Abstract
Prostate cancer is the most common malignancy and the second most common cause of cancer-associated mortality in males. Bone metastasis is frequent and generally multiple and osteoblastic. Presentation of a pure osteolytic and solitary metastasis from a prostate carcinoma is extremely rare. We report a case of prostate cancer in a 70-year-old man who presented with progressive severe right hip pain and stiffness with no urinary symptom. A whole-body bone scan revealed a solitary metastasis to the right hip. A prostate biopsy revealed prostate adenocarcinoma. We believe this is the first reported case of presentation of a solitary osteolytic bone metastasis in the pelvis from carcinoma of the prostate.
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; COPD, chronic obstructive pulmonary disease
Introduction
Prostate cancer constitutes 29% of all cancer cases and 9% of all cancer deaths in the United States. Currently, an estimated 91% of new cases of prostate cancer are diagnosed at local or regional stages, for which the five-year relative survival approaches 100%. Increased screening through prostate-specific antigen (PSA) testing has resulted in an increased rate of diagnosis of prostate cancer (1) and a considerable stage migration toward a higher proportion of localized tumors (2).
Bone metastasis is frequent, and is a serious and costly complication of cancer with an unknown exact annual incidence in the United States in patients with advanced prostate cancer. Once tumor metastasizes to bone, it is usually incurable (3). Metastatic bone lesions from cancer are generally multiple and osteoblastic (4); only approximately 10% are solitary (5). Bone metastases are more frequent in patients with high PSA levels and poorly differentiated tumors at biopsy, regardless of the patient’s age (6). Presentation of a pure osteolytic and solitary metastasis from a prostate carcinoma is extremely rare. There are a handful of reports of solitary osteoblastic bone lesions due to adenocarcinoma of the prostate. However, to our knowledge, only one other case of a solitary osteolytic bone metastasis has been reported from a prostatic primary, and it was located in the radial head (5). Brown et al also reported a solitary osteolytic bone lesion of the mandible in 1965, which was suspected to originate from a prostate carcinoma; however, this was never proved by a tissue biopsy (7).
Our case of prostate cancer presented with clinical symptoms of a solitary metastasis to the right hip. The combination of multiple radiologic studies such as plane x-rays, CT images, and MRI means that it is now possible to detect a solitary lesion with no other lesion very accurately. To the best of our knowledge, this is the first case report of an osteolytic solitary hip lesion that is a metastasis from prostate carcinoma.
Case report
A 70-year-old man was admitted to our hospital with a four-month history of gradually progressive severe right hip pain and stiffness, restricting physical activities and walking. Both passive and active movements aggravated the pain, causing a complete restriction in walking. The patient denied any urinary symptom. Past medical history included type II diabetes mellitus, atrial fibrillation, hypercholestrolemia, COPD, and coronary artery disease. The patient’s family history was significant for a history of unknown type of cancers; his father died of such a cancer at the age of 45. Significant findings on physical examination included tenderness over the anterolateral region of the right upper thigh. The prostate was mildly enlarged and appeared to be smooth, normal in texture, and with no nodules by digital rectal exam. Laboratory data revealed a normal complete blood count and chemistry panel. Alkaline phosphatase was elevated at 210 U/L. A plain AP-view radiograph of the pelvis and right hip showed a osteolytic lesion in the superior aspect of the right femoral head (Fig. 1).
Figure 1.
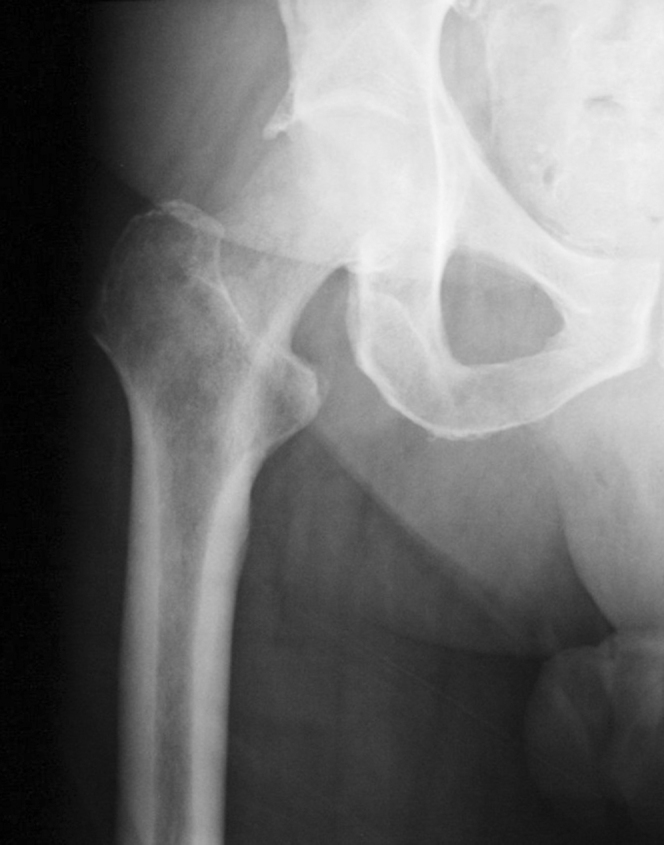
70-year-old male with prostate carcinoma. AP radiograph of the proximal right femur, showing an ill-defined osteolytic lesion of the right femoral neck and intertrochanteric ridge.
Whole-body bone scanning revealed a solitary focus of abnormal intense radiotracer uptake within the interotrochanteric region of the right hip as well as the head of the right femur (Fig. 2).
Figure 2.
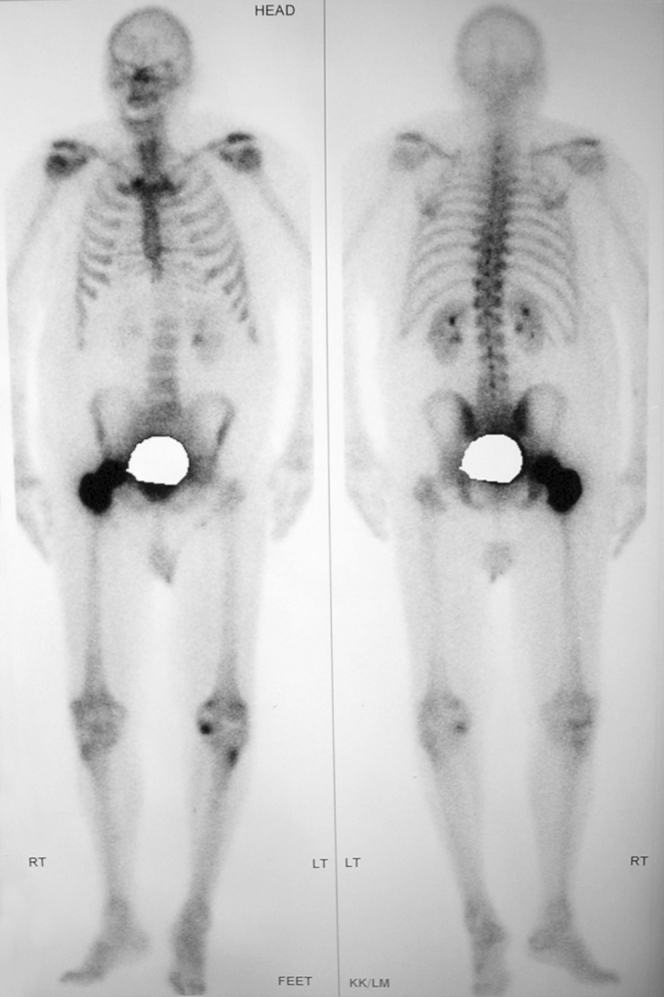
70-year-old male with prostate carcinoma. Radionuclide bone scan of the pelvis and right femur, revealing a solitary focus of abnormal intense radiotracer uptake within the interotrochanteric region of the right hip as well as the head of the right femur.
MRI imaging of the right hip pre- and post-gadolinium administration revealed markedly hypointense signal within the head, the intertrochanteric portion, and the proximal shaft of the right femur on T1- and T2-weighted sequences. This suggested lytic destructive bone lesions due to a malignancy (Fig. 3).
Figure 3.
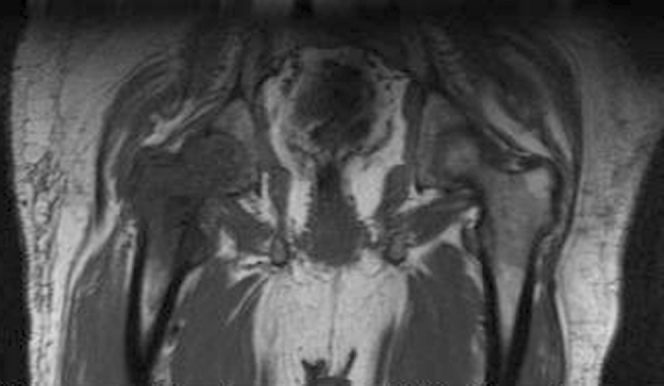
70-year-old male with prostate carcinoma. Coronal T1-weighted MR image of the lower pelvis, showing abnormally decreased T1 signal in the right proximal femur, indicating marrow fat replacement with tumor.
CT confirmed the presence of a single lytic lesion of the right femoral neck with a partially sclerotic margin (Fig. 4).
Figure 4.
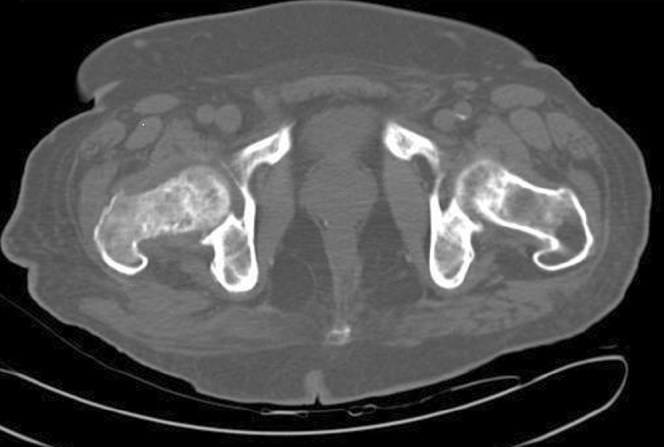
70-year-old male with prostate carcinoma. Axial CT scan of the pelvis, showing mixed osteolytic lesion with sclerotic margins in right femoral neck.
Tumor marker studies revealed an elevated level of PSA at 174 ng/ml. A transrectal ultrasound-guided prostate needle biopsy revealed prostate adenocarcinoma, with Gleason’s score of 3+4=7, involving multiple cores; approximately 50% of the total prostatic tissue and immunostain was positive for PSA. The patient was started on LHRH-agonist, Goserelin, along with antiandrogen Bicalutamide and radiation therapy. The patient was clinically doing fine; however, he sustained a massive pulmonary embolism on the 30th day of admission and unexpectedly expired.
Discussion
Prostate cancer is the most common malignancy in males over 50 years of age (5) and the second most common cause of cancer-associated mortality in the United States and Western Europe (6). Most newly diagnosed patients with prostate carcinoma are asymptomatic and have moderately differentiated and organ-confined disease. This migration has been attributed primarily to the impact of widespread prostate carcinoma screening initiatives (2).
Bone metastasis occurs in up to 70% of patients with advanced prostate cancer (3); approximately 30% of the cases have bone metastasis at the time of diagnosis. Cancer cells spread to bone by direct extension, arterial embolization, or venous dissemination (4). Data have confirmed that bone metastases are more frequent in patients with high prostate-specific antigen (PSA) levels and poorly differentiated tumors at biopsy, regardless of the patient’s age (6). The pretreatment level of PSA is the most important predictor of recurrence after radiotherapy (8). Overproduction of urokinase-type plasminogen activator (u-PA) by prostate-cancer cells increases bone metastasis. PSA can also cleave parathyroid hormone-related peptide at the N-terminal, which could block tumor-induced bone resorption. It may also activate osteoblastic growth factors, released in the bone microenvironment during the development of bone metastases (3). Bone scintigraphy is the most sensitive and commonly used technique for detecting skeletal metastases (9); the finding of multiple areas of increased uptake is diagnostic of metastatic disease (4). Metastatic tumors of prostate carcinoma in bone are generally multiple (5) and are of two distinct types; osteoblastic or osteolytic, or occasionally mixed (3), with the osteoblastic type of lesion occurring more frequently (7).
Skeletal complications from bone metastases include pathologic fractures, hypercalcemia, severe bone pain, and spinal cord compression. Cord compression occurs in approximately 7% of patients with prostate cancer and can lead to paraplegia, if surgical intervention is not performed immediately. Skeletal complications contribute to the erosion of quality of life in prostate cancer patients (10).
Brown et al reported two cases of solitary metastatic mandible cancer, suspected to be of prostatic origin. The nature of these lesions has not been mentioned in these reported articles (7). Ansari et al for the first time reported osteolytic solitary radial head metastases from prostate cancer in a 60-year old male (5).
Pure osteolytic lesions may occur, but are less common. Solitary expansile metastases in prostate carcinoma patients are a rare clinical presentation (5).
Footnotes
Published: November 15, 2009
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Miller DC, Hafez KS, Stewart A, Montie JE, Wei JT. Prostate Carcinoma Presentation, Diagnosis, and Staging. Cancer. 2003;98(6):1169–1178. doi: 10.1002/cncr.11635. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Roodman GD. Mechanisms of Bone Metastasis. NEJM. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Robey EL, Schellhammer PF. Solitary lesions on bone scan in genitourinary malignancy. J Urol. 1984;132(5):1000–1002. doi: 10.1016/s0022-5347(17)49986-1. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Ansari MS, Nabi G, Aron M. Solitary Radial Head Metastasis with Wrist Drop: A Rare Presentation of Metastatic Prostate Carcinoma. Urol Int. 2003;70(1):77–79. doi: 10.1159/000067696. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Salonia A, Gallina A, Camerota TC, Picchio M, DaPozzo MFLF, Guazzoni G, Fazio F, Rigatti P, Montorsi F. Bone metastases are infrequent in patients with newly diagnosed prostate cancer: Analysis of their clinical and pathologic features. Urology. 2006;68(2):362–366. doi: 10.1016/j.urology.2006.02.009. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Brown WF, Masson JK, Harrison EG, Jr, McConahey WM. Solitary Metastatic Cancer of the Mandible: three Cases with Apercalcemia. Mayo Clin Proc. 1965;40:392–396. [PubMed] [PubMed] [Google Scholar]
- 8.Roach M, III, Weinberg V, Sandler H, Thompson I. Staging for prostate cancer: time to incorporate pretreatment prostate-specific antigen and Gleason score? Cancer. 2007;109(2):213–220. doi: 10.1002/cncr.22403. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Maeda H, Koizumi M, Yoshimura K, Yamauchi T, Kawai T, Ogata E. Correlation between bone metabolic markers and bone scan in prostatic cancer. J Urol. 1997 Feb;157(2):539–543. [PubMed] [PubMed] [Google Scholar]
- 10.Saad F, Olsson C, Schulman CC. Skeletal morbidity in men with prostate cancer: Quality-of-life considerations throughout the continuum of care. Eur Urol. 2004;46(6):731–739. doi: 10.1016/j.eururo.2004.08.016. [PubMed] [DOI] [PubMed] [Google Scholar]


