Abstract
Background/Aim:
The study was carried out to compare the efficacy of Vitamin E versus Ursodeoxycholic acid (UDCA) in nondiabetic nonalcoholic fatty liver disease (NAFLD) patients.
Patients and Methods:
We randomized 250 non cirrhotic and non diabetic NAFLD patients diagnosed on ultrasound, with raised alanine aminotransferase (ALT) level. (>40 IU/L), to receive Vitamin E 400 mg twice a day (Group A) or UDCA 300 mg twice a day (Group B) for 52 weeks. Lifestyle modification to achieve at least 5% weight reduction and subsequent weight control and regular exercise was advised to both groups. The primary study endpoint was normalization of ALT. Secondary endpoints were the proportion of patients with reduction in ALT, relative reduction in the NAFLD Fibrosis score (NFS), symptomatic improvement and tolerability.
Results:
One hundred and fifty patients received UDCA as compared to 100 patients receiving Vitamin E. The treatment groups were comparable at entry with regard to age (44.1 vs 42.4 years), gender (67% vs 63% female), risk factors for nonalcoholic steatohepatitis, hypochondriac pain, serum liver biochemistries, and NAFLD Fibrosis score. The primary endpoint was achieved in 21 (14%) and 19 (19%) of patients in Group A and Group B, respectively (P = 0.2). The proportion of patients with reduction in ALT (56% vs 63%, P = 0.2), symptomatic improvement (78% vs 67%, P= 0.058), reduction in the NFS (44% vs 47%, P= 0.69), and tolerability (98% vs 95%, P= 0.2) were similar between Group A and Group B, respectively.
Conclusion:
UDCA is an effective and safe alternative to Vitamin E in nondiabetic–noncirrhotic Indian NAFLD patients.
Keywords: Fatty liver, nonalcoholic steatohepatitis, steatohepatitis, Ursodeoxycholic acid
Nonalcoholic fatty liver disease (NAFLD) is a disorder characterized by excess fat accumulation in the liver in the absence of significant amounts of alcohol consumption, usually defined as less than 20 g of ethanol per day.[1] Comparison of nonalcoholic steatohepatitis (NASH) and NAFLD in Eastern and Western showed a similar age at presentation, that is, 4th to 8th decade, prevalence of 20%-30% in the West, whereas 10% in the East.[2] In 2007, The Asia-Pacific Working Party formulated guidelines for NAFLD in the Asia-Pacific region. The Working Party estimated the prevalence of NAFLD in adult population from 5% to 30%.[3,4] There is a rapid change in the socioeconomic status in Asia, which has led to inappropriate diet and sedentary lifestyle. The management of patients with NAFLD consists of treating liver disease as well as the associated metabolic comorbidities such as obesity, hyperlipidemia, insulin resistance, and Type 2 Diabetes Mellitus. A variety of drugs have been tried for treatment of NASH, including metformin, pioglitazone, Vitamin E, Ursodeoxycholic Acid (UDCA), omega-3 fatty acids, anti-hypertensives, antiobesity drugs, antioxidants, and many more. The most controversial of these drugs is UDCA with some studies favoring its use and others not. UDCA, a secondary bile acid produced by intestinal bacteria as metabolic byproduct, has been shown to be effective in the nonsurgical treatment of cholesterol gallstones and primary biliary cirrhosis (PBC). Studies have investigated UDCA (conventional and high doses) to improve aminotransferases and steatosis in patients with NAFLD and liver histology in patients with NASH.[5,6,7,8,9] A single large multicenter randomized controlled trial (RCT) showed that UDCA offers no benefit over placebo in patients with NASH.[8] Chalasani et al. recommends against the use of UDCA for the treatment of NASH.[10] Recent Chinese studies favor UDCA as monotherapy or in combination. However, the American Association for the Study of Liver Diseases (AASLD) recommends the use of Vitamin E administered at daily doses of 800 IU/day in nondiabetic adults with biopsy-proven NASH and is considered as a firstline pharmacotherapy for this patient population.[10] We carried out this study to compare the efficacy of these two drugs for treatment of NAFLD.
PATIENTS AND METHODS
Study design
This is a prospective, single center, open-labeled, RCT carried out at Lokmanya Tilak Municipal General Hospital between December 2011 and December 2013. An informed consent was taken from all patients. The institutional Ethics Committee clearance was obtained.
Patients
Eligible patients were 18–80 years of age attending Outpatient Department of our tertiary care center (nonreferred patients) for dyspepsia and who were diagnosed as fatty liver on ultrasound (hyperechoic liver where the echotexture of the liver brighter than the kidney, blurring of vascular margins, and deep attenuation of ultrasound signal). Of these, patients with serum alanine aminotransferase (AST) or aspartate aminotransferase (ALT) greater than upper limit of normal (40 IU/mL) were included in the study.
Exclusion criteria were history of alcohol intake greater than 20 g per day (during previous 5 years), hepatitis B antigen (HBsAg) reactive, presence of antibody against hepatitis C (Anti-HCV) Human Immunodeficiency Virus (HIV) reactive, active hepatitis, biliary obstruction on ultrasound, diagnosed as cirrhosis at any time in the past, tuberculosis, malabsorption, chronic drug use, pregnancy, and those with any cardiorespiratory comorbid conditions. Alpha-1 Antitrypsin deficiency and hemochromatosis are rarely seen in Indian patients and were therefore not investigated for in our patients. Besides, those patients who fulfilled the inclusion criteria but did not give consent were excluded.
Screening and evaluation
We performed complete physical examination, height, weight, waist circumference, complete blood count, serum transaminases, prothrombin time, serum creatinine, fasting and 2-hour postprandial blood sugar levels (PPBS), Glycosylated Hemoglobin, complete lipid profile, antinuclear antibody, HBsAg, Anti-HCV, HIV, serum ceruloplasmin, thyroid function tests, serum ferritin, ultrasound, and upper gastrointestinal endoscopy. All these patients with raised transaminases were offered liver biopsy. Aspartate aminotransferase to platelet ratio (APRI), AST/ALT ratio (AAR), and NAFLD Fibrosis score (NFS) were calculated. NFS was calculated as per the following formula: −1.675 + 0.037 × Age in years + 0.094 × BMI (kg/m2) +1.13 × IFG/Diabetes (yes = 1, no = 0) +0.99 × AST/ALT ratio – 0.013 × platelet (×109/L) – 0.66 × Albumin (g/dL). Those patients who consented for liver biopsies underwent liver biopsy with 16G needle, and a specimen length of minimum 2 cms was obtained. All liver biopsies were assessed by a senior histopathologist and were graded and staged according to criteria given by Brunt and Tiniakos.[11] FibroScan (FibroScan 402, M Probe) was carried out at baseline in all patients before the beginning of therapy.
Treatment protocol and monitoring
All patients were advised lifestyle modification to achieve at least 5% weight reduction, subsequent weight control and regular exercise. Dietary modification included a low calorie diet prescribed by a dietician and the exercise included 30 min of brisk walking 5 days a week or 20 days a month. For those who had difficulty walking, swimming or aerobic exercise under the guidance of a trainer was advised for the same duration. The dietician assessed the regimen every month for adherence to the lifestyle modification and reinforced the diet and exercise. Group A patients received Vitamin E 400 IU twice a day, whereas Group B patients received UDCA 300 mg twice a day for a period of 52 weeks.
All patients were followed up at monthly intervals and lifestyle modification as well as drug compliance were reinforced. At the end of 8, 16, 24, 36, and 52 weeks, each patient was assessed in terms of clinical history and examination, complete blood count, serum transaminases, serum creatinine, fasting blood sugar levels, serum triglycerides, and cholesterol. FibroScan was carried out at the end of treatment at 52 weeks. Adherence was considered adequate if patients had taken 80% of the prescribed duration of treatment.
Endpoint
The primary study endpoint was normalization of ALT levels. Secondary endpoints were the proportion of patients with reduction in ALT, relative reduction in the NAFLD Fibrosis score (NFS), symptomatic improvement, and tolerability.
Statistical analysis
The continuous variables were expressed as mean (SD) or median (range) and the categorical variables as frequencies and percentages. Continuous variables between the three groups were compared using nonparametric test and categorical variables were compared using Chi-square test. Within each group, post-treatment AST and ALT were compared using Wilcoxon's signed rank test for paired observations. P < 0.05 was considered significant. All analyses were done using intention-to-treat basis.
RESULTS
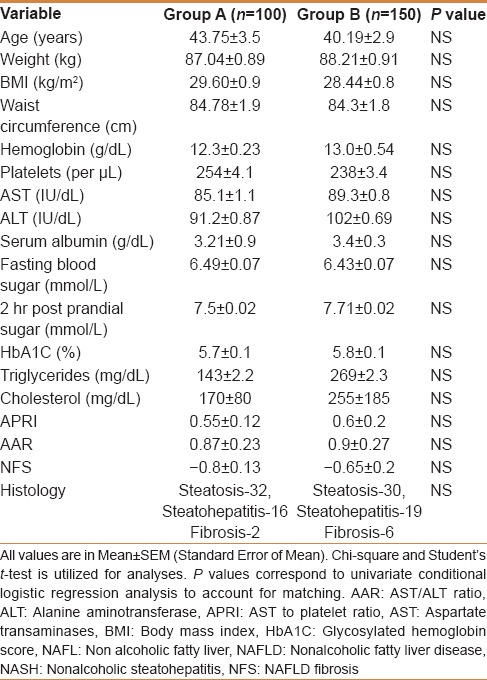
A total of 250 patients diagnosed as NAFLD and raised transaminases were included in the study. One hundred and fifty patients were enrolled in Group B (2:3 randomization, with random block sizes) [Figure 1]. The baseline characteristics of these patients are shown in Table 1. The two groups were similar in terms of age, gender distribution, frequency of right hypochondrial pain, body mass index, hypertension, lipid profile, transaminases, NFS, and AAR. Fifty patients (steatosis = 32, steatohepatitis without fibrosis = 6, steatohepatitis with fibrosis but no cirrhosis = 2) in Group A patients underwent liver biopsy at the onset of study as compared to 55 patients (steatosis = 30, steatohepatitis without fibrosis = 19, steatohepatitis with fibrosis but no cirrhosis = 6) in Group B (P > 0.05). Twelve patients in Group B and five patients in Group A did not finish the required 80% of total duration of treatment (P > 0.05). All patients who did not finish the study decided to stop medicines in view of absence of benefit of treatment in symptoms.
Figure 1.
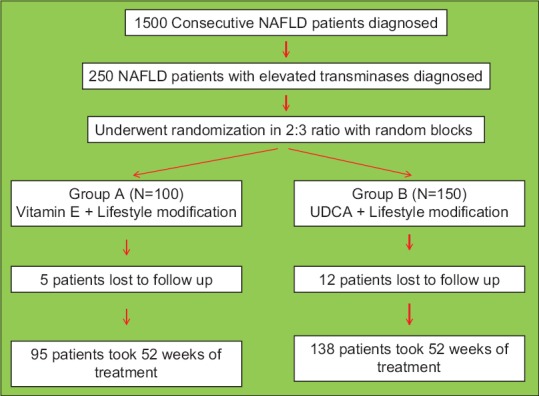
The randomization flow chart
Table 1.
The baseline characteristics of NAFLD patients in Group A (Vitamin E) and Group B (UDCA)
Lifestyle modification
Dietary advice was followed by 88% (n = 220) of patients. Desired weight reduction of 5% was achieved in 70% (n = 175) of patients following dietary advice. The weight reduction was seen in 67% in Group A, whereas it was 70% in Group B (P = 0.2). The median weight reduction in two groups (Group A = 8.2% vs Group B = 8.3%) was similar. The weight loss achieved by the patients in both groups was significant as compared to their baseline weight (P < 0.0001, paired Student's t-test). The maximum weight reduction seen was 17%. Weight reduction continued over 52 weeks for those who maintained dietary advice and lifestyle modification [Figure 2].
Figure 2.
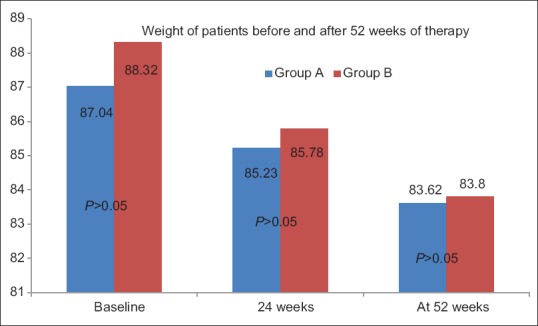
The figure shows the weight of patients in Group A and Group B before and after treatment of 52 weeks. The difference between Group A and Group B is not statistically significant at baseline as well as after 24 and 52 weeks of therapy (t test). The weight (kg) loss achieved by either group is significant at the end of 52 weeks (Paired Student's t test). Group A - Vitamin E plus lifestyle modification. Group B – UDCA plus lifestyle modification
Improvement in transaminases
All patients at the onset had elevated transaminases. The primary endpoint of normalization of transaminases was achieved in 21 (14%) and 19 (19%) patients in Group A and Group B, respectively (P = 0.2). The proportion of patients with reduction in ALT (56% vs 63%, P = 0.2) was similar in Group A and Group B. However, no significant improvement in transaminases was seen after treatment duration of 24 weeks. All patients with transaminases improvement had achieved the desired weight reduction [Figure 3a and b].
Figure 3a.
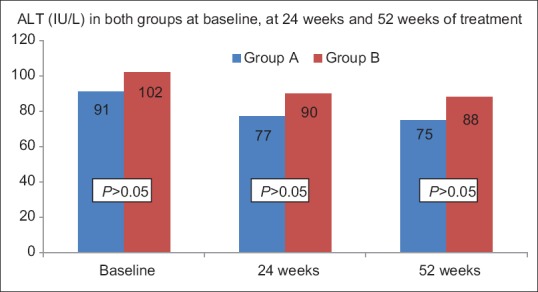
ALT values at baseline, 24 weeks, and at 52 weeks of treatment in both the groups. All values are in Mean. Student's t-test is utilized for analyses. P values correspond to univariate conditional logistic regression analyis to account for matching. The change in ALT levels from baseline at 24 weeks and 52 weeks is significant (P < 0.0001). However, the difference between ALT at 24 weeks and 52 weeks is not significant (P > 0.05). ALT, alanine aminotransferase
Figure 3b.
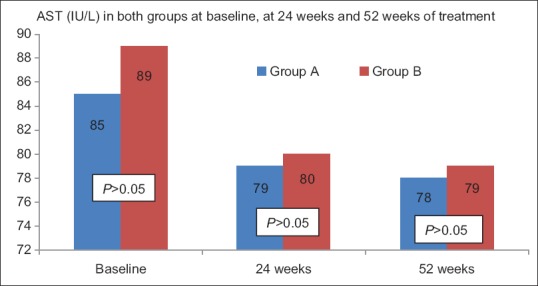
ALT values at baseline, 24 weeks, and at 52 weeks of treatment in both the groups. All values are in Mean. Student's t test is utilized for analyses. P values correspond to univariate conditional logistic regression analyis to account for matching. The change in ALT levels from baseline at 24 weeks and 52 weeks is significant (P < 0.0001). However, the difference between ALT at 24 weeks and 52 weeks is not significant (P > 0.05). AST, aspartate aspartate aminotransferase
Symptomatic improvement
All patients presented to outpatient department with dyspeptic (postprandial fullness, bloating, early satiation, epigastric discomfort and nausea) symptoms. Right hypochondrial pain was reported in 55% right hypochondriac pain was seen in 55% (n = 55) in group A and 60% (n = 90) in group B (P = 0.4). Symptomatic improvement in hypochondriac pain was seen in 78.1% (n = 43/55) in group A and 67% (n = 60/90) (P = 0.18) in Group B. There was no correlation between normalization of transaminases and improvement in symptoms. (r = 0.58, 95% CI-0.51-0.63).
Improvement in APRI, AAR, NFS and FibroScan
The median values of APRI, AAR, and NFS at the beginning of treatment was 0.55, 0.87, and − 0.8 for Group A and 0.6, 0.93, and − 0.65 for Group B, respectively (P > 0.05). On completing 52 weeks of treatment, the median values of APRI, AAR, and NFS were 0.44, 0.77, and − 0.55 for Group A and 0.53, 0.81, and − 0.50 for Group B, respectively. The difference between the two groups before and after the treatment was not significant (P > 0.05). However, each group showed a statistically significant improvement in APRI, AAR, and NFS at the end of 52 weeks (P < 0.001). Overall 44% in Group A and 47% in Group B demonstrated improvements in NFS (P > 0.05) [Figure 4]. The Median FibroScan value (in kPa) in Group A and Group B before the start of treatment was 7.9 ± 0.1 and 8.0 ± 0.1, respectively (P > 0.05). Repeat FibroScan could be done only in 70 patients of Group A and 114 patients of Group B. The Median FibroScan values (in kPa) at the end of therapy were 7.6 ± 0.1 and 7.7 ± 0.2, respectively, in groups A and B. There was significant reduction in the FibroScan values in both the groups (P < 0.05) [Figure 5].
Figure 4.
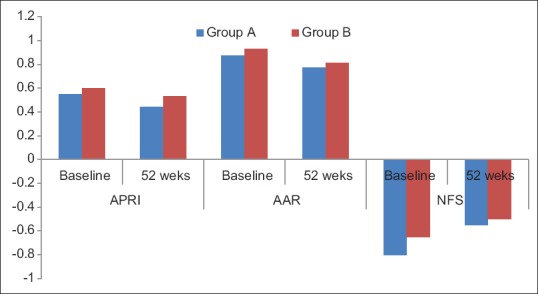
The figure shows APRI, AAR, and NFS in patients of Group A and Group B at baseline and at 52 weeks. All values are in Median. The difference between Group A and Group B is not statistically significant before and after the treatment. The improvement in APRI, AAR, and NFS is significant before and after the 52 weeks of treatment for both the groups (P < 0.05). APRI, AST to platelet ratio; AAR, AST to ALT ratio; NFS, NAFLD Fibrosis Score. Group A - Vitamin E plus lifestyle modification. Group B - UDCA plus lifestyle modification
Figure 5.
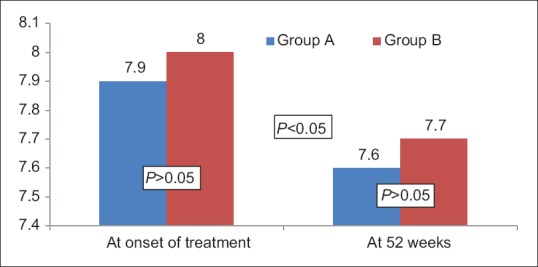
The figure shows Median FibroScan (in kPa) in patients of Group A and Group B at baseline and at 52 weeks. All values are in Median. The difference between Group A and Group B is not statistically significant before and after the treatment (P > 0.05). The improvement in Median FibroScan values is significant before and after the 52 weeks of treatment for both the groups (P < 0.05). Group A - Vitamin E plus lifestyle modification. Group B – UDCA plus lifestyle modification
Tolerability
Five percent in Group A and 8% in Group B did not finish the treatment for 52 weeks and they were considered to have lack of tolerability. Both the drugs were well tolerated by the rest of the patients. Eighty percent of the patients in Group A and 83% in Group B recorded no adverse events after taking the drug. The most common side effect seen in patients of both groups was worsening of dyspeptic symptoms (n = 15 in Group A, n = 18 in Group B). Headache was reported in five patients in Group A and 9 patients of Group B. Diarrhea was seen in four patients in Group B. No incidence of cardiac failure, stroke, bleeding, or rash was reported.
DISCUSSION
UDCA has on paper an impeccable track record of cytoprotection in vitro and in vivo due to its pleiotropic effects on many pathways leading to cell injury. Most of its hepatoprotective effects were demonstrated under experimental conditions. Early clinical studies suggested a potentially beneficial effect in NASH as well. Data on the efficacy of UDCA specifically in experimental models of steatosis/NASH is heterogenous. Between 1994 and 2008, four prospective randomized, double-blind, placebo-controlled studies of the treatment of NASH with UDCA were conducted and until 2013, 12 randomized, controlled studies investigating the effects of UDCA in patients with NASH have been carried out. Th first study, by Lindor et al.,[8] compared the impact of 13–15 mg/kg/day of UDCA to a placebo. The second study by Dufour et al.[5] had an additional third arm that administered a combination therapy with UDCA and vitamin E. The third and fourth studies by Leuschner et al.[7] and by Ratziu et al.[9] evaluated high doses of UDCA at 25–35 mg/kg/day, and used liver biopsies and serum liver enzyme levels to evaluate the impact of UDCA. With the exception of the Ratziu et al. study, none of these studies showed any significant differences in the treatment of NASH with UDCA compared with a placebo. However, Dufour et al. did observe a significant improvement of NASH with the combination (UDCA/VitE) versus placebo therapy. Nine of later RCTs, however, found that UDCA had positive effects in patients with NASH, whether as monotherapy or combined with other drugs. Six of these studies were Chinese.[12] Trials in China of UDCA therapy in patients with NASH have not been available to Western researchers because of language limitations, and there has been no complete overview of these data. Seven of these trials assessed the effects of UDCA monotherapy, with the other five testing combinations of UDCA with vitamin E, polyene phosphatidylcholine, silymarin, glycyrrhizin, and tiopronin. The duration of therapy ranged from 3 to 24 months, with two studies using high doses of UDCA (23-35 mg/kg/day).[12]
The PIVENS study by Sanyal et al.[13] showed that Vitamin E is effective in NAFLD and is considered the treatment of choice for nondiabetic noncirrhotic NASH patients by AASLD. The study compared Vitamin E with Pioglitazone and placebo and concluded that Vitamin E is the drug with significant benefit, with an efficacy of 40%. Pioglitazone, although beneficial, did not reach statistical significance. For this reason we included a Vitamin E-treated arm as a comparison group. Our study showed improvement in 56% of the patients.
The present study has has shown that in Indian patients UDCA is noninferior to Vitamin E in NASH patients. The two drugs were comparable in terms of normalization of transaminases, reduction in transaminases, improvement in symptoms, improvement in APRI, AAR, and NFS. Both the drugs are well tolerated. When the two drugs are accompanied by lifestyle modification, the improvement is equally efficacious.
The therapeutic effect of UDCA on NASH is biologically plausible. In an animal model, UDCA was found to improve hepatic steatosis and inflammation. Although its mechanism of action is still unclear, UDCA can protect hepatocytes by inhibiting the absorption of toxic hydrophobic bile salts from the small intestine, competing with toxic bile acids to bind to cell and organelle membranes and maintain cell membrane stability.[14] In addition, UDCA can reduce oxidative damage by inhibiting hydrophobic bile salt-induced Kupffer cell activation and increasing hepatic glutathione levels.[15] Finally, UDCA has immunomodulatory and antiapoptotic properties, as shown by its interaction with the glucocorticoid nuclear receptor at the hepatocyte level, its repression of IFN-gamma-induced MHC class II gene expression and its maintenance of mitochondrial membrane stability.[16,17,18,19,20,21]
Hence, UDCA is a well tolerated drug with minimal side effects, has a non-inferior efficacy as compared to Vitamin E and has a biologically plausible benefit. Clinical trials from China, like in our study, have also shown benefit. Hence, it is worthwhile to carry out double blinded studies with a larger sample size in Indian patients to prove or disprove the efficacy of UDCA, rather than following the trends of Western populations where UDCA has shown questionable benefit.
A major limitation of our study was that the benefit of both drugs was not documented histologically as a repeat biopsy was not permitted by the ethics committee.
CONCLUSION
UDCA is as effective as vitamin E in Indian patients when combined with adequate dietary guidance and lifestyle modification. UDCA can be utilized as an alternative to Vitamin E in the treatment of NASH as it has minimal side effects and equal tolerability.
Financial support and sponsorship
Nil.
Conflicts of interest
PP, PS, JP, PB, VP, and MI confirm that they have no conflicts of interest to declare.
REFERENCES
- 1.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8. [PubMed] [Google Scholar]
- 2.Chitturi S, Farrell GC, George J. Non-alcoholic steatohepatitis in the Asia-Pacific region: Future shock? J Gastroenterol Hepatol. 2004;19:368–74. doi: 10.1111/j.1440-1746.2003.03252.x. [DOI] [PubMed] [Google Scholar]
- 3.Chitturi S, Farrell GC, Hashimoto E, Saibara T, Lau GK, Sollano JD. Asia-Pacific Working Party on NAFLD. Non-alcoholic fatty liver disease in the Asia-Pacific region: Definitions and overview of proposed guidelines. J Gastroenterol Hepatol. 2007;22:778–87. doi: 10.1111/j.1440-1746.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- 4.Amarapurkar DN, Hashimoto E, Lesmana LA, Sollano JD, Chen PJ, Goh KL. Asia-Pacific Working Party on NAFLD. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J Gastroenterol Hepatol. 2007;22:788–93. doi: 10.1111/j.1440-1746.2007.05042.x. [DOI] [PubMed] [Google Scholar]
- 5.Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, et al. Swiss Association for the Study of the Liver. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin E in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537–43. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, et al. Ursodeoxycholic acid or clofibrate in the treatment of non-alcoholic-induced steatohepatitis: A pilot study. Hepatology. 1996;23:1464–7. doi: 10.1002/hep.510230624. [DOI] [PubMed] [Google Scholar]
- 7.Leushner U, Lindenthal B, Herrman G, Arnold JC, Rössle M, Cordes HJ, et al. NASH Study Group. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: A double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52:472–9. doi: 10.1002/hep.23727. [DOI] [PubMed] [Google Scholar]
- 8.Lindor KD, Kowldey KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: Results of a randomized trial. Hepatology. 2004;39:770–8. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 9.Ratziu V, de Ledinghen V, Oberti F, Mathurin P, Wartelle-Bladou C, Renou C, et al. FRESGUN. A randomized controlled trial of high-dose ursogeoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011–9. doi: 10.1016/j.jhep.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 11.Brunt EM, Tiniakos DG. Histopathology of nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16:5286–96. doi: 10.3748/wjg.v16.i42.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiang Z, Chen YP, Ma KF, Ye YF, Zheng L, Yang YD, et al. The role of Ursodeoxycholic acid in non-alcoholic steatohepatitis: A systematic review. BMC Gastroenterol. 2013;13:140. doi: 10.1186/1471-230X-13-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pathil A, Mueller J, Warth A, Chamulitrat W, Stremmel W. Ursodeoxycholic lysophosphatidylethanolamide improves steatosis and inflammation in murine models of nonalcoholic fatty liver disease. Hepatology. 2012;55:1369–78. doi: 10.1002/hep.25531. [DOI] [PubMed] [Google Scholar]
- 15.Buko VU, Kuzmitskaya-Nikolaeva IA, Naruta EE, Lukivskaya OY, Kirko SN, Tauschel HD. Ursodeoxycholic acid dose-dependently improves liver injury in rats fed a methionine- and choline-deficient diet. Hepatol Res. 2011;41:647–59. doi: 10.1111/j.1872-034X.2011.00820.x. [DOI] [PubMed] [Google Scholar]
- 16.Castro RE, Ferreira DM, Afonso MB, Borralho PM, Machado MV, Cortez-Pinto H, et al. miR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in rat liver and activated by disease severity in human non-alcoholic fatty liver disease. J Hepatol. 2012;58:119–25. doi: 10.1016/j.jhep.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Beuers U. Drug insight: Mechanisms and sites of action of Ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:318–28. doi: 10.1038/ncpgasthep0521. [DOI] [PubMed] [Google Scholar]
- 18.Sokolovic D, Nikolic J, Kocic G, Jevtovic-Stoimenov T, Veljkovic A, Stojanovic M, et al. The effect of Ursodeoxycholic acid on oxidative stress level and DNase activity in rat liver after bile duct ligation. Drug Chem Toxicol. 2012;36:141–8. doi: 10.3109/01480545.2012.658919. [DOI] [PubMed] [Google Scholar]
- 19.Bellentani S. Immunomodulating and anti-apoptotic action of ursodeoxycholic acid: Where are we and where should we go? Eur J Gastroenterol Hepatol. 2005;17:137–40. doi: 10.1097/00042737-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H, Makino Y, Miura T, Hirano F, Okamoto K, Komura K, et al. Ligand-independent activation of the glucocorticoid receptor by ursodeoxycholic acid. Repression of IFN-gamma-induced MHC class II gene expression via a glucocorticoid receptor-dependent pathway. J Immunol. 1996;156:1601–8. [PubMed] [Google Scholar]
- 21.Chun HS, Low WC. Ursodeoxycholic acid suppresses mitochondria-dependent programmed cell death induced by sodium Nitroprusside in SH-SY5Y cells. Toxicology. 2012;292:105–12. doi: 10.1016/j.tox.2011.11.020. [DOI] [PubMed] [Google Scholar]


