Abstract
Context: There is a paucity of data describing the impact of type of beverage (coffee versus energy drink), different rates of consumption and different temperature of beverages on the pharmacokinetic disposition of caffeine. Additionally, there is concern that inordinately high levels of caffeine may result from the rapid consumption of cold energy drinks. Objective: The objective of this study was to compare the pharmacokinetics of caffeine under various drink temperature, rate of consumption and vehicle (coffee versus energy drink) conditions. Materials: Five caffeine (dose = 160 mg) conditions were evaluated in an open-label, group-randomized, crossover fashion. After the administration of each caffeine dose, 10 serial plasma samples were harvested. Caffeine concentration was measured via liquid chromatography–mass spectrometry (LC–MS), and those concentrations were assessed by non-compartmental pharmacokinetic analysis. The calculated mean pharmacokinetic parameters were analyzed statistically by one-way repeated measures analysis of variance (RM ANOVA). If differences were found, each group was compared to the other by all pair-wise multiple comparison. Results: Twenty-four healthy subjects ranging in age from 18 to 30 completed the study. The mean caffeine concentration time profiles were similar with overlapping SDs at all measured time points. The ANOVA revealed significant differences in mean C max and V d ss/F, but no pair-wise comparisons reached statistical significance. No other differences in pharmacokinetic parameters were found. Discussion: The results of this study are consistent with previous caffeine pharmacokinetic studies and suggest that while rate of consumption, temperature of beverage and vehicle (coffee versus energy drink) may be associated with slightly different pharmacokinetic parameters, the overall impact of these variables is small. Conclusion: This study suggests that caffeine absorption and exposure from coffee and energy drink is similar irrespective of beverage temperature or rate of consumption.
Keywords: Caffeine, coffee, energy drinks, pharmacokinetics, rapid administration
Introduction
Caffeine is one of the most ubiquitously distributed and ingested compounds on the planet. While it has been used for centuries and is generally considered safe, some question its safety and call for more strict regulation. Generally, there have been concerns with the consumption of energy drinks and other “non-conventional” forms of caffeine. In reality, most acute toxic problems associated with caffeine arise from the use of high doses of caffeine in the form of tablets or pure caffeine powder in situations of abuse or attempted suicide.[1,2] Specifically, some have claimed that the short ingestion times (seconds to minutes) that can occur with chilled and palatable energy drinks may result in a potentially deleterious situation when compared with the longer ingestion times (minutes to a half an hour) typically encountered with hot caffeine-containing beverages such as coffee or tea.[3–6] While these concerns are not consistent with our current understanding of the pharmacokinetics of caffeine, the above cited conditions have not been specifically evaluated and there is a paucity of well-controlled studies demonstrating and comparing the pharmacokinetic parameters of caffeine in energy drinks to other types of caffeine delivery vehicles (e.g. coffee). This study was developed to evaluate the above cited concerns.
The objective of this trial was to evaluate the impact of drink temperature and rate of ingestion on caffeine pharmacokinetics after consumption of instant coffee versus sugar-free energy drink. The hypothesis was that caffeine exposure will not be significantly different when equal amounts of caffeine are consumed in equal volume beverages under conditions of different drink temperatures and/or consumption times. Specifically, this study characterized and compared the pharmacokinetics of caffeine after equivalent doses (160 mg) were administered as: rapidly consumed (2 min) chilled sugar-free energy drink, rapidly consumed (2 min) chilled coffee, slowly consumed (20 min) chilled coffee, slowly consumed (20 min) hot coffee and slowly consumed (20 min) chilled sugar-free energy drink. The dose of 160 mg was chosen because this amount of caffeine is typically found in a 16 ounce energy-drink, and in a standard cup of brew-house coffee. Additionally, we compared caffeine pharmacokinetics in men versus women and also compared female subjects who were concurrently being treated with low-dose estrogen containing oral contraceptives to those who were not because prior studies have suggested that low-dose estrogen containing oral contraceptives may inhibit the metabolism of caffeine.
Methods
This was an open-label, group-randomized, crossover study. The study was approved by the Washington State University Institutional Review Board (IRB number 13 945). Subjects were recruited via IRB-approved recruitment posters. Twenty-four (12 men, 12 women) non-caffeine naïve, healthy subjects aged 18–30, were recruited, provided their informed consent to participate, and were pre-screened. Two subjects were excluded due to poor venous access. Enrolled subjects had a history of consuming 1–3 caffeine containing beverages per day, an absence of any major or chronic illness, were non-smokers, were not taking any medications known to have an interaction with caffeine (e.g. medications metabolized via the CYP1A2 pathway) except for low-dose oral contraceptives, and had no known sensitivity to caffeine. Subjects who by history met these enrolment criteria underwent a full medical history and physical exam, a complete blood cell count with differential, electrolytes, liver function tests, serum creatinine, a routine urine analysis and human chorionic gonadotropin analysis (female subjects only).
Six subjects were assigned sequentially to one of four groups as they were consented. Subject groups were studied on five occasions, receiving one of the five dosage forms (as per their assigned block) on each study day. Group randomization of dosage form was assigned by a randomization generator. Study occasions for each individual were separated by at least three days. Each dosage form contained 160 mg of caffeine. The dosage forms included hot coffee consumed over 20 min (Arm A), cold coffee consumed over 2 min (Arm B), cold coffee consumed over 20 min (Arm C), sugar-free energy drink consumed over 2 min (Arm D) and sugar-free energy drink consumed over 20 min (Arm E).
Subjects were remunerated for their participation in the study. Once enrolled and screened, subjects were asked to present to the study area on study mornings in a fasting state and were also asked to not ingest caffeine after 9:00 a.m. on the previous day. Vital signs and weight were recorded and venous cannulae were placed in appropriate antecubital veins by trained personnel using accepted techniques. Cannulae were kept patent with normal saline flushes. The test beverage was administered at approximately 8:00 a.m. Whole blood samples (6 mL) were collected in EDTA tubes prior to the beginning of drink administration (t = 0) and then at 5, 10, 20, 40, 60, 90, 120, 240, and 480 min after consumption of the beverage had begun. The cannulae were removed at the completion of each study day. Subjects were not allowed to consume additional caffeine during study days but were provided with meals, snacks and water. They were allowed to eat a snack after the 120 min sample and were provided with lunch. They were provided with and were allowed to drink bottled water ad libitum 2 h following caffeine ingestion. After collection, the blood was immediately centrifuged, the plasma harvested, divided into aliquots and frozen. The samples were stored at −20 °C until analysis.
Caffeine dosage preparation and administration
Sugar-free energy drink: A single lot # of sugar-free energy drink (Red Bull) sufficient to carry out the study was procured prior to the study. Caffeine content was assayed (see below) in triplicate and the mean (356 μg/mL) of those three concentrations was used to calculate the volume of liquid needed to provide 160 mg of caffeine (450 mL). The sugar-free energy drink was refrigerated at 4 °C prior to each study day. On study days, 450 mL of the drink was measured and transferred to a 20 fluid ounce (591 mL) paper cup just prior to administration. The sugar-free energy drink was also assessed for pH (3.61).
Coffee: A single lot # of instant coffee (Folgers classic roast), sufficient to complete the study was procured prior to the study. Caffeine content was assayed (see below) in triplicate and the mean (38.7 mg/g) of those three concentrations was used to calculate the amount of instant coffee powder (4.1 g) needed to provide 160 mg of caffeine. The coffee solutions below were assessed for pH (6.04).
Hot coffee: On each study day (hot coffee condition days) 450 mL of hot water (85 °C) was mixed with 4.1 g of coffee powder in a 20 fluid ounce (591 mL) paper cup just prior to administration.
Cold coffee: The night prior to each study day (cold coffee condition days) 450 mL of warm water was mixed with 4.1 g of coffee powder in a disposable sealable plastic bottle and shaken. After the coffee was dissolved the bottle was refrigerated at 4 °C until the following day. Just prior to administration the solution was transferred to a 20 fluid ounce (591 mL) paper cup.
Administration: Each of the caffeinated beverages was placed in a 20 fluid ounce (591 mL) paper cup that had visible markings at approximate four ounce (118 mL) gradations. Subjects had timers set for either 2 or 20 min. Each subject was observed by a research team member. For 2 min consumption times, they were directed to consume the beverage over the course of 2 min. For 20 min consumption times, they were asked to consume approximately four ounces (118 mL) during each 5 min interval.
Quantification of caffeine in plasma
Plasma caffeine concentrations were measured by a newly developed LC–MS method. Frozen plasma was thawed and an aliquot of 10 μL plasma was spiked with 10 μL of 1 ppm 13C3-caffeine as an internal standard. Then, 180 μL of cold methanol was added. After mixing and centrifugation, the supernatant was transferred into a sample vial for analysis by LC–MS. The LC–MS system consisted of an ultra-pressure LC system (Waters Corporation, Millford, MA) connected to a Xevo G2S QTof (Waters Corporation, Millford, MA) mass spectrometer. An Acquity HSS T3 column (2.1 mm × 100 mm, 1.8 μm) was used for the chromatographic separation of caffeine. The mass spectrometer was operated under MS positive ion mode. The mass traces for quantitative analysis of caffeine and its internal standard 13C3-caffeine were m/z 195.089 and 198.098, respectively. A standard curve was constructed using serial standards ranging from 39 to 10,000 ng/mL of caffeine. TargetLynx software (Waters Corporation, Millford, MA) was used for quantitative analysis.
Pharmacokinetic analysis
Caffeine pharmacokinetic parameters were determined using standard non-compartmental methods; calculations were programmed into a spreadsheet (Microsoft Excel, 2010). Concentration maximum (Cmax) and time when the maximum concentration was reached (Tmax) were determined by identifying the highest measured concentration in each subject’s plasma caffeine concentration–time profile. All other parameters were determined by statistical moment analysis.[7] Mean absorption time (MAT) was calculated as the difference between mean residence time (MRT) and the inverse of the slope of the terminal elimination phase. The slope of the terminal elimination phase was determined by linear regression of the log-transformed concentration data. At minimum, the last three data points were included in the regression; decisions to include or exclude additional points were based on the absence or presence, respectively, of bias in the description of the terminal phase.
Statistical testing was conducted using SigmaPlot 10.0 software (Systat Software, Inc., San Jose, CA). Differences in mean parameter values among treatment groups were determined by one-way RM ANOVA. In cases where overall statistical significance was determined, the data were further analyzed by all pair-wise multiple comparison (Holm-Sidak method). The effects of gender on the mean parameter differences among treatment groups were determined by two-way RM ANOVA; the effects of oral contraceptive usage among female participants on parameter differences among treatment groups were determined similarly.
Results
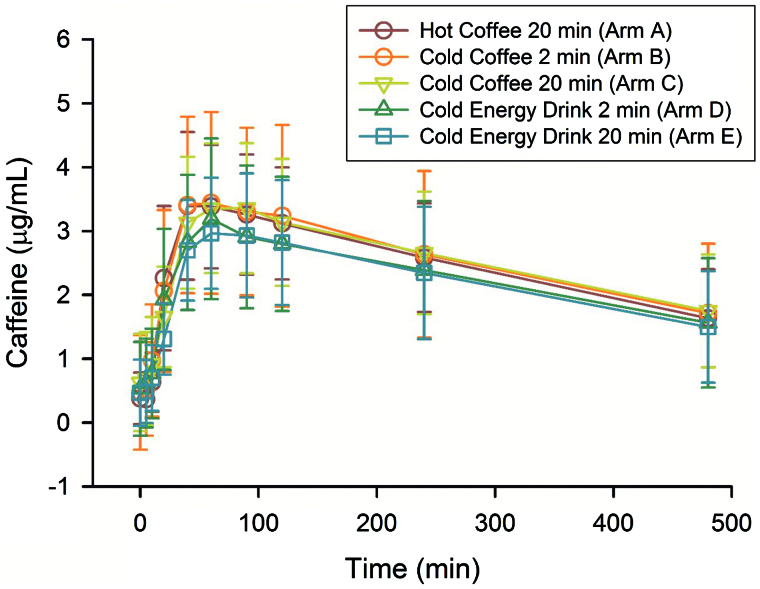
The mean age of the subjects was 25 (±2), mean height 67 in. (±4.6), mean weight 74 kg (±13) and mean BMI was 25.2 (±3.2). The mean caffeine concentration–time profiles of the five arms were similar, with overlapping SDs at all measured time-points (Figure 1). There were no significant differences in caffeine area under the curve from time zero to infinity (AUC0–∞), Tmax, MRT, MAT, half-life (t1/2) or clearance divided by assumed bioavailability (CL/F) between the five arms. The ANOVA revealed significant differences in mean Cmax (one-way RM ANOVA, p = 0.043), but no pair-wise comparisons reached statistical significance. Likewise, differences in mean steady-state volume of distribution divided by assumed bioavailability (Vd ss/F) were statistically significant (one-way RM ANOVA, p = 0.013), but no pair-wise comparison reached statistical significance. The mean values for all of the calculated pharmacokinetic parameters are provided in Table 1.
Figure 1.
Time–caffeine concentration profiles for five conditions.
Table 1.
Pharmacokinetic parameters for caffeine in five studied conditions – means and ranges.
| Mean values ± SD (range) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Condition | Cmax (μg/mL) a | Dose-normalized b Cmax (μg/mL) | Tmax (min) | AUC0–∞ (μg/mL min) | Dose-normalized b AUC0–∞ (μg/mL min) | MRT (min) | MAT (min) | CL/F (mL/min/kg) | Vd,ss/F (mL/kg) a | t1/2 (min) |
| A (hot coffee – 20 min) | 3.74 ± 1.09 (1.96–5.95) | 1.69 ± 0.43 (1.07–2.40) | 59 ± 27 (20–120) | 2312 ± 1338 | 1035 ± 541 | 601 ± 263 | 12 ± 11 | 1.2 ± 0.6 | 623 ± 129 | 408 ± 178 (210–990) |
| B (cold coffee – 2 min) | 3.64 ± 1.36 (1.81–8.17) | 1.57 ± 0.59 (0.981–3.13) | 62 ± 32 (40–120) | 2447 ± 1781 | 1047 ± 725 | 607 ± 267 | 14 ± 10 | 1.3 ± 0.7 | 626 ± 145 | 411 ± 183 (187–966) |
| C (cold coffee – 20 min) | 3.56 ± 1.06 (1.69–5.77) | 1.60 ± 0.44 (0.917–2.79) | 64 ± 20 (40–90) | 2625 ± 2177 | 1172 ± 882 | 673 ± 362 | 16 ± 6 | 1.2 ± 0.6 | 642 ± 151 | 455 ± 249 (217–1386) |
| D (energy drink – 2 min) | 3.36 ± 1.20 (1.40–5.78) | 1.51 ± 0.48 (0.782–2.81) | 70 ± 44 (20–240) | 2473 ± 2302 | 1059 ± 834 | 665 ± 427 | 15 ± 16 | 1.4 ± 0.8 | 712 ± 194 | 450 ± 295 (154–1386) |
| E (energy drink – 20 min) | 3.14 ± 0.95 (1.87–5.25) | 1.41 ± 0.32 (0.940–1.96) | 82 ± 42 (40–240) | 2107 ± 1504 | 925.1 ± 565 | 593 ± 258 | 17 ± 8 | 1.5 ± 0.8 | 708 ± 144 | 399 ± 177 (187–990) |
A: hot coffee 20 min; B: cold coffee 2 min; C: cold coffee 20 min; D: sugar-free energy drink 2 min; E: sugar-free energy drink 20 min.
-RM ANOVA p ≤ 0.05.
Values normalized by mg/kg dose of caffeine.
The differences in calculated pharmacokinetic parameters between men (n = 12) and women (n = 12) were evaluated. Statistically significant differences were found for Cmax (2.8 versus 4.2 μg/mL, p < 0.001), AUC0–∞ (1522 versus 3277 μg/mL min, p < 0.001), MRT (517 versus 741 min, p = 0.009), t1/2 (349 versus 502 min, p = 0.009), CL/F (1.6 versus 1.0 mL/min/kg, p = 0.015), and Vd ss/F (733 versus 591 mL/kg, p < 0.001) for men versus women, respectively. No significant differences in mean MAT (14 versus 16 min) or Tmax (63 versus 72 min) were found for men versus women. Gender comparisons for AUC0–∞ and Cmax were also analyzed after dividing by weight-normalized dose. While the absolute differences in parameter values were less than observed with the non-normalized values, they were still statistically different in both cases; AUC0–∞/weight-normalized dose (765 versus 1352 μg/mL min, P = 0.002), Cmax/weight-normalized dose (1.4 versus 1.7 μg/mL, p = 0.018) for males versus females, respectively.
Caffeine pharmacokinetic parameters from women being treated with low-dose oral contraceptives (n = 6) were compared with women not using oral contraceptives (n = 6). None of the differences between the groups was statistically significant. The mean values for the calculated parameters were Cmax (4.0 versus 4.4 μg/mL), Tmax (77 versus 67 min), AUC0–∞(2745 versus 3828 μg/mL min), MRT (658 versus 827 min), MAT (18 versus 14 min), t1/2 (444 versus 563 min), CL/F (1.3 versus 0.82 mL/min/kg) and Vd ss/F (629 versus 550 mL/kg), respectively, for those not being treated with low-dose oral contraceptives compared with those that were.
Discussion
Caffeine pharmacokinetic parameters in this study did not differ substantially from previously reported parameters under any of the conditions tested. Additionally, the differences in caffeine concentrations with the various conditions tested were in some cases statistically significant but would not be expected to result in clinically significant differences in effect.
Peak plasma concentrations of caffeine after oral administration have been reported to occur at a Tmax of 30–120 min.[8–10] There have been several studies comparing the pharmacokinetics of caffeine following the consumption of tea, coffee, cola, capsules, chocolate or sugar-free cola.[10–12] One trial evaluated a dose of 160 mg of caffeine and reported Tmax values of 0.5, 0.5 and 2.0 h for coffee, tea and cola, respectively.[11] This study did not control for volume of beverage (coffee – 2 cups, tea – 3 cups, cola – 4.5 cups) and only evaluated three subjects. Another study evaluating seven subjects reported Tmax values of 0.5 and 1.5–2 h, respectively, for caffeine capsules and cola.[10] This study did not control for volume for administered solution (capsules – administered with 360 mL, cola – 800 mL). Another trial which evaluated a 400 mg dose of caffeine in 13 subjects reported salivary caffeine Tmax values of 42, 39 and 67 min, respectively, for coffee, sugar-free cola and capsules.[12] This study also did not control for volume (coffee – 12 oz, sugar-free cola – 24 oz, capsules – volume of administered fluid not reported). Caffeine is well-distributed throughout the body, with an average Vd of 0.7 L/kg.[8] The clearance of caffeine is variable, but an average of 0.078 L/kg/h (1.3 mL/min/kg) has been reported.[13] While the average t1/2 of caffeine is generally reported as between 4 and 6 h, and may range from 1.5 to 9.5 h in adults, it may also be increased with pregnancy or reduced by cigarette smoking.[13,14] Studies evaluating the pharmacokinetics of caffeine when administered as energy drinks have not been published. Also, studies evaluating the impact of temperature and rate of ingestion on the pharmacokinetics of caffeine have not previously been published.
Mean Tmax values ranged from 59 to 82 min in this study and are consistent with previously reported values.[8,9] Mean clearance values for the five conditions were similar, ranging from 1.2 to 1.5 mL/min/kg, and are consistent with previously reported values.[13] The mean Vd ss values reported in this study ranged from 0.62 to 0.70 L/kg and were similar to previously reported values.[8] The mean t1/2 values in this trial ranged from 6.7 to 7.6 h and were not inconsistent with previously reported trials.[13] Additionally, Cmax values in this trial were consistent with predictions based on previously reported pharmacokinetic values and the given caffeine dose of 160 mg. Overall, the pharmacokinetics of caffeine in the five arms of this study are consistent with and similar to previously reported pharmacokinetic values.
The comparisons between the conditions revealed statistically significant differences in Cmax and Vd ss. The highest Cmax values were observed in the three coffee arms and the lowest values were found in the energy drink arms. However, no direct comparisons between individual arms were statistically significant. While there were no statistically significant differences in Tmax, this value was higher in the two sugar-free energy drink arms (70 and 82 min) than in the coffee arms (59, 62 and 64 min).
Taken together these findings run contrary to the belief/concern that “rapid” consumption of energy drinks might provide inordinately rapid and/or high concentrations of caffeine relative to more slowly consumed hot beverages such as coffee. In fact, trends in the data suggest that caffeine contained in hot coffee may be more rapidly absorbed and produce higher plasma concentration levels than the caffeine ingested at the same rate via cold coffee. While there were significant differences in Cmax, with the highest Cmax occurring in the hot coffee arm it is unlikely that this variability would result in significant effect differences. While overall there were statistically significant differences in Vd ss between the arms, no statistically significant differences were found when one arm was compared with another. Still, there was a suggestion that the Vd ss was slightly greater in the sugar-free energy drink arms although the Vd ss in the sugar-free energy drink arms was consistent with previously reported Vd values for caffeine. Caffeine exposure was very similar between the five conditions studied with no significant differences in Tmax, MRT, MAT or AUC0–∞. Additionally, the mean concentration time profiles of the five conditions were very similar.
The differences between gender groups in Cmax and AUC0–∞ are explained partially by the differences in body mass between the men and the women. Adding to the difference in exposure between the two groups was the lower CL/F in the women subjects. There is a paucity of comparative evaluations of caffeine pharmacokinetics in males versus females, making comparisons between this study and others difficult. While head-to-head male to female caffeine pharmacokinetic studies are not available, the findings of this study are similar but not identical to those that were previously reported in a caffeine pharmacokinetic comparison of females being treated with low-dose oral contraceptives versus females that were not.[15] Our study, like others, does suggest that low-dose estrogen containing oral contraceptives are associated with a reduction in caffeine clearance.
The results of this study delineate the pharmacokinetics of caffeine in a healthy young adult population in which the vehicle temperature and consumption time are variable and the volume of fluid and concentration of caffeine is constant. Specifically, this study evaluated a particular brand of instant coffee prepared without sugar or cream and a particular brand of sugar-free energy drink.
Although the sampling period for this study was relatively short, because the parameters derived from these data are consistent with published values, it is unlikely that the sampling schedule had a major influence on interpretation of the data. Variables not evaluated in this trial which might be associated with differences in caffeine pharmacokinetics include but are not limited to caffeine containing beverages with sugar, higher concentration/lower volume caffeine containing beverages or supplements (e.g. 5-h energy), other brands of energy drinks that contain different non-caffeine constituents, and other brands of instant coffee or various types of brewed coffee, and genomic differences.
Conclusions
Results from this study suggest that contrary to concerns about potential rapid absorption of caffeine from rapidly consumed cold energy drinks, caffeine absorption and exposure from instant coffee and sugar-free energy drink are similar irrespective of drink temperature or rapid versus slow administration times. While there were statistically significant differences in Cmax values, there were no statistically significant differences in other measures of caffeine exposure including Tmax, AUC0–∞, MRT and MAT. The highest Cmax value was associated with hot coffee consumed slowly and the lowest Cmax value was found in the slowly consumed sugar-free energy drink group. Overall, however, the findings of this study suggest that the exposure to caffeine relative to ingestion of coffee or sugar-free energy drink is very similar.
Disclosure statement
John R. White, Jr.: Consultant – American Beverage Association, Consultant – Red Bull Gmbh, Consultant – Monster Energy. The other authors report no declarations of interest
Funding information
This study was funded by a grant from the American Beverage Association.
References
- Higdon J, Frei B. Coffee and Health: a review of recent human research. Crit Rev Food Sci Nutr. 2006;46:101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- FDA Safety Alerts & Advisories . FDA consumer advice on pure powdered caffeine. 2015. http://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisories/ucm40787.htm [Google Scholar]
- Simon M, Mosher J. Eat drink politics. 2007. http://www.eatdrinkpolitics.com/wp-content/uploads/AEDReportSimon2007.pdf [Google Scholar]
- Ressig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks – a growing problem. Drug Alcohol Depend. 2009;99:1–3. doi: 10.1016/j.drugalcdep.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arria AM, O’Brien MC. The “high” risk of energy drinks. JAMA. 2011;305:600–601. doi: 10.1001/jama.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arria AM, O’Brien MC, Griffiths R, et al. The use of caffeine in energy drinks. Letter to FDA Commissioner Margaret Hamburg. March 19. 2013. http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=10&ved=0CF4QFjAJahUKEwjs89ayktLIAhVM4mMKHUIbBjQ&url=http%3A%2F%2Fiom.nationalacademies.org%2F∼%2Fmedia%2FFiles%2FActivity%2520Files%2FNutrition%2FPotentialEffectsofCaffeine%2FCaffeine-Items%2520Submitted%2520to%2520Committee%25202.pdf&usg=AFQjCNF8Iu0b8nd4lpuNmXH1wSxfor7Upg&bvm=bv.105454873,d.cGc [Google Scholar]
- Yamaoka K, Nakagawa T, Uno T. Statistical moments in pharmacokinetics. J Pharmacokinet Biopharm. 1978;6:547–558. doi: 10.1007/BF01062109. [DOI] [PubMed] [Google Scholar]
- Carrillo JA, Benitez J. Clinically significant pharmacokinetic interactions between dietary caffeine and medications. Clin Pharmacokinet. 2000;39:127–153. doi: 10.2165/00003088-200039020-00004. [DOI] [PubMed] [Google Scholar]
- Mandel HG. Update on caffeine consumption, disposition and action. Food Chem Toxicol. 2002;40:1231–1234. doi: 10.1016/s0278-6915(02)00093-5. [DOI] [PubMed] [Google Scholar]
- Mumford G, Benowitz N, Evans S. Absorption rate of methylxanthines following capsules, cola, and chocolate. Eur J Clin Pharmacol. 1996;51:319–325. doi: 10.1007/s002280050205. [DOI] [PubMed] [Google Scholar]
- Marks V, Kelly J. Absorption of caffeine from tea, coffee, and coca cola. Lancet. 1973;1:827. doi: 10.1016/s0140-6736(73)90625-9. [DOI] [PubMed] [Google Scholar]
- Liguori A, Hughes J, Grass J. Absorption and subjective effects of caffeine from coffee, cola, and capsules. Pharmacol Biochem Behav. 1997;58:721–726. doi: 10.1016/s0091-3057(97)00003-8. [DOI] [PubMed] [Google Scholar]
- Caffeine for the Sustainment of Mental Task, Performance: Formulations for Military Operations . Committee on Military Nutrition Research, Food, and Nutrition Board. National Academy of Sciences. 2015. https://iom.nationalacademies.org/Reports/2001/Caffeine-for-the-Sustainment-of-Mental-Task-Performance-Formulations-for-Military-Operations.aspx [Google Scholar]
- Parsons WD, Neims AH. Effect of smoking on caffeine clearance. Clin Pharmacol Ther. 1978;24:40–45. doi: 10.1002/cpt197824140. [DOI] [PubMed] [Google Scholar]
- Abernathy DR, Todd EL. Impairment of caffeine clearance by chronic use of low-dose oestrogen-containing oral contraceptives. Eur J Clin Pharmacol. 1985;28:425–428. doi: 10.1007/BF00544361. [DOI] [PubMed] [Google Scholar]