Abstract
Objectives: To compare the efficacy and safety of certolizumab pegol (CZP) with and without loading dose (LD) in a post-hoc analysis of two Japanese clinical studies.
Methods: Data from the double-blind trials (DBT) J-RAPID and HIKARI, and their open-label extension (OLE) studies, were used. Patients randomized to CZP 200 mg every 2 weeks (Q2W) groups starting with LD (400 mg Weeks 0/2/4; LD group; J-RAPID: n = 82, HIKARI: n = 116) and patients randomized to placebo groups who subsequently started CZP Q2W without LD in the OLEs (No-LD group; J-RAPID: n = 61, HIKARI: n = 99) were analyzed. Efficacy and pharmacokinetics were assessed during 24 weeks. Adverse events were reported from all studies.
Results: In both trials, the LD groups showed more rapid initial ACR20/50/70 kinetics, and maintained higher ACR50/70 responses until 24 weeks, compared with the No-LD groups. Anti-CZP antibody development was less frequent in the LD groups (J-RAPID: 1.2% versus 4.9%; HIKARI: 17.2% versus 27.3%). Similar safety profiles were reported between LD and No-LD groups (any AEs: 281.8 versus 315.7 [J-RAPID], 282.6 versus 321.3 [HIKARI] [incidence rate/100 patient-years]).
Conclusions: Despite limitations, including comparing DBT and OLE studies, these results suggest that a CZP LD improves clinical response in active rheumatoid arthritis without altering the safety profile.
Keywords: Certolizumab pegol, Loading dose, Randomized controlled trial, Rheumatoid arthritis, Tumor necrosis factor-alpha inhibitor
Introduction
The treatment of rheumatoid arthritis (RA) has seen remarkable changes over the last decade with the advent of anti-tumor necrosis factor (anti-TNF) agents. More patients are now able to achieve clinical remission, including those having incomplete response to methotrexate (MTX), which is the mainstay of RA treatment. In addition, biologic therapies have been shown to be efficacious in the prevention of structural damage progression and functional deterioration, especially when used in combination with MTX.
Certolizumab pegol (CZP) is an Fc-free anti-TNF, which has demonstrated rapid and sustained improvements in disease activity and quality of life in Japanese patients with active RA in placebo-controlled, double-blind (DB), randomized studies, both in combination with MTX (J-RAPID; NCT00791999) and without MTX (HIKARI; NCT00791921) [1,2]. Based on these and previous studies [3,4], including model-based simulations [5], the recommended dose of CZP includes an initial subcutaneous loading dose (LD) of 400 mg at Weeks 0, 2, and 4, followed by a maintenance dose of 200 mg every 2 weeks (Q2W). Nevertheless, the benefit of using the LD versus No-LD has never been demonstrated in a clinical study.
The patients who enrolled in J-RAPID and HIKARI were eligible to subsequently enter the respective open-label extension (OLE) studies (J-RAPID OLE; NCT00851318 and HIKARI OLE; NCT00850343) [6,7] either after completing 24 weeks of the DB trial, or at Week 16 if they were classified as non-responders. The OLE studies were not designed to include the LD, therefore patients who received placebo in the DB trials started CZP treatment without receiving the LD in the OLEs. We hereby report the efficacy and safety of CZP, with and without the initial LD, from two Japanese clinical trials and their respective OLE studies. In addition, pharmacokinetics (PK) data from these trials were analyzed to investigate the impact of the LD on anti-drug antibody (ADAb) formation.
Materials and methods
Study design
The study design and primary results of the placebo-controlled, randomized, DB J-RAPID (NCT00791999), and HIKARI (NCT00791921) trials have been reported previously [1,2]. Briefly, patients in the J-RAPID and HIKARI trials had a diagnosis of adult-onset RA as defined by the ACR (1987) criteria [8]. In J-RAPID, patients had to have at least nine swollen and nine tender joints at baseline while receiving methotrexate (MTX) for at least 6 months. Patients recruited to HIKARI were unable to receive MTX due to prior adverse events and/or safety concerns, and had previously failed treatment with at least one disease-modifying anti-rheumatic drug (DMARD). Patients enrolled in HIKARI had at least six swollen and six tender joints at baseline. J-RAPID consisted of four groups: CZP 100, 200, or 400 mg plus MTX, or saline placebo plus MTX, Q2W. HIKARI consisted of two groups: CZP 200 mg or saline placebo, Q2W. In both trials, patients randomized to CZP (±MTX) groups received loading doses of 200 mg (100 mg group) or 400 mg (200 and 400 mg groups) at Week 0, 2, and 4. The trial period was 24 weeks.
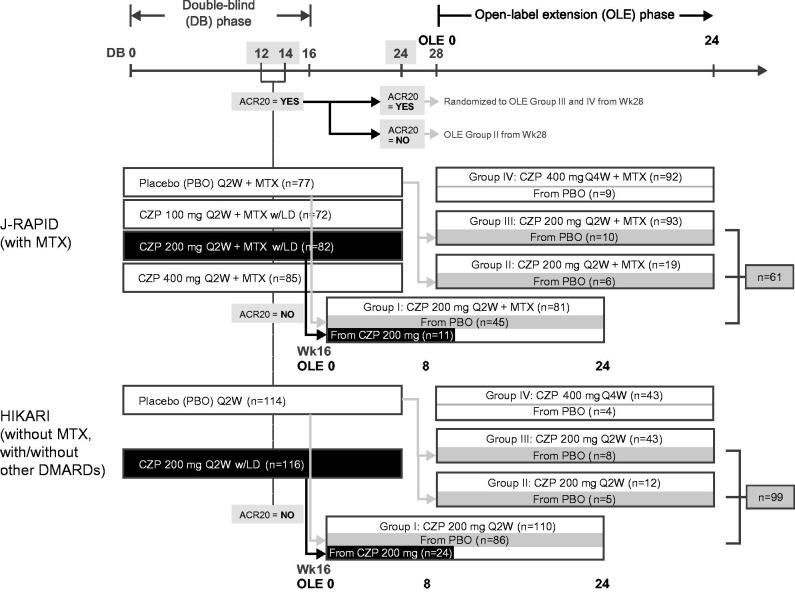
The J-RAPID and HIKARI OLE studies [6,7] were designed to re-group patients by efficacy during the DB trial period regardless of their initial assignment at DB randomization. Specifically, patients who did not achieve an ACR20 response at both Weeks 12 and 14 in the DB phase were withdrawn from the DB trial and were eligible to enter the respective OLE study at Week 16 (Group I; Figure 1). Patients who exhibited an ACR20 response at DB Week 12 and/or 14, but failed to achieve ACR20 at Week 24, were assigned to Group II in the OLE. Patients who had an ACR20 response at DB Weeks 12 and/or 14 and also at Week 24 were re-randomized to Groups III and IV in the OLE. Groups I, II, and III received CZP 200 mg Q2W, while Group IV received CZP 400 mg every 4 weeks (Figure 1).
Figure 1.
Schematic of the analysis design.
Plasma concentrations of CZP and antibodies to CZP (CZP-ADAb) were measured using a standard UCB in-house enzyme-linked immunosorbent assay (ELISA; Covance Immunochemistry Services). In the CZP-ADAb assay, the cut-off (>2.4 U/mL) was defined by twice the level of mean baseline CZP-ADAb before starting CZP among all the patients who entered a phase II PK trial (CDP870-004).
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and with the Pharmaceutical Affairs Law Standards for the Conduct of Clinical Trials on Drugs (Ministry of Health, Labour and Welfare Ordinance no. 28, 27 March 1997) and related notifications. Institutional review board approval was obtained at all centers and written informed consent was provided by all patients.
Post-hoc analyses
LD groups were defined as the DB CZP 200 mg groups in J-RAPID and HIKARI, in which patients received CZP 400 mg at Weeks 0, 2, and 4, and CZP 200 mg Q2W thereafter up to 24 weeks. For the patients who withdrew from the DB trial at Week 16 and entered OLE Group I (Figure 1), the clinical data from the first 8 weeks of the OLE (corresponding to Weeks 16–24 of continuous treatment) were analyzed. Since PK data at OLE Week 8 were not collected, data at OLE Week 10 (which corresponded to Week 26 of continuous treatment) were used as Week 24 data for these patients. No-LD groups were defined as the patients who were assigned to the placebo groups of J-RAPID and HIKARI, and received CZP 200 mg Q2W for the first time without the LD in the two OLE studies (Groups I + II + III; Figure 1).
The baseline disease activity status was defined at the time when CZP treatment was first initiated; i.e. Week 0 of the DB trials was used for the LD groups, while Week 0 of the OLEs was used for the No-LD groups. ACR responses were assessed using non-responder imputation (NRI) and DAS28(ESR) using last observation carried forward (LOCF) for patients who withdrew for any reason. Patients in the No-LD group who had missing data or a zero score in ACR core set measures at OLE Week 0 were excluded from the analysis of ACR responses (four patients in J-RAPID and four in HIKARI). The safety population consisted of patients in LD and No-LD groups. Event rates (ERs) per 100 patient-years (PY) were calculated as the number of cases reported during 24 weeks after starting CZP treatment, including repeat occurrences of the same adverse event (AE) in individual patients, with the denominator being the total duration of exposure. The safety analysis presented here focuses on overall AEs, infections, and injection site reactions.
Results
Patient characteristics and disposition
The patient demographics at DB baseline, and disease activity status at DB baseline and CZP baseline (LD: DB baseline, No-LD: at OLE entry), are summarized in Tables 1 and 2, respectively. The baseline DAS28(ESR) scores for the LD and the No-LD groups were 6.2 ± 0.8 and 5.9 ± 1.3 in J-RAPID, and 6.1 ± 0.9 and 6.2 ± 1.4 in HIKARI (mean ± SD), respectively. Several parameters showed small variations in the No-LD groups following exposure to placebo in the DB periods, but these baseline differences were considered acceptable within the current exploratory analysis.
Table 1. Patient demographics at DB trial baseline.
| J-RAPID |
HIKARI |
|||
|---|---|---|---|---|
| Characteristic | LD group (n = 82) | No-LD group (n = 61) | LD group (n = 116) | No-LD group (n = 99) |
| Mean age, years (SD) | 50.6 (11.4) | 51.7 (11.7) | 56.0 (10.2) | 55.2 (9.7) |
| Female, n (%) | 69 (84.1) | 54 (88.5) | 83 (71.6) | 78 (78.8) |
| Mean body weight, kg (SD) | 56.3 (11.3) | 55.6 (12.6) | 57.5 (11.7) | 56.9 (10.1) |
| Mean disease duration, years (SD) | 5.6 (4.2) | 6.0 (4.0) | 5.4 (4.0) | 5.8 (4.4) |
| Mean no. of prior DMARDs, including MTX (SD) | 1.7 (0.8) | 0.7 (0.9) | 1.9 (1.0) | 1.1 (0.9) |
| Mean MTX dose, mg/week (SD) | 7.6 (0.8) | 7.4 (0.9) | – | – |
| DMARDs at baseline, n (%) | – | – | 62 (53.4) | 55 (55.6) |
| Baseline corticosteroid use, n (%) | 56 (68.3) | 37 (60.7) | 77 (66.4) | 70 (70.7) |
| Prior anti-TNF use, n (%) | 11 (13.4) | 15 (24.6) | 8 (6.9) | 13 (13.1) |
DMARD, disease-modifying antirheumatic drug; LD, loading dose; MTX, methotrexate; SD, standard deviation; TNF, tumor necrosis factor.
Table 2. Disease activity status at RCT baseline and CZP baseline.
| J-RAPID |
HIKARI |
|||||
|---|---|---|---|---|---|---|
| LD group (n = 82) |
No-LD group (n = 61) |
LD group (n = 116) |
No-LD group (n = 99) |
|||
| Characteristics, mean (SD), unless otherwise stated | DB baseline | DB baseline | OLE entry | DB baseline | DB baseline | OLE entry |
| DAS28(ESR) score | 6.2 (0.8) | 6.5 (0.8) | 5.9 (1.3) a | 6.1 (0.9) | 6.3 (1.0) | 6.2 (1.4) |
| No. of tender joints (0–68) | 19.0 (9.0) | 19.4 (9.3) | 17.6 (11.7) | 16.2 (9.6) | 18.0 (10.5) | 17.4 (13.4) |
| No. of swollen joints (0–66) | 16.6 (8.4) | 16.8 (8.3) | 15.2 (11.1) | 13.8 (7.5) | 15.8 (8.7) | 15.0 (10.1) |
| Patient’s assessment of pain (100 mm VAS) | 55.6 (20.6) | 60.6 (22.3) | 50.9 (25.6) a | 56.6 (21.2) | 56.9 (21.2) | 56.3 (24.5) |
| Patient’s assessment of global disease activity (100 mm VAS) | 53.0 (19.6) | 57.4 (21.5) | 50.9 (25.7) a | 54.1 (20.7) | 55.7 (21.7) | 57.8 (23.5) |
| Physician’s assessment of global disease activity (100 mm VAS) | 61.2 (16.2) | 65.1 (15.5) | 54.0 (24.3) b | 58.8 (17.5) | 63.8 (17.6) | 56.7 (25.4) c |
| CRP (mg/dL), geometric mean (CV) | 1.4 (123.0) | 1.6 (141.9) | 1.3 (158.0) | 1.7 (139.8) | 1.7 (147.2) | 1.7 (179.4) |
| ESR (mm/h), geometric mean (CV) | 46.3 (60.9) | 48.8 (47.0) | 40.2 (61.1) | 49.0 (50.3) | 52.8 (52.8) | 50.5 (71.5) |
| HAQ-DI score | 1.13 (0.65) | 1.14 (0.62) | 0.95 (0.62) a | 1.05 (0.68) | 1.21 (0.69) | 1.32 (0.72) |
CRP, C-reactive protein; DAS28, 28-joint Disease Activity Score; ESR, erythrocyte sedimentation rate; HAQ-DI, Health Assessment Questionnaire – Disability Index; LD, loading dose; SD, standard deviation; VAS, visual analogue scale.
n = 60.
n = 59.
n = 98.
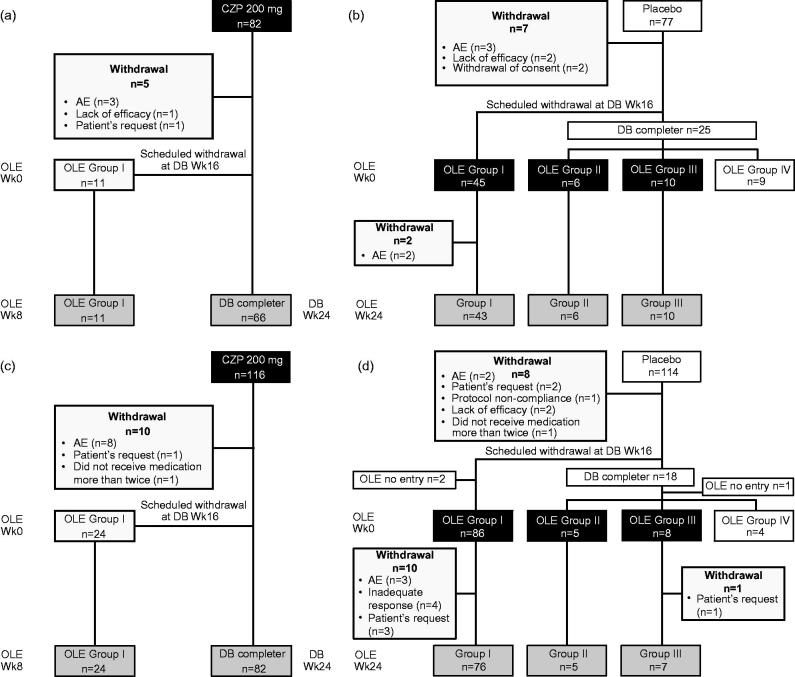
A total of 82 (J-RAPID; Figure 2a) and 116 (HIKARI; Figure 2c) patients were randomly assigned to the LD-group (CZP 200 mg group): 66 and 82 patients completed the DB trials, and 11 and 24 patients entered J-RAPID and HIKARI OLE Group I from Week 16 due to not achieving ACR20 response at Week 12 and 14. Five (J-RAPID) and 10 (HIKARI) patients withdrew from the studies during the first 24 weeks (Figure 2a and c).
Figure 2.
Patient disposition: (a) J-RAPID LD group, (b) J-RAPID No-LD group, (c) HIKARI LD group, and (d) HIKARI No-LD group.
A further 77 and 114 patients were assigned to the placebo group in J-RAPID and HIKARI, of which 61 (J-RAPID; Figure 2b) and 99 (HIKARI; Figure 2d) patients started CZP 200 mg Q2W without LD in the respective OLEs (No-LD groups). The No-LD groups consisted primarily of patients who were assigned to Group I of the OLE studies (73.8% [n = 45/61] in J-RAPID and 86.9% [n = 86/99] in HIKARI) who failed to achieve ACR20 at both Week 12 and 14 while receiving placebo during the DB trials. Two and 11 patients in the J-RAPID and HIKARI No-LD groups, respectively, withdrew during the first 24 weeks after starting CZP.
Delayed manifestation of response and sustained lower efficacy without LD
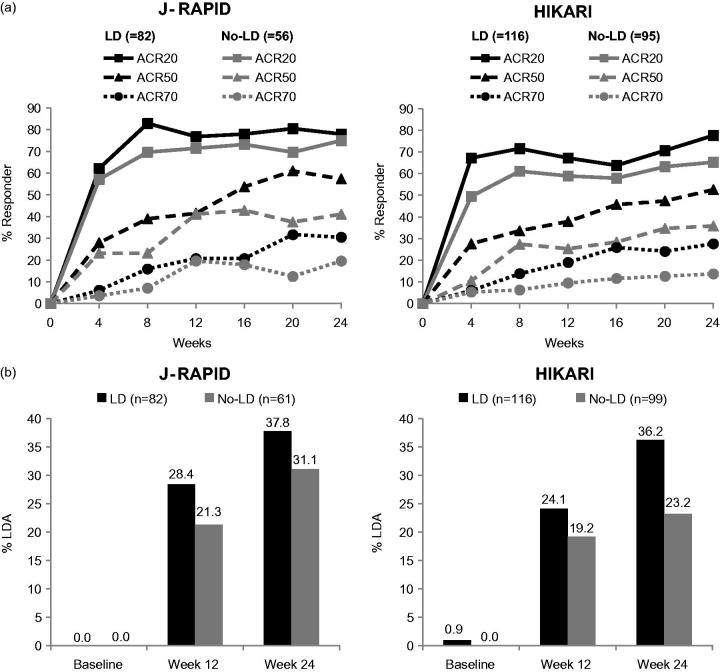
The No-LD groups showed a delay in the initial speed of ACR response, with ACR20 achieved by 57.1% and 49.5% in J-RAPID and HIKARI, respectively, at Week 4 compared to 62.2% and 67.2% in the LD groups (Figure 3a). At Week 8, ACR20 responder rates for the LD and No-LD groups were 82.9% and 69.6% in J-RAPID, and 71.6% and 61.1% in HIKARI, respectively. Area under the curve (AUC) of ACR-N during the initial 8 weeks for the LD versus No-LD groups was 207.0 ± 143.9 versus 158.3 ± 196.2, and 185.0 ± 180.7 versus 107.3 ± 194.9 (mean ± SD) in J-RAPID and HIKARI, respectively. Although these differences in the early kinetics for the ACR20 response seem to converge with time, the No-LD groups showed sustained lower ACR50/70 responses until Week 24, compared with the LD groups, in both studies. The ACR50/70 response rates for the LD versus No-LD groups at Week 24 were 57.3%/30.5% versus 41.1%/19.6% in J-RAPID, and 52.6%/27.6% versus 35.8%/13.7% in HIKARI, respectively. AUC of ACR-N over 24 weeks for the LD versus No-LD groups was 928.9 ± 640.7 versus 744.8 ± 651.8, and 793.8 ± 763.1 versus 549.6 ± 652.3 (mean ± SD) in J-RAPID and HIKARI, respectively.
Figure 3.
ACR response rates and DAS28(ESR) LDA/remission rates: (a) ACR20, ACR50 and ACR70 response rates over time up to Week 24 (NRI) and (b) DAS28(ESR) LDA/remission rates at Week 12 and 24 (LOCF).
Similarly, the proportion of patients with DAS28(ESR) low disease activity (LDA) was higher in the LD groups, compared to the No-LD groups, at Week 12 (J-RAPID: 28.4% versus 21.3%; HIKARI: 24.1% versus 19.2%) and Week 24 (J-RAPID: 37.8% versus 31.1%; HIKARI 36.2% versus 23.2%; Figure 3b). The AUC of change from baseline in DAS28(ESR) over 24 weeks for the LD versus No-LD groups was 48.0 ± 22.1 versus 40.9 ± 22.8, and 43.3 ± 22.1 versus 37.8 ± 24.0 (mean ± SD) in J-RAPID and HIKARI, respectively.
Immunogenicity, plasma CZP concentration and clinical efficacy
In the LD and the No-LD groups, the development of CZP-ADAb at any time during the first 24 weeks of CZP administration was observed in one (1.2%) and three (4.9%) patients in J-RAPID, and 20 (17.2%) and 27 (27.3%) patients in HIKARI, respectively (Table 3).
Table 3. Number of CZP-ADAb positive patients at any time during 24 weeks treatment.
| J-RAPID |
HIKARI |
|||||
|---|---|---|---|---|---|---|
| Total patients |
CZP-ADAb positive patients |
Total patients |
CZP-ADAb positive patients |
|||
| Groups | N | n | % | N | n | % |
| LD | 82 | 1 | 1.2 | 116 | 20 | 17.2 |
| No-LD | 61 | 3 | 4.9 | 99 | 27 | 27.3 |
CZP-ADAb, antibodies to CZP; LD, loading dose.
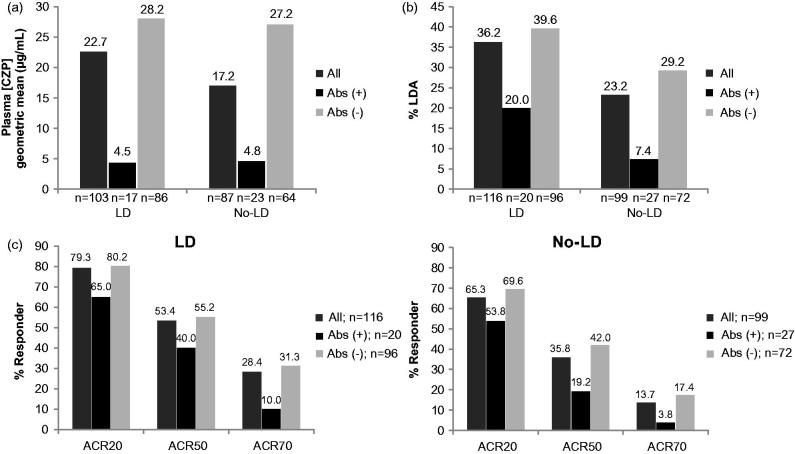
In HIKARI and the HIKARI OLE, CZP-ADAb-positive patients, in both the LD and No-LD groups, were found to have a lower CZP plasma concentration at Week 24 (Figure 4a). Analysis of efficacy in these patients revealed that only 20.0% of the CZP-ADAb-positive patients in the LD group (7.4% in the No-LD group) were in DAS28 (ESR) LDA at Week 24 compared with 39.6% of the CZP-ADAb-negative patients (29.2% in the No-LD group; Figure 4b). Likewise, CZP-ADAb positivity was associated with lower clinical response rates (ACR20/50/70) compared to CZP-ADAb-negative patients in both LD (65.0%/40.0%/10.0% versus 80.2%/55.2%/31.3%) and No-LD (53.8%/19.2%/3.8% versus 69.6%/42.0%/17.4%) groups, respectively (Figure 4c).
Figure 4.
Plasma CZP concentration, DAS28(ESR) LDA rates and ACR response rates in CZP-ADAb positive and negative patients: (a) Plasma CZP concentration at Week 24 (observed data) and (b) DAS28(ESR) LDA rates at Week 24 (LOCF), (c) ACR20, ACR50, and ACR70 response rates in LD and No-LD patients at Week 24 (NRI) in the HIKARI study.
To investigate further the clinical impact of CZP-ADAb positivity, an alternative analysis was undertaken to compare subgroups characterized by plasma CZP concentrations ([CZP]) in HIKARI, of which the CZP-ADAb positivity was assumed to be adequate to see any impact. Individual [CZP] at Week 24 in both LD and No-LD groups were gathered, sorted in ascending order and divided into quartiles of equal size. The number of patients included in the respective subgroup, mean [CZP], and LDA rates within individual groups (LD and No-LD) were then determined. The percentage of patients in LDA in the lowest [CZP] subgroup (subgroup 1) was lower in the LD group compared to the No-LD group (20.4% versus 29.9%). Of note, LDA rates of all the subgroups in the LD group were equal to or higher than in the No-LD group, despite equivalent mean [CZP] levels at Week 24 (Table 4).
Table 4. Subgroup analysis of plasma CZP concentration ([CZP]) at Week 24 (observed data) in HIKARI.
| LDA |
|||||||
|---|---|---|---|---|---|---|---|
| Groups | [CZP] Subgroup no. | [CZP] (μg/mL) | N | %Group | [CZP] geometric mean (μg/mL) | N | %Subgroup |
| LD (n = 103 a ) | 1 | <12.0 | 21 | 20.4 | 4.6 | 7 | 33.3 |
| 2 | ≤12.0, <26.6 | 27 | 26.2 | 19.2 | 9 | 33.3 | |
| 3 | ≤26.6, <38.1 | 32 | 31.1 | 31.8 | 12 | 37.5 | |
| 4 | ≤38.1 | 23 | 22.3 | 51.5 | 12 | 52.2 | |
| No-LD (n = 87 a ) | 1 | <12.0 | 26 | 29.9 | 3.8 | 4 | 15.4 |
| 2 | ≤12.0, <26.6 | 21 | 24.1 | 19.2 | 7 | 33.3 | |
| 3 | ≤26.6, <38.1 | 15 | 17.2 | 31.4 | 2 | 13.3 | |
| 4 | ≤38.1 | 25 | 28.7 | 52.9 | 8 | 32.0 | |
CZP-ADAb, antibodies to CZP; LD, loading dose; LDA, low disease activity.
Patients for whom data are available.
Similar adverse event profiles with or without LD
The rates of AEs were similar between the LD and the No-LD groups during the 24-week analysis period in both J-RAPID and HIKARI (Table 5). Infections and infestations was the most common AEs observed overall. There was a single death in the HIKARI LD (DB CZP 200 mg) group (ruptured aortic aneurysm in a female patient, aged 59 years) which was considered unlikely to have been related to study medication after the 24-week analysis period.
Table 5. Treatment-emergent adverse events (safety population) during 24 weeks after CZP initiation.
| Adverse events |
J-RAPID |
HIKARI |
||
|---|---|---|---|---|
| Event rates per 100 patient-years | LD (n = 82) | No-LD (n = 61) | LD (n = 116) | No-LD (n = 99) |
| Total exposure (patient-years) | 37.251 | 27.751 | 52.504 | 44.950 |
| Any adverse events | 359.72 | 479.26 | 447.59 | 444.94 |
| Infections and infestations | 107.38 | 158.55 | 102.85 | 131.26 |
| Bronchitis | 0 | 10.81 | 3.81 | 11.12 |
| Nasopharyngitis | 32.21 | 50.45 | 43.81 | 24.47 |
| Pharyngitis | 10.74 | 7.21 | 11.43 | 8.90 |
| Upper respiratory tract infection | 5.37 | 25.22 | 7.62 | 4.45 |
| Any serious adverse events a | 13.42 | 28.83 | 20.95 | 24.47 |
| Serious infections and infestations | 10.74 | 14.41 | 7.62 | 4.45 |
| Injection site reaction | 2.68 | 46.85 | 53.33 | 37.82 |
| Malignancies | 0 | 0 | 0 | 0 |
| AE leading to death | 0 | 0 | 0 | 0 |
AE, adverse event; LD, Loading dose.
Serious adverse events were those that resulted in death, were life-threatening, required or prolonged hospitalization, or resulted in significant disability or incapacity or were congenital anomalies/birth defects.
Discussion
The recommendation for treatment of RA with CZP includes an initial LD of 400 mg at Weeks 0, 2, and 4, followed by a maintenance dose of CZP 200 mg Q2W [9]. However, a comparison of CZP efficacy and safety with and without the LD has not previously been conducted in clinical studies. Therefore, post-hoc analyses to address these comparisons were undertaken, using data from the HIKARI and J-RAPID DB and OLE clinical trials, and are presented in this article.
Comparison of clinical response demonstrated that patients who received the LD (LD group) showed better initial kinetics for ACR response, followed by sustained ACR response and lower DAS28(ESR) disease activity up to 24 weeks, compared to patients who did not receive the LD (No-LD groups; Figure 3). These results support a previous report of a Markov mixed-effects model simulation, which suggested the use of the LD accelerates response to CZP [5]. Together, these results demonstrate the clinical impact of higher drug concentrations during early treatment time points.
Percentages of patients who had experience of prior anti-TNF treatment were lower in the LD groups compared with the No-LD groups (J-RAPID: 13.4% versus 24.6%: HIKARI: 6.9% versus 13.1%; Table 2). It should be noted that efficacy of 200 mg CZP in both anti-TNF naïve and experienced patient groups was similar in the J-RAPID and HIKARI DB trials (ACR20 response rates at Week 12: 77.5% [55/71] versus 72.7% [8/11] and 67.6% [73/108] versus 62.5% [5/8] in J-RAPID and HIKARI, respectively), suggesting there may be little, if any, impact of this variance on the better clinical outcomes in the LD group.
The immunogenicity of CZP was also compared between patients who received the LD and those who did not. Development of CZP-ADAb was slightly more frequently observed in the No-LD groups compared with the LD groups. Several reports have suggested that ADAb development is less common in patients who receive higher drug doses. In J-RAPID, development of CZP-ADAb over 24 weeks was more frequently observed in patients receiving CZP 100 mg plus MTX (9 CZP-ADAb-positive patients out of 72 patients [12.5%]) compared to those receiving CZP 200 mg plus MTX (1/82; [1.2%]) or CZP 400 mg plus MTX (1/85; [1.2%]) [2]. A similar observation was reported in a study of adalimumab, a human anti-TNF monoclonal antibody, where ADAb development was less common in the highest dosing group compared to the lower dosing group [10]. Furthermore, Ducourau et al. reported a correlation between the formation of ADAbs (due to low initial drug concentration) and poor long-term clinical outcomes with infliximab, a chimeric human-mouse anti-TNF monoclonal antibody [11]. This is in agreement with our observation that CZP-ADAb-positive patients in the HIKARI and J-RAPID studies had lower plasma CZP concentrations and higher disease activity at Week 24 compared to the CZP-ADAb-negative patients. Taken together, these results suggest that the higher rate of low plasma CZP concentration in CZP-ADAb-positive patients may be associated with low efficacy, which could, at least partially, explain the complexities experienced in controlling disease activity in patients who do not receive the CZP LD as per prescribing information.
However, considering that the number of CZP-ADAb positive patients is low in J-RAPID, the development of CZP-ADAb may not be the sole factor influencing differences in CZP efficacy between the LD and No-LD groups. Several other anti-TNFs also employ a boosted dosing regimen during the initial phase of treatment. For example, the infliximab dosing schedule for RA treatment specifies short intervals between the first three infusions (3 mg/kg at 0, 2, and 6 weeks), followed by a dosing interval of every 8 weeks during maintenance phase [12]. In addition, adalimumab requires an initial loading dose in several diseases including Crohn's disease, ulcerative colitis, and plaque psoriasis [13]. Although direct comparisons of these treatments with and without the initial boosted dosing regimen are not available, prescribed use of such dosing regimens suggests that sufficient inhibition of TNF signaling during initial therapy may affect long-term clinical outcomes. Previous reports have also suggested that early response predicts better long-term outcomes [14]. Achieving rapid response is not only important for patient satisfaction, but also perhaps for maintaining long-term efficacy and the prevention of secondary non-response.
Comparison of AE rates between patients who did and did not receive the LD demonstrated similar safety outcomes between the two groups (Table 5). This supports the original J-RAPID trial results, which demonstrated a similar safety profile between patients receiving different doses of CZP (CZP: 100, 200, and 400 mg, plus MTX) [2]. Repeated inquiries into this issue have continued to provide evidence that, as a class, the anti-TNFs have a large therapeutic window.
There were limitations to this study, including the exploratory nature of the post-hoc analysis and comparison of the data from the DB and OLE phases of the J-RAPID and HIKARI studies. In order to report clinical outcomes at Week 24 in the LD groups, OLE data up to 8 weeks (disease activity) or 10 weeks (PK) were used for patients who entered OLE at Week 16 due to lack of efficacy. Knowledge of the treatment assignment among those patients after entering OLE, in addition to the patients in the No-LD groups who started CZP in OLE, could have biased the results as patients from group III were in response when they started CZP (10 in J-RAPID and 8 in HIKARI). In addition, there was a delay in initiating CZP treatment in the No-LD group due to the DB placebo exposure period (16 weeks [OLE Group 1] or 24 weeks [OLE Group 2 and 3]). Furthermore, MTX was continued in the J-RAPID placebo group, and this could have been responsible for the slight improvement in disease activity in the No-LD group from DB baseline. In contrast, although 56% of patients used DMARDs other than MTX in HIKARI, a slight deterioration in disease activity was seen in the No-LD group. These minor variations in baseline parameters as well were presumed to be acceptable for performing this exploratory analysis.
Overall, while this analysis does have limitations, the results suggest that the use of the CZP LD improves clinical response and reduces development of CZP-ADAb in patients with active RA, without affecting the safety profile of the drug.
Acknowledgments
The authors thank the patients, the investigators and their teams who took part in this study. The authors also acknowledge Costello Medical Consulting, Cambridge, UK, for editorial assistance, which was funded by UCB Pharma. The UCB Publications Team reviewed the manuscript for consistency and accuracy.
Conflicts of interest
T. Takeuchi has received grants from Astellas Pharma, Bristol–Myers K.K., Chugai Pharmaceutical Co, Ltd., Daiichi Sankyo Co. Ltd., Eisai Co. Ltd., Mitsubishi Tanabe Pharma Co., Pfizer Japan Inc., Santen Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Teijin Pharma Ltd., AbbVie GK, Asahi Kasei Pharma Corp., and Taisho Toyama Pharmaceutical Co. Ltd., SymBio Pharmaceuticals Ltd; speaking fees from AbbVie GK., Bristol–Myers K.K., Chugai Pharmaceutical Co. Ltd., Eisai Co. Ltd., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Co., Pfizer Japan Inc., and Takeda Pharmaceutical Co. Ltd., Astellas Pharma, and Daiichi Sankyo Co. Ltd; consultant fees from Astra Zeneca K.K., Eli Lilly Japan K.K., Novartis Pharma K.K., Mitsubishi Tanabe Pharma Co., and Asahi Kasei Medical K.K., AbbVie GK, Daiichi Sankyo Co. Ltd., Bristol–Myers K.K. K. Yamamoto has served as a consultant for UCB Pharma, Pfizer, AbbVie, BMS, Roche, Chugai, Mitsubishi Tanabe and Eisai and has received research funding from UCB Pharma, Pfizer, Astellas, AbbVie, Santen, Mitsubishi-Tanabe, Takeda and Eisai. H. Yamanaka has received honoraria for lectures from AbbVie, Chugai, Daiichi Sankyo, Eisai, Mitsubishi Tanabe, Pfizer, Takeda, Teijin Pharma; has received research grant from AbbVie, Asahi Kasei Pharma, Astellas, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, MSD, Nippon Kayaku, Pfizer, Santen, Taisho Toyama, Takeda, Teijin Pharma, K. N. Ishiguro has received research funding from Takeda, Mitsubishi-Tanabe, Astellas, Chugai, AbbVie, Bristol-Myers Squibb, Eisai, Daiichi Sankyo, Janssen, Kaken, UCB Pharma and Pfizer and has served on speaker bureaus for Daiichi Sankyo, Takeda Pharmaceutical Co. Ltd., Hisamitsu Pharmaceutical Co. Inc., Otsuka Pharmaceutical Co. Ltd., Taisho Toyama Pharmaceutical Co. Ltd., Kaken Pharmaceutical Co. Ltd., Eisai Co. Ltd., Janssen Pharmaceutical K.K., Bristol-Myers Squibb, AbbVie, Chugai Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharmaceutical, UCB Pharma, Astellas Pharma Inc., and Pfizer Japan Inc. Y. Tanaka has received consulting fees, speaking fees, and/or honoraria from AbbVie, Chugai, Astellas, Takeda, Santen, Mitsubishi-Tanabe, Pfizer, Janssen, Eisai, Daiichi-Sankyo, UCB Pharma, GlaxoSmithKline, Bristol-Myers Squibb and has received research grants from Mitsubishi-Tanabe, Chugai, MSD, Astellas, Novartis. K. Eguchi has served as a consultant for UCB Pharma. A. Watanabe has received research support from Daiichi-Sankyo, Kyorin, Shionogi, Taisho, Dainippon-Sumitomo, Taiho, Toyama Chemical and Meiji Seika and has served on speaker bureaus for MSD, GlaxoSmithKline, Fuji Film Pharma, Shionogi, Daiichi-Sankyo, Taisho-Toyama, Mitsubishi-Tanabe, and Pfizer. H. Origasa has served on a member of data monitoring board of Astellas and consulted for UCB Pharma. M. Kobayashi is an employee of UCB Pharma. T. Shoji is an employee of UCB Pharma. O. Togo is an employee of UCB Pharma. N. Miyasaka has received research grants from Abbott Japan Co. Ltd., Astellas Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd., Dainihon-Sumitomo Pharma Co. Ltd., Daiichi-Sankyo, Eisai Co. Ltd., Mitsubishi Tanabe Pharma Co., Novartis Pharma K.K. Ltd., Takeda Pharmaceutical Co. Ltd., Teijin Pharma Ltd and received consulting fees or honoraria from Abbott Japan Co. Ltd., Bristol-Myers Squibb, Janssen Pharmaceutical KK, and Otsuka Pharmaceutical Co. Ltd. T. Koike has served on speaker bureaus for UCB Pharma, Pfizer, Chugai, AbbVie, Mitsubishi-Tanabe, Takeda, Eisai, Santen, Astellas, Taisho-Toyama, BMS, Teijin, and Daiichi-Sankyo.
References
- Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, et al. Efficacy and safety of certolizumab pegol without methotrexate co-administration in Japanese patients with active rheumatoid arthritis: the HIKARI randomized, placebo-controlled trial. Mod Rheumatol. 2014;24(4):552–60. doi: 10.3109/14397595.2013.843764. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: the J-RAPID randomized, placebo-controlled trial. Mod Rheumatol. 2014;24(5):715–24. doi: 10.3109/14397595.2013.864224. [DOI] [PubMed] [Google Scholar]
- Keystone E, Heijde D, Mason D, Jr., Landewe R, Vollenhoven RV, Combe B, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58(11):3319–29. doi: 10.1002/art.23964. [DOI] [PubMed] [Google Scholar]
- Smolen J, Landewe RB, Mease P, Brzezicki J, Mason D, Luijtens K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis. 2009;68(6):797–804. doi: 10.1136/ard.2008.101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix BD, Lovern MR, Stockis A, Sargentini-Maier ML, Karlsson MO, Friberg LE. A pharmacodynamic Markov mixed-effects model for determining the effect of exposure to certolizumab pegol on the ACR20 score in patients with rheumatoid arthritis. Clin Pharmacol Ther. 2009;86(4):387–95. doi: 10.1038/clpt.2009.136. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Eguchi K, et al. Long-term efficacy and safety of certolizumab pegol in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: 52-week results from an open-label extension of the J-RAPID study. Mod Rheumatol. 2014;24(5):734–43. doi: 10.3109/14397595.2014.881709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Eguchi K, et al. Long-term efficacy and safety of certolizumab pegol in Japanese rheumatoid arthritis patients who could not receive methotrexate: 52-week results from an open-label extension of the HIKARI study. Mod Rheumatol. 2014;24(5):725–33. doi: 10.3109/14397595.2013.865822. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Cimzia (certolizumab pegol) prescribing information. 2013. Available from: http://www.cimzia.com/assets/pdf/MedicationGuide.pdf [last accessed 21 Jul 2015].
- Miyasaka N. Clinical investigation in highly disease-affected rheumatoid arthritis patients in Japan with adalimumab applying standard and general evaluation: the CHANGE study. Mod Rheumatol. 2008;18(3):252–62. doi: 10.1007/s10165-008-0045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducourau E, Mulleman D, Paintaud G, Miow Lin DC, Lauferon F, Ternant D, et al. Antibodies toward infliximab are associated with low infliximab concentration at treatment initiation and poor infliximab maintenance in rheumatic diseases. Arthritis Res Ther. 2011;13(3):R105. doi: 10.1186/ar3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remicade (infliximab) prescribing information. 2013. Available from: http://www.remicade.com/shared/product/remicade/prescribing-information.pdf. [last accessed 21 Jul 2015].
- Humira (adalimumab) prescribing information. 2014. Available from: http://www.rxabbvie.com/pdf/humira.pdf. [last accessed 21 Jul 2015].
- Takeuchi T, Yamamoto K, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, et al. Early response to certolizumab pegol predicts long-term outcomes in patients with active rheumatoid arthritis: results from the Japanese studies. Mod Rheumatol. 2015;25:11–20. doi: 10.3109/14397595.2014.904475. [DOI] [PubMed] [Google Scholar]