity and activity of GO (11), and some authors have described milder disease phenotypes in TRAb-negative patients including milder degrees of hyperthyroidism, smaller goiters, and lower levels of radioactive iodine uptake than patients with classic disease (14). The cause of TRAb negativity in such individuals is unclear, but it has been suggested that antibody-negative individuals may harbor intrathyroidal TRAbs, which do not spill into the circulation (6). Furthermore, TRAbs are unlikely to be the sole pathogenetic agent in GO. Insulin-like growth factor 1 receptor (IGF1R), which is present on the surface of orbital fibroblasts, is another autoantigen that has been implicated in the pathogenesis of GO (2). Tsui and coworkers demonstrated that IGF1R forms functional and physical complexes with the TSH receptor with shared signaling pathways between the receptors (15). Other workers have argued for a pathogenetic role for other orbital muscle autoantibodies in GO such as calquestrin and collagen XIII. In longitudinal studies, McCorquodale and coworkers detected both antibodies in TRAb-negative patients with ophthalmopathy, implying a role for additional antigenic targets in the pathogenesis of Graves’ disease (16).
Our patient was initially managed with topical eye treatments before surgical resection of the inferior rectus. Steroid treatment was originally considered but was not instituted, as the diagnosis of Graves’ orbitopathy was not established at presentation due to the antibody-negative euthyroid status. Since there were a number of other diagnostic considerations, we opted to obtain a surgical biopsy before considering specific treatment by way of glucocorticoids or orbital radiotherapy. Glucocorticoids are indicated in the management of moderate-to-severe and active Graves’ orbitopathy (1, 2). Intravenous glucocorticoid therapy is associated with better response rates than oral glucocorticoids, but optimal dose regimens and treatment duration are still to be established (1). Orbital radiotherapy is a useful adjunct to treatment and may improve outcomes when combined with glucocorticoids (1). However, after surgery, our patient’s symptoms improved obviating the need for steroids or radiotherapy.
Our patient ultimately developed thyroid dysfunction 24 months after the initial presentation with eye disease. This is recognized the sequelae in some patients with EGO. In a series by Khoo and coworkers, 25% of patients with EGO developed thyroid dysfunction within 4 years of follow-up underpinning the need for long-term follow-up and continued patient and physician vigilance in patients who have been treated for EGO (17).
Conclusion
Clinicians should be aware of the variable temporal relationship between the clinical expression of thyroid dysfunction and orbital disease in the natural course of Graves’ disease. Patients with suggestive symptoms of ophthalmopathy should be carefully evaluated for GO with imaging studies even when thyroid function and autoantibodies are normal.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Patient consent
Patient’s consent has been obtained for the publication of this case report.
Author contribution statement
A T was responsible for writing the manuscript, researching the discussion, and reviewing and editing the manuscript; I K was responsible for researching the discussion and reviewing and editing the manuscript; P T was also responsible for writing the manuscript, researching the discussion and reviewing and editing the manuscript; G D was responsible for supervision and reviewing and editing the manuscript; O E O is the senior author and was responsible for supervision, obtaining patient’s consent, and reviewing and editing the final manuscript.
References
- 1.Bartalena L, Tanda ML. 2009. Clinical practice. Graves’ ophthalmopathy. New England Journal of Medicine 360 994–1001. ( 10.1056/NEJMcp0806317) [DOI] [PubMed] [Google Scholar]
- 2.Bahn RS. 2010. Graves’ ophthalmopathy. New England Journal of Medicine 362 726–738. ( 10.1056/NEJMra0905750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA, Gorman CA. 1996. Clinical features of Graves’ ophthalmopathy in an incidence cohort. American Journal of Ophthalmology 121 284–290. ( 10.1016/S0002-9394(14)70276-4) [DOI] [PubMed] [Google Scholar]
- 4.Cakir M. 2005. Euthyroid Graves’ ophthalmopathy with negative autoantibodies. Journal of the National Medical Association 97 1547–1549. [PMC free article] [PubMed] [Google Scholar]
- 5.Smith BR, Bolton J, Young S, Collyer A, Weeden A, Bradbury J, Weightman D, Perros P, Sanders J, Furmaniak J. 2004. A new assay for thyrotropin receptor autoantibodies. Thyroid 14 830–835. ( 10.1089/1050725042451248) [DOI] [PubMed] [Google Scholar]
- 6.Kamath C, Adlan MA, Premawardhana LD. 2012. The role of thyrotrophin receptor antibody assays in Graves’ disease. Journal of Thyroid Research 2012 525936 ( 10.1155/2012/525936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckstein AK, Lösch C, Glowacka D, Schott M, Mann K, Esser J, Morgenthaler NG. 2009. Euthyroid and primarily hypothyroid patients develop milder and significantly more asymmetrical Graves’ ophthalmopathy. British Journal of Ophthalmology 93 1052–1056. ( 10.1136/bjo.2007.137265) [DOI] [PubMed] [Google Scholar]
- 8.Chen DY, Schneider PF, Zhang XS, Luo XY, He ZM, Chen TH. 2014. Changes in Graves’ ophthalmopathy after radioiodine and antithyroid drug treatment of Graves’ disease from 2 prospective, randomized, open-label, blinded end point studies. Experimental and Clinical Endocrinology & Diabetes 122 1–6. ( 10.1055/s-0033-1358484) [DOI] [PubMed] [Google Scholar]
- 9.Mallika P, Tan AK, Aziz S, Alwi SS, Chong MS, Vanitha R, Intan G. 2009. Thyroid associated ophthalmopathy – a review. Malaysian Family Physician 4 8–14. [PMC free article] [PubMed] [Google Scholar]
- 10.García-Rojas L, Adame-Ocampo G, Alexánderson E, Tovilla-Canales JL. 2013. 18-fluorodeoxyglucose uptake by positron emission tomography in extraocular muscles of patients with and without Graves’ ophthalmology. Journal of Ophthalmology 2013 529187 ( 10.1155/2013/529187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckstein AK, Plicht M, Lax H, Neuhäuser M, Mann K, Lederbogen S, Heckmann C, Esser J, Morgenthaler NG. 2006. Thyrotropin receptor autoantibodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. Journal of Clinical Endocrinology & Metabolism 91 3464–3470. [DOI] [PubMed] [Google Scholar]
- 12.Theodoraki A, Jones G, Parker J, Woolman E, Martin N, Perera S, Thomas M, Bunn C, Khoo B, Bouloux PM, et al. 2011. Performance of a third-generation TSH-receptor antibody in a UK clinic. Clinical Endocrinology 75 127–133. ( 10.1111/j.1365-2265.2011.04022.x) [DOI] [PubMed] [Google Scholar]
- 13.Weetman AP, Byfield PG, Black C, Reimer CB. 1990. IgG heavy-chain subclass restriction of thyrotropin-binding inhibitory immunoglobulins in Graves’ disease. European Journal of Clinical Investigation 20 406–410. ( 10.1111/j.1365-2362.1990.tb01877.x) [DOI] [PubMed] [Google Scholar]
- 14.Mukuta T, Tamai H, Oshima A, Morita T, Matsubayashi S, Fukata S, Kuma K. 1994. Immunological findings and thyroid function of untreated Graves’ disease patients with undetectable TSH-binding inhibitor immunoglobulin. Clinical Endocrinology 40 215–219. ( 10.1111/j.1365-2265.1994.tb02471.x) [DOI] [PubMed] [Google Scholar]
- 15.Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A, Gianoukakis AG, Douglas RS, Smith TJ. 2008. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. Journal of Immunology 181 4397–4405. ( 10.4049/jimmunol.181.6.4397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCorquodale T, Lahooti H, Gopinath B, Wall JR. 2012. Long-term follow-up of seven patients with ophthalmopathy not associated with thyroid autoimmunity: heterogeneity of autoimmune ophthalmopathy. Clinical Ophthalmology 6 1063–1071. ( 10.2147/OPTH.S30704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoo DH, Eng PH, Ho SC, Tai ES, Morgenthaler NG, Seah LL, Fong KS, Chee SP, Choo CT, Aw SE. 2000. Graves’ ophthalmopathy in the absence of elevated free thyroxine and triiodothyronine levels: prevalence, natural history, and thyrotropin receptor antibody levels. Thyroid 10 1093–1100. ( 10.1089/thy.2000.10.1093) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a