Abstract
Monocytes and macrophages play a critical role in tissue development, homeostasis, and injury repair. These innate immune cells participate in guiding vascular remodeling, stimulation of local stem and progenitor cells, and structural repair of tissues such as muscle and bone. Therefore, there is a great interest in harnessing this powerful endogenous cell source for therapeutic regeneration through immunoregenerative biomaterial engineering. These materials seek to harness specific subpopulations of monocytes/macrophages to promote repair by influencing their recruitment, positioning, differentiation, and function within a damaged tissue. Monocyte and macrophage phenotypes span a continuum of inflammatory (M1) to anti-inflammatory or pro-regenerative cells (M2), and their heterogeneous functions are highly dependent on microenvironmental cues within the injury niche. Increasing evidence suggests that division of labor among subpopulations of monocytes and macrophages could allow for harnessing regenerative functions over inflammatory functions of myeloid cells; however, the complex balance between necessary functions of inflammatory versus regenerative myeloid cells remains to be fully elucidated. Historically, biomaterial-based therapies for promoting tissue regeneration were designed to minimize the host inflammatory response; although, recent appreciation for the roles that innate immune cells play in tissue repair and material integration has shifted this paradigm. A number of opportunities exist to exploit known signaling systems of specific populations of monocytes/macrophages to promote repair and to better understand the biological and pathological roles of myeloid cells. This review seeks to outline the characteristics of distinct populations of monocytes and macrophages, identify the role of these cells within diverse tissue injury niches, and offer design criteria for immunoregenerative biomaterials given the intrinsic inflammatory response to their implantation.
Keywords: Monocyte, macrophage, biomaterial, regeneration, wound healing, inflammation
Introduction
The innate immune system plays a critical role in tissue development, homeostasis, and repair of injured tissues. Monocytes and macrophages are among the first responders to tissue injury and are required for successful tissue regeneration.1–7 The striking evolutionary examples of complex tissue regeneration in the salamander limb and zebrafish tailfin require the presence of myeloid cells and regeneration fails when macrophages are depleted prior to injury.2,3 Increasing evidence suggests that division of labor among subpopulations of monocytes and macrophages could allow for harnessing regenerative functions over inflammatory functions of myeloid cells;7–11 however, the complex balance between necessary functions of inflammatory versus regenerative myeloid cells remains to be fully elucidated.12 Engineering exogenous control of the myeloid-driven repair system may be the key to developing regenerative therapies in adult tissues and provides the foundation for the new area of “immunoregenerative” biomaterial strategies. These materials seek to harness specific subpopulations of monocytes/macrophages to promote repair by influencing their recruitment, positioning, differentiation, and function within an injured tissue.9,13,14 In order to best implement this powerful repair system, an understanding of the affordances of each cell type, their function within specific injury contexts, and their interaction with implanted biomaterials is necessary. This review seeks to outline the features of distinct populations of monocytes and macrophages, to identify the role of these cells within diverse tissue injury contexts, and offer design criteria for materials given the intrinsic inflammatory response to their implantation.
Heterogeneity among monocytes
Monocytes (CD115+CD11b+SSClowcells) are found in the bone marrow, blood, and spleen of vertebrates during homeostasis and can be recruited to tissue injury or infection as effectors and as progenitors of macrophages and dendritic cells.1,15,16 As effectors, monocytes participate in coordinating efforts between the innate and adaptive immune response,10,11,17,18 killing microbial pathogens and parasites such as Leishmania major,19 and promoting repair of damaged tissues.4,8,20,21 Myeloid cells responding to injuries typically secrete inflammatory cytokines and chemokines, phagocytose necrotic debris, secrete proteolytic and matrix remodeling proteins, and produce growth factors upon entry to the tissue.1,22 Cell surface markers and functional activities distinguish multiple subsets of monocytes that exist on a spectrum from inflammatory (classical) to anti-inflammatory (alternatively activated, non-classical, or resident) (Table 1).
Table 1.
Monocyte and macrophage subset characteristics
| Phenotype | Polarizing cues | Common surface markers | Distinguishing surface markers | Protein/gene expression | References |
|---|---|---|---|---|---|
| IM | Unknown | CD115+ CD11b+ SSClow | Mouse: Ly6Chigh, CX3CR1low, CCR2high Human: CD14+CD16− Rat: CD43low | TNFα, IL-1β | Arnold et al.,4 Geissmann et al.18 |
| AM | Unknown | Mouse: Ly6Clow, CX3CR1high, CCR2− Human: CD14low/−CD16+ Rat: CD43high | TGFβ, IL-10 | Arnold et al.,4 Geissmann et al.18 | |
|
| |||||
| M1 | IFNγ, LPS, TNFα, GM-CSF | MerTK+ CD64+ | CCR7, CD80, MHC II | IL-1β, TNFα, IL-6, IL-8, IL-12, IL-23, iNOS, CXCL10 (IP-10), CXCL11 (IP-9), IFNγ, CCL1, CCL5, VEGF, FGF | Mosser and Edwards,10 Spiller et al.,23 Mantovani et al.,24 Spiller et al.,25 Martinez et al.26 |
| M2a | IL-4, IL-13 | CD206 | CCL17, CCL18, CCL22, PDGF-BB, TIMP-3, Arginase 1, YM1, Fizz1/RELMα, IL-27Rα, CXCR4, IGF-1 | Mosser and Edwards,10 Gautier et al.,27 Spiller et al.,23 Mantovani et al.,24 Spiller et al.,25 Martinez et al.,26 Sironi et al.28 | |
| M2b | Immune complexes plus TLR or IL-1 agonist | CD86, MHC II | IL-10, CCL1, CXCL3, IL-6, TNF, SPHK1, iNOS | Edwards et al.,11 Mantovani et al.,24 Sironi et al.28 | |
| M2c | IL-10, glucocorticoid hormones | CD163, MHC II | IL-10, MMP9, IL-1β, | Spiller et al.,23 Mantovani et al.,24 Spiller et al.,25 Knipper et al.29 | |
TNF: Tumor necrosis factor; IL: interleukin; iNOS: inducible nitric oxide synthase; CCL: CC-chemokine ligand; CXCL: C-X-C motif ligand; IFN: interferon; VEGF: vascular endothelial growth factor; FGF: fibroblast growth factor; PDGF-BB: platelet-derived growth factor; TIMP: tissue inhibitor of metalloproteinase; YM1: chitinase-like 3; Fizz1/RELMα: resistin-like molecule alpha1; IGF1: insulin-like growth factor 1; SPHK1: sphingosine kinase 1; MMP9: matrix metalloproteinase 9.
Each monocyte subset responds to distinct chemotactic and trophic cues and equivalent subsets have been identified across multiple vertebrate species, including human, mouse, and rat.18,30–32 Dynamic and biphasic recruitment of monocyte subsets is a key feature of the early inflammatory response. Monocytes either persist in tissue as monocytes, repolarize to a different monocyte subset, or differentiate into macrophages.21 Heterogeneity in the function and differentiation potential of monocyte subsets is important to consider in designing therapies to manipulate the innate immune microenvironment, particularly which subset to recruit and how to educate the cells upon recruitment to perform a desired function.
Inflammatory monocytes (IMs) are characterized by high surface expression of Ly6C and CCR2, but low expression of CX3CR1 in mice.18 Low expression of CD43 in rat indicates an equivalent population.31 The human classical inflammatory subset is CD14+CD16−18,30 and an intermediate population expresses both CD14 and CD16.1 IMs are derived from a dividing macrophage-dendritic cell precursor (MDP) or common monocyte progenitor (cMoP) in the bone marrow33,34 and have a half-life of about 20 h in the blood at homeostasis.16 They are elevated in circulation following inflammatory injury with a peak concentration near 48 h, and the magnitude of mobilization scales with the severity of the inflammatory injury.35 The source of this increase is likely mobilization from primary reservoirs in the bone marrow and spleen36 and requires expression of CCR2.16 Early during the innate immune response, IMs are recruited from blood to sites of inflammation utilizing integrin α4β1 and CD62L to arrest on the endothelium37–39 and chemokine receptors CCR2 and CCR5 to migrate toward gradients of inflammatory cytokines such as monocyte chemoattractant protein-1 (MCP-1).1,40 While IMs are sparsely adherent and motile on the endothelium during steady state, they rapidly adhere and transmigrate through the endothelium when the vessel is activated with inflammatory cytokines such as tumor necrosis factor α (TNFα) or interferon gamma (IFNγ).38 The MCP-1/CCR2 signaling axis is a major experimental tool for exploring the role of IMs in tissue injury and could be utilized in biomaterials to specifically target IM recruitment.
Anti-inflammatory monocytes (AMs) have recently emerged as pro-regenerative cells that support behaviors such as matrix remodeling, arteriogenesis, and prevention of fibrosis.1,8,9,22 The AM cell surface is characteristically Ly6Clow, CCR2− and CX3CR1high in mice, CD14lowCD16+ and CX3CR1+ in human, and CD43high in rat18,30,31 (Table 1). In addition, AMs express a particular signature of adhesion molecules, including high lymphocyte function-associated antigen 1 (LFA-1 or αLβ2 integrin) and low L-selectin (CD62L)1 that allow them to “patrol” or crawl on blood vessel walls during homeostasis.38,39 At steady state, AMs maintain a half-life in the blood of greater than two days, which is more than double the half-life of IMs.16 Interestingly, IM depletion from the blood in the CCR2 knockout mouse increases the circulation half-life of AMs to approximately 11 days, suggesting that IMs limit the lifespan of AMs at steady state.16 Two models exist for the origin of blood AMs: they may differentiate from IMs or directly from a MDP in the bone marrow.16,33,41 Fate mapping, adoptive transfer, and new intravital imaging studies have elegantly shown progression of labeled IMs to AMs; however, these studies do not exclude the possibility that some AMs are derived directly from progenitors in the bone marrow.4,16,18,21,33,41 Further evidence suggests that AMs do not give rise to IMs, and differentiation is unidirectional.18 The signals controlling the switch from IM to AM are not clear, although recent work suggests that the transcription factor NR4A1 controls AM development and knockout leads to an absence of AMs in blood.41
Chemokine receptors that distinguish AMs from IMs may be particularly useful in impacting selective recruitment or activity of this regenerative subset. AMs exhibit higher migration compared with IMs to gradients of CX3CL1, due their higher expression of the cognate receptor CX3CR1.18,42 Additional chemotactic receptors, including the sphingosine-1-phosphate receptor 3 (S1PR3) and C-X-C chemokine receptor 4 (CXCR4), are higher on AMs and may contribute to enhanced sensitization to regenerative chemotactic signals.9,13,14,18 We have recently shown that localized release of SDF-1α, a CXCR4 ligand, or the S1PR3-targeting small molecule FTY720 can increase the recruitment of AMs into injured skin tissue and position the cells along a gradient from the biomaterial source.9,13,14 SDF-1α may also work in tandem with vascular endothelial growth factor (VEGF) to position monocytes in a peri-vascular niche in order to facilitate their role in vascular repair.43,44 Localized signals from biomaterials provide a powerful in situ opportunity to recruit cells along an engineered gradient and also “educate” or modulate the response of recruited cells without impacting systemic inflammatory responses.9,13,14,44,45 A clear elucidation of the signals to which different monocytes respond in vivo could provide valuable tools for engineering their recruitment and activity within injured tissues and biomaterial implants.
Origin and heterogeneity of macrophages
Macrophages (MerTK+CD64+)27 have many important tissue functions in development, homeostasis, and tissue repair.46–49 Some macrophages are the descendants of circulating monocytes and are replenished at steady state and during inflammation by blood-derived monocytes.15,50 The monocyte to macrophage transition, particularly the phenotypes each monocyte subset produces and under what circumstances is the target of much recent investigation. Some studies support a model where monocytes are recruited to tissues as IMs, then convert to AMs, and subsequently to macrophages.4 Others contend that the AMs are directly recruited from blood to supply monocyte-derived macrophages.9,13 This distinction could be attributed to differences in tissue injury context; however, experiments to directly test this idea have not been performed.50 Considerable evidence also supports a model of multiple origins for macrophages, where some macrophages derived from embryonic sources and self-maintain locally in tissues throughout adulthood.16,51,52 Specifically, populations of tissue macrophages of the heart, lung, brain, skin, and liver are derived prenatally from the yolk-sac endothelium, fetal liver monocytes, and early erythroid-myeloid progenitors and are not adult monocyte decendants.16,53–55 Some injury contexts, such as Th2-linked infection, can increase local tissue macrophage populations specifically through proliferation as opposed to monocyte recruitment.51 Characterization of gene expression signatures among macrophage populations from different tissues, species, and polarization and activation states reveal marked heterogeneity based on all these factors.23,27 These studies suggest that “macrophage” is an umbrella term covering cells that have remarkable diversity in gene and protein expression and function23,27,50,56 (Table 1). Despite the diversity of macrophage origin,50 tissue environment,57 and secondary pathologies,12 all macrophages (tissue resident and monocyte-derived) seem to share one common expression signature of MerTK and CD64 across populations.27 For biomaterial design, it will be critical to account for properties of the specific population of macrophage resident in the tissue of interest. Like monocytes, macrophage functional diversity is described as a continuum from inflammatory to anti-inflammatory macrophages, and plasticity is believed to be retained in order to rapidly respond to microenvironmental changes.56 As current dogma continues to evolve concerning the origin of tissue macrophages, evidence suggests that the diversity in macrophage phenotypes likely extends beyond stimulating factors to cell ontogeny, tissue context, and other environmental factors.
Inflammatory macrophages (M1) are the “classically activated” subset of macrophage. In vitro, naïve macrophages can be polarized to an M1 phenotype by treatment with IFNγ, microbial stimuli such as lipopolysaccharide (LPS), and/or inflammatory cytokines such as TNFα.24 These cells produce many inflammatory cytokines, reactive oxygen species, and growth factors such as VEGF and FGF225,58 upon stimulation (summarized in Table 1). IFNγ can be provided in inflamed tissue by natural killer cells and T-helper 1 (TH1) cells to activate or polarize macrophages.10 The inflammatory cytokines produced by M1s support host defense through pathogen clearance, necrotic tissue clearance, and activation of additional immune populations. Over-stimulation or failure to resolve the M1 response can be detrimental through activation and propagation of pathogenic TH17 cells, which can contribute to tissue damage and autoimmune disease pathologies when not properly regulated.10
Anti-inflammatory macrophages (M2) are also known as “alternatively activated” macrophages due to differing activation signals compared with the M1 subset. This group of macrophages can be further subdivided into M2a, M2b, and M2c based on activation signals, cell surface receptors, and functional diversity (Table 1). In vitro, naïve macrophages can be polarized to an M2a phenotype by treatment with IL-4 receptor ligands IL-4 and/or IL-13; an M2b phenotype can be achieved by treatment with immune complexes combined with toll-like receptor (TLR) or IL-1R ligands; and M2c macrophages are generated by IL-10 stimulation.24 M2a macrophages, also called wound healing macrophages, express high levels of arginase -1 in response to IL-4, which allows them to generate precursors for collagen and fibroblast stimulating factor, thus supporting their role in extracellular matrix deposition and wound closure.10 This matrix-remodeling role of M2a cells allows them to contribute to wound stabilization; however, they must be carefully coordinated to avoid creating undesirable fibrotic changes in an injured tissue or surrounding a biomaterial implant.10,29 M2b and M2c contribute to suppressing inflammation through secretion of IL-10.10 M2 macrophages retain a great deal of plasticity and are thought to be able to shift through various transition states between M2a-c. A greater understanding of their specific functions in tissue has been difficult due to the complexity of signal integration in vivo and the lack of defined set of distinguishing markers for each class of macrophage.
Functions of monocytes and macrophages in tissue repair
The “injury niche”
A niche is a specialized microenvironment that instructs and supports the behavior of its resident cells through localized paracrine signaling, cell–cell interactions, cell–matrix interactions, and environmental factors such as hypoxia.59 The site of tissue injury can be functionally equated to a niche or “injury niche,” based on localized complex signaling that orchestrates the progression of inflammation and healing. After injury, rapid and spatially controlled changes occur within the tissue through the evolutionarily conserved endogenous programs of clean-up, repair, and regeneration. The tissue microenvironment becomes rich with signals to recruit, position, and functionally instruct cells of the inflammatory system to program repair and inflammation resolution. Recruited cells further propagate the response by releasing their own paracrine signals that continue to guide inflammation even after their apoptotic clearance.
Each tissue injury niche has a unique set of damage, recruitment, and education signals coordinating the repair response that arise from specialization within the tissue composition and the type of injury. Unique microenvironmental cues are created by sterile inflammation versus pathogen-mediated inflammation due to the damage-associated molecular patterns recognized by inflammatory cells rather than pathogen-associated molecular patterns. Similarly, ischemic injury can activate signaling divergent from traumatic injury based on induction of hypoxia signaling and reactive oxygen species. Upon recruitment from the vasculature, monocytes differentiate into macrophages based on signals they encounter within the injury niche. Physiology/biology of the particular tissue also significantly impacts the way the injury niche orchestrates repair. For example, repair of a bony structure requires vastly different activities than regeneration of skeletal muscle, as reflected by the differences in cytokine, chemokine, and growth factors present during homeostasis and wound healing in these two tissues. The local stromal cell and tissue-resident macrophage populations, mechanical properties and organization of the tissue, and extent of vascularization and oxygenation could all contribute to differential engagement of immune cell populations in situ. Tissue-resident macrophage populations show remarkable heterogeneity in gene expression due to epigenetic programing by their surrounding tissue microenvironment to assume tissue-specific functions.23,27,57 In addition, chronic conditions such as diabetes,12 atherosclerosis, obesity, or sickle cell disease60 may pose singular challenges during pathogenesis and healing of an injury. Perhaps, these distinctions underlie some of the controversies in the literature regarding dynamics of monocyte recruitment, origin and fate tracking of subpopulations of cells, and differentiation sequence into macrophages. These factors contribute to the diversity of macrophage phenotypes and repair programs produced by the innate immune system.50
For biomaterial-mediated tissue repair strategies seeking to leverage endogenous monocyte/macrophage populations, the inflammatory injury microenvironment may contribute significantly to material design criteria. Immunoregenerative materials can be designed to release molecules to boost or interfere with certain features of the injury niche to facilitate repair,9,13,14 but should also prioritize the overall healing goals of a particular tissue. In order to mimic and take advantage of this powerful natural repair system, we need to understand what cells are required to support healing and what cues direct them within a particular wound environment. A better understanding of these signals and processes will allow us to design therapeutics that can push repair processes in a desirable direction.
Monocytes/macrophages in vascular remodeling
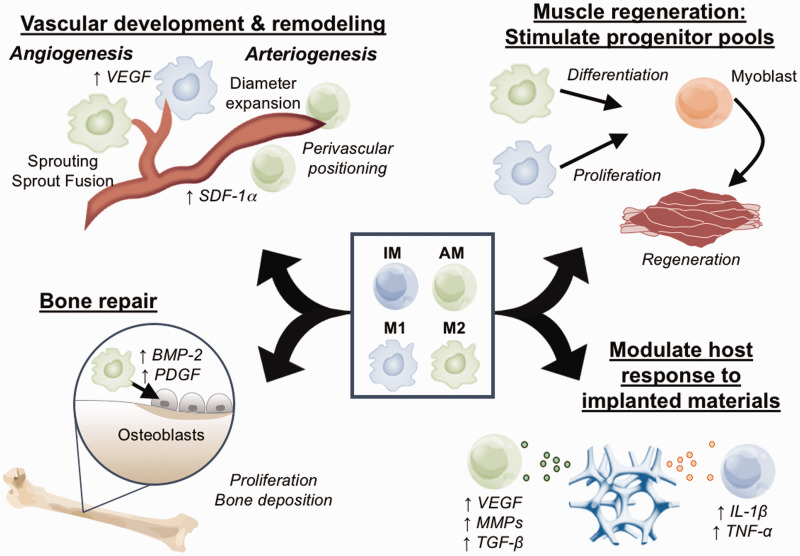
Vasculature is a universal necessity for the delivery of oxygen and nutrients to tissue and vascular damage is a common feature of many injury niches. Injuries ranging from ischemia to trauma produce vascular damage and induce the two major processes of vascular remodeling: angiogenesis and arteriogenesis. Regenerative or reparative therapies, including the implantation of biomaterials or tissue-engineered constructs, similarly must ensure proper oxygen and nutrient supply through remodeling or expansion of existing vascular networks.61 Monocytes and macrophages have been implicated in supporting vascular network remodeling and growth in development, homeostasis, and repair7,13,14,43,47,62–64 (Figure 1). During development, embryonic macrophages similar to an M2 phenotype physically associate with sprouting angiogenic tip cells to guide connections between fusing vessels in both murine and zebrafish models.47 This direct association of macrophage and endothelial cell suggests that the positioning of myeloid cells relative to vessels is important for their pro-angiogenic function, and therefore implies the importance of spatially guiding recruited myeloid cells with immunoregenerative material strategies. Genetic mutation of colony stimulating factor receptor 1 (CSFR1) in the op/op mouse, which is essential to normal myeloid development, causes not only a reduction in circulating monocytes and tissue macrophages but also aberrant collateral anastomoses, reduced collateral tortuosity, smaller diameter arteries, and immature hindbrain vasculature.7,47
Figure 1.
During injury, heterogeneous populations of monocytes (IM and AM) are recruited from circulation, where they can persist transiently as monocytes or rapidly differentiate into macrophages ranging in phenotype from M1-type to M2-type. Within the specific injury niche, monocytes and macrophages can guide tissue repair by promoting angiogenesis and arteriogenesis, secreting growth factors that guide progenitor cell differentiation and proliferation, regulating the secretome of parenchymal cells, and releasing inflammatory mediators that guide other immune cells. (A color version of this figure is available in the online journal.)
Monocyte recruitment to sites of vessel injury and their ability to adhere to the endothelium65 have been correlated with the extent of collateral arterial growth9,13,63,66 and effective angiogenesis.67 Depletion of myeloid cells after myocardial cryoinjury leads to regression of capillary structures and reduces the formation of vascularized granulation tissue.5,68 In a typical biphasic injury response, IMs are the first monocyte responders, followed by AMs around three days later. Loss of CCR2, which leads to depletion of IMs from the blood and decreased tissue myeloid cells,16 impairs the development of collateral arteriogenesis in mice after ischemic hindlimb occlusion.69 MCP-1 infusion into an ischemic hindlimb causes an increase in total monocyte accumulation in parallel with increase in the density of collateral arteries, the number of sprouting capillaries increase and an overall significant enhancement of bulk and collateral conductance within an ischemic hindlimb.64 MCP-1 is a key chemotactic molecule for IMs, so this may suggest that IMs play a role in arteriogenesis following ischemic damage. Constitutive overexpression of MCP-1 in heart tissue leads to severe damage to the myocardium that is related to persistent monocyte infiltration and macrophage metalloelastase-mediated tunneling within cardiac tissue.70 Collectively, these studies suggest that IM/M1 cells may be essential for effective vascularization, perhaps through matrix degradation, although overzealous activation of these cells can lead to tissue damage.
Connecting the IM and AM response, some studies suggest that cells on the IM/M1 end of the myeloid spectrum produce VEGF.25 Pathologically, VEGF can be upregulated by endothelial cells or pericytes in a stress response to hypoxia to stimulate angiogenesis. Transgenic organ-specific VEGF induction causes robust tissue infiltration of circulating CX3CR1+ monocytes.43 Increased transgenic VEGF expression causes peri-vascular cells to produce the chemotactic factor SDF-1α, which supports the positioning of CXCR4+ monocytes in proximity to vascular structures, allowing for reciprocal paracrine signaling between the monocytes and the vasculature.43 While this study did not characterize the subset of monocyte that was recruited, the expression of CX3CR1 and CXCR4 suggests that these cells may be AMs.13 Release of SDF-1α from a biomaterial implant in a dermal inflammatory wound causes an increase in the early recruitment of AMs, which correlated with later parameters of vascular remodeling.13 Intravascularly, AMs patrol the endothelial layer for signs of damage and assist in clearing apoptotic endothelial cells.20 Loss of IL-4 receptor α, which plays a major role in M2a polarization, leads to massive hemorrhage and delayed epithelialization in a skin injury model, highlighting the critical role these cells play in supporting repair.29 Monocyte/macrophage subset discrimination analysis has suggested that an enhanced pool of AM/M2 cells or accelerated recruitment correlates with increased vascular remodeling.9,13 The precise mechanisms by which each subset of myeloid cell contributes to vessel development and stabilization remains to be fully elucidated; however, secretion of soluble factors, matrix deposition/remodeling, and stabilization of nascent endothelial sprouts is likely to mediate the process.
Monocytes/macrophages in muscle repair
The dynamics of myeloid cell recruitment and the concepts of local differentiation and local education in the muscle hold many unanswered questions. During skeletal muscle toxin-induced injury, the myeloid response is characterized by an early IM/M1 infiltration for the first four days after injury, followed by a substantial boost in AM infiltration beginning around four days after injury.4 Upon depletion of AMs from the blood with clodronate liposomes, IMs infiltrate the muscle and convert to AM/M2s in situ,4 suggesting that one mechanism of AM/M2 deposition in the tissue is differentiation. Similar biphasic kinetics of early M1 cells followed by M2 cells in the muscle tissue have been observed in other skeletal muscle injury models.71 In vitro studies suggest that M1-polarized macrophages can re-polarize toward an M2 phenotype upon phagocytosis of necrotic muscle debris and induce secretion of TGF-β1 while downregulating TNF-α secretion.4 Blockade of M2 polarization by transgenic knockout of IL-10 causes an increased/sustained M1 response and poor regeneration in an injured muscle.71 This phenotype switching in the muscle may be a key step in normal healing and may suggest that both polarization states are important for muscle repair. Muscle tissue maintains the ability to regenerate in the adult through maintenance of a quiescent-resident stem cell population known as satellite cells or myogenic precursor cells. Macrophages in injured muscle can produce pro-regenerative growth factors such as IGF-1 to stimulate the activity of satellite cells (Figure 1). CCR2-deficient muscle had a significantly dampened macrophage response and coordinately reduced IGF-1 expression. Injection of exogenous IGF-1 was able to partially rescue the healing defect in the absence of recruited monocytes.49 In vitro, M1 macrophages stimulate proliferation of myogenic precursor cells but inhibit their fusion into myotubes. M2a and M2c cells enhance differentiation of in vitro progenitor cells by increasing myogenin expression and large myotube formation.4,72 The role of myeloid cells in the repair process is emphasized by depletion studies showing delay or failure of skeletal muscle regeneration in the absence of myeloid cells.4 Loss of CCR2, which is an essential receptor for IM trafficking,21,40 causes a deficit in inflammatory cell infiltrate and impaired regeneration, suggesting that the recruitment of IMs is critical to muscle healing.49 Interestingly, two recent studies support roles for CX3CR1 that are not related to AM trafficking; however, these studies do not agree on a positive or negative role for CX3CR1 in regeneration of toxin-induced injury.73,74 Nevertheless, CX3CR1 was important for macrophage phagocytosis functions, regulation of apolipoprotein E (ApoE) production, secretion of the inflammatory cytokine IL-6, and the trophic factor IGF-1.73,74
The inflammatory response to ischemic injury in the heart requires a phase for clearance of debris balanced with a structural repair phase that must avoid overzealous remodeling of the ventricular wall leading to dysfunction. The myeloid response to myocardial infarction has been characterized as predominantly monocytic within the first week of injury. IMs and AMs respond dynamically in two phases with IM recruitment peaking at day 3 through elevated MCP-1 and AM recruitment dominating from day 5 onward in parallel to decreased MCP-1 and increased CX3CL1 and VCAM-1 expression.8 Monocyte recruitment appears to be ongoing throughout this acute phase of injury, with a significant contribution of spleen-derived monocytes, and a turnover rate estimated to be 20 h.29,68 Both monocyte subsets were found critical to proper repair with IMs contributing to debris clearance and AMs supporting healing through granulation tissue formation and vascular remodeling.5,8,68 The differentiation of these monocytes into macrophages within the post-ischemic heart is not fully elucidated. Tissue-resident CX3CR1+ macrophages are found within the myocardium in contact with vessel structures and cardiomyocytes during homeostasis.75 This resident population at steady-state expresses a number of M2-associated genes (including CD206, CD163, MMP13, Ym1, Fizz1) with enrichment compared with brain- or spleen-derived CX3CR1+ cells.75 Age-dependent changes in ability to respond to injury couples to age-dependent differences in resident and recruited myeloid populations. In the neonatal mouse, response to toxin-induced myocardial injury resulted in a greater response of a MCH-IIlowCCR2− (AM/M2-like) embryonic macrophage compared with a dominant MHC-IIhighCCR2+ (IM/M1-like) monocyte-derived macrophage response in the adult.49 The neonatal heart has a higher capacity for repair and less deleterious remodeling requiring MCH-IIlowCCR2− embryonic macrophages associated with less T cell activation, inflammasome activity, and lower secretion of IL-1β.53 When cardiac macrophage subsets are further defined in the adult heart, multiple distinct classes of myeloid cells are present.53 The relative role of resident macrophages in muscle versus recruited monocytes and monocyte-derived macrophages is incompletely understood and may further inform a desirable engineered immune response.
As in other tissues, myeloid cells responding to the ischemic heart are janus-faced. They support clean-up and regeneration, but unchecked activity can also produce proteolytic tissue damage by IMs or fibrotic scar formation and undesirable remodeling by AMs. Co-morbidities such as atherosclerotic disease can shift the inflammatory balance towards the deleterious IM response and thus may call for intervention in recruitment or survival of IMs. Indeed, partial reduction of IM recruitment to myocardial infarction in the ApoE−/− mouse by RNAi-mediated silencing of the CCR2 receptor promoted considerable repair benefits,76 while massive depletion of early monocyte response by clodronate liposomes or splenectomy exacerbates injury in wild-type mice.5,36 Therefore, the design of immunomodulatory therapies for the heart and other tissues must walk a fine line to allow sufficient debris clearance in the inflammatory phase, while supporting effective regeneration without over-activation of matrix deposition by AM/M2 cells. Additional investigation into the M2 macrophage component of repair may lend further therapeutic targets given the diverse functions of the M2 subsets (M2a, M2b, and M2c). Further considerations include the presence of additional disease states or risk factors such as atherosclerosis, diabetes, age, and obesity that may contribute to differential signaling or response in myeloid cells.12,76
Role of monocytes/macrophages in bone repair
A role for monocytes and macrophages in bone repair has become increasingly recognized in recent years.77 During homeostasis, osteal macrophages form a canopy structure along the endosteal bone surface, and their depletion either with clodronate liposomes or macrophage Fas-induced apoptosis (Mafia) transgenic mice rapidly eliminates bone-forming osteoblasts.78,79 Macrophages appear to directly signal to osteogenic cells, as they promote mineralization during in vitro co-culture through soluble cues.78 Long-term depletion of macrophages in the Mafia mouse results in severe osteopenia that is not rescued by parathyroid hormone, which is used clinically to promote bone anabolism.80 Genetic depletion of macrophages using transgenic mice expressing diphtheria toxin A in lysozyme-M-expressing cells results in shorter bone length and reduced bone density by three months of age.81 While bone-resorbing osteoclasts are derived from circulating monocytic progenitors, osteoclasts can be retained during macrophage depletion strategies.81 During bone injury, monocytes and macrophages play diverse roles in repair, modulating the acute inflammatory response,46 producing growth factors such as BMP-282 and PDGF-BB,83 and inducing ostegeonesis of mesenchymal progenitor cells78,79 (Figure 1). Impaired monocyte trafficking induced by genetic knockout of CCR2 does not affect osteoclast numbers but impairs vascular remodeling and delays bone repair.84 Interestingly, the observed vascular defects were due to impaired arteriogenesis (decreased vascular diameter) and not angiogenesis (no change in total vascular length). Macrophages are required for callous formation after fracture, and they localize to regions undergoing early endochondral ossification.85 Depletion of macrophages significantly impairs intramembranous bone repair86 and prevents fracture repair, resulting in fibrotic tissue formation instead of endochondral bone.81 Taken together, monocytes and macrophages are multi-faceted cellular regulators of the healing response after bone injury, and likely represent a powerful target for immunoregenerative therapies.
Innate immune response to engineered materials
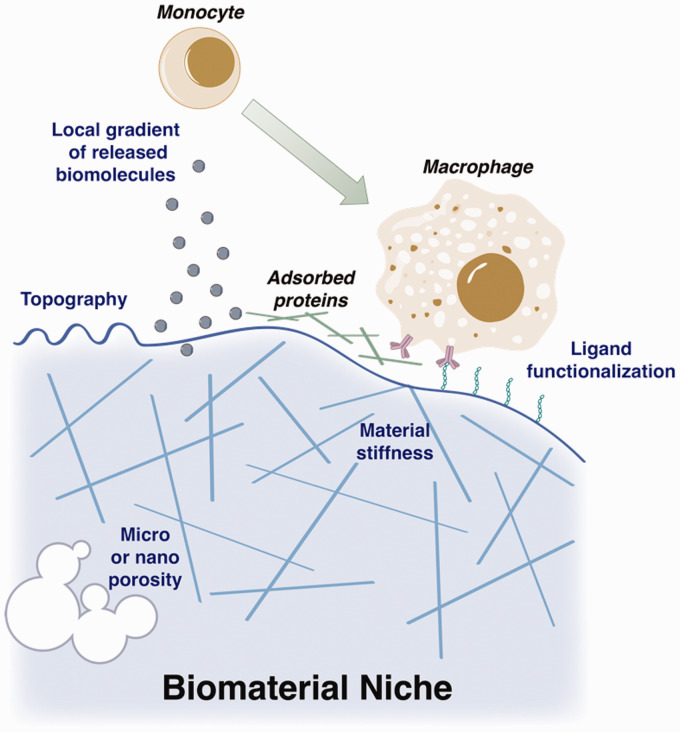
Biomaterials afford many advantages as a system to exploit the repair functions of monocytes/macrophages; however, foreign materials unavoidably elicit a biological response when implanted in vivo. The immune response must be carefully considered in the material design in order to avoid unwanted cell recruitment/attachment, heightened secretion of inflammatory cytokines, fibrous encapsulation, or chronic inflammation.87,88 Physical properties such as geometry, topography, and porosity and biochemical properties such as surface chemistry, ligand functionalization, and degradation mode are critical factors in the host response to the material (Figure 2). Immunomodulatory or immunoregenerative materials seek to alter this inflammatory response or tune the immune response for regeneration typically through biomolecule or small molecule delivery (Figure 1). As the innate immune response is highly dynamic, spatiotemporal control of delivery is critical to target specific phases of inflammation and repair in vivo. Localized release can be utilized to generate a gradient of a particular compound, which is important for providing spatial information to monocytes in order to recruit them to the desired site. Materials can also be used to provide a substrate or scaffold to organize complex tissue reconstruction, however, can face challenges such as proper integration into host tissue, vascularization, and circumventing fibrosis and the foreign body response. By engineering material properties and biomolecule delivery, the biological response can be tuned to maximally promote repair, while not prolonging the inflammatory response.
Figure 2.
The fate of monocytes and macrophages surrounding implants can be greatly affected by material properties, both those inherent to the selected material and those engineered to confer additional functionality. Properties with effects on macrophages include topographical cues, bulk mechanical properties such as elasticity and stiffness, porosity, functionalization with ligands, and release of biomolecules. (A color version of this figure is available in the online journal.)
Geometry and topography
Material geometry on the bulk, micro-, and nanoscale, can greatly impact material–host interactions and presents an opportunity to design features of a material to best hone the inflammatory response. Phagocytic cells like monocytes and macrophages are primary responders to foreign materials, and the ability of these cells to interact with the material is greatly impacted by the shape and size. Pathophysiologically, myeloid cells use size and shape of microbes they encounter to dictate the immune response they signal.89 Geometry can influence the ability of cells to adhere to a surface, phagocytose, or align with one another. Spherical materials appear to induce far less fibrous encapsulation than cylindrical or materials containing sharp angles.90 Moreover, a spherical diameter around 1.5 mm is superior to smaller spheres across a wide range of materials (including alginate hydrogels, stainless steel, and glass) for reducing foreign body response, perhaps due to reduced cell attachment to the particle surface.90 Sub-micron silicon particles induce a robust inflammatory response in naïve bone marrow-derived macrophages by secretion of IL-1β and TNF-α due to disruption of the lysosomal machinery in the cells.91 Macrophages are most able to phagocytose microspheres around 2 µm in diameter, both the number of microspheres internalized, and their total volume.92 Microsphere shape is equally important, as macrophages detect a “local shape” at the initial point of contact that determines whether they will spread along or initiate phagocytosis of the material.93 Interestingly, a wide range of shapes can be engulfed by macrophages if the initial orientation is correct and total size primarily affects completion of phagocytosis. Further, the sub-micron size of particles can determine whether a particle is engulfed by endocytosis (∼150 nm) versus phagocytosis (∼500 nm), a distinction that could impact the extent of macrophage activation.94 Liposomal material strategies can take advantage of the phagocytic nature of monocytes and macrophages to specifically target drug delivery to myeloid cells. Phagocytosis of unilamellar nano-liposomes (∼200 nm) has been exploited experimentally as a tool to specifically deplete myeloid cells with clodronate-loaded liposomes.2
Topographical cues at the micro- or nano-scale direct macrophage responses to materials after implantation, including adhesion, spreading, activation, migration, and polarization. Murine macrophages appear unable to detect nano-topographical features less than 150 nm, whereas cells such as fibroblasts and endothelial cells are able to detect smaller topographies and display decreased spreading as feature size increases from 55 nm to 200 nm.95 Parallel gratings made of different polymers ranging from 250 to 2000 nm promote spreading and elongation of unpolarized macrophages and impact secretion of M1-associated cytokines such as TNF-α, MCP-1, and VEGF.96 Electrospun fibers, which can create scaffolds mimicking natural extracellular matrix, support differing amounts of macrophage response based on diameter, packing, and alignment features.97–99 The topographical orientation appears to also be important, as randomly oriented nanofibers reduce fibrous encapsulation in vivo compared with aligned nanofibers or non-patterned substrates.99 Three-dimensional (3D) microstructure similarly reduces inflammatory cytokine production compared with surface-restricted geometries.100 Material geometry and topography can also be used to engineer desired inflammatory outcomes. Micro-patterned lines of fibronectin (20 µm in width) promote macrophage elongation, reduce inflammatory cytokine production, and prime these cells for differentiation into M2 macrophages compared with larger patterns or no patterning.101 Generally, porous implants are more readily vascularized and can exhibit lower fibrous encapsulation than their non-porous counterparts.102, 103 PTFE materials with pores 4.4 µm in width increase secretion of IL-1β by human monocytes in vitro compared with non-porous materials or those with smaller pores (1.2 or 3.0 µm) but demonstrate reduced fibrous encapsulation in vivo.104
Chemical properties
The chemical makeup of the material impacts properties such as surface charge and hydrophobicity. For small cellulose microspheres, macrophages are less able to phagocytose non-ionic hydrophilic particles, demonstrating an eight-fold increase in phagocytosis for particles that are highly anionic or a four-fold increase for highly cationic particles.92 Negatively charged particles induce apoptotic clearance of IMs following scavenger receptor-dependent uptake in the circulation. A negative charge was required for therapeutic efficacy in preventing IM accumulation and reducing disease symptoms during myocardial infarction, encephalitis, colitis, and other experimental inflammatory diseases.105 The surface properties of macro-materials impact the foreign body response, with hydrophilic or anionic surfaces causing macrophage apoptosis and inhibiting macrophage fusion in vivo compared with unmodified, cationic, or hydrophobic surfaces.106 Effects on macrophage function by chemical properties of the material may be due to variations in the type107 and conformation108 of adsorbed proteins or related to the charged regions on macrophage membranes.109
Mechanical properties
Like many other cell types, macrophages are able to detect mechanical properties of their substrate such as stiffness.110–112 Soft PEG hydrogels (modulus of 130 kPa) prevent macrophage spreading and formation of actin stress fibers, as well as prevent inflammatory gene expression in vitro and fibrous encapsulation in vivo.111 Stiffer hydrogels (up to 840 kPa) prime macrophages for higher expression of IL-1β and IL-6, and promote thicker capsules. A decrease in inflammatory response with decreasing material stiffness applies to even softer substrates such as polyacrylamide hydrogels below 150 kPa.110 Interestingly, inflammatory mediators such as IFN-γ or LPS increase the elasticity of the macrophages.110 Consequently, cellular mechanics can be regulated both by substrate properties and through biochemical cues in macrophages.
Functionalization
The emergence of chemical conjugation strategies that enable biomaterials scientists to have precise control over ligand presentation and degradation mode, while paving the way for the next generation of biomaterials. For example, bioorthogonal click chemistry has increased opportunities to develop diverse and complex materials that are both biocompatible and biofunctional. Hydrogels have been increasingly utilized in tissue engineering for their ease of tunability to specific applications, relative inertness in vivo, and compatibility for cell and biomolecule delivery. Inflammatory cell interactions with materials are mediated by adsorption of proteins such as albumin, fibrinogen, and components of the complement system,113,114 which is dictated by properties such as surface chemistry and hydrophobicity. Due to their protein-fouling nature, hydrogels often must be functionalized with adhesive ligands such as the integrin recognition site arginylglycylaspartic acid (RGD) to reduce protein aggregation on their surface. Poly(ethylene glycol) (PEG) hydrogels functionalized with RGD adsorb less protein than unfunctionalized hydrogels following subcutaneous implantation in vivo, although the relative composition of adsorbed protein remains approximately 90% the same.114 Functionalization with RGD reduces inflammatory gene expression by macrophages in vitro,115 and reduces the inflammatory response to non-degradable PEG hydrogels in vivo.116 Interestingly, a delay in RGD presentation via in vivo light-triggered activation reduces the fibrous capsule thickness surrounding implanted PEG hydrogels,62 highlighting the potential importance of spatiotemporal control of biomaterial features. Components of the extracellular matrix may inherently be immunomodulatory, as coating polypropylene meshes with various types of extracellular matrices shifts the macrophage response from M1 to M2 and reduces the foreign body response.117 Taken together, adhesive ligands enable better integration with surrounding tissue, which may reduce the material-mediated inflammatory response.
Incorporation of degradation sites has enabled more precise control over the kinetics and mechanism of degradation, as well as release rates of embedded biomolecules that are diffusion limited. Protease-sensitive linkers allow a material to be environmentally responsive in the sense that degradation depends on the abundance of proteases surrounding the material. Proteases such as MMP-2, MMP-9, and cathepsins are upregulated during the early stages of inflammation. Materials that degrade in response to MMP activity promote better integration with host tissue and healing compared with non-degradable materials.118 The rate of degradation can be further controlled by tuning the affinity, avidity, and protease specificity of crosslinkers used.119 Growth factor delivery can be engineered to be environmentally responsive through conjugation to PEG monomers with a protease-sensitive linker.120 While protease-sensitive materials, as well as similar pH-sensitive materials, are able to dynamically respond to their environment, they are not able to respond to user-defined stimuli. Consequently, an emerging field in regenerative medicine that will likely be a critical component of immunoregenerative therapies is the application of materials that can be regulated temporally by external stimuli such as light, ultrasound, and heat.121 For example, macrophage and neutrophil adhesion to hydrogels can be activated by transdermal application of ultraviolet light due to functionalization with “caged” adhesive ligands. Non-invasive adhesive ligand activation seven days post-implantation reduces fibrous encapsulation, providing control over the inflammatory response in both space and time.62
Proof-of-principle experiments have demonstrated that changes to specific material properties alter the biological response to implanted materials, but this may become more complicated when multiple parameters are simultaneously varied. For example, the foreign body response to materials varying significantly in surface chemistry, stiffness, and topography (polymers, ceramics, metals, and plastics) is most controlled by the material's size (0.5 mm or 1.5–2 mm),90 despite previous studies demonstrating that each of these properties can impact the inflammatory response. Consequently, high-throughput and empirical studies may be required when optimizing material design. While a set of unified principles describing the impact that material properties have on inflammatory cell response have yet to be developed, the design of next-generation immunomodulatory materials will likely rely on a deeper understanding of how specific material properties impact immune cell function in vivo.
Biomolecule delivery
Delivery of synthetic and natural molecules from implanted materials can be used to further modulate the host response. Generally, biomolecules embedded to regulate monocyte and macrophage function can target the following biological processes: (1) recruitment of monocytes and macrophages from blood or surrounding tissue, (2) monocyte/macrophage polarization state, and (3) macrophage proliferation. Recruitment can be controlled by locally delivery of chemokines that selectively target monocytes and macrophages, such as MCP-1, SDF-1, CX3CL1, or bioactive sphingolipids. This strategy can be further fine-tuned by selecting biomolecules that target monocyte/macrophage subsets. For example, SDF-1 delivery from heparin-PEG hydrogels can selectively enhance recruitment of AMs, but not IMs, by exploiting their relative overexpression of CXCR4.13 Delivery of MCP-1, which broadly recruits monocytes through CCR2, increased the frequency of arginase-positive M2 macrophages and improved endothelial cell transplantation.122 Delivery of PDGF-BB and FGF from PEG hydrogels recruits more macrophages to the cornea and better supports vascularization than VEGF,123 further indicating that targeting specific cell recruitment can improve repair. Polarizing cytokines can also be released to condition the local macrophages to a particular phenotype. Delivery of IL-4 from an agarose-based scaffold enhanced M2 macrophage bias in the local tissue and correlated with increase in peripheral nerve regeneration.124 Sequential delivery of IFN-γ and IL-4 can be used to guide the kinetics of macrophage polarization on decellularized bone matrices to achieve a biphasic inflammatory to regenerative macrophage profile.45 Delivery of macrophage polarizing factors has been achieved on other types of matrices, such as silk, either through adsorption, or direct conjugation.125 Inflammation-induced macrophage proliferation has only recently been appreciated and may provide a new therapeutic target, given that tissue macrophage expansion depends on IL-4 and M-CSF.126 Material-based biomolecule delivery has the unique advantage of the ability to control both the spatial and temporal profiles of released molecules, both of which are critical aspects of the immunological response after injury.
Summary
Historically, biomaterial-based therapies for promoting tissue regeneration were designed to minimize the host inflammatory response; however, recent appreciation for the roles that innate immune cells play in tissue repair and material integration has shifted this paradigm. As our understanding of the affordances of monocyte and macrophage roles in repair continues to sharpen, a great opportunity arises to meet the challenge of engineering regeneration utilizing immunoregenerative strategies. In this pursuit, we must balance complex goals of cell recruitment, cell phenotype, dynamic shifts in polarization, and material-immune interactions. Biomaterials afford many advantages as a drug delivery system to coordinate this innate immune response. Design features could include sustained or tunable release, tandem or sequential release of different molecules, and localized versus systemic release. Temporal control of drug delivery is critical to target specific phases of inflammation and repair in vivo. Localized release can be utilized to generate a gradient of a particular compound, which is important for providing spatial information to monocytes in order to recruit them to the desired site. Materials can also be used to provide a substrate or scaffold to organize complex tissue reconstruction; however, they can face challenges such as proper integration into host tissue, vascularization, and circumventing fibrosis and the foreign body response. By engineering material properties and biomolecule delivery, the biological response can be tuned to maximally promote repair, while not prolonging the inflammatory response leading to “immunologically smart” materials that better integrate with biological systems.
Acknowledgements
This work was supported by NIH 5R01AR056445, American Heart Association Grant 15PRE25090024 (CES), and P.E.O. Scholar Award (CES).
Authors' contributions
All authors participated in the design and content of the manuscript. MEO, CES, and SS wrote the manuscript and generated figures and tables. All authors revised and approved the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011; 11: 762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci USA 2013; 110: 9415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Yan B, Shi YQ, Zhang WQ, Wen ZL. Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J Biol Chem 2012; 287: 25353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 2007; 204: 1057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol 2007; 170: 818–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlundt C, El Khassawna T, Serra A, Dienelt A, Wendler S, Schell H, van Rooijen N, Radbruch A, Lucius R, Hartmann S, Duda GN, Schmidt-Bleek K. Macrophages in bone fracture healing: their essential role in endochondral ossification. Bone 31 October 2015. DOI: 10.1016/j.bone.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann CE, Hoefer IE, Meder B, Roth H, van Royen N, Breit SM, Jost MM, Aharinejad S, Hartmann S, Buschmann IR. Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J Leukoc Biol 2006; 80: 59–65. [DOI] [PubMed] [Google Scholar]
- 8.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007; 204: 3037–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awojoodu AO, Ogle ME, Sefcik LS, Bowers DT, Martin K, Brayman KL, Lynch KR, Peirce-Cottler SM, Botchwey E. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci USA 2013; 110: 13785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8: 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol 2006; 80: 1298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nassiri S, Zakeri I, Weingarten MS, Spiller KL. Relative expression of proinflammatory and antiinflammatory genes reveals differences between healing and nonhealing human chronic diabetic foot ulcers. J Invest Dermatol 2015; 135: 1700–3. [DOI] [PubMed] [Google Scholar]
- 13.Krieger JR, Ogle ME, McFaline-Figueroa J, Segar CE, Temenoff JS, Botchwey EA. Spatially localized recruitment of anti-inflammatory monocytes by SDF-1alpha-releasing hydrogels enhances microvascular network remodeling. Biomaterials 2016; 77: 280–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogle ME, Sefcik LS, Awojoodu AO, Chiappa NF, Lynch K, Peirce-Cottler S, Botchwey EA. Engineering in vivo gradients of sphingosine-1-phosphate receptor ligands for localized microvascular remodeling and inflammatory cell positioning. Acta Biomater 2014; 10: 4704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med 1968; 128: 415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013; 38: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slaney CY, Toker A, La Flamme A, Backstrom BT, Harper JL. Naive blood monocytes suppress T-cell function. A possible mechanism for protection from autoimmunity. Immunol Cell Biol 2011; 89: 7–13. [DOI] [PubMed] [Google Scholar]
- 18.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003; 19: 71–82. [DOI] [PubMed] [Google Scholar]
- 19.Goncalves R, Zhang X, Cohen H, Debrabant A, Mosser DM. Platelet activation attracts a subpopulation of effector monocytes to sites of Leishmania major infection. J Exp Med 2011; 208: 1253–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 2013; 153: 362–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dal-Secco D, Wang J, Zeng Z, Kolaczkowska E, Wong CH, Petri B, Ransohoff RM, Charo IF, Jenne CN, Kubes P. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2 + monocytes at a site of sterile injury. J Exp Med 2015; 212: 447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 2010; 121: 2437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiller KL, Wrona EA, Romero-Torres S, Pallotta I, Graney PL, Witherel CE, Panicker LM, Feldman RA, Urbanska AM, Santambrogio L, Vunjak-Novakovic G, Freytes DO. Differential gene expression in human, murine, and cell line-derived macrophages upon polarization. Exp Cell Res. Epub ahead of print 20 October 2015. DOI: 10.1016/j.yexcr.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25: 677–86. [DOI] [PubMed] [Google Scholar]
- 25.Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014; 35: 4477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 2006; 177: 7303–11. [DOI] [PubMed] [Google Scholar]
- 27.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma'ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ. Immunological Genome, C. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 2012; 13: 1118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sironi M, Martinez FO, D'Ambrosio D, Gattorno M, Polentarutti N, Locati M, Gregorio A, Iellem A, Cassatella MA, Van Damme J, Sozzani S, Martini A, Sinigaglia F, Vecchi A, Mantovani A. Differential regulation of chemokine production by Fcgamma receptor engagement in human monocytes: association of CCL1 with a distinct form of M2 monocyte activation (M2b, Type 2). J Leukoc Biol 2006; 80: 342–9. [DOI] [PubMed] [Google Scholar]
- 29.Knipper JA, Willenborg S, Brinckmann J, Bloch W, Maass T, Wagener R, Krieg T, Sutherland T, Munitz A, Rothenberg ME, Niehoff A, Richardson R, Hammerschmidt M, Allen JE, Eming SA. Interleukin-4 receptor alpha signaling in myeloid cells controls collagen fibril assembly in skin repair. Immunity 2015; 43: 803–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989; 74: 2527–34. [PubMed] [Google Scholar]
- 31.Yrlid U, Jenkins CD, MacPherson GG. Relationships between distinct blood monocyte subsets and migrating intestinal lymph dendritic cells in vivo under steady-state conditions. J Immunol 2006; 176: 4155–62. [DOI] [PubMed] [Google Scholar]
- 32.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol 2004; 172: 4410–7. [DOI] [PubMed] [Google Scholar]
- 33.Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol 2013; 14: 821–30. [DOI] [PubMed] [Google Scholar]
- 34.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med 2007; 204: 171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruparelia N, Godec J, Lee R, Chai JT, Dall'Armellina E, McAndrew D, Digby JE, Forfar JC, Prendergast BD, Kharbanda RK, Banning AP, Neubauer S, Lygate CA, Channon KM, Haining NW, Choudhury RP. Acute myocardial infarction activates distinct inflammation and proliferation pathways in circulating monocytes, prior to recruitment, and identified through conserved transcriptional responses in mice and humans. Eur Heart J 2015; 36: 1923–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009; 325: 612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rzeniewicz K, Newe A, Rey Gallardo A, Davies J, Holt MR, Patel A, Charras GT, Stramer B, Molenaar C, Tedder TF, Parsons M, Ivetic A. L-selectin shedding is activated specifically within transmigrating pseudopods of monocytes to regulate cell polarity in vitro. Proc Natl Acad Sci USA 2015; 112: E1461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collison JL, Carlin LM, Eichmann M, Geissmann F, Peakman M. Heterogeneity in the locomotory behavior of human monocyte subsets over human vascular endothelium in vitro. J Immunol 2015; 195: 1162–70. [DOI] [PubMed] [Google Scholar]
- 39.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007; 317: 666–70. [DOI] [PubMed] [Google Scholar]
- 40.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest 2007; 117: 185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol 2011; 12: 778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16 + monocytes. J Exp Med 2003; 197: 1701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 2006; 124: 175–89. [DOI] [PubMed] [Google Scholar]
- 44.Avraham-Davidi I, Yona S, Grunewald M, Landsman L, Cochain C, Silvestre JS, Mizrahi H, Faroja M, Strauss-Ayali D, Mack M, Jung S, Keshet E. On-site education of VEGF-recruited monocytes improves their performance as angiogenic and arteriogenic accessory cells. J Exp Med 2013; 210: 2611–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015; 37: 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu AC, Raggatt LJ, Alexander KA, Pettit AR. Unraveling macrophage contributions to bone repair. Bonekey Rep 2013; 2: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 2010; 116: 829–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci USA 2014; 111: 16029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu H, Huang D, Saederup N, Charo IF, Ransohoff RM, Zhou L. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J 2011; 25: 358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol 2016; 17: 34–40. [DOI] [PubMed] [Google Scholar]
- 51.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 2011; 332: 1284–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013; 38: 792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 2014; 40: 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JK, Greter M, Becher B, Samokhvalov IM, Merad M, Ginhoux F. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 2015; 42: 665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015; 518: 547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005; 5: 953–64. [DOI] [PubMed] [Google Scholar]
- 57.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 2014; 159: 1312–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crane MJ, Daley JM, van Houtte O, Brancato SK, Henry WL, Jr, Albina JE. The monocyte to macrophage transition in the murine sterile wound. PLoS One 2014; 9: e86660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature 2001; 414: 98–104. [DOI] [PubMed] [Google Scholar]
- 60.Awojoodu AO, Keegan PM, Lane AR, Zhang Y, Lynch KR, Platt MO, Botchwey EA. Acid sphingomyelinase is activated in sickle cell erythrocytes and contributes to inflammatory microparticle generation in SCD. Blood 2014; 124: 1941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griffith CK, Miller C, Sainson RC, Calvert JW, Jeon NL, Hughes CC, George SC. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng 2005; 11: 257–66. [DOI] [PubMed] [Google Scholar]
- 62.Lee TT, Garcia JR, Paez JI, Singh A, Phelps EA, Weis S, Shafiq Z, Shekaran A, Del Campo A, Garcia AJ. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat Mater 2015; 14: 352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nickerson MM, Burke CW, Meisner JK, Shuptrine CW, Song J, Price RJ. Capillary arterialization requires the bone-marrow-derived cell (BMC)-specific expression of chemokine (C-C motif) receptor-2, but BMCs do not transdifferentiate into microvascular smooth muscle. Angiogenesis 2009; 12: 355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res 1997; 80: 829–37. [DOI] [PubMed] [Google Scholar]
- 65.Hoefer IE, van Royen N, Rectenwald JE, Deindl E, Hua J, Jost M, Grundmann S, Voskuil M, Ozaki CK, Piek JJ, Buschmann IR. Arteriogenesis proceeds via ICAM-1/Mac-1-mediated mechanisms. Circ Res 2004; 94: 1179–85. [DOI] [PubMed] [Google Scholar]
- 66.Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol 2002; 283: H2411–9. [DOI] [PubMed] [Google Scholar]
- 67.Melgar-Lesmes P, Edelman ER. Monocyte-endothelial cell interactions in the regulation of vascular sprouting and liver regeneration in mouse. J Hepatol 2015; 63: 917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med 2012; 209: 123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heil M, Ziegelhoeffer T, Wagner S, Fernandez B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann G, Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking CC-chemokine receptor-2. Circ Res 2004; 94: 671–77. [DOI] [PubMed] [Google Scholar]
- 70.Moldovan NI, Goldschmidt-Clermont PJ, Parker-Thornburg J, Shapiro SD, Kolattukudy PE. Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloelastase-positive tunnels in ischemic myocardium. Circ Res 2000; 87: 378–84. [DOI] [PubMed] [Google Scholar]
- 71.Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol 2012; 189: 3669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, Kjaer M, Chazaud B. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 2013; 31: 384–96. [DOI] [PubMed] [Google Scholar]
- 73.Zhao W, Lu H, Wang X, Ransohoff RM, Zhou L. CX3CR1 deficiency delays acute skeletal muscle injury repair by impairing macrophage functions. FASEB J 2016; 30: 380–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arnold L, Perrin H, de Chanville CB, Saclier M, Hermand P, Poupel L, Guyon E, Licata F, Carpentier W, Vilar J, Mounier R, Chazaud B, Benhabiles N, Boissonnas A, Combadiere B, Combadiere C. CX3CR1 deficiency promotes muscle repair and regeneration by enhancing macrophage ApoE production. Nat Commun 2015; 6: 8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, Bilbao-Cortes D, Godwin JW, Rosenthal NA. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One 2012; 7: e36814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF, Gorbatov R, Iwamoto Y, Dutta P, Wojtkiewicz G, Courties G, Sebas M, Borodovsky A, Fitzgerald K, Nolte MW, Dickneite G, Chen JW, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation 2013; 127: 2038–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sinder BP, Pettit AR, McCauley LK. Macrophages: their emerging roles in bone. J Bone Miner Res 2015; 30: 2140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol 2008; 181: 1232–44. [DOI] [PubMed] [Google Scholar]
- 79.Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, Levesque JP. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 2010; 116: 4815–28. [DOI] [PubMed] [Google Scholar]
- 80.Cho SW, Soki FN, Koh AJ, Eber MR, Entezami P, Park SI, van Rooijen N, McCauley LK. Osteal macrophages support physiologic skeletal remodeling and anabolic actions of parathyroid hormone in bone. Proc Natl Acad Sci USA 2014; 111: 1545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vi L, Baht GS, Whetstone H, Ng A, Wei Q, Poon R, Mylvaganam S, Grynpas M, Alman BA. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res 2015; 30: 1090–102. [DOI] [PubMed] [Google Scholar]
- 82.Champagne CM, Takebe J, Offenbacher S, Cooper LF. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone 2002; 30: 26–31. [DOI] [PubMed] [Google Scholar]
- 83.Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L, Li C, Xie L, Crane J, Wan M, Zhen G, Bian Q, Yu B, Chang W, Qiu T, Pickarski M, Duong LT, Windle JJ, Luo X, Liao E, Cao X. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med 2014; 20: 1270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xing Z, Lu C, Hu D, Yu YY, Wang X, Colnot C, Nakamura M, Wu Y, Miclau T, Marcucio RS. Multiple roles for CCR2 during fracture healing. Dis Model Mech 2010; 3: 451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raggatt LJ, Wullschleger ME, Alexander KA, Wu AC, Millard SM, Kaur S, Maugham ML, Gregory LS, Steck R, Pettit AR. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am J Pathol 2014; 184: 3192–204. [DOI] [PubMed] [Google Scholar]
- 86.Alexander KA, Chang MK, Maylin ER, Kohler T, Muller R, Wu AC, Van Rooijen N, Sweet MJ, Hume DA, Raggatt LJ, Pettit AR. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res 2011; 26: 1517–32. [DOI] [PubMed] [Google Scholar]
- 87.Goodman SB. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials 2007; 28: 5044–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol 2008; 20: 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rettig L, Haen SP, Bittermann AG, von Boehmer L, Curioni A, Kramer SD, Knuth A, Pascolo S. Particle size and activation threshold: a new dimension of danger signaling. Blood 2010; 115: 4533–41. [DOI] [PubMed] [Google Scholar]
- 90.Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, Jhunjhunwala S, Chiu A, Siebert S, Tang K, Hollister-Lock J, Aresta-Dasilva S, Bochenek M, Mendoza-Elias J, Wang Y, Qi M, Lavin DM, Chen M, Dholakia N, Thakrar R, Lacik I, Weir GC, Oberholzer J, Greiner DL, Langer R, Anderson DG. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat Mater 2015; 14: 643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kusaka T, Nakayama M, Nakamura K, Ishimiya M, Furusawa E, Ogasawara K. Effect of silica particle size on macrophage inflammatory responses. PLoS One 2014; 9: e92634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tabata Y, Ikada Y. Effect of the size and surface charge of polymer microspheres on their phagocytosis by macrophage. Biomaterials 1988; 9: 356–62. [DOI] [PubMed] [Google Scholar]
- 93.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA 2006; 103: 4930–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Underhill DM, Goodridge HS. Information processing during phagocytosis. Nat Rev Immunol 2012; 12: 492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Padmanabhan J, Kinser ER, Stalter MA, Duncan-Lewis C, Balestrini JL, Sawyer AJ, Schroers J, Kyriakides TR. Engineering cellular response using nanopatterned bulk metallic glass. ACS Nano 2014; 8: 4366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen S, Jones JA, Xu Y, Low HY, Anderson JM, Leong KW. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials 2010; 31: 3479–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garg K, Pullen NA, Oskeritzian CA, Ryan JJ, Bowlin GL. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 2013; 34: 4439–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sanders JE, Stiles CE, Hayes CL. Tissue response to single-polymer fibers of varying diameters: evaluation of fibrous encapsulation and macrophage density. J Biomed Mater Res 2000; 52: 231–7. [DOI] [PubMed] [Google Scholar]
- 99.Cao H, McHugh K, Chew SY, Anderson JM. The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J Biomed Mater Res A 2010; 93: 1151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bartneck M, Heffels KH, Pan Y, Bovi M, Zwadlo-Klarwasser G, Groll J. Inducing healing-like human primary macrophage phenotypes by 3D hydrogel coated nanofibres. Biomaterials 2012; 33: 4136–46. [DOI] [PubMed] [Google Scholar]
- 101.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci USA 2013; 110: 17253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feng B, Jinkang Z, Zhen W, Jianxi L, Jiang C, Jian L, Guolin M, Xin D. The effect of pore size on tissue ingrowth and neovascularization in porous bioceramics of controlled architecture in vivo. Biomed Mater 2011; 6: 015007. [DOI] [PubMed] [Google Scholar]
- 103.Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, Ratner BD. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci USA 2010; 107: 15211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bota PC, Collie AM, Puolakkainen P, Vernon RB, Sage EH, Ratner BD, Stayton PS. Biomaterial topography alters healing in vivo and monocyte/macrophage activation in vitro. J Biomed Mater Res A 2010; 95: 649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Getts DR, Terry RL, Getts MT, Deffrasnes C, Muller M, van Vreden C, Ashhurst TM, Chami B, McCarthy D, Wu H, Ma J, Martin A, Shae LD, Witting P, Kansas GS, Kuhn J, Hafezi W, Campbell IL, Reilly D, Say J, Brown L, White MY, Cordwell SJ, Chadban SJ, Thorp EB, Bao S, Miller SD, King NJ. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci Transl Med 2014; 6: 219ra217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brodbeck WG, Patel J, Voskerician G, Christenson E, Shive MS, Nakayama Y, Matsuda T, Ziats NP, Anderson JM. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proc Natl Acad Sci USA 2002; 99: 10287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Muller RH. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B Biointerfaces 2000; 18: 301–13. [DOI] [PubMed] [Google Scholar]
- 108.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J Biomed Mater Res A 2003; 66: 247–59. [DOI] [PubMed] [Google Scholar]
- 109.Mutsaers SE, Papadimitriou JM. Surface charge of macrophages and their interaction with charged particles. J Leukoc Biol 1988; 44: 17–26. [DOI] [PubMed] [Google Scholar]
- 110.Patel NR, Bole M, Chen C, Hardin CC, Kho AT, Mih J, Deng L, Butler J, Tschumperlin D, Fredberg JJ, Krishnan R, Koziel H. Cell elasticity determines macrophage function. PLoS One 2012; 7: e41024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blakney AK, Swartzlander MD, Bryant SJ. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A 2012; 100: 1375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fereol S, Fodil R, Labat B, Galiacy S, Laurent VM, Louis B, Isabey D, Planus E. Sensitivity of alveolar macrophages to substrate mechanical and adhesive properties. Cell Motil Cytoskeleton 2006; 63: 321–40. [DOI] [PubMed] [Google Scholar]
- 113.Riedel T, Riedelova-Reicheltova Z, Majek P, Rodriguez-Emmenegger C, Houska M, Dyr JE, Brynda E. Complete identification of proteins responsible for human blood plasma fouling on poly(ethylene glycol)-based surfaces. Langmuir 2013; 29: 3388–97. [DOI] [PubMed] [Google Scholar]
- 114.Swartzlander MD, Barnes CA, Blakney AK, Kaar JL, Kyriakides TR, Bryant SJ. Linking the foreign body response and protein adsorption to PEG-based hydrogels using proteomics. Biomaterials 2015; 41: 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lynn AD, Bryant SJ. Phenotypic changes in bone marrow-derived murine macrophages cultured on PEG-based hydrogels activated or not by lipopolysaccharide. Acta Biomater 2011; 7: 123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lynn AD, Kyriakides TR, Bryant SJ. Characterization of the in vitro macrophage response and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A 2010; 93: 941–53. [DOI] [PubMed] [Google Scholar]
- 117.Wolf MT, Dearth CL, Ranallo CA, LoPresti ST, Carey LE, Daly KA, Brown BN, Badylak SF. Macrophage polarization in response to ECM coated polypropylene mesh. Biomaterials 2014; 35: 6838–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, Hubbell JA. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol 2003; 21: 513–8. [DOI] [PubMed] [Google Scholar]
- 119.Patterson J, Hubbell JA. SPARC-derived protease substrates to enhance the plasmin sensitivity of molecularly engineered PEG hydrogels. Biomaterials 2011; 32: 1301–10. [DOI] [PubMed] [Google Scholar]
- 120.Phelps EA, Headen DM, Taylor WR, Thule PM, Garcia AJ. Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes. Biomaterials 2013; 34: 4602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hoffman AS. Stimuli-responsive polymers: biomedical applications and challenges for clinical translation. Adv Drug Deliv Rev 2013; 65: 10–16. [DOI] [PubMed] [Google Scholar]
- 122.Jay SM, Shepherd BR, Andrejecsk JW, Kyriakides TR, Pober JS, Saltzman WM. Dual delivery of VEGF and MCP-1 to support endothelial cell transplantation for therapeutic vascularization. Biomaterials 2010; 31: 3054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hsu CW, Poche RA, Saik JE, Ali S, Wang S, Yosef N, Calderon GA, Scott L Jr, Vadakkan TJ, Larina IV, West JL, Dickinson ME. Improved angiogenesis in response to localized delivery of macrophage-recruiting molecules. PLoS One 2015; 10: e0131643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials 2012; 33: 8793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Reeves AR, Spiller KL, Freytes DO, Vunjak-Novakovic G, Kaplan DL. Controlled release of cytokines using silk-biomaterials for macrophage polarization. Biomaterials 2015; 73: 272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jenkins SJ, Ruckerl D, Thomas GD, Hewitson JP, Duncan S, Brombacher F, Maizels RM, Hume DA, Allen JE. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J Exp Med 2013; 210: 2477–91. [DOI] [PMC free article] [PubMed] [Google Scholar]