ABSTRACT
Objective: To explore the treatment response, tolerability and safety of once-monthly paliperidone palmitate (PP1M) in non-acute patients switched from oral antipsychotics, stratified by time since diagnosis as recently diagnosed (≤3 years) or chronic patients (>3 years).
Research design and methods: Post hoc analysis of a prospective, interventional, single-arm, multicentre, open-label, 6-month study performed in 233 recently diagnosed and 360 chronic patients.
Main outcome measures: The proportion achieving treatment response (defined as ≥20% improvement in Positive and Negative Syndrome Scale [PANSS] total score from baseline to endpoint) and maintained efficacy (defined as non-inferiority in the change in PANSS total score at endpoint [Schuirmann’s test]).
Results: 71.4% of recently diagnosed and 59.2% of chronic patients showed a ≥20% decrease in PANSS total score (p = 0.0028 between groups). Changes in PANSS Marder factors, PANSS subscales, and the proportion of patients with a Personal and Social Performance scale (PSP) total score of 71–100 were significantly greater in recently diagnosed compared with chronic patients. PP1M was well tolerated, presenting no unexpected safety findings.
Conclusion: These data show that recently diagnosed patients treated with PP1M had a significantly higher treatment response and improved functioning, as assessed by the PSP total score, than chronic patients.
KEYWORDS: Long-acting injectable antipsychotic, LAI, paliperidone palmitate, schizophrenia, treatment response
1. Introduction
Long-term clinical outcomes in schizophrenia are variable but the majority of patients will experience multiple exacerbations and relapses.[1] A high proportion of patients are likely to relapse if they discontinue antipsychotic (AP) treatment [2] and relapse rates in patients continuing AP medication after stabilization are reported as 27% over 7–12 months.[3] In first-episode patients who were stabilized on a second generation long-acting injectable antipsychotic treatment (LAT) for 2 years, 79% and 94% relapsed within 1 and 2 years, respectively, when allowed to discontinue their treatment.[4] Moreover, evidence shows that frequent relapses are associated with clinical deterioration.[5–7] As such, relapse prevention remains a key therapeutic aim in the treatment of schizophrenia.[8]
Since clinical and psychosocial deterioration associated with schizophrenia often occur within the first few years following the onset of the illness,[9] this early phase is a critical period for treatment. Early and effective intervention can be decisive in improving long-term patient outcomes.[10] Patients with early psychosis can achieve optimal long-term outcomes if attempts are made to overcome barriers such as non-adherence to medication.[11] However, evidence has shown that 42% of patients failed to collect their prescription within 30 days of discharge, following a first hospitalization for schizophrenia.[12]
LATs were designed as an option to address common partial or non-adherence in patients with schizophrenia. Meta-analyses assessing the benefits of LATs are sensitive to research design, and results differ depending on the method used.[13–15] Nevertheless, studies that are more reflective of clinical practice demonstrate that the use of LATs may help to enhance transparency of non-adherence and has been associated with improvements in adherence,[16] relapse prevention,[14,17] and reduced rates of re-hospitalization [12,17] compared with oral formulations of APs. Thus, compared with oral APs, LATs are likely to reduce the burden to the patient and the healthcare system.[18] Despite the observed benefits,[12,14,16,17] many psychiatrists are reluctant to discuss LATs with patients, possibly due to concerns regarding patients’ perceptions of injectable APs. Many clinical guidelines still recommend that treatment with LATs be reserved for patients with recurrent relapses related to partial/non-adherence or for patients who have a preference for the LAT regimen,[1,8] while other more recent guidelines suggest considering LATs at any time, including very early stages of schizophrenia.[19,20]
Altogether, growing evidence suggests that the critical period of the disease encompasses the first few years following an initial psychotic episode.[21–23] Therefore, it will be important to assess the response to treatment, safety and tolerability, and functional outcomes of recently diagnosed versus chronic patients in routine clinical practice.
Paliperidone Palmitate Flexible Dosing in Schizophrenia (PALMFlexS) was a large international, prospective 6-month, open-label trial that explored treatment response, functional outcomes, safety and tolerability in patients switched to once-monthly long-acting paliperidone palmitate (PP1M) following previously unsuccessful treatment with oral or other depot APs. The patient population and the flexible dosing of PP1M in PALMFlexS were designed to resemble routine clinical practice, compared with previous randomized controlled trials (RCTs). The PALMFlexS study comprised samples from three distinct patient populations: patients with non-acute schizophrenia switching to PP1M from oral APs,[24] non-acute patients switching to PP1M from other depot APs,[25] and acute patients switching to PP1M from oral APs.[26] The data presented here are from a subset with particular clinical interest, non-acute patients switched to PP1M from oral APs stratified according to the time since diagnosis as recently diagnosed (≤3 years) or chronic patients (>3 years).
2. Patients and methods
2.1. Study design
The aim of this post hoc analysis from a single-arm, multicentre, open-label, 6-month interventional study in non-acute but symptomatic patients with schizophrenia previously unsuccessfully treated with oral APs (Clinical trials.gov number: NCT01281527) was to explore treatment response, functional outcomes, safety and tolerability of flexible doses of PP1M in recently diagnosed (≤3 years) and chronic (>3 years) adult non-acute but symptomatic patients with schizophrenia. Prior to trial initiation, the protocol was reviewed and approved by an independent ethics committee in all participating countries. The study was performed in accordance with the Declaration of Helsinki, and was consistent with Good Clinical Practice of the International Conference on Harmonisation and applicable regulatory requirements. All patients provided their signed consent to participate in the trial and were free to withdraw at any time. Methods and overall results have previously been reported in detail [24] and are briefly summarized as follows.
2.2. Patients
Patients aged ≥18 years were eligible for study enrolment if they had a diagnosis of schizophrenia (Diagnostic and Statistical Manual of Mental Disorder [DSM]-IV), were non-acute but symptomatic and were switched to PP1M from an unsuccessful treatment with a previous oral AP medication. Participants were required to be stable (i.e. have been on the same oral AP given for the treatment of schizophrenia in an adequate therapeutic dose and with a change in Clinical Global Impression-Severity [CGI-S] score ≤1 in the 4 weeks before enrolment). Their current treatment was considered to have been unsuccessful due to one or more of the following: lack of efficacy (baseline Positive and Negative Syndrome Scale [PANSS] total score ≥70 or ≥2 items scoring ≥4 in the PANSS positive or negative subscale or ≥3 items scoring ≥4 in the PANSS general psychopathology subscale, as judged by the investigator), lack of tolerability or safety (the presence of clinically relevant adverse effects), lack of compliance or patient’s wish. Patients were categorized according to the main reason for switching, either due to lack of efficacy or due to other reasons (lack of tolerability, lack of compliance or patient’s wish). There were no exclusions based on body mass index (BMI). Drug abuse, specifically nicotine, caffeine, and cannabis, is frequent in patients with schizophrenia and therefore represents a naturalistic trait of the schizophrenia population. A consistent, more frequent and regular drug use as seen with intravenous drug use, i.e. heroin and derivatives, is significantly less common in schizophrenia and does not represent a naturalistic trait of schizophrenia populations. Therefore, to select a more naturalistic population within this study, we included patients with current substance use or abuse but excluded patients with intravenous drug use.
2.3. Treatment
PP1M was initiated at 150 mg eq on Day 1 and 100 mg eq on Day 8 (±2 days) intramuscularly, both given in the deltoid muscle. At initiation of PP1M, patients were tapered off their previous oral AP, preferably within a maximum of 4 weeks. Thereafter, PP1M was administered once monthly (±7 days) in either the deltoid or gluteal muscle, using flexible maintenance dosages, preferably within the range of 50 to150 mg eq, based upon the clinical judgement of the treating physician.
2.4. Study assessments
The primary efficacy outcome for stable but symptomatic patients switched due to unsuccessful treatment with the previous oral AP was the percentage of patients achieving treatment response, defined as ≥20% improvement in PANSS total score from baseline (Day 1) to last-observation-carried-forward (LOCF) endpoint (at 6 months or early discontinuation). Maintained efficacy (defined as non-inferiority in the change in PANSS total score at endpoint versus baseline, as measured by means of Schuirmann’s test) was the primary efficacy outcome for patients switched to PP1M for other reasons.
Secondary outcomes encompassed actual values and changes from baseline to endpoint in the following clinician-rated scales: CGI-S; Clinical Global Impression-Change (CGI-C); PANSS subscales and Marder factors; the Personal and Social Performance (PSP) total score;[27] the Mini International Classification of Functionality, Disability, and Health (ICF) Rating for Activity and Participation Disorders in Psychological Illnesses (Mini-ICF-APP) scale;[28,29] sleep and daytime drowsiness. Carer burden was measured according to the Involvement Evaluation Questionnaire (IEQ) [30] and the Subjective Well-being under Neuroleptics Scale (Short form) (SWN-S) [31] and Treatment Satisfaction Questionnaire for Medication (TSQM) [32] were completed by the patients.
Safety assessments included the recording and monitoring of treatment-emergent adverse events (TEAEs) and measures of alcohol and substance use (Clinician Rating Alcohol Use Scale [CRAUS], Clinician Rating Substance Use Scale [CRSUS]).[33] In addition, extrapyramidal motor symptoms (EPMS) were assessed by the Extrapyramidal Symptom Rating Scale (ESRS) total score.[34] Body weight was recorded and BMI calculated. A threshold of ≥7% was used to determine clinically significant weight gain or loss.[35] In this pragmatic study, there were no obligatory protocol-based prolactin measurements, yet investigators were allowed to measure prolactin levels at any time during the study at their own discretion.
2.5. Data analysis
The intent-to-treat (ITT) population comprised all subjects who received PP1M at least once. LOCF endpoint analysis in addition to observed case analysis of treatment response was performed on all ITT subjects with at least one post-baseline assessment on any efficacy parameter. Actual values and changes from baseline were summarized descriptively at each assessment time point and at endpoint, and categorical variables were summarized with frequency and percentage. Within-group differences for both primary and secondary endpoints were tested by means of the Wilcoxon signed-rank test. The McNemar test was used to test within-group changes in binomial variables and between-group comparisons were tested by means of the Wilcoxon two-sample test or Fisher’s exact test. Safety and tolerability were evaluated throughout the study on the safety ITT population, which comprised all ITT patients who had at least one post-baseline observation on any safety parameter. TEAE frequency distributions included severity of events (i.e. mild, moderate, or severe) and causal relationship to treatment (i.e. not related, doubtful, possible, probable, or very likely).
3. Results
3.1. Demographics and patient disposition
The ITT population consisted of 593 patients and included 233 (39.3%) recently diagnosed and 360 (60.7%) chronic patients. Overall, 77.7% and 72.5% of recently diagnosed and chronic patients, respectively, completed the study (Table 1). In both groups, the most common reason for early study discontinuation was withdrawal of consent. The main reason for transition from prior oral AP treatment was patient’s wish (44%) followed by lack of efficacy (24%), lack of compliance (23%), and lack of tolerability (9%) with previous AP medication.
Table 1.
Patient disposition.
| Recently diagnosed patients (≤3 years) | Chronic patients (>3 years) | |
|---|---|---|
| Patients enrolled (n) | 234 | 361 |
| ITT population* (n) | 233 | 360 |
| ↓ | ↓ | |
| Most common reasons for early study discontinuation (≥2% in any group) (%) | ||
| Withdrawal of consent | 6.4 | 12.5 |
| Lost to follow-up | 3.9 | 2.8 |
| Adverse event | 3.4 | 7.8 |
| Lack of efficacy | 3.0 | 2.2 |
*Patients who received at least one dose of study drug.
ITT: Intent-to-treat.
In both groups, the majority of patients were male (63.1%) and had a diagnosis of schizophrenia, paranoid subtype (78.6%). Noticeable baseline differences in recently diagnosed compared with chronic patients were younger age, a higher rate of paranoid subtype schizophrenia, lower baseline body weight and BMI, and a lower rate of vascular disorders (particularly hypertension), metabolism and nutrition disorders. In addition, most (83.3%) recently diagnosed patients had a history of ≤3 psychiatric hospitalizations while more than half (51.7%) of chronic patients had a history of ≥4 previous psychiatric hospitalizations (Table 2).
Table 2.
Patient demographics and dosing information.
| Recently diagnosed (≤3 years) (n = 233) |
Chronic (>3 years) (n = 360) |
|
|---|---|---|
| Mean age, years (SD) | 32.2 (10.3) | 42.4 (11.0) |
| Mean age at diagnosis, years (SD) | 32.0 (10.3) | 29.5 (9.4) |
| Male, (%) | 63.9 | 62.5 |
| Schizophrenia subtype, n (%) | ||
| Paranoid | 195 (83.7) | 271 (75.3) |
| Disorganized | 13 (5.6) | 30 (8.3) |
| Catatonic | 1 (0.4) | 4 (1.1) |
| Undifferentiated | 19 (8.2) | 35 (9.7) |
| Residual | 5 (2.1) | 20 (5.6) |
| Mean baseline weight, kg (SD) | 77.6 (17.1) | 83.3 (17.8) |
| Mean BMI, kg/m2 (SD) | 26.2 (5.7) | 28.6 (5.9) |
| Patients with ≥1 co-morbidity, %* | 60.9 | 61.4 |
| Relevant co-morbidities reported in ≥5% of patients in any group and >4% difference between groups, n (%) | ||
| Metabolism and nutrition disorders | 22 (9.4) | 69 (19.2) |
| Vascular disorders | 11 (4.7) | 41 (11.4) |
| Hypertension | 10 (4.3) | 31 (8.6) |
| Number of patients with previous psychiatric hospitalizations, n (%) | ||
| None | 59 (25.3) | 54 (15.0) |
| 1–3 | 135 (57.9) | 120 (33.3) |
| ≥4 | 39 (16.7) | 186 (51.7) |
| PP1M dosing | ||
| Patients receiving PP1M initiation regimen at Day 1 and Day 8 according to protocol, n (%)† | 219 (94.0) | 338 (93.9) |
| Mean modal PP1M maintenance dose, mg eq (SD)‡ | 98.5 (34.2) | 103.3 (32.9) |
| Last PP1M maintenance dose received, n (%) of patients | ||
| 50 mg eq | 26 (11.2) | 27 (7.5) |
| 75 mg eq | 78 (33.5) | 90 (25.0) |
| 100 mg eq | 71 (30.5) | 140 (38.9) |
| 150 mg eq | 58 (24.9) | 103 (28.6) |
| Relevant co-medications | ||
| Patients using benzodiazepines, n (%) | ||
| At baseline | 43 (18.5) | 95 (26.4) |
| Newly initiated during study | 42 (18.0) | 83 (23.1) |
| At endpoint | 39 (16.7) | 84 (23.3) |
| At 6 months for completers | 25 (13.8) | 60 (23.0) |
| Patients using anticholinergics, n (%) | ||
| At baseline | 28 (12.0) | 39 (10.8) |
| Newly initiated during study | 22 (9.4) | 26 (7.2) |
| At endpoint | 18 (7.7) | 28 (7.8) |
| At 6 months for completers | 13 (7.2) | 18 (6.9) |
*Individual patients can be labelled for >1 co-morbidity.
†The recommended initiation regimen was PP1M 150 mg eq on Day 1 and 100 mg eq on Day 8 given in the deltoid muscle.
‡Excluding the initiation regimen (Day 1/Day 8).
BMI: body mass index; PP1M: once-monthly paliperidone palmitate; SD: standard deviation.
Nearly two-thirds (61.2%) of recently diagnosed and chronic patients had at least one co-morbidity at baseline. The body systems for which ≥10% of patients overall reported co-morbidities were psychiatric disorders (22.1%, of which the most frequent were: insomnia, 8.1%; depression, 7.8%; anxiety, 3.9%), metabolism and nutrition disorders (15.3%), and nervous system disorders (14.0%). Higher incidences (≥2% difference) of hepatobiliary disorders (3.4% vs. 0.8%), laboratory tests (4.7% vs. 2.2%), psychiatric disorders (24.5% vs. 20.6%), and skin and subcutaneous tissue disorders (3.9% vs. 1.7%) were reported in patients who were recently diagnosed compared with chronic patients, respectively. Other disorders of body systems that were more frequently reported (≥2% difference) in chronic compared with recently diagnosed patients included vascular disorders (11.4% vs. 4.7%), infections and infestations (6.9% vs. 4.7%), gastrointestinal disorders (9.7% vs. 6.9%), and metabolism and nutrition disorders (19.2% vs. 9.4%).
The mean modal PP1M maintenance doses (standard deviation [SD]) (excluding the Day 1/Day 8 initiation regimen) were numerically lower, but overall comparable for recently diagnosed (98.5 [34.2] mg eq; 95% confidence interval [CI] 94.1, 102.9) and chronic patients (103.3 [32.9] mg eq; 95% CI 99.7, 106.9) (Table 2). The majority of patients received PP1M according to the recommended initiation regimen on Day 1 (150 mg eq; 98.3% recently diagnosed, 99.2% chronic) and Day 8 (100 mg eq; 97.8% recently diagnosed, 96.9% chronic). Of those who did not receive the recommended initiation regimen, most either missed their Day 8 dose (n = 15), received a higher Day 8 dose (n = 8) after receiving the correct Day 1 dose, or received a lower dose on Day 1 before receiving the correct dose on Day 8 (n = 6). Around half (51.5%) of recently diagnosed and 46.4% of chronic patients required only one maintenance dose adjustment after the third dose.
3.2. Efficacy outcomes
In total, 71.4% of all recently diagnosed patients and 59.2% of all chronic patients showed a treatment response, as measured by a decrease of ≥20% in the PANSS total score at endpoint (p = 0.0028 between groups, Fisher’s exact test). Furthermore, over one-third (39.4%) of recently diagnosed patients and nearly one-quarter (24.6%) of chronic patients achieved ≥50% improvement in PANSS total score (p < 0.001 between groups, Fisher’s exact test). Mean PANSS total score (SD) decreased from 72.6 (14.8) and 70.7 (14.4) at baseline to 57.5 (16.9) and 61.2 (18.7) at endpoint in recently diagnosed and chronic patients, respectively, with a statistically significant and clinically relevant improvement (SD; 95% CI of mean change) observed in both recently diagnosed (−15.1 [15.6; −17.1, −13.1]; p < 0.0001) and chronic patients (−9.6 [15.7; −11.2; −8.0]; p < 0.0001) (change from baseline to endpoint; between-group test, p < 0.0001) (Figure 1).
Figure 1.
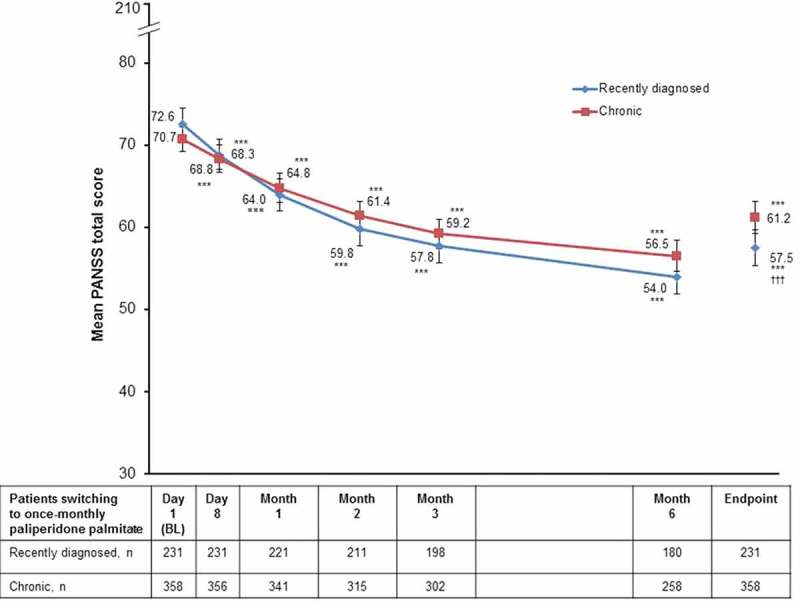
Mean PANSS total score over time (efficacy ITT population).
***p < 0.0001 vs. baseline.††† p < 0.0001 for mean change from baseline in PANSS total score for recently diagnosed vs. chronic patients.Error bars represent 95% confidence intervals.BL: baseline; ITT: intent-to-treat; PANSS: Positive and Negative Syndrome Scale.
The changes from baseline to endpoint for recently diagnosed and chronic patients were statistically significant for mean PANSS positive subscale, negative subscale, and general psychopathology subscale score (Table 3) as well as Marder factor scores (p < 0.0001, Wilcoxon signed-rank test). At baseline, 69.9% of recently diagnosed and 67.1% of chronic patients were rated as moderately, markedly, or severely ill based on CGI-S score. At endpoint, the proportion of patients in these categories decreased from baseline by 36.7% and 28.1%, respectively (p < 0.0001 for both groups, McNemar’s test). At Month 6 and endpoint, disease severity was rated as much improved or very much improved in 59.4% and 52.4% of recently diagnosed patients compared with 48.6% and 38.0% in the chronic group, respectively (CGI-C; p = 0.0320 and p = 0.0008 between groups, Fisher’s exact test) (Figure 2).
Table 3.
Efficacy outcome (efficacy ITT population)*.
| Recently diagnosed (≤3 years) (n = 231) |
Chronic (>3 years) (n = 358) |
|
|---|---|---|
| Mean PANSS positive subscale score, (SD) | ||
| Baseline | 15.6 (4.8) | 15.4 (5.0) |
| Endpoint | 12.1 (4.7) | 13.1 (5.4) |
| Mean change from baseline to endpoint | −3.6 (4.9) | −2.3 (4.9) |
| 95% CI of mean change; p value† | −4.2, −2.9; <0.0001 | –2.8, −1.8; <0.0001 |
| Change from baseline to endpoint p value between subgroup test‡ | 0.0010 | |
| Mean PANSS negative subscale score, (SD) | ||
| Baseline | 20.4 (5.3) | 20.1 (5.5) |
| Endpoint | 16.0 (5.5) | 17.2 (6.1) |
| Mean change from baseline to endpoint | −4.4 (5.1) | −2.9 (5.5) |
| 95% CI of mean change; p value† | −5.1, −3.8; <0.0001 | –3.4, −2.3; <0.0001 |
| Change from baseline to endpoint p value between subgroup test‡ | 0.0003 | |
| Mean PANSS general psychopathology score, (SD) | ||
| Baseline | 36.6 (8.4) | 35.2 (7.7) |
| Endpoint | 29.5 (8.6) | 30.8 (9.6) |
| Change from baseline to endpoint | −7.1 (8.4) | −4.4 (8.2) |
| 95% CI of mean change; p value† | −8.2, −6.0; <0.0001 | −5.3, −3.6; <0.0001 |
| Change from baseline to endpoint p value between subgroup test‡ | 0.0001 | |
*Only patients with a valid baseline measurement and at least one valid follow-up assessment were included.
†Within-group difference was tested using the Wilcoxon signed-rank test.
‡Between-group difference was tested using the Wilcoxon two-sample test.
CI: confidence interval; ITT: intent-to-treat; PANSS: Positive and Negative Syndrome Scale; SD: standard deviation.
Figure 2.
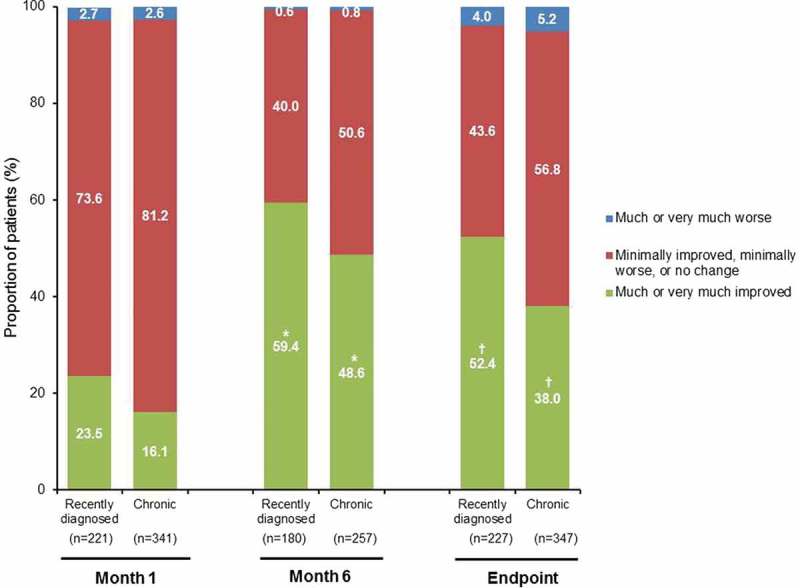
CGI–C according to proportion of patients by category over time (efficacy ITT population).
*p = 0.0320; † p = 0.0008 between-group comparison, Fisher’s exact test.CGI–C: Clinical Global Impression–Change; ITT: intent-to-treat.
3.3. Subjective well-being, treatment satisfaction and caregiver involvement
Measures of subjective patient well-being (SWN-S; p < 0.0001 for both groups), treatment satisfaction (TSQM global satisfaction; p < 0.0001 for both groups), sleep quality (p < 0.0001 for recently diagnosed and p = 0.0346 for chronic patients), and daytime drowsiness (p < 0.0001 for recently diagnosed and p = 0.0008 for chronic patients) also showed statistically significant improvements from baseline to endpoint in both recently diagnosed and chronic patients (Table 4). There were no significant between-group differences for mean change in SWN-S total score and no between-group differences in TSQM total or individual domain scores except for ‘convenience’ for which the mean change from baseline to endpoint was significantly greater in recently diagnosed compared with chronic patients (p < 0.05). Statistically significant increases from baseline to endpoint in all four TSQM domain scores (effectiveness, side effects, convenience, and global satisfaction) were observed in both groups, indicating improved satisfaction with treatment compared with baseline medication. Sleep quality and daytime drowsiness were significantly better in recently diagnosed compared with chronic patients (p < 0.05 for both).
Table 4.
Selected secondary endpoints*.
| Recently diagnosed (≤3 years) | Chronic (>3 years) | |
|---|---|---|
| Mean SWN-S total score, n | 211 | 310 |
| Baseline, (SD) | 80.7 (16.7) | 79.7 (17.6) |
| Endpoint, (SD) | 86.6 (17.7) | 84.7 (17.1) |
| Mean change from baseline to endpoint, (SD) | 5.9 (16.6) | 5.0 (15.0) |
| 95% CI; p value† | 3.6, 8.2; <0.0001 | 3.4, 6.7; <0.0001 |
| Change from baseline to endpoint p value between subgroup test‡ | NS | |
| Mean TSQM total global satisfaction score, n | 195 | 299 |
| Baseline, (SD) | 56.6 (20.3) | 55.4 (22.4) |
| Endpoint, (SD) | 68.3 (23.4) | 62.9 (26.0) |
| Mean change from baseline to endpoint (SD) | 11.7 (27.9) | 7.5 (29.7) |
| 95% CI; p value† | 7.7, 15.6; <0.0001 | 4.1, 10.9; <0.0001 |
| Change from baseline to endpoint p value between subgroup test‡ | NS | |
| §Mean quality of sleep score, n | 229 | 353 |
| Baseline, (SD) | 6.9 (2.4) | 6.7 (2.6) |
| Endpoint, (SD) | 7.7 (2.2) | 7.0 (2.4) |
| Mean change from baseline to endpoint, (SD) | 0.8 (2.6) | 0.3 (2.8) |
| 95% CI; p value† | 0.5, 1.1; <0.0001 | 0.0, 0.6; 0.0346 |
| Change from baseline to endpoint p value between subgroup test‡ | 0.0397 | |
| ¶Mean drowsiness score, n | 229 | 353 |
| Baseline, (SD) | 4.0 (2.8) | 3.9 (2.9) |
| Endpoint, (SD) | 2.8 (2.7) | 3.3 (2.8) |
| Mean change from baseline to endpoint, (SD) | −1.3 (3.4) | −0.6 (3.4) |
| 95% CI; p value† | −1.7, −0.8; <0.0001 | −1.0, −0.2; 0.0008 |
| Change from baseline to endpoint p value between subgroup test‡ | 0.0118 | |
*Only patients with a valid baseline measurement and at least one valid follow-up assessment were included.
†Within-group difference was tested using the Wilcoxon signed-rank test.
‡Between-group difference was tested using the Wilcoxon two-sample test.
§A higher score indicates improvements in the quality of sleep.
¶A lower score indicates improvements in the level of drowsiness.
CI: confidence interval; NS: not significant; SD: standard deviation; SWN-S: Subjective Well-being under Neuroleptics (Short form); TSQM: Treatment Satisfaction Questionnaire for Medication.
There was a statistically significant (p = 0.0113) reduction in the IEQ total score from baseline to endpoint in the total population of non-acute patients group as a whole, indicating a reduction in carer burden during the study. Reductions in the IEQ total score were also observed in recently diagnosed and chronic patients but these changes did not reach statistical significance; this may be partly due to lack of statistical power because of the small number of carers assessed.
3.4. Functioning outcomes
From baseline to endpoint, the mean PSP total score increased from 59.2 to 67.7 in recently diagnosed patients and from 57.4 to 65.0 in chronic patients (p < 0.0001 for both groups, Wilcoxon signed-rank test). There was no significant between-group change from baseline in PSP total score (p = 0.27, Wilcoxon two-sample test). The proportion of patients with mild or no functional impairment (PSP total score of 71–100) increased from 15.1% at baseline to 47.1% at endpoint (recently diagnosed) and from 15.5% at baseline to 36.7% at endpoint (chronic) (p < 0.0001 for both groups, McNemar’s test) and differed significantly between groups (p = 0.0058, Fisher’s exact test) (Figure 3).
Figure 3.
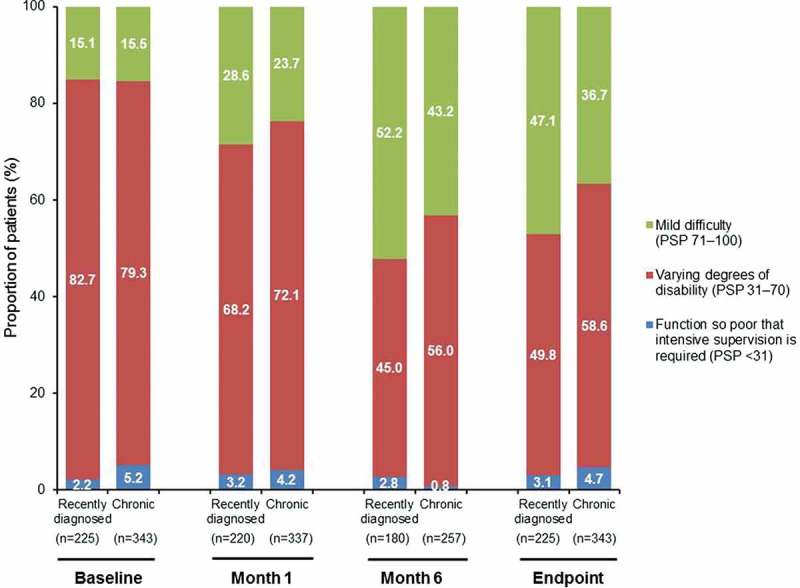
Functioning outcomes in non-acute schizophrenia patients. Changes in frequency distribution by PSP category over time in recently diagnosed and chronic patients.
PSP: Personal and Social Performance
Illness-related disabilities of activity and participation in Mini-ICF-APP were significantly lower in recently diagnosed compared with chronic patients at baseline (p < 0.01 between-groups comparison, Wilcoxon two-sample test) and mean (SD) Mini-ICF-APP total scores improved significantly from baseline, including all domains of activity and participation, to endpoint in both recently diagnosed (18.7 [7.6]–14.6 [8.8] [p < 0.0001]) and chronic patients (20.6 [7.9]−16.7 [8.8] [p < 0.0001]) (Figure 4A and 4B). The mean change from baseline was not statistically significantly different between groups.
Figure 4.
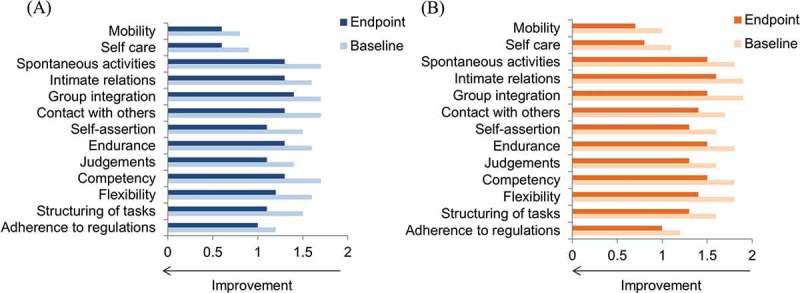
Functioning outcomes in non-acute schizophrenia patients: Mini-ICF-APP domain scores* at baseline and endpoint for recently diagnosed (A, n = 218) and chronic (B, n = 325) patients.
*Change from baseline to Month 6, p < 0.0001 for all domains in both groups.Mini-ICF-APP: Mini International Classification of Functionality, Disability and Health (ICF) Rating for Activity and Participation Disorders in Psychological Illnesses.
3.5. Tolerability and safety
In the safety ITT population, 61.4% of recently diagnosed and 58.6% of chronic patients had at least one TEAE; 12.0% and 17.2% had at least one serious TEAE, respectively. Most TEAEs were rated as mild or moderate in intensity (93.1% in both patient groups). Thirteen (5.6%) recently diagnosed patients and 29 (8.1%) chronic patients had at least one TEAE that led to early termination of the study. TEAEs reported in ≥5% of subjects in at least one group are summarized in Table 5.
Table 5.
TEAEs occurring in ≥5% of patients in any group (safety ITT population).
| Recently diagnosed (≤3 years) (n = 233) |
Chronic (>3 years) (n = 360) |
|
|---|---|---|
| Number of patients (%) | ||
| Injection-site pain | 30 (12.9) | 43 (11.9) |
| Insomnia | 23 (9.9) | 28 (7.8) |
| Anxiety | 15 (6.4) | 25 (6.9) |
| Headache | 14 (6.0) | 19 (5.3) |
| Akathisia | 13 (5.6) | 15 (4.2) |
| Somnolence | 12 (5.2) | 14 (3.9) |
| Weight increased | 12 (5.2) | 7 (1.9) |
| Depression | 12 (5.2) | 6 (1.7) |
| Psychotic disorder | 11 (4.7) | 25 (6.9) |
ITT: intent-to-treat; TEAEs: treatment-emergent adverse events.
Although there were no obligatory protocol-based laboratory tests during this pragmatic study; at least one potentially prolactin-related TEAE was reported by 3.0% (n = 7) of recently diagnosed patients and 3.1% (n = 11) of chronic patients, and increased prolactin levels were reported in one patient in each group. The mean (SD) ESRS total score at baseline was 2.0 (3.4) for recently diagnosed and 3.4 (5.76) for chronic patients. The mean change in ESRS (SD) from baseline to endpoint was −0.9 (3.4) for recently diagnosed and −1.4 (4.5) for chronic patients (p < 0.0001 for both groups). There was a reduction in the proportion of patients receiving benzodiazepines and anticholinergics from baseline to endpoint and end of study in both recently diagnosed and chronic patients (Table 2). The mean weight change (SD) from baseline to endpoint was 1.4 (5.0) kg (95% CI 0.8, 2.1) for recently diagnosed and 1.0 (5.0) kg (95% CI 0.4, 1.6) for chronic patients. Overall, 18% of recently diagnosed patients and 13.7% of chronic patients had a weight gain of ≥7% at endpoint. At baseline, 89.5% (n = 187) of recently diagnosed and 92.0% (n = 287) of chronic patients were assessed as abstinent using the CRSUS; while at endpoint 91.4% (n = 191) of recently diagnosed and 94.2% (n = 294) of chronic patients were assessed as abstinent.
4. Discussion
In this post hoc analysis of recently diagnosed and chronic non-acute but symptomatic patients with schizophrenia, both groups showed clinically relevant improvement of their symptoms; yet a significantly greater improvement was observed in the recently diagnosed group. The change in PANSS total score, PANSS Marder factors and PANSS subscales were all statistically significantly greater in recently diagnosed patients compared with chronic patients, consistent with the established view that newly and more recently diagnosed patients tend to be more responsive to APs compared with multiple-episode patients.[36,37] These results are of particular interest since, according to current guidelines, LATs are largely reserved for patients with recurrent relapses related to nonadherence.[1,8] However, it should be noted that guidelines are based primarily on RCTs, and patients included in RCTs are not fully representative of the whole spectrum of patients encountered in clinical practice. Measurement of the comparative effectiveness of APs has been shown to be sensitive to study design [13] and therefore more pragmatic trials are recommended for the evaluation of treatment efficacy in a naturalistic clinical practice setting.[38] As such, treatment guidelines do not always offer the level of detail that might be expected and/or required, particularly around issues of illness management in newly and more recently diagnosed patients,[39,40] highlighting a need for more open-label or double-blind RCTs in early phases of schizophrenia.[40] A pertinent example is the current study, in which a more diverse population of patients with schizophrenia (with higher rates of co-morbidities, substance abuse, and/or comedications) was included in the study population to provide data in a more representative setting.
The results from this analysis are in line with a prospective, single-site, open-label study in patients with recent-onset psychosis who were treated with risperidone LAT (RLAT) over 2 years.[4,41] A clinical response of at least 20% reduction in PANSS total scores was obtained by 92% (46/50) of patients, and a reduction of at least 50% was obtained by 84% (42/50) of patients,[41] while 84.8% of patients achieved remission.[4] Thus, the results from the current study provide further evidence that a substantial proportion of patients at an early stage of schizophrenia who receive LAT are able to achieve considerable and sustained symptomatic and functional improvement. A post hoc analysis of a 13-week, randomized, double-blind, double-dummy comparative study of PP1M (n = 161) and RLAT (n = 173) in adults with schizophrenia, which focused specifically on a subset of patients with recently diagnosed schizophrenia (≤5 years before study entry), showed that the tolerability (including rates of EPMS and prolactin-related TEAEs) and efficacy of PP1M and RLAT were generally similar over 13 weeks.[42] While the recently diagnosed patients described in the current study had a lower baseline PANSS total score than those in this 13-week study, they achieved a similar reduction at study endpoint, highlighting that further clinically relevant symptom improvements are possible, even in this more stabilized population. Baseline value and mean change from baseline in PSP total score were also similar between studies. Furthermore, in a study by Lieberman et al., data collected over 5 years suggested that patients with schizophrenia should be treated as early as possible to improve long-term prognosis.[36] Taken together, these results support the view that patients should be treated as early as possible in the course of the disorder for better long-term outcomes.
Patients in this study were non-acute but symptomatic and considered as stable by their treating physician for at least 1 month prior to enrolment. Nevertheless, 39.4% of recently diagnosed patients and 24.6% of chronic patients achieved a ≥50% improvement in PANSS total score. These data are clinically relevant since this result demonstrates that continuous effective treatment in patients who are considered clinically stable still offers the opportunity for further clinically relevant symptom improvement.
Higher incidences of weight gain and depression were reported in recently diagnosed compared with more chronic patients. The greater incidence of weight gain in recently diagnosed patients is in line with another study of early schizophrenia in which most weight gain occurred within the first 3 to 6 months of treatment [43] as well as data showing that patients with a higher baseline BMI gain less weight than those with a lower BMI.[44] Moreover, the finding that a relatively higher proportion of recently diagnosed patients reported depression over the study period (5.2% vs. 1.7% of chronic patients) is in line with depressive symptoms being frequent among newly diagnosed patients at baseline but decreasing during follow-up periods of up to 10 years.[45] Overall, in the present study, clinically relevant TEAEs such as EPMS, sedation, potentially prolactin-related TEAEs, and weight gain with PP1M were low and consistent with the randomized controlled studies,[46–48] which suggests that PP1M is also generally well tolerated in a patient population more representative of routine clinical practice with higher rates of co-morbidities, co-medications, and substance abuse.
Patients with schizophrenia are known to have a shorter life expectancy than the general population;[49,50] likely linked to the increased incidence of cardiovascular risk factors as well as metabolic co-morbidities reported in these patients.[51] Consistent with the latter observation, chronic patients in the current study population had a greater incidence of metabolic and vascular disorders at baseline. Early initiation of continuous treatment may benefit patients, as a lack of AP treatment has been associated with greater all-cause mortality.[50]
Despite the potential advantages of using LAT demonstrated in this and other studies,[12,14,16,17] they are considered underused in practice [40] and negative attitudes towards their use early in the treatment of schizophrenia among psychiatrists are common.[52] Furthermore, there is some debate in relation to the use of LAT in the early phases of schizophrenia.[9] Yet, recommendations for their use earlier in the disease course are emerging, not least during the critical period of the first 2 to 5 years following diagnosis [20] and as maintenance treatment following the first episode of psychosis.[19]
These were post hoc analyses and should therefore be considered exploratory in nature. Study limitations include the unblinded treatment and the lack of a comparator group, resulting in a potential for reporting bias. Therefore, these data do not provide a head-to-head comparison between treatments but suggest that suboptimal treatment with one AP does not predict failure with other APs, including PP1M, and that recently diagnosed patients may show a higher treatment response than more chronic patients. Comparison between treatments was not the primary aim of this pragmatic trial, which was designed to capture data in a setting mirroring daily clinical practice, not normally achieved in RCTs. Nevertheless, the method used in this study is consistent with current standards used in clinical trials and therefore allows for some indirect comparisons with data from interventional studies of a similar design. In addition, the present study did not exclude patients with current substance use or abuse, with the exception of intravenous drug use; this is in contrast to the pivotal randomized controlled studies in PP1M that excluded patients with a DSM-IV diagnosis of active substance dependence within 3 months prior to screening.[47,48,53] Nonetheless, rates of alcohol/substance use in the current group of patients were low, and it cannot be ruled out that psychiatrists selected patients who they felt would be most likely to complete the study.
5. Conclusions
Data from the current study demonstrate that recently diagnosed patients treated with PP1M had a significantly higher treatment response and better functioning, as assessed by the PSP total score, compared with more chronic patients. These data support current discussions that earlier continuous and effective AP treatment may be associated with better outcomes in patients with schizophrenia.
Declaration of interest
L Hargarter, P Bergmans, P Cherubin, S Keim, and A Schreiner are employees of Janssen Cilag. A Conca has received consulting fees from Lundbeck, Janssen, and Eli Lilly and speaking fees and travel grants from the University of Theology in Brixen and the Psychiatric Associations of Italy and Germany. A Serrano-Blanco has received research support from Ferrer International and Otsuka. N Bilanakis has no conflicts of interest to declare. I Bitter has received research support from Eli Lilly and honoraria or consultation fees from EGIS, EGRIS, Eli Lilly, Gedeon Richter, Janssen Cilag, Lundbeck, MedAvante, Pierre Fabre, PSI (CRO) and Servier. Medical writing support was provided by ApotheCom and funded by Janssen Cilag. This study was funded by Janssen Cilag.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- American Psychiatric Association [cited 2016 Mar 09;];Practice Guideline For The Treatment of Patients With Schizophrenia. 2004 http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf Available at.
- Zipursky RB, Menezes NM, Streiner DL. Risk of symptom recurrence with medication discontinuation in first-episode psychosis: a systematic review. Schizophr Res. 2014;152:408–414. doi: 10.1016/j.schres.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Leucht S, Tardy M, Komossa K. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379:2063–2071. doi: 10.1016/S0140-6736(12)60239-6. [DOI] [PubMed] [Google Scholar]
- Emsley R, Oosthuizen PP, Koen L. Symptom recurrence following intermittent treatment in first-episode schizophrenia successfully treated for 2 years: a 3-year open-label clinical study. J Clin Psychiatry. 2012;73:e541–e547. doi: 10.4088/JCP.11m07138. [DOI] [PubMed] [Google Scholar]
- Robinson D, Woerner MG, Alvir JM. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56:241–247. doi: 10.1001/archpsyc.56.3.241. [DOI] [PubMed] [Google Scholar]
- Schennach R, Obermeier M, Meyer S. Predictors of relapse in the year after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2012;63:87–90. doi: 10.1176/appi.ps.201100084. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Liu D, Ziebell S. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170:609–615. doi: 10.1176/appi.ajp.2013.12050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health & Care Excellence [cited 2016 Mar 09;];Psychosis and schizophrenia in adults. The NICE guideline on treatment and management. National Clinical Guideline Number CG178 2014. https://www.nice.org.uk/guidance/cg178 Available at.
- Kim B, Lee S-H, Yang YK. Long-acting injectable antipsychotics for first-episode schizophrenia: the pros and cons. Schizophr Res Treatment. 2012;2012:560836. doi: 10.1155/2012/560836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Perkins D, Belger A. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50:884–897. doi: 10.1016/s0006-3223(01)01303-8. [DOI] [PubMed] [Google Scholar]
- Emsley R, Oosthuizen P, Koen L. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23:325–331. doi: 10.1097/YIC.0b013e32830c2042. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Haukka J, Taylor M. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603–609. doi: 10.1176/appi.ajp.2011.10081224. [DOI] [PubMed] [Google Scholar]
- Kirson NY, Weiden PJ, Yermakov S. Efficacy and effectiveness of depot versus oral antipsychotics in schizophrenia: synthesizing results across different research designs. J Clin Psychiatry. 2013;74:568–575. doi: 10.4088/JCP.12r08167. [DOI] [PubMed] [Google Scholar]
- Leucht C, Heres S, Kane JM. Oral versus depot antipsychotic drugs for schizophrenia–a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res. 2011;127:83–92. doi: 10.1016/j.schres.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Robenzadeh A, Leucht C. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40:192–213. doi: 10.1093/schbul/sbs150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JM. Review of treatments that can ameliorate nonadherence in patients with schizophrenia. J Clin Psychiatry. 2006;67(Suppl 5):9–14. [PubMed] [Google Scholar]
- Kishimoto T, Nitta M, Borenstein M. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74:957–965. doi: 10.4088/JCP.13r08440. [DOI] [PubMed] [Google Scholar]
- Lin J, Wong B, Offord S. Healthcare cost reductions associated with the use of LAI formulations of antipsychotic medications versus oral among patients with schizophrenia. J Behav Health Serv Res. 2013;40:355–366. doi: 10.1007/s11414-013-9329-z. [DOI] [PubMed] [Google Scholar]
- Llorca PM, Abbar M, Courtet P. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry. 2013;13:340. doi: 10.1186/1471-244X-13-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malla A, Tibbo P, Chue P. Long-acting injectable antipsychotics: recommendations for clinicians. Can J Psychiatry. 2013;58:30S–35S. doi: 10.1177/088740341305805s05. [DOI] [PubMed] [Google Scholar]
- Birchwood M, Todd P, Jackson C. Early intervention in psychosis. The critical period hypothesis. Br J Psychiatry Suppl. 1998;172:53–59. [PubMed] [Google Scholar]
- Crumlish N, Whitty P, Clarke M. Beyond the critical period: longitudinal study of 8-year outcome in first-episode non-affective psychosis. Br J Psychiatry. 2009;194:18–24. doi: 10.1192/bjp.bp.107.048942. [DOI] [PubMed] [Google Scholar]
- Carrión RE, McLaughlin D, Goldberg TE. Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA Psychiatry. 2013;70:1133–1142. doi: 10.1001/jamapsychiatry.2013.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner A, Bergmans P, Cherubin P. A prospective flexible-dose study of paliperidone palmitate in nonacute but symptomatic patients with schizophrenia previously unsuccessfully treated with oral antipsychotic agents. Clin Ther. 2014;36(10):1372–1388.e1. doi: 10.1016/j.clinthera.2014.08.014. [DOI] [PubMed] [Google Scholar]; •• The PALMFlexS study was a pragmatic prospective 6-month interventional study designed specifically to mimic real-world clinical situations. Patients switched to flexibly dosed once monthly paliperidone palmitate showed significant improvements in symptom control and functioning.
- Schreiner A, Bergmans P, Cherubin P. Paliperidone palmitate in non-acute patients with schizophrenia previously unsuccessfully treated with risperidone long-acting therapy or frequently used conventional depot antipsychotics. J Psychopharm. 2015;29:910–922. doi: 10.1177/0269881115586284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargarter L, Cherubin P, Bergmans P. Intramuscular long-acting paliperidone palmitate in acute patients with schizophrenia unsuccessfully treated with oral antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2015;58:1–7. doi: 10.1016/j.pnpbp.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Morosini PL, Magliano L, Brambilla L. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101:323–329. [PubMed] [Google Scholar]
- Linden M, Baron S. The “Mini-ICF-Rating for Mental Disorders (Mini-ICF-P)” . A short instrument for the assessment of disabilities in mental disorders. Rehabilitation (Stuttg) 2005;44:144–151. doi: 10.1055/s-2004-834786. [DOI] [PubMed] [Google Scholar]
- Molodynski A, Linden M, Juckel G. The reliability, validity, and applicability of an English language version of the Mini-ICF-APP. Soc Psychiatry Psychiatr Epidemiol. 2013;48:1347–1354. doi: 10.1007/s00127-012-0604-8. [DOI] [PubMed] [Google Scholar]; • The mini-ICF-APP is being used increasingly to measure patient functioning in both clinical and research settings. Thirteen domains of capacity are assessed: (1) adherence to regulations, (2) planning and structuring of tasks, (3) flexibility, (4) competency, (5) endurance, (6) assertiveness, (7) contact with others, (8) group integration, (9) intimate relationships, (10) non-work activities, (11) self care, (12) mobility, and (13) competence to judge and decide.
- Van Wijngaarden B, Schene AH, Koeter M. Caregiving in schizophrenia: development, internal consistency and reliability of the Involvement Evaluation Questionnaire–European Version. EPSILON Study 4. European Psychiatric Services: Inputs Linked to Outcome Domains and Needs. Br J Psychiatry Suppl. 2000:s21–s27. doi: 10.1192/bjp.177.39.s21. [DOI] [PubMed] [Google Scholar]
- Naber D, Moritz S, Lambert M. Improvement of schizophrenic patients’ subjective well-being under atypical antipsychotic drugs. Schizophr Res. 2001;50:79–88. doi: 10.1016/s0920-9964(00)00166-3. [DOI] [PubMed] [Google Scholar]
- Atkinson MJ, Sinha A, Hass SL. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. doi: 10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey KB, Cocco KM, Simons JS. Concurrent validity of clinicians’ ratings of substance abuse among psychiatric outpatients. Psychiatr Serv. 1996;47:842–847. doi: 10.1176/ps.47.8.842. [DOI] [PubMed] [Google Scholar]
- Chouinard G, Margolese HC. Manual for the Extrapyramidal Symptom Rating Scale (ESRS) Schizophr Res. 2005;76(2–3):247–265. doi: 10.1016/j.schres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Parsons B, Allison DB, Loebel A. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;110:103–110. doi: 10.1016/j.schres.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Alvir JM, Koreen A. Psychobiologic correlates of treatment response in schizophrenia. Neuropsychopharmacol. 1996;14:13S–21S. doi: 10.1016/0893-133X(95)00200-W. [DOI] [PubMed] [Google Scholar]
- Jäger M, Riedel M, Messer T. Psychopathological characteristics and treatment response of first episode compared with multiple episode schizophrenic disorders. Eur Arch Psychiatry Clin Neurosci. 2007;257:47–53. doi: 10.1007/s00406-006-0683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphs L, Schooler N, Lauriello J. How study designs influence comparative effectiveness outcomes: the case of oral versus long-acting injectable antipsychotic treatments for schizophrenia. Schizophr Res. 2014;156:228–232. doi: 10.1016/j.schres.2014.04.024. [DOI] [PubMed] [Google Scholar]; • A review of key methodological considerations for clinical trials examining the differences between exploratory and pragmatic trial designs and the impact of these features when comparing LAT and oral AP treatments.
- Kane JM, Garcia-Ribera C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry Suppl. 2009;52:SS63–SS67. doi: 10.1192/bjp.195.52.s63. [DOI] [PubMed] [Google Scholar]
- Parellada E, Velligan DI, Emsley R. Long-acting injectable antipsychotics in first-episode schizophrenia. Schizophr Res Treatment. 2012;2012:318535. doi: 10.1155/2012/318535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley R, Medori R, Koen L. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28:210–213. doi: 10.1097/JCP.0b013e318167269d. [DOI] [PubMed] [Google Scholar]
- Fu D-J, Bossie CA, Sliwa JK. Paliperidone palmitate versus oral risperidone and risperidone long-acting injection in patients with recently diagnosed schizophrenia: a tolerability and efficacy comparison. Int Clin Psychopharmacol. 2014;29:45–55. doi: 10.1097/YIC.0000000000000006. [DOI] [PubMed] [Google Scholar]
- Liu J, Sun J, Shen X. Randomized controlled trial comparing changes in serum prolactin and weight among female patients with first-episode schizophrenia over 12 months of treatment with risperidone or quetiapine. Shanghai Arch Psychiatry. 2014;26:88–94. doi: 10.3969/j.issn.1002-0829.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah HA. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008;13:27–35. doi: 10.1038/sj.mp.4002066. [DOI] [PubMed] [Google Scholar]
- Sönmez N, Røssberg JI, Evensen J. Depressive symptoms in first-episode psychosis: a 10-year follow-up study. Early Interv Psychiatry. 2016 doi: 10.1111/eip.12163. [DOI] [PubMed] [Google Scholar]
- Hough D, Gopal S, Vijapurkar U. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2010;116:107–117. doi: 10.1016/j.schres.2009.10.026. [DOI] [PubMed] [Google Scholar]
- Pandina GJ, Lindenmayer J-P, Lull J. A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychopharmacol. 2010;30:235–244. doi: 10.1097/JCP.0b013e3181dd3103. [DOI] [PubMed] [Google Scholar]
- Pandina G, Lane R, Gopal S. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:218–226. doi: 10.1016/j.pnpbp.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Ösby U, Correia N, Brandt L. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ. 2000;321:483–484. doi: 10.1136/bmj.321.7259.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump C, Winkleby MA, Sundquist K. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170:324–333. doi: 10.1176/appi.ajp.2012.12050599. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150:1115–1121. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Heres S, Reichhart T, Hamann J. Psychiatrists’ attitude to antipsychotic depot treatment in patients with first-episode schizophrenia. Eur Psychiatry. 2011;26:297–301. doi: 10.1016/j.eurpsy.2009.12.020. [DOI] [PubMed] [Google Scholar]; • An examination of psychiatrists’ attitudes to LATs, emphasizing the importance of shared decision making when prescribing LATs to first-episode patients.
- Gopal S, Hough DW, Xu H. Efficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response study. Int Clin Psychopharmacol. 2010;25:247–256. doi: 10.1097/YIC.0b013e32833948fa. [DOI] [PubMed] [Google Scholar]


