Abstract
Nonketotic hyperglycinemia (NKH) is a rare metabolic disorder caused by a defect in the glycine cleavage enzyme system, resulting in high glycine concentrations in the brain. We report a neonate in which proton magnetic resonance spectroscopy provided biochemical evidence of elevated brain glycine levels and facilitated early diagnosis of NKH and guided clinical management.
Abbreviations: CT, computed tomography; MR, magnetic resonance
Introduction
Nonketotic hyperglycinemia (NKH) is a rare, autosomal-recessive, inborn error of glycine degradation caused by a defect of the glycine cleavage system (1). This defect causes large quantities of glycine to accumulate in all body tissues, particularly in the central nervous system (CNS). In classical neonatal-onset NKH, excessive CNS glycine levels lead to lethargy, poor feeding, hypotonia, and hiccups that develop within hours to days after birth. Symptoms progress to seizures, apnea, respiratory insufficiency, and coma, and typically lead to death between 3 and 5 months of age (2). Complicating the diagnosis and management of classical NKH in neonates are atypical and transient NKH variants that present with milder clinical symptoms and have variable neurological outcome, including complete resolution of neurologic symptoms in the transient form (2, 3, 4).
Proton (1H) magnetic resonance (MR) spectroscopy allows noninvasive measurement of brain glycine concentrations and has been described as a useful tool for diagnosis as well as for monitoring of the effects of therapeutic interventions in classical NKH (5, 6, 7, 8, 9). However, glycine is not reliably detected with MR spectroscopy in healthy brains or in medical conditions not associated with excessive glycine, such as psychiatric illnesses. We report a case in which 1H-MR spectroscopy facilitated early diagnosis of neonatal-onset NKH that was subsequently confirmed with metabolic studies and genetic testing. In medical centers where metabolic studies are not readily available, 1H-MR spectroscopy may assist with early diagnosis and management of NKH.
Case report
A male neonate delivered spontaneously at full term to a 23-year-old gravida 2, para 1 mother with an unremarkable pregnancy. The parents were from Somalia and were nonconsanguineous. There was no family history of metabolic disease, and the neonate’s male sibling was developmentally normal. The neonate did not appear dysmorphic. He had a birth weight of 4.098kg (84th percentile) and head circumference of 35 cm (44th percentile). At 3 days, he was noted to feed poorly, with decreased tone and reflexes noted on exam. He was admitted to the neonatal intensive care unit for a sepsis workup. Cranial ultrasonography, performed to exclude the possibility of hemorrhage, revealed a small right-sided choroid plexus cyst and partial agenesis of corpus collosum. Spontaneous and stimulus-provoked myoclonic jerks and intermittent hiccups were observed. He became progressively lethargic and unresponsive, with central hypotonia. At 4 days, amino acid analysis of the cerebral spinal fluid (CSF) and plasma was requested to evaluate for a metabolic disorder. Liver studies and an ammonia level were normal. The septic workup, which included a blood culture and CSF bacterial and viral cultures, was negative. Bedside electroencephalographic monitoring was abnormal, demonstrating diffuse cortical dysfunction and partial seizures. The neonate displayed progressive encephalopathy and respiratory insufficiency and so was placed on mechanical ventilation at 6 days of age.
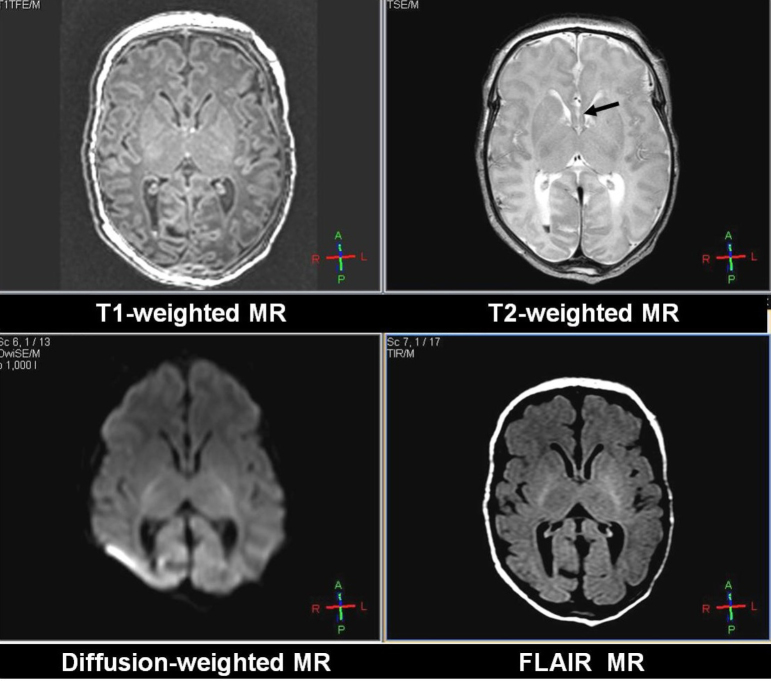
MR imaging of the brain was performed at 6 days of age, on a Philips Acheiva Scanner (version 2.6.1, 3 Tesla) using a standard quadrature head coil. Since the neonate was encephalopathic, anesthesia was not required to minimize movement during the imaging study. Sagittal 3D gradient-echo T1-weighted images were acquired with TR/TE of 8.2/3.8 ms, FOV 150 × 150 mm, 80 slices, section thickness of 1mm, no gap, and an imaging matrix of 160×160. Axial fast spin-echo T2-weighted 4800/90 ms (TR/TEeff) images were obtained with an echo-train length of 11, a field of view of 150×150 mm, an in-plane imaging matrix of 480 × 480, and a 3-mm section thickness with a 0.3-mm gap. Fluid-attenuated inversion-recovery images were acquired with TR/TE/TI 12000/140/2850 ms, and with the same resolution and positioning as with the T2-weighted images. Axial diffusion-weighted MR images were acquired with an in-plane field of view of 166 × 166 mm, 128 × 128 matrix size, section thickness/gap 4/0.4 mm, 2 diffusion gradient b values of 0 and 1000, and TR/TE 2738/57.75 ms; section positions were the same as in the axial T1- and T2-weighted sequences. From the acquired data, the average apparent diffusion coefficient was calculated. The MR imaging revealed a small intraventricular hemorrhage in the lateral ventricles and partial agenesis of the corpus callosum (Fig. 1).
Figure 1.
Neonate with nonketotic hyperglycinemia. T1-weighted (upper left), T2-weighted (upper right), diffusion-weighted (lower left), and FLAIR (lower right) images of this patient, all at the same slice location. These images reveal partial agenesis of the corpus callosum (black arrow).
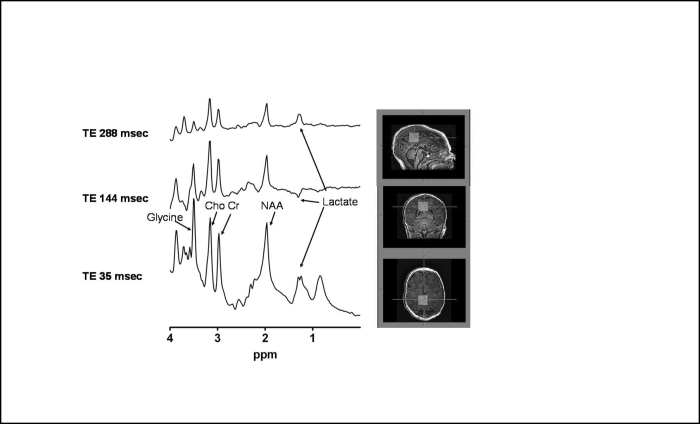
Due to clinical suspicion for NKH, 1H-MR spectroscopy to look for elevated brain glycine was performed. Single-voxel spectroscopy (using the PRESS pulse sequence with voxel localization [TR 2000 ms, 1024 FID points, spectral width 2000 Hz, 128 averages, voxel size 20 × 20 × 20 mm] over the midline superior parietal lobe) was performed with TE 35, 144, and 288.
1H-MR spectroscopy data reconstruction was conducted offline using software developed by one of the authors (TR) in Fortran, which prepares the data for LCModel processing (10). Spectra fit was conducted using LCModel, an automated technique that uses prior knowledge for model fits. A water spectrum of the same region was also acquired (without water suppression) for quantification used in the LCModel software. LCModel was used using a basis set that was prepared using phantoms with known concentrations of brain metabolites, as detailed in the LCModel manual (11). The absolute concentrations (millimoles; mmol) of choline, N-acetyl asparate, glycine, and creatine were computed. Tissue fractions were calculated by summing the CSF voxels within the 1H-MR spectroscopy voxel, which were segmented out (segmentation was performed using FSL’s FAST software, Oxford, UK, with 4 classification model) using the high-resolution three-dimensional anatomical image and then dividing the brain volume (total volume−CSF volume) by the total 1H-MR spectroscopy voxel volume. The results of this segmentation within the MRS voxel are reported as a fraction to the whole voxel volume as follows: gray matter 0.35, white matter 1 (partly myelinated) 0.30, white matter 2 (unmyelinated) 0.29, CSF 0.048. Free-induction decays (FIDs) were used as input into the LCModel software and were filtered with a finite discrete convolution to account for field inhomogeneities and eddy currents. FIDs were zero- and first-order phase-corrected. Absolute concentrations (mmol/kg wet weight) were obtained by scaling the in vivo spectrum to the unsuppressed water peak. The absolute concentrations of the 1H-MR spectroscopy metabolites seen in our patient were glycine (11.8), choline (2.47), creatine (7.66), N-acetylaspartate (5.74), and lactate (2.45). There could have been overestimation of the glycine concentration in the LCmodel output due to overlap of the inositol resonance at 3.55 ppm using TE=35 ms. The glycine concentration was also estimated from the spectrum at TE 288ms, and this resulted in a much smaller concentration of glycine ∼ 6 mM (assuming a similar T2 relaxation time as creatine). An elevated glycine peak at 3.55 ppm was noted (Fig. 2 and Fig. 3), supporting the diagnosis of NKH.
Figure 2.
Neonate with nonketotic hyperglycinemia. Proton MRIs (right) and spectra (left) obtained from an infant with nonketotic hyperglycinemia. T1-weighted images with voxel location (white boxes) of the MR spectroscopy showing all three reconstructed planes (top, sagittal; middle, coronal; bottom, axial). The T1-weighted images were acquired with a 3D gradient-echo pulse sequence with TR/TE of 8.2/3.8 ms, FOV 150 × 150 mm, 80 slices, section thickness of 1mm, no gap, and an imaging matrix of 160 × 160. Short (TE 35 ms) and long (TE 144 ms) proton (1H) MR spectra obtained from the parietal show a prominent singlet at 3.55 ppm (arrow), which is assigned to glycine and an elevated peak at 1.28 ppm (arrow), which is assigned to lactate. Lactate is visible at TE 35 as an upright peak, at TE 144 as an inverted peak, and at TE 288 ms as an upright peak. This change in lactate with echo time is a way to identify lactate due to its j-modulation properties as a function of echo time (which causes the inversion of the peak at 1.33ppm at TE 144 ms).
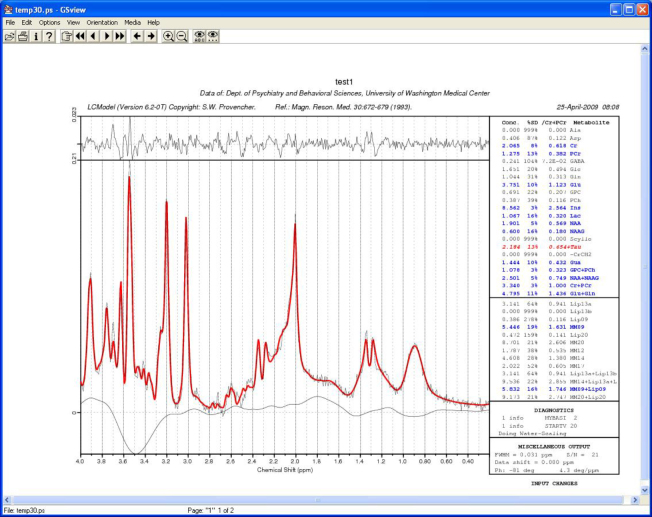
Figure 3.
Neonate with nonketotic hyperglycinemia. Graphic output from LCmodel showing the actual spectral data and the fitted data at TE 35 ms.
CSF and plasma amino acid analysis, which became available after the 1H-MR spectroscopy study, revealed glycine levels of 342 (normal, 1–11) μmol/mL and 1419 (normal, 106–318) μmol/mL, respectively. The CS- to-plasma glycine concentration ratio was 0.24 (normal, < 0.08), confirming the diagnosis of NKH. Urine organic acid analysis was negative. The neonate was treated with dextromethorphan (30 mg/kg/day), phenobarbital (7 mg/kg/day), and sodium benzoate (500 mg/kg/day). His level of consciousness improved slowly over several days, and at 19 days of age he was extubated from mechanical ventilation. Follow-up weekly amino acid analysis revealed overall decreased plasma glycine concentrations with periodic elevations. DNA testing demonstrated that the infant had a homozygous missense mutation (c. 2846C>T) in the GLDC gene, encoding the enzyme glycine decarboxylase (P-protein), namely p.P949L in exon 24. He is currently five months old, on a glycine-restricted diet, with ongoing medical issues including feeding problems, poor growth, recurrent vomiting, and seizures.
Discussion
The neonate in our report had partial agenesis of the corpus callosum, initially noted on cranial ultrasound. This finding, along with his clinical presentation, particularly the recurrent episodes of hiccups, was highly suspicious for the diagnosis of NKH. This prompted us to perform early 1H-MR spectroscopy to look for an elevated glycine peak, which has been previously reported (5, 6, 7, 8, 9). Glycine is normally not observed in 1H-MR spectroscopy of the brain, but in our case it was the highest peak shown (Fig. 2). The glycine peak at 3.55 ppm was most elevated (as compared with nearby metabolites) at a short TE of 35 ms, compared to longer TEs of 144 and 288 ms.
The absolute concentrations of the 1H-MR spectroscopy metabolites choline, creatine, and N-acetylaspartate seen in our patient were abnormal. These values resemble those seen in neonates with encephalopathy from cerebral hypoxic-ischemic injury associated with severely abnormal/fatal outcomes (death or major impairment with disability on neurologic assessment and/or developmental quotient < 75 based on examination at one year) compared to control infants (12). In addition to elevated brain glycine, 1H-MR spectroscopy demonstrated an elevated lactate peak. The increased lactate peak observed may reflect anaerobic glycolysis of brain tissue, probably caused by respiratory insufficiency and hypoxia (8). The absolute concentration of lactate was lower than values reported by Cheong et al (12) in neonates with encephalopathy secondary to perinatal cerebral hypoxic-ischemic injury; however, this may be secondary to differences in the underlying etiology of brain injury and the timing of the of 1H-MR spectroscopy studies.
1H-MR spectroscopy may be useful in providing valuable diagnostic information and early treatment in patients with clinical presentations suspicious for NKH. The diagnosis of NKH was supported by the 1H-MR spectroscopy findings in our case report, which were available before metabolic study results returned. 1H-MR spectroscopy has also been advocated as a noninvasive diagnostic tool for monitoring brain glycine levels in patients with NKH (13) and in differentiating the diagnosis of NKH in patients with other disorders associated with elevated plasma glycine levels (9). Elevated glycine levels are seen in other disorders on 1H-MR spectroscopy, such as central neurocytoma (14) and in oligodendroglial gliomatosis (15), so clinical correlation and metabolic studies are also needed to confirm the diagnosis of NKH.
Despite diagnosis with 1H-MR spectroscopy and metabolic studies, prognostication can be difficult in neonatal NKH. This is due to transient and atypical variants that display similar clinical presentations to classical NKH with hypotonia, seizures, and apneic episodes that may require assisted ventilation (3, 16). Unlike neonates with classical NKH, neonates with atypical and transient NKH may have improvement of symptoms over time, with subsequent normal psychomotor development in some cases. It is unclear if early therapeutic intervention affects long-term outcomes in these cases. The occurrence of patients with apparent transient forms of NKH adds uncertainty to the prediction of clinical outcome, especially regarding decisions of withholding or withdrawing therapy in affected neonates (4).
In summary, our case demonstrates that 1H-MR spectroscopy may facilitate early diagnosis of NKH before the availability of metabolic and genetic test results. Timely use of 1H-MR spectroscopy may identify and help guide clinical management in neonates with suspected NKH.
Acknowledgments
We gratefully acknowledge the help of Jeff Stevenson, who helped with MR spectroscopic analysis.
Footnotes
Published: November 15, 2009
References
- 1.Nakai T, Nakagawa N, Maoka N, Masui R, Kuramitsu S, Kamiya N. Structure of P-protein of the glycine cleavage system: implications for nonketotic hyperglycinemia. EMBO J. 2005;24:1523–1536. doi: 10.1038/sj.emboj.7600632. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang TF, Parr JR, Matthews EE, Gray RG, Bonham JR, Kay JD. Practical difficulties in the diagnosis of transient non-ketotic hyperglycinaemia. Dev Med Child Neurol. 2008;50:157–159. doi: 10.1111/j.1469-8749.2007.02003.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Dinopoulos A, Matsubara Y, Kure S. Atypical variants of nonketotic hyperglycinemia. Mol Genet Metab. 2005;86:61–69. doi: 10.1016/j.ymgme.2005.07.016. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Boneh A, Allan S, Mendelson D, Spriggs M, Gillam LH, Korman SH. Clinical, ethical and legal considerations in the treatment of newborns with non-ketotic hyperglycinaemia. Mol Genet Metab. 2008;94:143–147. doi: 10.1016/j.ymgme.2008.02.010. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Sener RN. Nonketotic hyperglycinemia: diffusion magnetic resonance imaging findings. J Comput Assist Tomogr. 2003;27:538–540. doi: 10.1097/00004728-200307000-00015. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Bekiesiniska-Figatowska M, Rokicki D, Walecki J. MRI in nonketotic hyperglycinaemia: case report. Neuroradiology. 2001;43:792–793. doi: 10.1007/s002340100577. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Viola A, Chabrol B, Nicoli F, Confort-Gouny S, Viout P, Cozzone PJ. Magnetic resonance spectroscopy study of glycine pathways in nonketotic hyperglycinemia. Pediatr Res. 2002;52:292–300. doi: 10.1203/00006450-200208000-00024. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Choi CG, Lee HK, Yoon JH. Localized proton MR spectroscopic detection of nonketotic hyperglycinemia in an infant. Korean J Radiol. 2001;2:239–242. doi: 10.3348/kjr.2001.2.4.239. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huisman TA, Thiel T, Steinmann B, Zeilinger G, Martin E. Proton magnetic resonance spectroscopy of the brain of a neonate with nonketotic hyperglycinemia: in vivo-in vitro (ex vivo) correlation. Eur Radiol. 2002;12:858–861. doi: 10.1007/s003300101073. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Provencher SW. LcModel and LcMgui User's Manual 2000. Available from: http://s-provencher.com/pages/lcm-manual.shtml
- 12.Cheong JL, Cady EB, Penrice J, Wyatt JS, Cox IJ, Robertson NJ. Proton MR spectroscopy in neonates with perinatal cerebral hypoxic-ischemic injury: metabolite peak-area ratios, relaxation times, and absolute concentrations. AJNR Am J Neuroradiol. 2006;27:1546–1554. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki Y, Ueda H, Toribe Y. [Proton MR spectroscopy of nonketotic hyperglycinemia] No To Hattatsu. 2001;33:422–425. [PubMed] [PubMed] [Google Scholar]
- 14.Yeh IB, Xu M, Ng WH, Ye J, Yang D, Lim CC. Central neurocytoma: typical magnetic resonance spectroscopy findings and atypical ventricular dissemination. Magn Reson Imaging. 2008;26:59–64. doi: 10.1016/j.mri.2007.04.005. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Gutowski NJ, Gomez-Anson B, Torpey N, Revesz T, Miller D, Rudge P. Oligodendroglial gliomatosis cerebri: (1)H-MRS suggests elevated glycine/inositol levels. Neuroradiology. 1999;41:650–653. doi: 10.1007/s002340050818. [PubMed] [DOI] [PubMed] [Google Scholar]
- 16.Aliefendioglu D, Tana Aslan A, Coskun T, Dursun A, Cakmak FN, Kesimer M. Transient nonketotic hyperglycinemia: two case reports and literature review. Pediatr Neurol. 2003;28:151–155. doi: 10.1016/s0887-8994(02)00501-5. [PubMed] [DOI] [PubMed] [Google Scholar]