Abstract
Objectives: The objective of this study is to evaluate the effects of a soy drink with a high concentration of isoflavones (ViveSoy®) on climacteric symptoms.
Methods: An open-label, controlled, crossover clinical trial was conducted in 147 peri- and postmenopausal women. Eligible women were recruited from 13 Spanish health centers and randomly assigned to one of the two sequence groups (control or ViveSoy®, 500 mL per day, 15 g of protein and 50 mg of isoflavones). Each intervention phase lasted for 12 weeks with a 6-week washout period. Changes on the Menopause Rating Scale and quality of life questionnaires, as well as lipid profile, cardiovascular risk and carbohydrate and bone metabolism were assessed. Statistical analysis was performed using a mixed-effects model.
Results: A sample of 147 female volunteers was recruited of which 90 were evaluable. In both sequence groups, adherence to the intervention was high. Regular consumption of ViveSoy® reduced climacteric symptoms by 20.4% (p = 0.001) and symptoms in the urogenital domain by 21.3% (p < 0.05). It also improved health-related quality life by 18.1%, as per the MRS questionnaire (p <0.05).
Conclusion: Regular consumption of ViveSoy® improves both the somatic and urogenital domain symptoms of menopause, as well as health-related quality of life in peri- and postmenopausal women.
Keywords: Estrogens, menopause, urogenital system
Chinese abstract
研究目的:本研究的目的是评价含有高浓度异黄酮(ViveSoy®)的大豆饮料对更年期症状的影响。
研究方法:对147名围绝经及绝经后妇女进行开放、对照、交叉临床试验。从13个西班牙医疗中心招募符合条件的女性受试者进行随机分配(对照组或ViveSoy®组,每天500毫升ViveSoy摄入,其中含有15g的蛋白质和50mg的异黄酮)。每个干预阶段持续12周其中包括6周的洗脱期。填写更年期评定量表和生活质量调查问卷,并且评估血脂高低、心血管疾病患病风险和碳水化合物和骨质新陈代谢情况。依据混合效应模型进行统计分析。
研究结果:147名女性志愿者中90人是可评价的。这两组成员的依从性都较高。经常食用ViveSoy®的小组成员更年期的症状减少了20.4%(p = 0.001),泌尿生殖系统症状减少了21.3%(p<0.05)。依据更年期评定量表问卷健康相关生活质量提高了18.1%(p<0.05)。
研究结论:规律食用ViveSoy®可以减轻躯体和泌尿生殖系统的更年期的症状,提高围绝经期和绝经期妇女的健康相关生活质量。
Introduction
Over the last decades, consumption of plant-based beverages has become popular in Western countries [1,2]. The phytoestrogenic effects of soy isoflavones and isoflavone supplements have been proposed as alternatives to conventional hormone therapy [3].
Several studies showed that the regular consumption of isoflavones may be beneficial for peri- and postmenopausal women when it comes to the reduction of climacteric symptoms [4], protection against bone decalcification [1,5,6], and reduction of markers of ischemic heart disease [3].
A Cochrane systematic review identified six studies that found a significant reduction in the frequency and severity of hot flushes and night sweats in perimenopausal and postmenopausal women [7–13].
Isoflavones may lower LDL-C levels [14–16], improve endothelial function, and slow the progression of atherosclerosis [3], although there is still controversy about their impact on hypercholesterolemia [17] and cardiovascular risk [18].
In vitro studies also showed that isoflavones may inhibit the mechanisms involved in adipose tissue growth and regulate adipogenesis [19].
The benefits of soy drink can not only be attributed to isoflavones but also to its modification of diet through a reduction in the saturated fat intake [2,20].
The objective of our study was to assess the effects of a commercially available soy drink (ViveSoy®) on climacteric symptoms in peri- and postmenopausal women.
Materials and methods
Study design and setting
A randomized, open-label, controlled, crossover clinical trial was conducted to assess the effects of including a commercially available soy drink with a high concentration of isoflavones (ViveSoy®) in the diet of peri- and postmenopausal women.
Participants were selected and followed up by their primary care physician at 13 primary care centers. Subject assignment to the intervention type was conducted using a block randomization list with four subjects in each block. They were assigned in a 1:1 ratio to two sequence groups. Every participant was assigned to the intervention type (group 1: control – ViveSoy®; group 2: ViveSoy® – control). Each phase (control or ViveSoy®) lasted for 12 weeks followed by a 6-week washout period.
Women in both groups were instructed by their physicians to follow a balanced diet without soy-based products or soy supplements. In the ViveSoy® phase, women added a daily consumption of 500 mL of soy drink (two 250 mL packs, which provided a minimum of 50 mg of isoflavones and 15 g of protein).
After the washout period, the second phase started. At the beginning and the end of each phase, daidzein and genistein concentrations were evaluated to assess the consumption of soy isoflavones.
Participants
Perimenopausal or postmenopausal women according the STRAW criteria [21] aged 45 or more, with climacteric symptoms (hot flushes and/or sweating) and non-consumers of soy-based foods or soy supplements were selected. The exclusion criteria included hormone replacement therapy at any time within 6 months prior to the study, hysterectomy and/or ovariectomy, among others.
Sample size calculation
It was assumed that regular consumption of soy protein or soy isoflavones may result in an approximately 30% reduction in climacteric symptoms [9]. Due to the lack of previous studies, a standard deviation of σ = 1.2 was assumed, aiming at achieving a power of 90% at a significance level of 5% while allowing for an expected dropout rate of 20%. The estimated sample size to be recruited was 152 patients.
Study variables
The primary variable, climacteric symptoms, was analyzed using the specific MRS quality of life questionnaire [22–24]. The scores for the urogenital domain and the psychological domain were also evaluated as secondary variables as well as MRS total score. The Short Form Health Survey (SF-12) questionnaire was performed for generic quality of life assessment [25].
The effects of regular consumption of ViveSoy® soy drink on anthropometric and clinical parameters, cardiovascular risk (SCORE) [26–28], total cholesterol, LDL and HDL-cholesterol, triglycerides, C-reactive protein, glucose and HbA1c, S-equol [29,30], and bone resorption metabolites were evaluated.
At the end of each period (visits 3 and 6), a structured interview was conducted to assess the dietary habits.
Monitoring of adherence to the recommended diet and to regular consumption of ViveSoy® was performed using a leading question adapted from the Haynes–Sackett test and by comparing the quantity of drink supplied with the number of barcodes returned.
Statistical analysis
All the analyses were conducted on the intention-to-treat (ITT) population, while the main analysis was also conducted in the per-protocol (PP) population.
The Chi-square test or Fisher's exact test was used to evaluate any potential association between categorical variables. Relationships between quantitative and categorical variables were assessed using T-tests, ANOVA, or the non-parametric Wilcoxon and Kruskal–Wallis tests.
Change analysis was performed using a mixed-effects model that accounted for the assigned sequence, period of consumption, variability between individuals and their baseline values.
All statistical analyses were performed using SAS® statistical package version 9.3 (SAS Inc., Cary, NC).
Results
A total of 147 volunteers were recruited between January and June 2012, 57 of whom were excluded from the analysis primarily because they dropped out or failed to meet the selection criteria. A total of 90 women were randomized to a sequence group (45 to group 1: control – ViveSoy® and 45 to group 2: ViveSoy® – control) and constituted the ITT evaluable population. Of these, 60 constituted the PP population.
Baseline demographic, clinical and laboratory characteristics as well as the MRS questionnaire scores were homogeneous in both sequence groups with no significant differences between them (Table 1).
Table 1. Demographic data and baseline clinical and analytical characteristics.
| Control – ViveSoy® | ViveSoy® – control | p values | |
|---|---|---|---|
| Demographic data | |||
| Age (years); mean (SD) | 51.5 (3.5) | 51.8 (3.1) | 0.5665 |
| Weight (kg); mean (SD) | 64.8 (9.1) | 65.4 (10.7) | 0.9822 |
| Anthropometric measurements | |||
| BMI; mean (SD) | 25.55 (3.22) | 26.70 (4.26) | 0.3617 |
| Overweight or obese patients; n (%) | 26 (57.8) | 24 (53.3) | 0.6714 |
| Neck circumference (cm); mean (SD) | 32.8 (1.8) | 33.6 (2.6) | 0.0750 |
| Waist circumference (cm); mean (SD) | 83.0 (9.4) | 87.0 (12.2) | 0.0997 |
| Hip circumference (cm); mean (SD) | 100.5 (8.4) | 101.6 (10.5) | 0.6931 |
| Body fat (%); mean (SD) | 38.4 (4.4) | 40.2 (5.4) | 0.1145 |
| Lifestyle and habits | |||
| Smoking | 0.5749 | ||
| Ex-smoker for less than 5 years; n (%) | 1 (2.2) | 3 (6.7) | |
| Smoker (at least 1 cigarette in the last month); n (%) | 15 (33.3) | 12 (26.7) | |
| Non-smoker/Ex-smoker for at least 5 years; n (%) | 29 (64.4) | 30 (66.7) | |
| Alcohol consumption (AU/week; mean (SD) | 0.3 (0.4) | 0.1 (0.3) | 0.1158 |
| Physical activity | 0.2244 | ||
| Vigorous; n (%) | 2 (4.4) | 1 (2.2) | |
| Light; n (%) | 21 (46.7) | 29 (64.4) | |
| Moderate; n (%) | 22 (48.9) | 15 (33.3) | |
| Energy expenditure (kcal/day); mean (SD) | 2148.77 (253.49) | 2135.48 (253.37) | 0.9039 |
| Clinical data | |||
| SBP (mmHg); mean (SD) | 120.5 (13.3) | 123.6 (14.1) | 0.4164 |
| DBP (mmHg); mean (SD) | 74.7 (9.1) | 77.3 (9.7) | 0.2607 |
| Heart rate (bpm.); mean (SD) | 71.8 (9.2) | 72.3 (8.4) | 0.6373 |
| Analytical data | |||
| Glucose (mg/dL); mean (SD) | 88.8 (8.4) | 89.6 (9.7) | 0.5302 |
| Cholesterol (mg/dL); mean (SD) | 233.5 (33.1) | 241.6 (31.6) | 0.2788 |
| Triglycerides (mg/dL); mean (SD) | 98.3 (53.2) | 104.9 (48.1) | 0.2307 |
| HDL Cholesterol (mg/dL); mean (SD) | 66.4 (15.2) | 64.4 (11.7) | 0.7111 |
| LDL Cholesterol (mg/dL); mean (SD) | 147.5 (29.7) | 156.1 (29.3) | 0.1930 |
| Hemoglobin A1c (%); mean (SD) | 5.51 (0.28) | 5.43 (0.37) | 0.0985 |
| Hemoglobin A1C (mmol/mol); mean (SD) | 36.9 (3.1) | 35.8 (4.1) | 0.0985 |
| C-reactive protein (mg/L); mean (SD) | 2.37 (4.14) | 2.66 (3.41) | 0.9519 |
| Atherogenic index; mean (SD) | 3.67 (0.88) | 3.87 (0.84) | 0.2182 |
| Total cholesterol > 200 mg/dL; n (%) | 37 (82.2) | 38 (84.4) | 1.0000 |
| LDL cholesterol > 100 mg/dL; n (%) | 42 (93.3) | 45 (100.0) | 0.2416 |
| HDL cholesterol < 40 mg/dL; n (%) | 0 | 0 | - |
| Triglycerides > 150 mg/dL; n (%) | 5 (11.1) | 6 (13.3) | 1.0000 |
| Cardiovascular risk | |||
| SCORE score; mean (SD) | 0.52 (0.44) | 0.56 (0.38) | 0.5680 |
| Bone metabolism | |||
| BAP | 11.50 (4.19) | 11.55 (3.73) | 0.6653 |
| MRS questionnaire | |||
| Total score; mean (SD) | 15.7 (6.9) | 15.8 (7.5) | 0.9836 |
| Symptoms subscale; mean (SD) | 7.1 (2.5) | 7.2 (2.7) | 0.7736 |
| Psychological subscale; mean (SD) | 5.5 (3.6) | 5.8 (3.5) | 0.4025 |
| Urogenital subscale; mean (SD) | 3.2 (2.3) | 2.7 (2.8) | 0.1579 |
| SF-12 quality of life questionnaire | |||
| Mental health component (MCS); mean (SD) | 45.56 (9.65) | 43.07 (10.86) | 0.3664 |
| Physical health component (PCS); mean (SD) | 46.81 (10.39) | 45.00 (10.26) | 0.2202 |
MRS, Menopause Rating Scale; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; BAP, Bone alkaline phosphatase.
No significant differences in S-equol levels were found in both groups (Kruskal–Wallis test p = 0.1035) at the end of each phase.
Women in the ITT sample ranged from 45 to 62 years of age, with a mean of 51.6 (SD = 3.3) years and a mean BMI of 26.12 (SD = 3.80).
Results from the follow-up surveys showed that 77.3% of women from group 1 (control-ViveSoy®) followed the recommended diet during the control phase and 73.3% during the ViveSoy® phase (p = 0.8067). In group 2 (ViveSoy®-control), 66.7% correctly followed the recommended diet during the ViveSoy® phase and 65.9% during the control phase (p = 1.0000). When it came to consuming the soy drink on a regular basis during the ViveSoy® phase, the adherence rate was 66.7% regardless of which sequence group participants had been assigned to (p = 1.000).
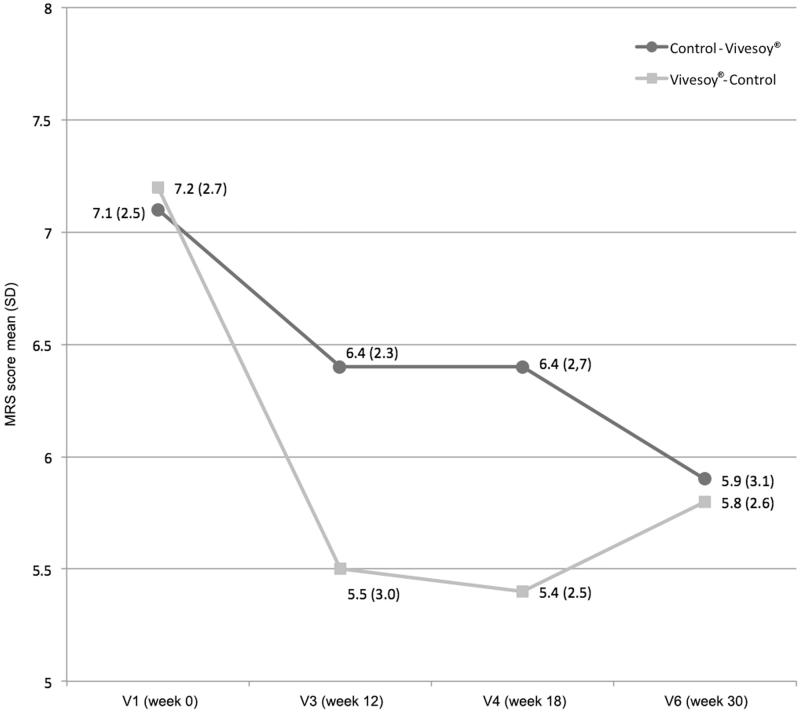
In the ViveSoy® phases, significant decreases were recorded in the climacteric symptom scores, while in both washing periods, scores remained stable (Figure 1).
Figure 1.
Changes in climacteric symptoms (symptoms subscale on the MRS questionnaire, items 1, 2, 3, and 11).
In the ViveSoy® phase, a mean percentage reduction of climacteric symptoms of 4.4% (median 10%) and 25% was observed in group 1 (control – ViveSoy®) and group 2 (ViveSoy® – control), respectively.
Analysis of the mixed-effects model results revealed that the effect soy drink had on climacteric symptoms was statistically significant (p < 0.001), regardless of the temporal order of soy drink consumption in the sequence (p = 0.479). Regular consumption of ViveSoy® soy drink during 12 weeks reduced climacteric symptoms by 20.4% (Figure 1). Similar results were observed in the PP population.
In participants with urogenital symptoms, the regular consumption of soy drink caused a statistically significant (21.3%) reduction in symptoms (p = 0.019).
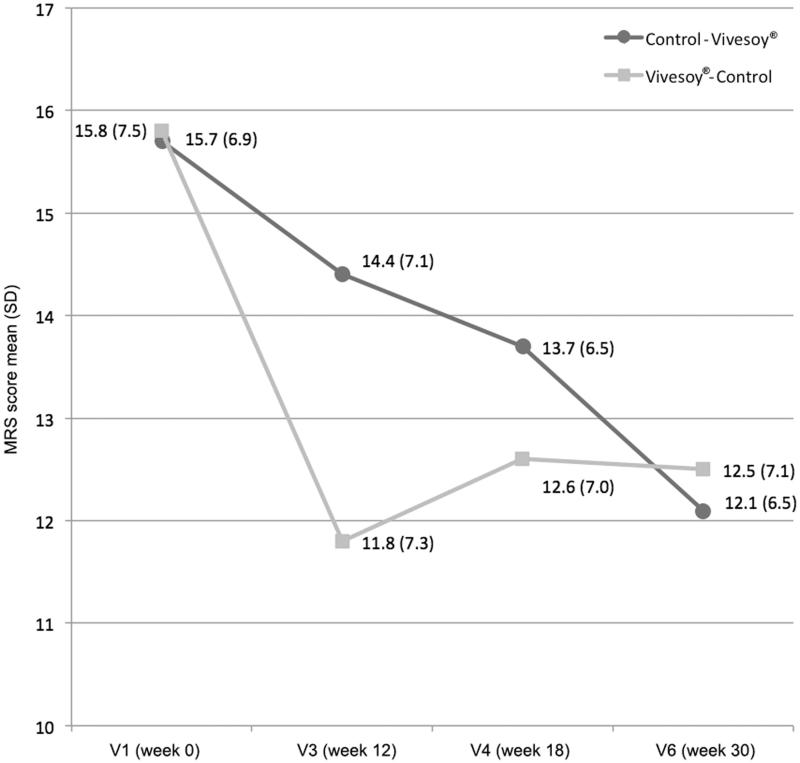
Analysis of the mixed-effects model results indicated that regular consumption of ViveSoy® soy drink improved the health-related quality of life of women, as per the total scores on the MRS questionnaire, regardless of the order of consumption. Thus, total scores on the questionnaire decreased by 18.1% when ViveSoy® was consumed (p = 0.005) (Figure 2).
Figure 2.
Changes in total score on the MRS questionnaire.
No significant changes were observed in the psychological subscale scores (p = 0.205) as well as in the mental (MCS) and physical health (PCS) dimension scores on the SF-12 questionnaire (MCS, p = 0.196; PCS, p = 0.900) when ViveSoy® was consumed.
Regular consumption of ViveSoy® soy drink for 12 weeks did not cause any statistically significant changes in anthropometric measurements, lipid parameters, and the atherogenic index.
Discussion
Although the effects of soy isoflavones on menopause are well known [31], few studies have evaluated the efficacy of commercially available soy-based products.
This study aimed to assess, for the first time, the effect of soy drink (ViveSoy®) on peri- and postmenopausal women using a balanced diet-controlled design with the objective of minimizing potential interindividual variability.
The results showed the homogeneity of the populations enrolled and no significant differences were found in the different variables.
With respect to the primary variable, the soy drink under study significantly reduced climacteric symptoms in both evaluated sequences. Also consistent with these results, consumption of soy drink improved women's health-related quality of life and reduced scores in the urogenital domain.
These data are consistent with the results of previous studies in which placebo was compared with soy isoflavone capsules [4,14,32–41] and are also in line with the results of placebo-controlled studies that evaluated consumption of soy protein by postmenopausal women at a dose of 40 g per day for 12 weeks [9].
In a previous study with a randomized parallel group design and other sources of soy protein, no statistically significant changes in climacteric symptoms were found [30]. In this regard, the choice of soy drink as the source of soy protein and isoflavones and the crossover design may explain the favorable results of our study on climacteric symptoms, especially with respect to hot flushes.
Hot flushes have a negative impact on quality of life in peri- and postmenopausal women [42] and are a frequent reason for consultation in primary care and in gynecology clinics [43,44]. It is important for health professionals who care for peri- and postmenopausal women to consider a holistic approach in the management of climacteric symptoms that also includes dietary measures. The results of this study support recommending soy drinks to minimize climacteric symptoms during these periods.
Although the protective effect of soy isoflavones against cardiovascular disease has been described [18–20], we did not find significant differences in total cholesterol or cardiovascular risk (SCORE) after consumption of soy drink. Nevertheless, it is possible that, based on the findings of previous studies [14–16], statistically significant differences would be observed if consumption of soy drink was maintained over a longer period of time. Similarly, these differences would perhaps also appear in HbA1C levels, anthropometric measurements (weight and BMI) or bone metabolism markers (BAP) in certain subpopulations of women.
Despite the methodology used in the study being the same as in drug clinical trials, results should be interpreted taking into account the limitations of an open-label study; therefore, the placebo effect cannot be entirely discarded. Because of the limited evaluable sample size, further studies with a larger sample are needed in order to extrapolate our conclusions. Moreover, as the intervention was implemented only in Spanish primary care centers, it will be important to evaluate the effects of soy drink with other ethnically and nutritionally diverse populations.
In summary, the results of this study demonstrate the favorable effect of ViveSoy® soy drink on menopause-related symptoms and health-related quality of life in peri- and postmenopausal women, particularly on symptoms that are most bothersome to women, such as hot flushes. Further studies are needed to confirm our findings, including the effect of soy drink on lipid, glucose, and bone metabolism.
Acknowledgements
The study was performed under the auspices of the Spanish Society of Family and Community Medicine (semFYC).
Declaration of interest
The authors report no conflicts of interest. The study was funded by Calidad Pascual S.A.U. (formerly Grupo Leche Pascual).
References
- Matthews VL, Knutsen SF, Beeson WL, Fraser GE. Soy milk and dairy consumption is independently associated with ultrasound attenuation of the heel bone among postmenopausal women: the Adventist Health Study-2. Nutr Res. 2011;31:766–75. doi: 10.1016/j.nutres.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Carty MF. The origins of western obesity: a role for animal protein? Med Hypotheses. 2000;54:488–94. doi: 10.1054/mehy.1999.0882. [DOI] [PubMed] [Google Scholar]
- Messina M. Soy foods, isoflavones, and the health of postmenopausal women. Am J Clin Nutr. 2014;100:423S–30. doi: 10.3945/ajcn.113.071464. [DOI] [PubMed] [Google Scholar]
- Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057–71. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco C, Cancelo Hidalgo MJ. Isoflavones: effects on bone health. Climacteric. 2011;14:204–11. doi: 10.3109/13697137.2010.529198. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco C, Soveral I. Phytoestrogens and bone health at different reproductive stages. Gynecol Endocrinol. 2013;29:735–43. doi: 10.3109/09513590.2013.801441. [DOI] [PubMed] [Google Scholar]
- Lethaby A, Marjoribanks J, Kronenberg F, et al. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013;12:CD001395. doi: 10.1002/14651858.CD001395.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski A, Adlercreutz H, Shaoul R, et al. Short-term effects of phytoestrogen-rich diet on postmenopausal women. Menopause. 1997;4:89–94. [Google Scholar]
- Albertazzi P, Pansini F, Bonaccorsi G, et al. The effect of dietary soy supplementation on hot flushes. Obstet Gynecol. 1998;91:6–11. doi: 10.1016/s0029-7844(97)00597-8. [DOI] [PubMed] [Google Scholar]
- Cheng G, Wilczek B, Warner M, et al. Isoflavone treatment for acute menopausal symptoms. Menopause. 2007;14:468–73. doi: 10.1097/GME.0b013e31802cc7d0. [DOI] [PubMed] [Google Scholar]
- Hanachi P, Golkho S. Assessment of soy phytoestrogens and exercise on lipid profiles and menopause symptoms in menopausal women. J Biol Sci. 2008;8:789–93. [Google Scholar]
- Radhakrishnan G, Agarwal N, Vaid N. Evaluation of isoflavone rich soy protein supplementation for postmenopausal therapy. Pak J Nutr. 2009;8:1009–17. [Google Scholar]
- Carmigiani LO, Pedro AO, Cost-Paiva LH, Pinto-Neto AM. The effect of dietary soy supplementation compared to estrogen and placebo on menopausal symptoms: a randomized controlled trial. Maturitas. 2010;67:262–9. doi: 10.1016/j.maturitas.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Han KK, Soares JM, Jr, Haidar MA, et al. Benefits of soy isoflavone therapeutic regimen on menopausal symptoms. Obstet Gynecol. 2002;99:389–94. doi: 10.1016/s0029-7844(01)01744-6. [DOI] [PubMed] [Google Scholar]
- Matthan NR, Jalbert SM, Ausman LM, et al. Effect of soy protein from differently processed products on cardiovascular disease risk factors and vascular endothelial function in hypercholesterolemic subjects. Am J Clin Nutr. 2007;85:960–6. doi: 10.1093/ajcn/85.4.960. [DOI] [PubMed] [Google Scholar]
- Gardner CD, Messina M, Kiazand A, et al. Effect of two types of soy milk and dairy milk on plasma lipids in hypercholesterolemic adults: a randomized trial. J Am Coll Nutr. 2007;26:669–77. doi: 10.1080/07315724.2007.10719646. [DOI] [PubMed] [Google Scholar]
- Qin Y, Niu K, Zeng Y, et al. Isoflavones for hypercholesterolaemia in adults. Cochrane Database Syst Rev. 2013;6:CD009518. doi: 10.1002/14651858.CD009518.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZM, Ho SC, Chen YM, et al. Cardiovascular risks in relation to daidzein metabolizing phenotypes among Chinese postmenopausal women. PLoS One. 2014;9:e87861. doi: 10.1371/journal.pone.0087861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørgaard A, Jensen L. The effects of soy isoflavones on obesity. Exp Biol Med (Maywood) 2008;233:1066–80. doi: 10.3181/0712-MR-347. [DOI] [PubMed] [Google Scholar]
- Deibert P, König D, Schmidt-Trucksaess A, et al. Weight loss without losing muscle mass in pre-obese and obese subjects induced by a high-soy–protein diet. Int J Obes Relat Metab Disord. 2004;28:1349–52. doi: 10.1038/sj.ijo.0802765. [DOI] [PubMed] [Google Scholar]
- Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19:387–95. doi: 10.1097/gme.0b013e31824d8f40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser GA, Huber IC, Keller PJ, et al. Evaluation of climacteric symptoms (Menopause Rating Scale). Zentralbl Gynakol. 1994;116:16–23. [PubMed] [Google Scholar]
- Heinemann LA, Potthoff P, Schneider HP. International versions of the Menopause Rating Scale (MRS). Health Qual Life Outcomes. 2003;1:28. doi: 10.1186/1477-7525-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann K, Ruebig A, Potthoff P, et al. The Menopause Rating Scale (MRS) scale: a methodological review. Health Qual Life Outcomes. 2004;2:45. doi: 10.1186/1477-7525-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- De Backer G, Ambrosioni E, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice; Third Joint Task Force of European and other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of eight societies and by invited experts). Eur J Cardiovasc Prev Rehabil. 2003;10:S1–10. doi: 10.1097/01.hjr.0000087913.96265.e2. [DOI] [PubMed] [Google Scholar]
- Graham I, Atar D, Borch-Johnsen K, et al. Fourth Joint Task Force of the European Society of Cardiology and other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur J Cardiovasc Prev Rehabil. 2007;14:S1–113. doi: 10.1097/01.hjr.0000277983.23934.c9. [DOI] [PubMed] [Google Scholar]
- Cassidy A. Dietary phyto-oestrogens: molecular mechanisms, bioavailability and importance to menopausal health. Nutr Res Rev. 2005;18:183–201. doi: 10.1079/NRR2005102. [DOI] [PubMed] [Google Scholar]
- Liu ZM, Ho SC, Woo J, et al. Randomized controlled trial of whole soy and isoflavone daidzein on menopausal symptoms in equol-producing Chinese postmenopausal women. Menopause. 2014;21:653–60. doi: 10.1097/GME.0000000000000102. [DOI] [PubMed] [Google Scholar]
- Juliá MD, Ferrer J, Allué J, et al. Posicionamiento de la Asociación Española para el Estudio de la Menopausia sobre el uso clínico de las isoflavonas en el climaterio. Prog Obstet Ginecol. 2008;51:146–61. [Google Scholar]
- Quella SK, Loprinzi CL, Barton DL, et al. Evaluation of soy phytoestrogens for the treatment of hot flashes in breast cancer survivors: a North Central Cancer Treatment Group Trial. J Clin Oncol. 2000;18:1068–74. doi: 10.1200/JCO.2000.18.5.1068. [DOI] [PubMed] [Google Scholar]
- Crisafulli A, Marini H, Bitto A, et al. Effects of genistein on hot flushes in early postmenopausal women: a randomized, double-blind EPT and placebo-controlled study. Menopause. 2004;11:400–4. doi: 10.1097/01.gme.0000109314.11228.e5. [DOI] [PubMed] [Google Scholar]
- Duffy R, Wiseman H, File SE. Improved cognitive function in postmenopausal women after 12 weeks of consumption of a soya extract containing isoflavones. Pharmacol Biochem Behav. 2003;75:721–9. doi: 10.1016/s0091-3057(03)00116-3. [DOI] [PubMed] [Google Scholar]
- Faure ED, Chantre P, Mares P. Effects of a standardized soy extract on hot flushes: a multicenter, double-blind, randomized, placebo-controlled study. Menopause. 2002;9:329–34. doi: 10.1097/00042192-200209000-00005. [DOI] [PubMed] [Google Scholar]
- Mac Gregor CA, Canney PA, Patterson G, et al. A randomised double-blind controlled trial of oral soy supplements versus placebo for treatment of menopausal symptoms in patients with early breast cancer. Eur J Cancer. 2005;41:708–14. doi: 10.1016/j.ejca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Nikander E, Kilkkinen A, Metsä-Heikkilä M, et al. A randomized placebo-controlled crossover trial with phytoestrogens in treatment of menopause in breast cancer patients. Obstet Gynecol. 2003;101:1213–20. doi: 10.1016/s0029-7844(03)00232-1. [DOI] [PubMed] [Google Scholar]
- Penotti M, Fabio E, Modena AB, et al. Effect of soy-derived isoflavones on hot flushes, endometrial thickness, and the pulsatility index of the uterine and cerebral arteries. Fertil Steril. 2003;79:1112–17. doi: 10.1016/s0015-0282(03)00158-4. [DOI] [PubMed] [Google Scholar]
- Scambia G, Mango D, Signorile PG, et al. Clinical effects of a standardized soy extract in postmenopausal women: a pilot study. Menopause. 2000;7:105–11. doi: 10.1097/00042192-200007020-00006. [DOI] [PubMed] [Google Scholar]
- Upmalis DH, Lobo R, Bradley L, et al. Vasomotor symptom relief by soy isoflavone extract tablets in postmenopausal women: a multicenter, double-blind, randomized, placebo-controlled study [published correction appears in Menopause 2000;7:422]. Menopause. 2000;7:23642. doi: 10.1097/00042192-200007040-00005. [DOI] [PubMed] [Google Scholar]
- Secreto G, Chiechi LM, Amadori A, et al. Soy isoflavones and melatonin for the relief of climacteric symptoms: a multicenter, double-blind, randomized study. Maturitas. 2004;47:11–20. doi: 10.1016/s0378-5122(03)00219-6. [DOI] [PubMed] [Google Scholar]
- Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes. 2005;3:47. doi: 10.1186/1477-7525-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MK. Alternatives to hormone therapy for hot flashes: many choices but science is lacking. Menopause. 2013;20:980–2. doi: 10.1097/GME.0b013e3182982436. [DOI] [PubMed] [Google Scholar]
- Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005;294:183–93. doi: 10.1001/jama.294.2.183. [DOI] [PubMed] [Google Scholar]