Abstract
Recent studies have highlighted the fact that cancer cells have an altered metabolic phenotype, and this metabolic reprogramming is required to drive biosynthesis pathways necessary for rapid replication and proliferation. Specifically, the importance of tricarboxylic acid (TCA) cycle-generated intermediates in the regulation of cancer cells proliferation has been recently appreciated. One function of monocarboxylate transporters (MCTs) is to transport the TCA cycle substrate pyruvate across the plasma membrane and into mitochondria, and inhibition of MCTs has been proposed as a therapeutic strategy to target metabolic pathways in cancer. Here, we examined the effect of different metabolic substrates (glucose and pyruvate) on mitochondrial function and proliferation in breast cancer cells. We demonstrated that cancer cells proliferate more rapidly in the presence of exogenous pyruvate when compared to lactate. Pyruvate supplementation fueled mitochondrial oxygen consumption and the reserve respiratory capacity, and this increase in mitochondrial function correlated with proliferative potential. In addition, inhibition of cellular pyruvate uptake using the MCT inhibitor α-cyano-4-hydroxycinnamic acid impaired mitochondrial respiration and decreased cell growth. These data demonstrate the importance of mitochondrial metabolism in proliferative responses and highlight a novel mechanism of action for MCT inhibitors through suppression of pyruvate-fueled mitochondrial respiration.
Keywords: reserve capacity, mitochondria, α-cyano-4-hydroxycinnamic acid, extracellular flux technology, Warburg effect
1. Introduction
Otto Warburg first described the increased utilization of anaerobic metabolism in the presence of adequate oxygen by cancer cells compared to their normal counterparts – termed the ‘Warburg effect’ [37]. Since these initial observations, it is now clear that classic oncogene activity not only regulates proliferation, but also leads to alterations in metabolic pathways (e.g. glutaminolysis, glycolysis, and mitochondrial function) which may play a causative role in tumor development [7]. Recent studies have highlighted the fact that metabolic reprogramming of cancer cells is required to drive biosynthesis pathways which enables rapid replication and proliferation [7]. As such, the targeting of metabolic pathways is emerging as a novel strategy in the treatment of many malignancies.
An emerging concept in the field of cancer metabolism is the importance of mitochondrial metabolism, particularly tricarboxylic acid (TCA) cycle activity, in providing intermediates required for the biosynthesis of cellular macromolecules (e.g. fatty acids, non-essential amino acids). It is now clear that metabolism of mitochondrial substrates such as glutamine and pyruvate is necessary to support the rapid proliferation of multiple cancer cell types (e.g. colon, glioblastoma), and a functional link between mitochondrial respiration and proliferative capacity has been established [24;38].
In the current study, we have examined the role of monocarboxylate transporters (MCTs) because of their critical role in transportation of multiple monocarboxylate molecules, in particular pyruvate, across cell membranes [4;13]. In normal physiology, MCTs play a crucial role in ‘lactate shuttles’ where they function to transport lactate between cells (e.g. white-glycolytic and red-oxidative fibers in working muscle) or between intracellular compartments (e.g. lactate uptake into mitochondria) [2]. At least 14 members of this transporter family have been identified and have unique tissue expression patterns and kinetic properties; however in the context of cancer, expression of MCT1 and MCT4 has been best-characterized. Expression of both MCT1 and MCT4 has been shown to be elevated in several tumor types when compared to matched, normal tissue (e.g. breast, prostate, ovarian, cervix, and gastrointestinal tract), and high levels of these proteins often correlate with poor prognosis and disease progression [5;14;26–28]. MCT expression is also thought to underpin aspects of the Warburg effect. Since highly glycolytic cancer cells produce increased levels of lactate, MCT-dependent lactate efflux from cells is required to maintain intracellular pH and avoid cytotoxic accumulation of lactate. A central role for MCTs in cancer is further supported by clinical evidence which demonstrates that tumor-produced lactate correlates with poor prognosis and resistance to radiotherapy [29;35;36]. For these reasons, inhibition of MCTs has been proposed as a therapeutic strategy targeting metabolic pathways in cancer. Preclinical studies have demonstrated that inhibition of MCTs using the compound α-cyano-4-hydroxycinnamic acid (CHC) decreases tumor size and sensitizes hypoxic tumor regions to radiotherapy [31].
The effects of MCT inhibitors on cancer cell growth have largely been attributed to their ability to block lactate efflux; however, since MCTs also transport other monocarboxylates such as pyruvate, MCT inhibition will likely result in changes in the influx and/or efflux of other metabolically important molecules. Increasing evidence shows that energy substrates metabolized through mitochondria (e.g. glutamine, pyruvate) are required for biosynthesis of macromolecules in rapidly dividing cells [6]; thus, here, we examined the effect of metabolic substrates (glucose and pyruvate) on breast cancer cell proliferation and mitochondrial function. We demonstrated that cancer cells proliferate more rapidly when provided pyruvate when compared to glucose, but this effect was not observed with lactate. In addition, inhibition of cellular pyruvate uptake using the MCT inhibitor CHC decreases cell growth.
Pyruvate supplementation fueled mitochondrial oxygen consumption, and altered mitochondrial function correlated with proliferative potential. These data demonstrate that metabolism through mitochondria drives cancer cell proliferation and highlight a novel mechanism of action for MCT inhibitors in cancer.
2. Materials and Methods
2.1 Materials
All chemicals were of analytical grade and purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Dimethyl sulfoxide (DMSO; 0.1%) was used as a vehicle control for CHC in all studies.
2.2 Cell culture
MDA-MB231 (MB231) and T-47D human mammary adenocarcinoma cells were obtained from American Type Culture Collection (Manassas, VA), and MCF7 human mammary adenocarcinoma cells were a generous gift from Dr. B. Kalyanaraman. All cells were maintained in low glucose (5.56 mM) DMEM media containing sodium pyruvate (1 mM) and glutamine (4 mM) supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), 200 U/mL penicillin, and 200 µg/mL streptomycin. Cultures were incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
In some experiments, media containing different metabolic substrates was used. In these experiments the base media was DMEM powder (CellGro, Manassas, VA), and metabolic substrates were supplemented to the final concentrations denoted. Sodium bicarbonate (44 mM) was added to media for all assays except extracellular flux analysis. FBS was added to media for all assays except extracellular flux analysis and glucose/pyruvate uptake assays.
2.3 MTT assay
Cells were seeded at a density of 5,000 cells/well in 48-well cluster plates and allowed to attach and grow for 24 h. Cells were then washed with sterile PBS, and DMEM medium supplemented with 10% FBS and specified metabolic substrates (glucose, sodium pyruvate, and lactate) were added. Media were replaced daily to ensure metabolic substrates did not become limiting. Cell proliferation was monitored every 24 h for growth kinetics assays or after 72 h treatment by MTT assay as described previously [18]. Treatment media were replaced with complete culture medium containing 0.4 mg/mL thiazoyl blue tetrazolium, and the cells were incubated at 37°C for 2 h. The medium was then removed, the resulting formazan crystals were solubilized in DMSO, and the absorbance was read at 590 nm with background reference at 620 nm. MTT assay absorbance values correlated with total number of cells per well (result not shown).
2.4 Trypan Blue exclusion assay
After indicated treatment, cells were trypsin-harvested and diluted 1:1 with 0.4% Trypan Blue. The Countess Automated Cell Counter (Invitrogen) was then used to score the percentage of cells which excluded Trypan Blue.
2.5 Colony formation assay
For acute treatment studies, colony formation was measured after treatment in indicated metabolic substrate containing media. Cells from experimental dishes were trypsinized and collected. All cells from each dish were then centrifuged, resuspended in fresh medium, and counted using a Countess Automated Cell Counter. Cells were then plated in 6-well cluster plates at low density (100–200 cells per well), and clones were allowed to grow for 7 days in complete culture medium in the presence of 0.1% gentamycin. Cells were subsequently fixed with 70% ethanol and stained with Coomassie blue for analysis of colony formation as previously described [32]. For chronic treatment studies, routinely cultured cells were collected and counted as described above and then 200–400 cells per well plated into 6-well cluster plates containing DMEM media supplemented with 10% FBS, 0.1% gentamycin, and indicated metabolic substrates (5.56 mM glucose, 1 mM sodium pyruvate, or both). Clones were allowed to grow for 14 days prior to fixation, staining, and scoring of colony formation.
2.6 Extracellular flux technology
To measure mitochondrial function in intact breast cancer cells, a Seahorse Bioscience XF24 Extracellular Flux Analyzer (North Billerica, MA) was used [10;11;20;39]. This instrument allows for the sensitive measurement of oxygen consumption rates (OCRs) from adherent, intact cultured cells. The mitochondrial function assay employed in the present study used sequential injection of oligomycin (1 µg/mL) and carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP; 3 µM) to define the mitochondrial function parameters basal OCR, maximal OCR, and reserve respiratory capacity as described previously [9]. All assays were conducted using a seeding density of 20,000 cells/well in unbuffered DMEM supplemented with specified metabolic substrates (5.56 mM glucose, 1 mM lactate, and/or 0.1–5 mM sodium pyruvate), and the AKOS algorithm was used. Cells were switched to assay media 1 h prior to the beginning of the assay and maintained at 37°C. Values were normalized to the total protein per well after the completion of the XF assay by the Bradford protein assay (Bio-Rad, Hercules, CA).
2.7 Glucose and pyruvate consumption assays
The consumption of glucose and pyruvate by cells was monitored by assaying media levels of these metabolic substrates before and after 4 h incubation with cells using commercially available kits (BioVision, Mountain View, CA). Substrate consumption was then normalized to total cell number. For analysis of glucose consumption and pyruvate consumption, cells were incubated in 0.75 mL DMEM media without FBS supplemented with 5.56 mM glucose or 1 mM sodium pyruvate, respectively. Media lactate levels were measured using a commercially available kit (BioVision).
2.8 Western blot analysis of MCT expression
The protein levels of MCT1, MCT4, and β-actin were assessed using Western blot analysis after reducing SDS-PAGE. Briefly, the cells were harvested in lysis buffer consisting of 50 mM Tris·HCl (pH 8.0), 150 mM NaCl, 0.5% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, and protease inhibitors, and 20 µg of cell lysate proteins were separated by 10% SDS-PAGE and then transferred onto nitrocellulose membranes. Membranes were blocked with 5% nonfat milk/TBS-T solution for 1 h at room temperature, and then incubated with antibodies specific for MCT1 (Santa Cruz Biotechnology, Santa Cruz, CA), MCT4 (Santa Cruz Biotechnology), or β-actin (Cell Signaling, Danvers, MA) overnight at 4°C. After washing with TBS-T, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies, and proteins were visualized using SuperSignal West Pico or Femto chemiluminescence substrate on film (Pierce, Rockford, IL). Adobe Photoshop 7.0 software (San Jose, CA) was used for image processing.
2.9 HPLC analysis of nucleotides
Adenine nucleotides (ATP and ADP) and NAD+ were extracted using perchloric acid precipitation as described previously [23]. Solvent A (75 µL; 0.1 mol/L potassium phosphate, 4 mmol/L tetrabutylammonium bisulfate, pH 6.0, and water 64:36) was added to supernatants, and they were filtered prior to HPLC analysis. Protein pellets were resuspended in 0.5 N NaOH (200 µL), and protein concentration was determined by the method of Bradford. HPLC analysis of nucleotides based on a previously described method [40] was performed on Kinetex C-18 column (2.6 µm, 100 mm × 4.6 mm I.D.) using solvent A and solvent B (0.1 mol/L potassium phosphate, 4 mmol/L tetrabutylammonium bisulfate, pH 6.0, and methanol 64:36) with a flow rate of 1 mL/min. For NADH measurements, samples were analyzed using the same chromatographic conditions but cells were harvested under alkaline conditions (0.5 N KOH/Hank’s Balanced Salts Solution,3:1). The pH of lysates was adjusted to ~8 using 6 N HCl and ammonium acetate (1 M, pH 4.7) and samples were filtered prior to HPLC analysis. ATP, ADP, NAD+, and NADH peaks levels were measured for each sample, compared to the standard, and expressed in nanomoles per milligram of protein.
2.10 Statistical analysis
Results are reported as means ± standard error of the mean (SEM) for n ≥ 3 as indicated in the figure legends. Statistical significance was evaluated by Student’s t-test. The minimum level of significance was set at p < 0.05.
3. Results
3.1 Effect of metabolic substrates on breast cancer cell proliferation
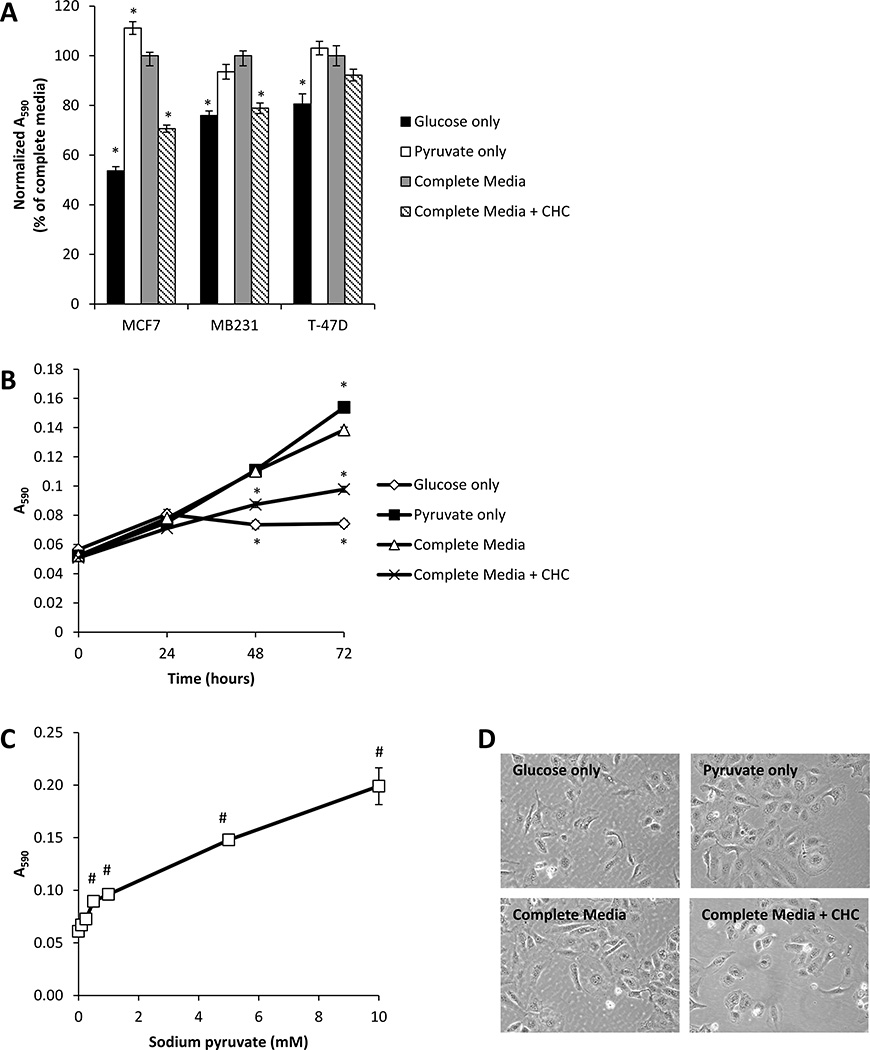
To determine on the effect of metabolic substrates on breast cancer cell proliferation, MCF7, MB231, and T-47D breast cancer cells were supplied with either glucose-only (5.56 mM), pyruvate-only (1 mM), or both glucose and pyruvate (referred to as ‘complete media’) in DMEM media, and cell proliferation was determined after 72 h (Fig. 1A). In all three cell lines, more rapid proliferation was observed for cells provided pyruvate-only when compared to glucose-only. In addition, growth of cells on pyruvate-only was comparable to that of cells provided complete media. We also examined the effect of the classical MCT inhibitor CHC on cell growth. Treatment with CHC (500 µM, a concentration 3 times higher than the IC50 [4]) in complete media resulted in a significant decrease in the total number of cells after 72 h incubation for MCF7 and MB231 cells, but not for T-47D cells.
Figure 1. Effect of metabolic substrates on breast cancer cell proliferation.
MCF7, MB231, and T-47D cells were cultured in media containing glucose only (5.56 mM), pyruvate only (1 mM), glucose and pyruvate (complete media), or complete media containing CHC (500 µM) for 72 h. Total cell number was then assessed using the MTT assay (A). MCF7 cells were exposed to the same media conditions for 24–72 h, and total cell number was assessed using the MTT assay (B). Media containing glucose (5.56 mM) and increasing concentrations of pyruvate (0.1–10 mM) were used to culture MCF7 cells for 72 h prior to assessment of total cell number (C). Light micrographs of cells cultured in media containing specified metabolic substrates for 72 h (D). Values represent means ± SEM, n = 6. * p < 0.05 compared to complete media. # p < 0.05 compared to 0 mM sodium pyruvate.
The growth kinetics of MCF7 cells cultured under similar conditions are shown in Fig. 1B. Over 72 h, cells proliferated most rapidly when supplied either pyruvate-only or complete media when compared to the glucose-only condition. CHC also significantly decreased proliferation of MCF7 cells at the 48 and 72 h time points. In addition, cell proliferation in presence of pyruvate was concentration-dependent (Fig. 1C). Light micrographs of MCF7 cells cultured in different metabolic substrates for 72 h are shown in Fig. 1D, and marked morphological changes were not observed between any conditions.
Since supplementation of pyruvate clearly promoted proliferation, we examined whether another physiologic monocarboxylate, lactate, could also promote cell growth. MCF7 cells were cultured for 72 h in media containing glucose (5.56 mM) and increasing concentrations (0.1–10 mM) of lactate; however, lactate supplementation had no effect on proliferative responses (Supplemental Fig. 1A). To further understand the difference between lactate and pyruvate supplementation, we examined whether there were limitations in the ability to convert lactate to pyruvate in the cell through lactate dehydrogenase (LDH). We measured both the activity of LDH and levels of NAD+ and NADH in MCF7 cells provided different metabolic substrates for 72 h. LDH catalyzes the conversion of lactate to pyruvate using NAD+ as a cofactor. There was no difference in LDH activity or levels of NAD+ and NADH in cells cultured in glucose + lactate or glucose + pyruvate media (Supplemental Fig. 1B,C). Moreover, NAD+ levels were always significantly higher than NADH, a condition which should promote the conversion of lactate to pyruvate.
3.2 Effect of metabolic substrates on MCF7 cell viability and colony formation
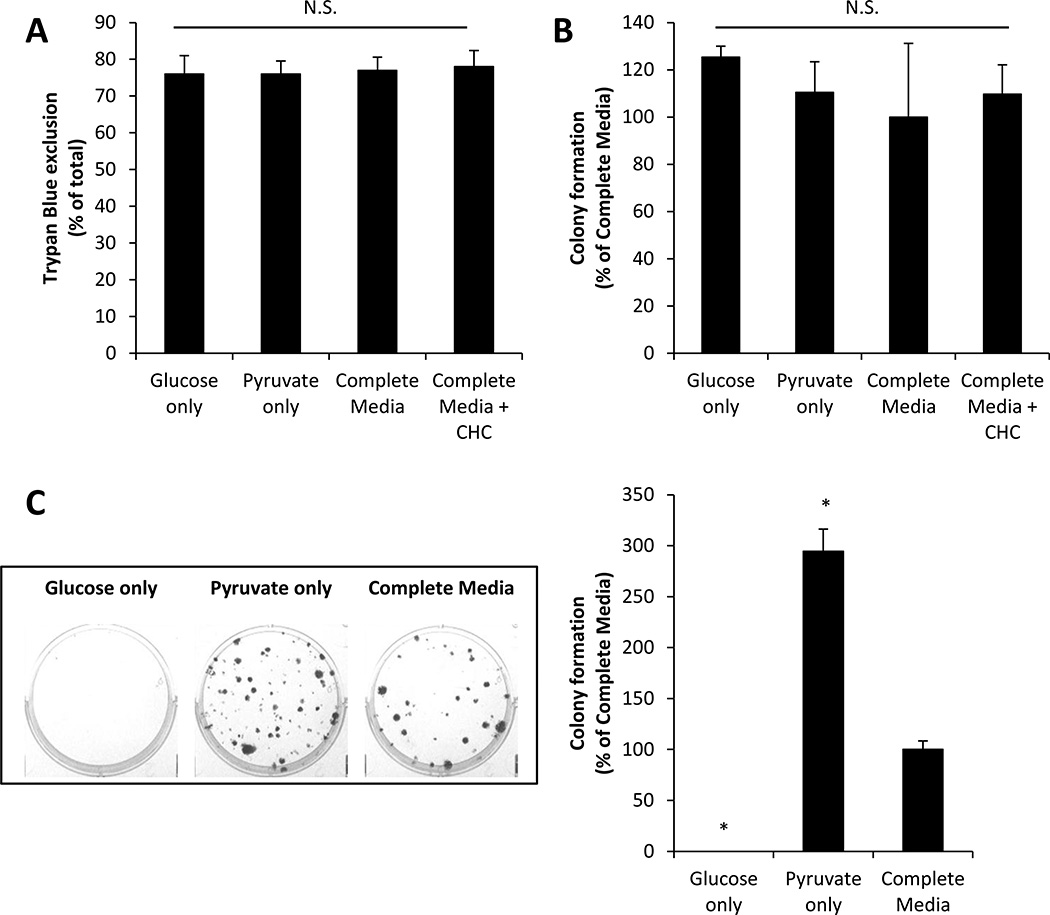
Our results suggest that pyruvate may be a more preferable metabolic substrate than glucose for promoting cell proliferation; however, it is also possible that alterations in metabolic substrates may by cytotoxic. To examine this, MCF7 cells were incubated in glucose-only, pyruvate-only, or complete media for 48 h and then cell viability was assessed by Trypan Blue exclusion assay. There was no significant difference in the number of cells which excluded Trypan Blue (scored as viable) in any condition (Fig. 2A). Exposure of cells to CHC (500 µM) in complete media (48 h) also had no effect on cell viability.
Figure 2. Effect of metabolic substrates on MCF7 cell viability and colony formation.
MCF7 cells were cultured in media containing glucose only (5.56 mM), pyruvate only (1 mM), glucose and pyruvate (complete media), or complete media containing CHC (500 µM) for 48 h. The number of cells which exclude Trypan Blue (A) and colony formation (B) was quantified. MCF7 cells were also seeded at low density in media containing glucose only (5.56 mM), pyruvate only (1 mM), or glucose and pyruvate (complete media), and the number of colonies formed was measured. Representative images of the cloning wells and quantification are shown (C). Values represent means ± SEM, n = 6. * p < 0.05 compared to complete media. N.S. denotes no significant difference between groups.
To confirm these observations, we exposed MCF7 cells to the different metabolic substrate-containing media, and their ability to form colonies was assessed. Following incubation in glucose-only, pyruvate-only, complete media, or complete media + CHC (500 µM) for 48 h, MCF7 cells were seeded at low density (100–200 cells/well) and allowed to form colonies for 7 days in DMEM culture media (described in Materials and Methods section). Pre-exposure (48 h) to different metabolic substrates or CHC had no effect on colony formation (Fig. 2B). This result further supports the conclusion that alterations in metabolic substrate supply do not cause overt cell death.
To examine the substrate-dependence of proliferative capacity of cancer cells, MCF7 cells were seeded at low density in glucose-only, pyruvate-only, or complete media, and colony formation was quantified after 14 days. Figure 2C shows both representative images from cloning plates as well as quantification of these data. MCF7 cells were unable to form colonies in glucose-only media; however, colony formation was observed in both the pyruvate-only and complete media conditions. Surprisingly, significantly more colonies were formed in pyruvate-only media when compared to cloning in complete media. Taken together, these data indicate that metabolic substrates regulate cancer cell proliferation and do not cause overt cell death. Moreover, pyruvate appears to be a key metabolic substrate to support cell proliferation.
3.3 MCT expression and effects of CHC on metabolic substrate transport into cells
In the present study, CHC is used as an inhibitor of MCTs which function, in part, to transport lactate and pyruvate across cellular membranes [4]. To confirm that CHC does in fact inhibit pyruvate transport into cells without interfering with glucose transport into cells, we treated MCF7 cells with increasing concentrations of CHC (50–500 µM) for 4 h, and assayed media glucose and pyruvate concentration before and after this incubation. As expected, there was no effect of CHC on glucose consumption by MCF7 cells after 4 h (Fig. 3A); however, treatment with CHC caused a concentration-dependent inhibition of pyruvate consumption by these cells (Fig.3B). In the concentration-range examined here, CHC was not capable of blocking lactate excretion from these cells (Fig. 3C). In fact, treatment with 500 µM CHC for 4 h resulted in a small, but significant increase in media lactate levels. These results confirm that CHC is an effective inhibitor of cellular pyruvate consumption presumably through the inhibition of MCTs; however, lactate export from cells was not blocked.
Figure 3. Effects of CHC on metabolic substrate transport into cells.
Cellular consumption of glucose (A) and pyruvate (B) from the media after exposure to increasing concentrations of CHC (50–500 µM) was determined in MCF7 cells. Lactate production from cells was assessed under the same experimental conditions (C). Glucose, pyruvate, and lactate levels in media were determined before and after 4 h incubation with cells. Values were normalized to total cell number/well and represent means ± SEM, n=3–5. * p < 0.05 compared to control. N.S. denotes no significant difference between groups.
3.4 Regulation of mitochondrial function by metabolic substrate supply
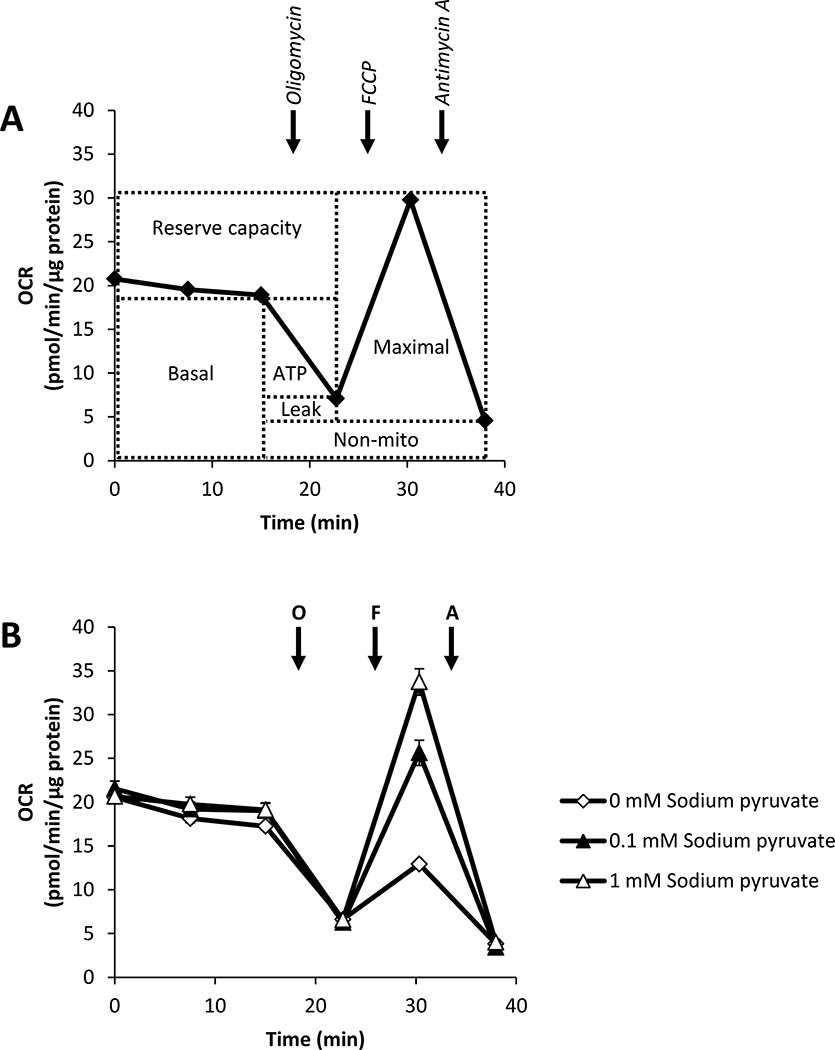
While a large literature supports the integral role of glucose metabolism in cancer (reviewed in [17]), our results indicate that pyruvate may also be important for rapid proliferation of cancer cells. Since pyruvate is a key substrate for mitochondrial function, we next examined the effects of pyruvate and glucose supplementation on mitochondrial function in MCF7 cells. We used novel extracellular flux technology [10] to assess multiple parameters of mitochondrial function: 1. basal oxygen consumption rate (OCR), 2. maximal OCR, 3. ATP-linked respiration, 4. proton leak, 5. reserve capacity, and 6. oxygen consumption independent of Complex IV (termed ‘non-mitochondrial’) in MCF7 cells. These parameters can be assessed using a protocol of OCR measurement after the sequential injection of oligomycin (ATP synthase inhibitor), FCCP (uncoupling agent), and Antimycin A (Complex III inhibitor). A typical OCR trace from this mitochondrial function assay and how each parameter is derived is shown in Fig. 4A. Using this protocol, MCF7 cells were assayed in media supplemented with glucose (5.56 mM) and increasing concentrations of pyruvate (0.1–5 mM). Representative OCR traces from cells assayed in media containing 0, 0.1, and 1 mM pyruvate are shown in Fig. 4B. Results from the entire concentration-dependency are shown in Fig. 5.
Figure 4. Assessment of mitochondrial function using extracellular flux technology.
A schematic representation of the mitochondrial function assay is shown in Panel A. After establishment of baseline oxygen consumption rate (OCR), sequential injection of oligomycin (O), FCCP (F), and Antimycin A (A) allows for the determination of multiple mitochondrial function parameters including basal OCR, maximal OCR, ATP-linked OCR (ATP), proton leak (leak), reserve capacity, and OCR independent of Complex IV (non-mito). MCF7 cells were seeded in specialized microplates and cultured for 24 h. Cells were then switched to unbuffered DMEM media supplemented with glucose (5.56 mM) and increasingly concentrations of pyruvate (0.1–5 mM) and mitochondrial function was assessed (B). OCRs were normalized to total protein/well after completion of assay, and representative OCR traces are shown. Values represent means ± SEM, n=3–5.
Figure 5. Regulation of mitochondrial function by pyruvate.
MCF7 cells were seeded in specialized microplates and cultured for 24 h. Cells were then switched to unbuffered DMEM media supplemented with glucose (5.56 mM) and increasingly concentrations of pyruvate (0.1–5 mM) and mitochondrial function was assessed using sequential injection of oligomycin, FCCP, and Antimycin A. Basal and maximal oxygen consumption rate (OCR) (A), ATP-linked OCR (B), proton leak (C), reserve capacity (D), and non-mitochondrial OCR (E) are shown. Extracellular acidification rate (ECAR) was measured concomitantly (F). OCR and ECAR were normalized to total protein/well after completion of assay. Values represent means ± SEM, n=3–5. * p < 0.05 compared to 0 mM sodium pyruvate. N.S. denotes no significant difference between groups.
Interestingly, while the addition of pyruvate had little effect on basal OCR, there was a concentration-dependent increase in the maximal OCR with increasing concentrations of pyruvate (Fig. 5A). This stimulation of maximal OCR by pyruvate also translates to a concentration-dependent increase in the reserve capacity (Fig. 5D). Pyruvate had little effect on the mitochondrial function parameters ATP-linked OCR, proton leak, and non-mitochondrial OCR (Fig. 5B,C,E). Extracellular acidification rate (ECAR), an indicator of glycolytic flux, was measured under the same condition, and a slight decrease was observed with pyruvate supplementation (Fig. 5F).
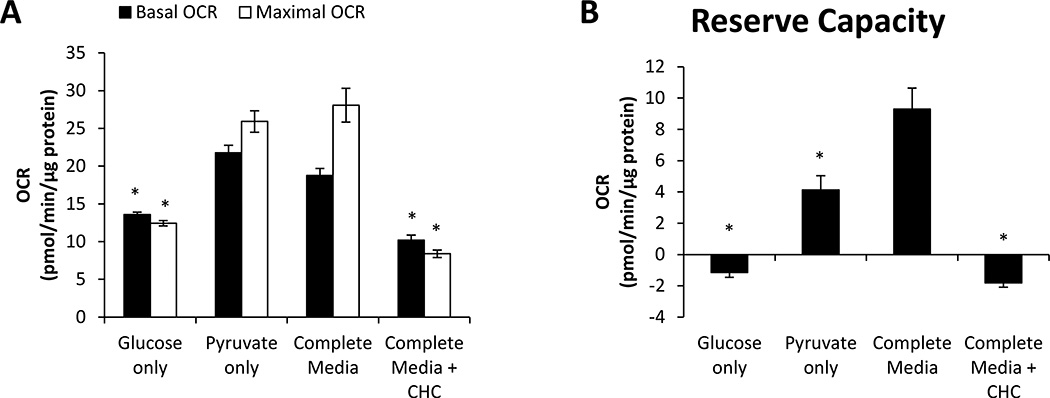
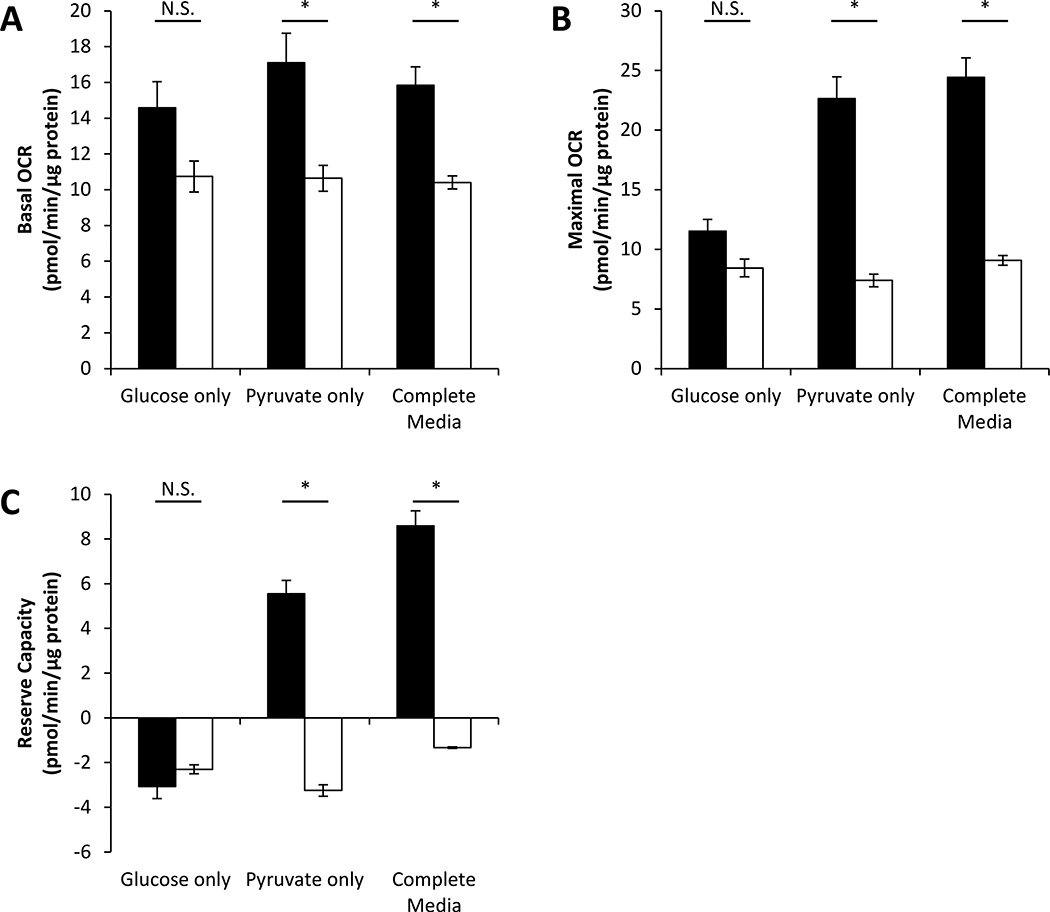
We next determined the effect of culture in glucose-only, pyruvate-only, and complete media on these mitochondrial function parameters. After 1 h incubation, basal and maximal OCR were significantly lower in cells provided only glucose in the media; however, there was little difference in either of these parameters for cells provided only pyruvate or complete media (Fig. 6A). Treatment with CHC, the MCT inhibitor, also caused a significant decrease in basal and maximal OCR when compared to complete media. Calculation of the reserve capacity from these data showed that cells provided complete media have the largest reserve capacity (Fig. 6B). Cells provided pyruvate-only have a significantly smaller reserve capacity than complete media, and cells provided only glucose or complete media + CHC have no reserve capacity. The time-dependent OCR traces from this experiment as well as the effects on other mitochondrial function parameters are shown in Supplemental Figure 2, and the effects on ATP-linked OCR largely mirror the effects on basal and maximal OCR (Fig. 6A). Not surprisingly, ECAR was also higher under conditions where glucose was present in the assay media.
Figure 6. Regulation of mitochondrial function by metabolic substrate supply.
MCF7 cells were seeded in specialized microplates and cultured for 24h. Cells were then switched to unbuffered DMEM containing glucose only (5.5.6 mM), pyruvate only (1 mM), glucose and pyruvate (complete media), or complete media with CHC (500 µM) 1 h prior to measuring mitochondrial function. Basal and maximal OCR (A) and the reserve capacity (B) were measured using sequential ingjection of oligomycin, FCCP, and Antimycin A. OCRs were normalized to total protein/well after completion of assay. Values represent means ± SEM, n=3–5. * p < 0.05 compared to complete media.
In additional studies, we compared the effect of pyruvate and lactate on mitochondrial function. Using a similar approach as described above, MCF7 cells that were provided pyruvate in the assay media had a high reserve capacity both in the presence and absence of glucose (Supplemental Fig. 3). In contrast, lactate was not able to support the reserve capacity independent on the presence of glucose in the assay media. Moreover, to relate our mitochondrial function results to cellular energy balance, we assessed ATP and ADP levels after culture in different metabolic substrates for 72 h. Interestingly, steady-state levels of these adenine nucleotides were unchanged by supplementation with different substrates (Supplemental Fig. 4). Thus, the changes in mitochondrial function observed here do not equate with changes in the steady-state levels of adenine nucleotide pools.
These data indicate that pyruvate uptake via pyruvate transporters (MCTs) contributes to mitochondrial respiration and is essential if cells need to access their respiratory reserve, as may be required for robust proliferation. Moreover, lactate, unlike pyruvate, does not support the reserve capacity in MCF7 cells.
3.5 Effect of CHC on mitochondrial function in the presence of different metabolic substrates
The primary mechanism of action for MCT inhibitors in cancer is thought to be through blocking lactate efflux in highly glycolytic cancer cells; however, we have shown that MCT inhibition also limits pyruvate uptake (Fig. 3). To disentangle whether CHC-dependent regulation of mitochondrial function occurs through deregulation of lactate efflux pathways or through limiting pyruvate uptake, we examined the effect of CHC on mitochondrial function in MCF7 cells. In this experiment, cells were supplied with glucose-only, pyruvate-only, or complete media. We reasoned that inhibition of lactate efflux would be the predominant mechanism of action in cells cultured in glucose-only media whereas inhibition of pyruvate uptake would predominate in pyruvate-only media. CHC (500 µM) significantly decreased basal and maximal OCR in cells provided pyruvate-only or complete media (Fig. 7A,B). CHC treatment did not significantly decrease these parameters in cells provided glucose-only. A similar pattern was observed for the effect of CHC on the reserve capacity. CHC treatment inhibited the reserve capacity only in cells cultured under pyruvate-only or complete media conditions (Fig. 7C). These results suggest that inhibition of pyruvate uptake by CHC regulates mitochondrial function in MCF7 cells. The time-dependent OCR traces from this experiment as well as the effects on other mitochondrial function parameters are shown in Supplemental Figure 5, and the effects on ATP-linked OCR largely mirror the effects on basal OCR (Fig. 7A). Interestingly, ECAR was stimulated by CHC in conditions where glucose was present in the assay media suggesting that at this concentration lactate efflux in not readily inhibited by CHC.
Figure 7. Effect of CHC on mitochondrial function in the presence of different metabolic substrates.
MCF7 cells were seeded in specialized microplates and cultured for 24 h. 1 h prior to assessment of mitochondrial function, cells were switched to unbuffered DMEM containing glucose only (5.56 mM), pyruvate only (1 mM), or glucose and pyruvate (complete media) in the absence (closed bars) or presence (open bars) of CHC (500 µM). Basal oxygen consumption rate (OCR) (A), maximal OCR stimulated with 3 µM FCCP (B), and reserve capacity (C) were measured. OCRs were normalized to total protein/well after completion of assay. Values represent means ± SEM, n=3–4. * p < 0.05 compared to media condition without CHC. N.S. denotes no significant difference between groups.
3.6 Effect of CHC on cell proliferation in the presence of different metabolic substrates
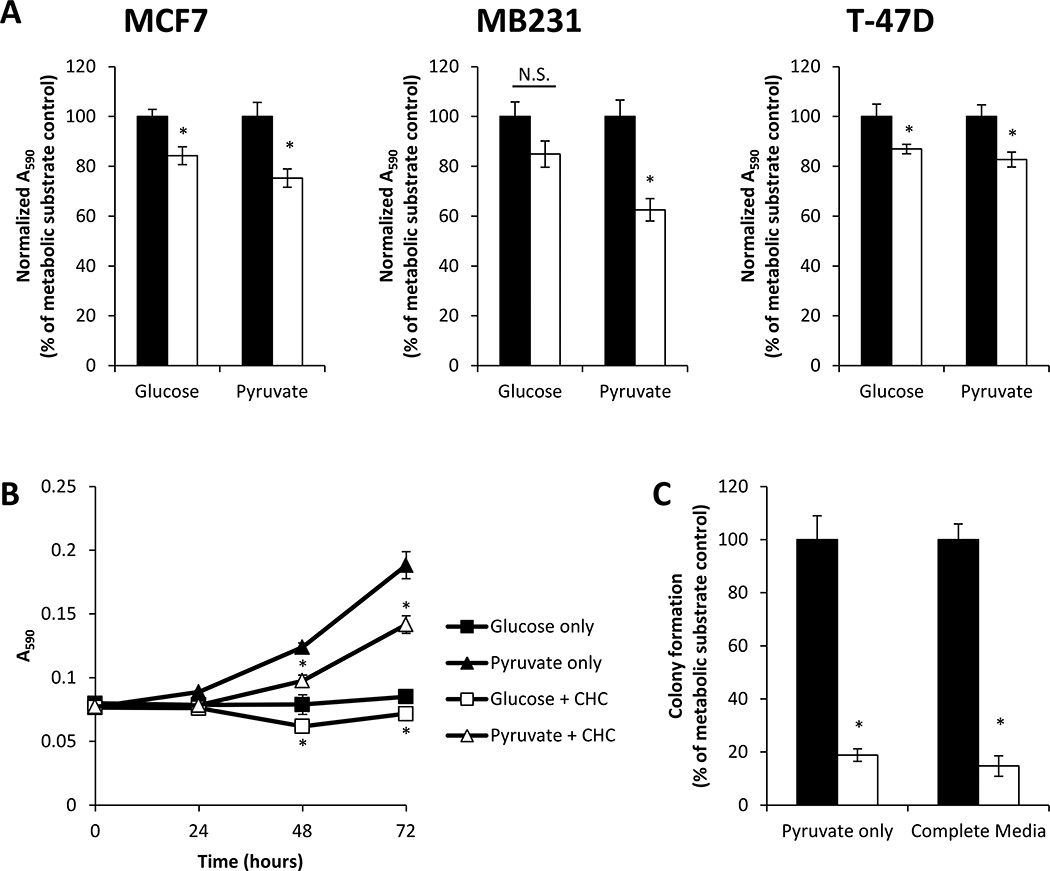
A similar approach was employed to examine whether inhibition of lactate efflux or pyruvate uptake by a MCT inhibitor is important in the regulation of cancer cell proliferation. MCF7, MB231, and T-47D breast cancer cells were treated with CHC (500 µM) in media containing only glucose or only pyruvate for 72 h. CHC decreased total cell number after treatment in all three cell types when cells were cultured in pyruvate-only media (Fig. 8A). Additionally, for both MCF7 and T-47D cells, but not MB231 cells, CHC treatment significantly decreased total cell number after incubation in glucose-only media.
Figure 8. Effect of CHC on cell proliferation in the presence of different metabolic substrates.
MCF7, MB231, and T-47D cells were cultured in media containing glucose only (5.56 mM) or pyruvate only (1 mM) in the absence (closed bars) or presence (open bars) of CHC (500 µM) for 72 h. Total cell number was then assessed using the MTT assay (A). MCF7 cells were exposed to the same media conditions for 24–72 h, and total cell number was assessed using the MTT assay (B). MCF7 cells were seeded at low density in media containing glucose only (5.56 mM), pyruvate only (1 mM), or glucose and pyruvate (complete media) in the absence (closed bars) or presence (open bars) of CHC (500 µM), and the number of colonies formed was measured (C). Values represent means ± SEM, n = 3–6. * p < 0.05 compared to media condition without CHC. N.S. denotes no significant difference between groups.
These findings were confirmed by examining the growth kinetics of MCF7 cells provided glucose or pyruvate with or without CHC (500 µM). CHC decreased cell proliferation in both media conditions (Fig. 8B). We also examined the effect of CHC on the ability of MCF7 cells to form colonies in different metabolic substrate containing media. There was significant inhibition of colony formation upon exposure to CHC in both pyruvate-only and complete media (Fig. 8C). No colonies were observed in glucose-only media with or without CHC (data not shown). In sum, these results demonstrate that MCT inhibition limits proliferation in the presence of only glucose or only pyruvate and imply that both lactate efflux and pyruvate uptake through MCTs are mechanistically linked to proliferative responses.
4. Discussion
In this study, we have examined the effect of different metabolic substrates on cancer cell proliferation and mitochondrial function. It is well-established that metabolic pathways are deregulated in cancer in a manner which supports rapid proliferation and provides a growth advantage over normal cells [3]. Since Otto Warburg’s early observations that cancer cells utilize glycolytic metabolism even in the presence of adequate oxygen, much of the research focus has been on the role of glycolysis in cancer. It is now becoming clear that while glycolysis is a less efficient means to generate ATP when compared to mitochondrial OXPHOS (2 ATP molecules per glucose molecule using glycolysis compared to 38 ATP molecules using OXPHOS), glycolytic metabolism provides a growth advantage through several different mechanisms (reviewed in [3]). These include generating ATP at a faster rate than OXPHOS, providing biosynthetic substrates needed for rapid proliferation (e.g. NADPH and ribose-5-phosphate) [19;33], and supporting cell growth under hypoxic conditions [30]. Though less well-understood, recent literature also demonstrates the important role mitochondria play in cancer cell proliferation through the metabolism of glutamine in the mitochondrial TCA cycle to supply additional biosynthetic substrates required for cell growth [6;33]. Because metabolic reprogramming is a nearly universal cancer phenotype, cancer cell metabolism is now thought to be an important therapeutic target in the treatment of many malignancies [7].
Here, we show that breast cancer cells (MCF7, MB231, and T-47D) proliferate more rapidly when provided pyruvate as a metabolic substrate when compared to glucose (Fig. 1), and this occurs in the absence of overt cell death (Fig. 2). This result is somewhat surprising given that glycolysis is preferentially upregulated in cancer cells when compared to their normal counterparts [37], and thus, our data imply that glycolysis alone is not sufficient to support rapid proliferation. This concept is consistent with recent reports that indicate that supplementation of energy substrates which can be metabolized through the mitochondrial TCA cycle and OXPHOS pathways (e.g. glutamine and pyruvate) support more rapid cancer cell growth when compared to glucose in several cell types (e.g. K-Ras transformed HCT-116 colon carcinoma cells and glioblastoma) [25;38]. We also compared the proliferative responses after supplementation of pyruvate versus lactate, another monocarboxylate that is transported through MCTs. While pyruvate supplementation led to a concentration-dependent increase in proliferation, lactate supplementation was unable to support cell growth (Supplemental Fig. 1). The different effects observed between these two monocarboxylates may result from alterations in their uptake into cells or their intracellular metabolism. Lactate can be converted to pyruvate by LDH using NAD+ as a cofactor. We found that LDH activity was not significantly different in cells cultured in lactate versus pyruvate (Supplemental Fig. 1). Moreover, there was no difference in the total levels of NAD+ or NADH in cells which were provided pyruvate or lactate, and NAD+ levels were higher than NADH in both substrate conditions which would allow for conversion of lactate to pyruvate (Supplemental Fig. 1). Therefore, neither of these findings clarifies why lactate was unable to promote proliferation, and further studies are warranted. Other possible explanations include that lactate is not readily transported into MCF7 cells or the rate at which lactate is metabolized to pyruvate is not sufficiently rapid to fuel mitochondrial respiration.
Recently, significant focus has been put on understanding the role of mitochondrial metabolism in cancer cells. New literature has highlighted the important ‘biosynthetic activity’ of mitochondria which generate substrates for lipid, protein, and nucleic acid biosynthesis in rapidly dividing cells [33]. Since pyruvate is a substrate for the TCA cycle, and thus drives mitochondrial function, we examined the effect of pyruvate on respiration in MCF7 cells. We showed that titration of pyruvate in the presence of glucose has little effect on the basal OCR; however, it clearly stimulated the maximal OCR elicited by the mitochondrial uncoupler FCCP (Fig. 5). In addition, cells cultured in media which contain pyruvate have high basal OCR, maximal OCR, and reserve capacity than those which are cultured in the absence of pyruvate (Fig. 6). Interestingly, there are no significant differences in the steady-state levels of ATP or ADP after culture in media containing varied metabolic substrates (Supplemental Fig. 4), so these results do not equate to general changes in cellular energy balance. Our results clearly demonstrate that pyruvate is required for MCF7 cells to have a reserve capacity, and there is also a correlation between the inability of lactate to promote cell growth (Fig. 1) and support the reserve capacity (Fig. 5,6). These findings are particularly interesting since it is thought that the reserve capacity is critical for maintaining cellular function in response to multiple stressors [9;16]. In the context of cancer, loss of the reserve capacity in breast cancer cells correlates with activation of cell death through mitochondrial apoptotic signaling pathways [8]. Drug-resistant glioma cells have also been shown to have increased reserve capacity and are not sensitive to cell killing by multiple agents [21]. Taken together, these data indicate that supplying metabolic substrates which drive mitochondrial respiration and, in specific support the reserve capacity of MCF7, correlates with more rapid proliferation of these cells.
Given the important role of pyruvate in fueling mitochondrial respiration and promoting cell growth, we also examined the effect of the inhibitor of pyruvate uptake into cells, CHC. First, to demonstrate this selective MCT inhibitor functions as expected in our system, we showed that treatment of MCF7 cells with CHC (50–500 µM) concentration-dependently inhibits pyruvate transport into cells without affecting glucose uptake (Fig. 3). Treatment of cells with CHC in Complete Media also slowed cancer cell growth in two of the three breast cancer cell lines examined (MCF7 and MB231; Fig. 1) and significantly decreased basal OCR, maximal OCR, and the reserve capacity (Fig. 6). Together, with the results from cells cultured with different metabolic substrates, this points to a link between mitochondrial function parameters and proliferative responses.
The primary mechanism of action for the regulation of cell growth by MCT inhibition is thought to be through blocking lactate efflux from highly glycolytic cancer cells. This inhibition of lactate efflux results in intracellular acidification and deregulation of cancer cell growth [34]. Our results suggest that pyruvate is an important metabolic substrate for cancer cell proliferation, and MCT inhibitors may work though blocking pyruvate metabolism in addition to their effects on lactate efflux pathways. To further investigate this concept, we examined the effects of CHC in cells provided glucose or pyruvate as metabolic substrates. We reasoned that in cells provided only glucose, the inhibition of lactate efflux would be the predominant mechanism of action for CHC; however, in cells provided only pyruvate, inhibition of pyruvate uptake into cells would dominate. Examining the effects of CHC on mitochondrial function showed that CHC decreased basal OCR, maximal OCR, and the reserve capacity of MCF7 cells supplied only pyruvate or complete media; however, there was not a significant effect of CHC on these parameters in cells provided only glucose (Fig. 7). Consistent with this, in both MCF7 and T-47D cells, inhibition of proliferation was observed after treatment with CHC in media containing either glucose or pyruvate. For MB231 cells, while there was a trend towards inhibition of growth with CHC in the glucose-only condition, statistically significant inhibition of growth was observed only when cells were provided pyruvate (Fig. 8). CHC also attenuated colony formation when cloning media contained only pyruvate.
Since CHC is known to be orders of magnitude more effective at inhibiting mitochondrial pyruvate transport than transport at the level of the plasma membrane [12], we believe the site of action for CHC in our model system is likely the mitochondrion. This is further supported by our results that indicate the ECAR and lactate efflux are, in fact, increased with CHC treatment (Fig. 3, Supplemental Fig. 5), not decreased as one would expect if the plasma membrane transporters were targeted.
In the current study, we have used CHC as a classical inhibitor of MCTs. Evidence suggests that CHC is selective for the MCT1 isoform and is an effective, non-competitive inhibitor of lactate uptake in tumor cells at concentrations in the low mircomolar range (IC50 for lactate (5 mM) uptake = 166 µM) [4]. While the bulk of enzyme kinetics studies using this compound have focused on the inhibitory action of CHC with respect to lactate uptake, since the Km values for lactate and pyruvate uptake in tumor cells are in the same range (Km for lactate = 4.54 mM; Km for pyruvate = 0.72 mM) [4], we believe they may be extrapolated to some extent to our findings. Recent studies have also investigated the intracellular distribution of MCT isoforms and have found differential expression patterns and differential distribution to the plasma and mitochondrial membranes in breast cancer cells [15]. Further, MCT1 was recently reported to reside in the mitochondria (MitoCarta; [22]). Thus, there are clearly multiple levels of regulation of monocarboxylate transport within cells.
We believe our findings may be particularly relevant in the context of the proposed ‘metabolic symbiosis’ model in the tumor microenvironment, first described by Sonveaux et. al [31]. They suggest that both glucose and lactate are metabolic substrates for cancer cells within solid tumors; however, their use as substrates is spatially distinct [31]. This process is somewhat analogous to the cell-cell lactate shuttle observed in working muscle bed [1]. In the absence of adequate oxygen, hypoxic tumor cells rely on glucose as a metabolic substrate and export lactate through MCT4. This effluxed lactate is then transported into oxygenated cancer cells through MCT1 and is used to fuel oxidative metabolism. Inhibition of MCTs disrupts the ‘metabolic symbiosis’ and results in decreased tumor size in vivo.
Additional studies are certainly needed to determine the effects of MCT inhibitors on the metabolic pathways under hypoxic stress. Pyruvate-driven respiration is unlikely to provide a growth advantage under hypoxia. Nonetheless, our study highlights the important role of mitochondrial function, and in particular the reserve capacity in cancer cell proliferation and describes an alternative mechanism of action for MCT inhibitors which may give renewed impetus for their development as chemotherapeutic agents.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr. B. Kalyanaraman for important support for these studies.
Grant Support
This research was supported by the Redox Biology Program at the Medical College of Wisconsin (N.H.), an Interdisciplinary Cancer Research Post-Doctoral Fellowship from the Cancer Center of the Medical College of Wisconsin (A.R.D.), and National Institutes of Health grant R01-GM-55792 (N.H.).
Non-standard abbreviations
- CHC
α-cyano-4-hydroxycinnamic acid
- DMSO
dimethyl sulfoxide
- ECAR
extracellular acidification rate
- FBS
fetal bovine serum
- FCCP
carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone
- MCT
monocarboxylate transporter
- OCR
oxygen consumption rate
- OXPHOS
oxidative phosphorylation
- SEM
standard error of the mean
- TBS-T
Tris buffered saline with Tween 20
Footnotes
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Brooks GA. Mammalian fuel utilization during sustained exercise. Comp Biochem.Physiol B Biochem.Mol.Biol. 1998;120:89–107. doi: 10.1016/s0305-0491(98)00025-x. [DOI] [PubMed] [Google Scholar]
- 2.Brooks GA. Cell-cell and intracellular lactate shuttles. J.Physiol. 2009;587:5591–5600. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat.Rev.Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter L, Halestrap AP. The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells studied with the intracellular pH indicator BCECF. Biochem.J. 1994;304(Pt 3):751–760. doi: 10.1042/bj3040751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Wang L, Beretov J, Hao J, Xiao W, Li Y. Co-expression of CD147/EMMPRIN with monocarboxylate transporters and multiple drug resistance proteins is associated with epithelial ovarian cancer progression. Clin.Exp.Metastasis. 2010;27:557–569. doi: 10.1007/s10585-010-9345-9. [DOI] [PubMed] [Google Scholar]
- 6.Dang CV. MYC, microRNAs and glutamine addiction in cancers. Cell Cycle. 2009;8:3243–3245. doi: 10.4161/cc.8.20.9522. [DOI] [PubMed] [Google Scholar]
- 7.Dang CV, Hamaker M, Sun P, Le A, Gao P. Therapeutic targeting of cancer cell metabolism. J.Mol.Med. 2011;89:205–212. doi: 10.1007/s00109-011-0730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diers AR, Higdon AN, Ricart KC, Johnson MS, Agarwal A, Kalyanaraman B, Landar A, Darley-Usmar VM. Mitochondrial targeting of the electrophilic lipid 15-deoxy-Delta12,14-prostaglandin J2 increases apoptotic efficacy via redox cell signalling mechanisms. Biochem.J. 2010;426:31–41. doi: 10.1042/BJ20091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dranka BP, Hill BG, Darley-Usmar VM. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radic.Biol.Med. 2010;48:905–914. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov.Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal.Chem. 2009;81:6868–6878. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halestrap AP, Denton RM. The specificity and metabolic implications of the inhibition of pyruvate transport in isolated mitochondria and intact tissue preparations by alpha-Cyano-4-hydroxycinnamate and related compounds. Biochem.J. 1975;148:97–106. doi: 10.1042/bj1480097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem.J. 1999;343(Pt 2):281–299. [PMC free article] [PubMed] [Google Scholar]
- 14.Hao J, Chen H, Madigan MC, Cozzi PJ, Beretov J, Xiao W, Delprado WJ, Russell PJ, Li Y. Co-expression of CD147 (EMMPRIN), CD44v3-10, MDR1 and monocarboxylate transporters is associated with prostate cancer drug resistance and progression. Br.J.Cancer. 2010;103:1008–1018. doi: 10.1038/sj.bjc.6605839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussien R, Brooks GA. Mitochondrial and plasma membrane lactate transporter and lactate dehydrogenase isoform expression in breast cancer cell lines. Physiol Genomics. 2011;43:255–264. doi: 10.1152/physiolgenomics.00177.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jekabsons MB, Nicholls DG. In situ respiration and bioenergetic status of mitochondria in primary cerebellar granule neuronal cultures exposed continuously to glutamate. J.Biol.Chem. 2004;279:32989–33000. doi: 10.1074/jbc.M401540200. [DOI] [PubMed] [Google Scholar]
- 17.Kim JW, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 18.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J.Immunol.Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 19.Newsholme EA, Crabtree B, Ardawi MS. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci.Rep. 1985;5:393–400. doi: 10.1007/BF01116556. [DOI] [PubMed] [Google Scholar]
- 20.Nicholls DG, Darley-Usmar VM, Wu M, Jensen PB, Rogers GW, Ferrick DA. Bioenergetic profile experiment using C2C12 myoblast cells. J.Vis.Exp. 2010;6 doi: 10.3791/2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliva CR, Nozell SE, Diers A, McClugage SG, III, Sarkaria JN, Markert JM, Darley-Usmar VM, Bailey SM, Gillespie GY, Landar A, Griguer CE. Acquisition of temozolomide chemoresistance in gliomas leads to remodeling of mitochondrial electron transport chain. J.Biol.Chem. 2010;285:39759–39767. doi: 10.1074/jbc.M110.147504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez J, Hill BG, Benavides GA, Dranka BP, Darley-Usmar VM. Role of cellular bioenergetics in smooth muscle cell proliferation induced by platelet-derived growth factor. Biochem.J. 2010;428:255–267. doi: 10.1042/BJ20100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pike LS, Smift AL, Croteau NJ, Ferrick DA, Wu M. (11 A.D.) Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim.Biophys.Acta. 1807:726–734. doi: 10.1016/j.bbabio.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Pike LS, Smift AL, Croteau NJ, Ferrick DA, Wu M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim.Biophys.Acta. 2010 doi: 10.1016/j.bbabio.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Pinheiro C, Albergaria A, Paredes J, Sousa B, Dufloth R, Vieira D, Schmitt F, Baltazar F. Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology. 2010;56:860–867. doi: 10.1111/j.1365-2559.2010.03560.x. [DOI] [PubMed] [Google Scholar]
- 27.Pinheiro C, Longatto-Filho A, Pereira SM, Etlinger D, Moreira MA, Jube LF, Queiroz GS, Schmitt F, Baltazar F. Monocarboxylate transporters 1 and 4 are associated with CD147 in cervical carcinoma. Dis.Markers. 2009;26:97–103. doi: 10.3233/DMA-2009-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinheiro C, Longatto-Filho A, Simoes K, Jacob CE, Bresciani CJ, Zilberstein B, Cecconello I, Alves VA, Schmitt F, Baltazar F. The prognostic value of CD147/EMMPRIN is associated with monocarboxylate transporter 1 co-expression in gastric cancer. Eur.J.Cancer. 2009;45:2418–2424. doi: 10.1016/j.ejca.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Quennet V, Yaromina A, Zips D, Rosner A, Walenta S, Baumann M, Mueller-Klieser W. Tumor lactate content predicts for response to fractionated irradiation of human squamous cell carcinomas in nude mice. Radiother.Oncol. 2006;81:130–135. doi: 10.1016/j.radonc.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr.Opin.Genet.Dev. 2010;20:51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelley MJ, Gallez B, Wahl ML, Feron O, Dewhirst MW. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J.Clin.Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spitz DR, Malcolm RR, Roberts RJ. Cytotoxicity and metabolism of 4-hydroxy-2-nonenal and 2-nonenal in H2O2-resistant cell lines. Do aldehydic by-products of lipid peroxidation contribute to oxidative stress? Biochem.J. 1990;267:453–459. doi: 10.1042/bj2670453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wahl ML, Owen JA, Burd R, Herlands RA, Nogami SS, Rodeck U, Berd D, Leeper DB, Owen CS. Regulation of intracellular pH in human melanoma: potential therapeutic implications. Mol.Cancer Ther. 2002;1:617–628. [PubMed] [Google Scholar]
- 35.Walenta S, Schroeder T, Mueller-Klieser W. Lactate in solid malignant tumors: potential basis of a metabolic classification in clinical oncology. Curr.Med.Chem. 2004;11:2195–2204. doi: 10.2174/0929867043364711. [DOI] [PubMed] [Google Scholar]
- 36.Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfor K, Rofstad EK, Mueller-Klieser W. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60:916–921. [PubMed] [Google Scholar]
- 37.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 38.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc.Natl.Acad.Sci.U.S.A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick DA. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am.J.Physiol Cell Physiol. 2007;292:C125–C136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- 40.Zur NS, Eason R, Doney AS, Frenguelli BG. An ion-pair reversed-phase HPLC method for determination of fresh tissue adenine nucleotides avoiding freeze-thaw degradation of ATP. Anal.Biochem. 2009;388:108–114. doi: 10.1016/j.ab.2009.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.