Abstract
Peripheral arterial disease (PAD) produces abnormal gait and disproportionately affects older individuals. The current study investigated PAD gait biomechanics in young and older subjects. Sixty-one (31 < 65 years, age: 57.4 ± 5.3 years and 30 ≥ 65 years; age: 72.2 ± 5.4 years) patients with PAD and 52 healthy age matched controls were included. Patients with PAD were tested during pain free walking and compared to matched healthy controls. Joint kinematics and kinetics (torques) were compared using a 2 × 2 ANOVA (Groups: PAD vs. Control, Age: Younger vs. Older). Patients with PAD had significantly increased ankle and decreased hip range of motion during the stance phase as well as decreased ankle dorsiflexor torque compared to controls. Gait changes in older individuals are largely constrained to time-distance parameters. Joint kinematics and kinetics are significantly altered in patients with PAD during pain free ambulation. Symptomatic PAD produces a consistent ambulatory deficit across ages definable by advanced biomechanical analysis. The most important finding of the current study is that gait, in the absence of PAD and other ambulatory comorbidities, does not decline significantly with age based on advanced biomechanical analysis. Therefore, previous studies must be examined in the context of potential PAD patients being present in the population and future ambulatory studies must include PAD as a confounding factor when assessing the gait function of elderly individuals.
Keywords: joint torques, peripheral vascular disease, aging, biomechanics, gait, joint angles, peripheral arterial disease
INTRODUCTION
Peripheral Arterial Disease (PAD) results in a significant decrease of blood flow to the lower extremities secondary to atherosclerosis within the pelvic and leg arteries. The most common symptom is intermittent claudication, a cramping pain occurring in the calves, thighs and/or buttocks brought on by physical activity and relieved with rest. The end result of PAD is reduced mobility, reduced physical functioning and poor health outcomes [1-4]. The prevalence of PAD increases as individuals age, with 4.3% of all Americans over 40 years old having PAD and 20% of individuals over age 75 having PAD according to Hirsch et al., 2001 [5]. Based on this disproportionate occurrence of PAD in older adult populations, the total number of patients affected by PAD is expected to increase according to current demographics predictions [6]. PAD will therefore significantly impact our older adult population by causing increased pain, discomfort, and fatigue. In addition, persons with PAD experience significant gait deficits, which result in reduced daily activity and loss of independence.
Gait changes in older adult individuals have been well-documented using biomechanical measures [7-11]. Older adults tend to walk slower and have shorter strides than young adults [7]. Older adults use their hip extensors more and their ankle plantar flexors and knee extensors less than healthy young individuals, showing an overall reorganization of joint torques [12]. In addition, older adults tend to have a decreased range of motion at the hip, knee and ankle joints and spend more time in double support, which also increases overall stance time [10]. Despite a known high prevalence of PAD in the older adult population [13], no previous study in older individuals has taken into account that these measured changes in older adults may be due to the impact of PAD within the tested population. Our groups’ work on PAD and aging [7, 11, 14, 15, 17, 18] led us to a recognition that the gait of older adult patients is very similar to the gait changes described for patients with PAD [14, 15]. It is our concern therefore that elderly patients with unrecognized PAD have likely “contaminated” previous studies of older adult gait [8, 16].
Our research group has noted that many gait alterations previously identified in patients with PAD are similar to the gait changes documented in older adult subjects. Specifically, slower walking speeds, shorter strides [7, 14, 15, 17], increased stance time [10, 11, 17, 18], decreased range of motion at the hip and knee during the stance phase, increased ankle range of motion during the stance phase [10, 19, 20], and reduced propulsion forces and push-off power [11, 15, 18, 21] have been noted in both populations. Our group has also reported kinetic changes in PAD gait. Specifically, patients with PAD have decreased joint torques [15, 22, 23], and powers [24] at the ankle and hip, and exhibit reduced external work [25] compared to healthy age matched controls. However, because many patients with PAD are also older adults, the independent effects of age and the disease are unknown. It is also unknown if the effects of PAD and aging are additive. If gait truly worsens as patients age, then intervention in patients with PAD becomes even more important to preserve physical function and overall quality of life for these patients. This creates a need to examine the effects of age on gait parameters in patients with PAD.
The current study investigated the effect of age and PAD on gait mechanics using kinematics and kinetics in younger (< 65 years) and older (≥ 65 years) patients with PAD and healthy age-matched control groups. We hypothesized that patients with PAD would exhibit altered gait characteristics compared to healthy controls. We also hypothesized that gait characteristics would be different between younger and older groups. Finally, we hypothesized that if age affects gait parameters, then age and PAD would act synergistically with the most significant gait alterations found in older adult patients with PAD.
METHODS
Subject inclusion and exclusion criteria
The study consisted of 61 patients with PAD (Table 1) diagnosed with Rutherford Class 2/Fontaine Class II arterial occlusive disease with classic Rose intermittent claudication. Subjects were arbitrarily considered younger (N=31) if less than 65 years of age and older (N=30) if greater than 65 years of age based on the definition of the older adult population by the Center for Disease Control and the National Center for Health Statistics [26]. Patients with PAD were recruited from the vascular surgery clinics of the Nebraska and Western Iowa Veterans’ Affairs Medical Center and the University of Nebraska Medical Center. In addition 52 gender, age, height and body mass matched healthy control subjects (Table 1) were recruited from the community. The Institutional Review Boards of the Nebraska Western Iowa Veterans Affairs Medical Center and the University of Nebraska Medical Center approved the study. Written informed consent was obtained from all subjects before data collection.
Table 1.
Subject demographics and clinical characteristics for younger (Y) and older (O) Peripheral Arterial Disease (PAD) and Control (CON) groups.
| Y-PAD (N=31) | O-PAD (N=30) | Y-CON (N=27) | O-CON (N=25) | |
|---|---|---|---|---|
| Gender (Male/Female) | 26/5 | 29/0 | 20/7 | 19/4 |
| Age (years) | 57.4 ± 5.28 | 71.9 ± 5.20 | 55.4 ± 6.30 | 70.3 ± 3.17 |
| Body mass (kg) | 84.2 ± 15.75 | 83.9 ± 17.4 | 85.04 ± 18.25 | 82.29 ± 13.16 |
| Body height (cm) | 172.8 ± 6.71 | 173.3 ± 5.46 | 173.6 ± 8.49 | 170.9 ± 8.61 |
| Ankle Brachial Index | 0.56 ± 0.47 | 0.53 ± 0.67 | 1.1 ±0.11 | 1.1 ± 0.10 |
| Smokers (%) | 83.9 | 33.3 | 7.4 | 12.0 |
| Hypertension (%) | 74.2 | 76.7 | 18.5 | 20.0 |
| Diabetes mellitus (%) | 9.7 | 36.7 | 0 | 12.0 |
| Hyperlipidemia (%) | 64.5 | 86.7 | 14.8 | 20.0 |
| Body Mass Index | 28.08 ± 4.37 | 27.84 ± 5.20 | 28.08 ± 4.63 | 28.14 ± 3.75 |
Note: values are shown Mean ± Standard deviation
Patients and controls were screened and evaluated by one of two board certified vascular surgeons (authors JJ & IP). Patient evaluation included resting ankle-brachial index (a resting measurement below 0.90 was present in all subjects with claudication), detailed history, and physical examination. Standard ankle-brachial index measurement was performed and consisted of blood pressure measurement at the brachial artery in the arm and the posterior tibial and dorsalis pedis arteries at the ankle. Control subjects had an ankle-brachial index greater than 0.90 and absence of subjective or objective ambulatory dysfunction. Controls were screened in a similar fashion as patients with PAD and were excluded for the same ambulation-limiting problems or if pain was experienced during walking. All participants were screened to exclude any underlying cardiac, pulmonary, neuromuscular, or musculoskeletal disease that would affect gait or make data collection unsafe. Therefore, both patients with PAD and healthy controls utilized for this study were somewhat unique in that they were excluded if they experienced pain or discomfort during walking for the following conditions: arthritis, low back pain, musculoskeletal problems, and neuropathy.
Experimental procedure and data collection
Prior to data collection, reflective markers were placed at specific anatomical locations of each subject's lower limbs based on the marker systems of Nigg et al [27] and Vaughan et al [28]. Each subject was directed to walk using their self-selected pace over a ten-meter pathway, while three-dimensional marker trajectories and ground reaction force data were simultaneously collected. The marker trajectories were captured with a twelve-camera motion capture system (Motion Analysis Corp., Santa Rosa, CA) sampling at 60Hz. The ground reaction force data were acquired with a Kistler force platform (Kistler Instrument Corp., Amhurst, NY) sampling at 600 Hz. To assess baseline ambulatory functions, patients were tested in a pain free condition. To ensure patients did not experience pain during walking, a minimum of one minute rest was required between each walking trial. Subjects completed five successful walking trials on each leg, with a successful trial being defined as the subject's foot being completely on the force platform. Subjects were positioned on the pathway so that they would step on the force platform while walking naturally.
Data analysis
Time-distance gait variables were calculated using custom software in Matlab (The Mathworks Inc., Natick, Mass). Total range of motion of the joint kinematics and peak values of the joint kinetics in the sagittal plane during the stance phase were analyzed (Figures 1 and 2). All marker trajectories were smoothed using a low-pass, fourth order Butterworth filter (7 Hz cutoff) during post data processing. Joint kinetics and kinematics were calculated for the stance phase from exported raw forces and marker trajectories using Visual3D software (C-Motion Inc., Germantown, MD). The joint torque values were scaled to body mass [11].
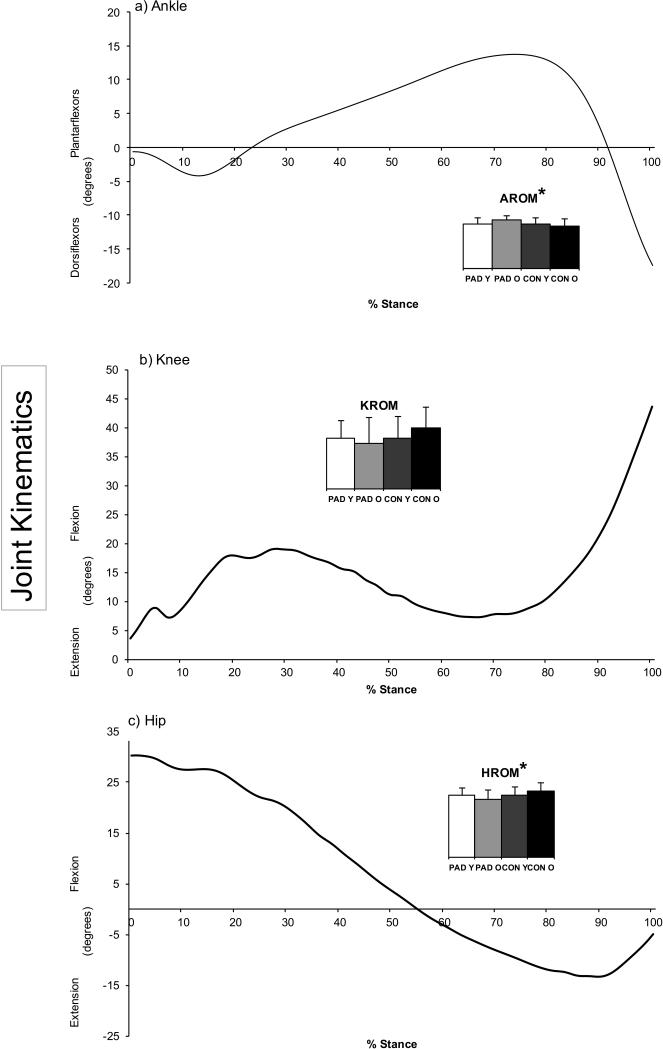
Figure 1.
Representative joint angle curves from a younger healthy control for the a) Ankle, b) Knee, and c) Hip during the stance phase. The bar graphs represent total flexion-extension range of motion of the corresponding curve. It is obvious that the peripheral arterial disease patients have more ankle range of motion while also exhibiting less knee and hip range of motion than controls.
Note: AROM: Maximum ankle range of motion during the stance phase. KROM: Maximum knee range of motion during the stance phase. HROM: Maximum hip range of motion during the stance phase. Y-PAD: Peripheral arterial disease patients less than 65 years of age. O-PAD: Peripheral arterial disease patients 65 years of age or older. Y-CON: Healthy control subjects less than 65 years of age. O-CON: Healthy control subjects 65 years of age or older.
*p < 0.5, significant main effect for group (PAD groups vs. CON groups).
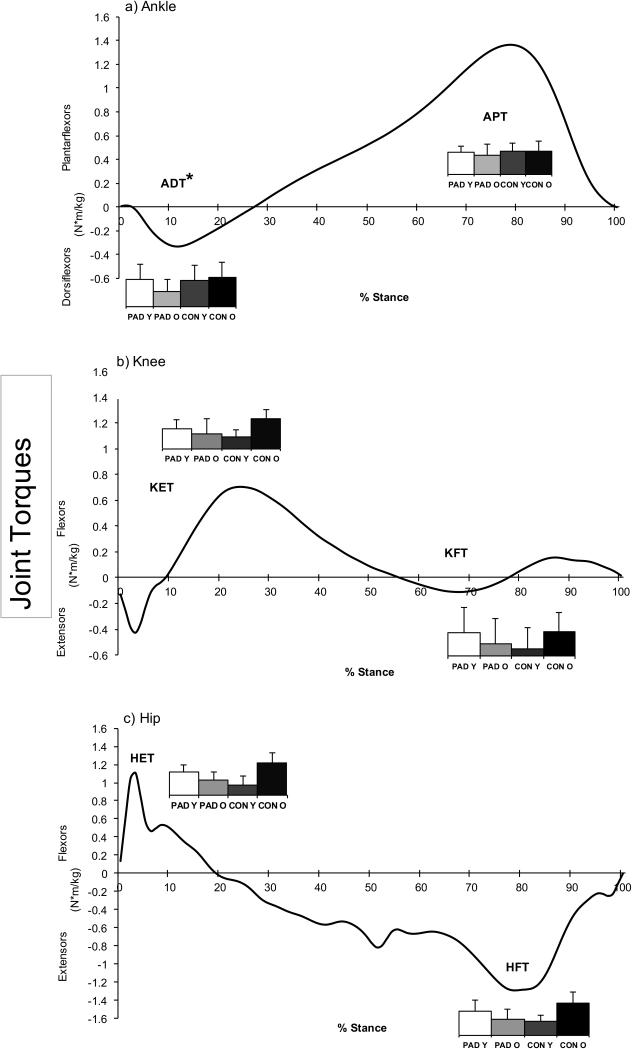
Figure 2.
Representative joint torque curves from a younger healthy control for the a) Ankle, b) Knee, and c) Hip during the stance phase. A positive (+) value indicates a net internal extensor/plantarflexor torque, and a negative (−) value indicates a net internal flexor/dorsiflexor torque. Torques are normalized to body mass. The bar graph values represent the peak torque of the corresponding curves (units = %BW X BH). It is obvious that patients with PAD have lower response of the hip extensors during early stance, suggesting weakness of the hip extensor muscles. Patients with PAD also have reduced ankle dorsiflexor and knee extensor torque during early stance and decreased hip flexor torque during late stance.
Note: ADT: Maximum Ankle Dorsiflexor Torque, APT: Maximum Ankle Plantarflexor Torque. KET: Maximum Knee Extensor Torque, KET: Maximum Knee Flexor Torque. HET: Maximum Hip Extensor Torque, HFT: Maximum Hip Flexor Torque. Y-PAD: Peripheral arterial disease patients less than 65 years of age. O-PAD: Peripheral arterial disease patients 65 years of age or older. Y-CON: Healthy control subjects less than 65 years of age. O-CON: Healthy control subjects 65 years of age or older.
*p < 0.5, significant main effect for group (PAD groups vs. CON groups).
Statistical analysis
Group means of the joint kinetics and kinematics were calculated by using the affected leg of unilateral patients and the side with the lowest respective ankle-brachial index for bilateral patients in the PAD group, as well as the dominant legs of healthy control groups (Younger PAD (Y-PAD), N=31; Older PAD (O-PAD), N=30; Younger Control (Y-CON), N=27; and Older Control (O-CON), N=25). For each dependent variable, a 2×2 ANOVA was used with the factors being group (PAD versus CON) and age (Younger versus Older). Group means for age, height, body mass, body mass index, and time-distance gait parameters were also compared using a 2×2 ANOVA. When a significant interaction was identified, Tukey tests were used for post-hoc analysis to identify significant differences between the group/age combinations. Statistical comparisons were performed using SPSS (SPSS Inc., 22.0). The level of significance was set at 0.05.
RESULTS
Demographics
The baseline clinical characteristics of the Y-PAD, O-PAD, Y-CON and O-CON are presented in Table 1. Group means for age (p=.310), height (p=.255), body mass (p=.266) and body mass index (p=.624) did not differ between PAD and CON groups. Thus, PAD and CON groups were well matched for anthropometric data, whereas clinical characteristics of the patient versus control groups were significantly different, as was expected.
Time-Distance Gait Parameters
Significant main effect of group (PAD versus CON) was found for cadence (F = 5.39, p=.024)(Table 2). When compared to CON, patients with PAD had significantly decreased walking velocity (p<.001), cadence (p<.001), stride length (p<.001), and stance phase % of the gait cycle (p=.024). Significant differences for age (Younger versus Older) also exist. Older had decreased walking velocity (p=.012), decreased step width (p=.046), and decreased stride length (p=.002). There were significant interactions between group and age. Specifically, Y-PAD had decreased stride length (p=.003) compared to Y-CON as well as increased step width (p=.030) and decreased cadence (p=.009) compared to O-CON. O-PAD also had decreased stride length (p=.002), cadence (p<.001), and stance phase % of the total gait cycle (p=.025) compared to O-CON. Y-PAD exhibited increased walking velocity (p=.001), stride length (p=.005), step length (p=.002), and stance phase % of the total gait cycle (p=.033) compared to O-PAD.
Table 2.
Group means for selected time-distance gait parameters for younger (Y) and older (O) Peripheral Arterial Disease (PAD) and Control (CON) groups.
| Y-PAD (N=31) | O-PAD (N=30) | Y-CON (N=27) | O-CON (N=25) | Significance | |
|---|---|---|---|---|---|
| Gait velocity (m/s) | 1.11 ± 0.18 | 0.98 ± 0.22 | 1.25 ± 1.74 | 1.19 ± 0.22 | *^çδ |
| Stride length (m) | 1.33 ± 0.13 | 1.21 ± 0.17 | 1.43 ± 0.13 | 1.36 ± 0.17 | *^ŧ§çδ |
| Cadence | 102.1 ± 8.46 | 100.2 ± 6.15 | 105.99 ± 8.61 | 108.63 ± 9.66 | *‡§ç |
| Step length | 0.64 ± 0.11 | 0.53 ± 0.15 | 0.67 ± 0.13 | 0.58 ± 0.17 | ^çδ |
| Step width | 0.13 ± 0.04 | 0.11 ± 0.04 | 0.12 ± 0.03 | 0.10 ± 0.05 | ^‡ |
| Stance phase (% of gait cycle) | 66.27 ± 2.12 | 62.70 ± 8.84 | 66.30 ± 1.53 | 66.85 ± 1.53 | *§çδ |
| Swing phase (% of gait cycle) | 33.66 ± 1.36 | 33.56 ± 1.69 | 33.70 ± 1.53 | 33.15 ± 1.91 | |
| Double support (% of gait cycle) | 16.46 ± 1.44 | 17.02 ± 1.15 | 16.46 ± 1.33 | 16.70 ± 1.69 |
Note: values are shown Mean ± Standard deviation
p < .05, significant differences between groups (CON vs. PAD)
p < .05, significant differences between groups (Younger vs. Older)
p < .05, significant interaction between Y-PAD and O-CON
p < .05, significant interaction between Y-PAD and Y-CON
p < .05, significant interaction between O-PAD and O-CON
p < .05, significant interaction between O-PAD and Y-CON
p > .05 significant interactions between Y-PAD and O-PAD
¶ p > .05 significant interactions between Y-CON and O-CON
Joint Angles
Significant main effect of groups (PAD versus CON) were detected for ankle ROM (F = 8.82, p=.004) Compared to controls, patients with PAD had increased ankle range of motion (p=.036) and decreased hip range of motion (p=.005) during stance (Table 3, Figure 1). No significant main effects for joint angles were observed for comparing Y-CON vs. O-CON. Additionally, there were significant interactions between group and age for joint angles. Specifically, Y-PAD had decreased hip range of motion (p=.032) compared to O-CON and decreased knee range of motion (p=.013) compared to O-PAD. O-PAD had increased ankle range of motion compared to O-CON (p=.004) and decreased knee (p=.019) and hip (p=.001) range of motion compared to O-CON (Table 3).
Table 3.
Group means for selected kinematic and kinetic parameters for younger (Y) and older (O) Peripheral Arterial Disease (PAD) and Control (CON) groups.
| Y-PAD (N=31) | O-PAD (N=30) | Y-CON (N=27) | O-CON (N=25) | Significance | |
|---|---|---|---|---|---|
| Ankle ROM (deg) | 18.48 ± 2.90 | 20.46 ± 3.13 | 18.75 ± 3.10 | 17.57 ± 3.96 | *§çδ |
| Knee ROM (deg) | 11.53 ± 4.01 | 10.45 ± 5.44 | 11.61 ± 5.0 | 13.84 ± 4.85 | § |
| Hip ROM (deg) | 40.42 ± 4.59 | 37.73 ± 6.62 | 40.84 ± 5.47 | 43.28 ± 5.12 | *‡§ |
| Ankle dorsiflexor torque (N*m/Kg) | 0.36 ± 0.11 | 0.31 ± 0.11 | 0.38 ± 0.11 | 0.38 ± 0.11 | *§çδ |
| Ankle plantarflexor torque (N*m/Kg) | 1.42 ± 0.12 | 1.32 ± 0.24 | 1.41 ± 0.17 | 1.43 ± 0.16 | δ |
| Knee extensor torque (N*m/ Kg) | 0.51 ± 0.45 | 0.38 ± 0.66 | 0.29 ± 0.69 | 0.77 ± 0.31 | ‡§¶ |
| Knee flexor torque (N*m/Kg) | 0.15 ± 0.16 | 0.08 ± 0.19 | 0.05 ± 0.21 | 0.16 ± 0.15 | ∓ ¶ |
| Hip extensor torque (N*m/Kg) | 0.46 ± 0.49 | 0.30 ± 0.56 | 0.21 ± 0.62 | 0.65 ± 0.27 | §¶ |
| Hip flexor torque (N*m/Kg) | 0.58 ± 0.55 | 0.39 ± 0.71 | 0.34 ± 0.96 | 0.79 ± 0.38 | §¶ |
Note: values are shown Mean ± Standard deviation
p < .05, significant differences between groups (CON vs. PAD)
p < .05, significant differences between groups (Younger vs. Older)
p < .05, significant interaction between Y-PAD and O-CON
p < .05, significant interaction between Y-PAD and Y-CON
p < .05, significant interaction between O-PAD and O-CON
p < .05, significant interaction between O-PAD and Y-CON
p > .05 significant interactions between Y-PAD and O-PAD
p > .05 significant interactions between Y-CON and O-CON
Joint Torques
A significant main effect for groups (PAD versus CON) was detected for hip flexor torque (F = 6.31, p<.014). Patients with PAD had significantly decreased ankle dorsiflexor torque in late stance compared to CON (p=.039, Table 3, Figure 2). No differences were observed for age (Younger versus Older). There were significant interactions between group and age for joint torques. Specifically, Y-PAD had greater knee flexor torque (p=.044) during stance compared to Y-CON and decreased knee extensor torque (p=.016) during stance compared to O-CON. Y-PAD also had increased ankle plantarflexor torque (p=.041) compared to O-PAD. O-PAD had decreased ankle dorsiflexor torque (p=.024) during stance compared to Y-CON. O-PAD also had decreased ankle dorsiflexor torque (p=.025), knee extensor torque (p=.009), hip extensor torque (p=.006), and hip flexor torque (p=.012) during stance compared to O-CON.
DISCUSSION
The purpose of our study was to investigate the effect of age and PAD independently on gait mechanics using kinematics and kinetics in younger (< 65 years) and older (≥ 65 years) patients. Because PAD occurs primarily in older individuals, it is imperative to determine the impact of aging and PAD on gait independently. Our hypothesis that gait characteristics would differ between PAD and CON groups was supported by the current study and our previous studies where time-distance parameters and joint kinematic and kinetic variables were different between PAD versus CON groups. Interestingly, gait biomechanics did not decline significantly with age but only declined for time distance variables suggesting the impact of aging is not nearly as significant as the changes seen due to PAD. This suggests the previous literature examining the gait of older patients failed to take into account the presence of PAD in the subjects and likely contaminated previous studies overemphasizing the impact of natural aging on gait function.
Previous studies have used joint kinematics [14, 19], ground reaction forces [18], joint torques [15, 23], and joint powers [22, 29] to study gait disability in patients with PAD. The present study is unique being the first to utilize advanced biomechanical measures to examine the independent effect of aging and PAD on gait parameters. In our study, time-distance parameters, joint kinematics and kinetics were evaluated while younger and older patients with PAD walked without claudication pain and were compared to those of younger and older healthy controls who were representative in gender, height, and body mass characteristics. Our results demonstrated that gait in patients with PAD is significantly affected prior to the onset of claudication pain. The time-distance parameters demonstrate a lethargic gait in patients with PAD, with reduced gait velocity, stride length, step length, cadence and increased step width. These findings, like previous PAD studies [30], show altered time-distance parameters for patients with PAD. The joint angle and joint torque data combined suggests that muscular weakness is present across all three joints and is likely the fundamental source of altered gait patterns in PAD. Muscle weaknesses in the plantar flexors (gastrocnemius, soleus) knee extensors (quadriceps), hip flexors (illiopasoas and anterior thigh muscles) and hip extensors (gluteal and posterior thigh muscles) resulted in decreased plantar flexor torque, knee extensor torque, hip extensor torque in early stance, and hip flexor torque during late stance in patients with PAD [22]. Thus, PAD has adverse gait effects that are of primary importance regardless of patient age. These findings supported the hypothesis that age and PAD would act synergistically, with the most significant gait alterations found among the older patient group with PAD. The only confounding issue is whether the amount of time the patient had PAD present contributed to the worsening of the muscular function. The findings coincide with weakening of the muscles driving movement at the ankle as patients with PAD age, a likely result of the documented myopathy found in the muscles of patients with PAD [40]. As the myopathy progresses, scar tissue becomes present, resulting in what amounts to the overall findings of our study. An alternative explanation for slower walking speed and reduced spatiotemporal variables could be that patients with PAD are using these alterations as a compensatory strategy to avoid claudication pain.
Perhaps the most notable and important finding of the current study is that gait, in the absence of PAD and other ambulatory comorbidities, does not decline significantly with age based on advanced biomechanical analysis. As previous studies have not excluded patients with underlying PAD and other co-morbidities, our study is important in that all ambulatory co-morbidities were excluded in the control population without selecting for high functioning elderly control patients. Time-distance parameters exhibit a shortening and slowing of gait, but the joint angles and joint kinetics remain mostly unchanged. This lack of differences between age groups differs from the generally accepted idea that it is normal for gait to decline as people age due to muscular changes such as sarcopenia. Previous studies that investigated kinematics in older adults have demonstrated a decreased knee range of motion, ankle plantar flexion maximum, and hip extension maximum for older adult individuals [20, 21]. Similarly, previous studies have found differences in joint torques in healthy older adults as compared to younger controls [12, 20, 31]. Interestingly and calling into question previous studies, the findings of gait decline in the aged from previous studies are consistent with findings in the current study for both younger and older patients with PAD. We believe this is due to the majority of studies examining the decline of older adult gait not excluding patients with PAD based on a simple ankle-brachial index, despite the known high prevalence of PAD in the older adult population [12, 20, 21, 31]. In contrast, significant evidence exists for marked ambulatory decline and function in older adult patients with PAD [32-34]. Therefore, it is likely that some of the older adult subjects in previous gait studies had unrecognized PAD, as 14-30% of people over 65 years of age have PAD [5,13]. Our results show that in healthy controls without health conditions affecting mobility, aging does not necessarily produce significant changes in gait mechanics. Therefore, our study calls into question the results of previous studies of gait dysfunction in older adults and whether gait actually does decline with age in the absence of movement pathologies.
The source of gait disability in patients with PAD, as suggested by previous studies, points to a definable muscular weakness resulting in decreased propulsion forces [18], decreased lower extremity strength [34-37], decreased hip and ankle joint torques [15, 23, 29], and reduced hip and ankle joint powers [22-24]. The presence of these gait deficits in a pain free state combined with recent biochemical and histological studies of PAD muscle supports the idea of an acquired neuromuscular weakness. Specifically, there is increasing evidence that a significant muscle metabolic myopathy [38] and an axonal polyneuropathy in the lower extremities exists in patients with PAD [39]. Ultimately, the metabolic myopathy present in the PAD muscle is likely related to the chronic repetitive cycles of ischemia reperfusion. This chronic ischemia leads to mitochondriopathy of PAD muscle and is linked with oxidative damage to the muscle components [40] and axonal nerve loss [39]. These biochemical changes appear to contribute to myopathic and neurologic changes in patients with PAD. Future work will need to correlate these biochemical changes in PAD muscle with biomechanical gait changes and overall physical function in patients with PAD.
For this study, we chose to evaluate subjects while walking at their self-selected pace. We took this approach to closely replicate real world conditions. Many studies have investigated the varying effects of speed during biomechanical analysis and the effect of speed is controversial even within the biomechanics community [21]. However, it is important to note that gait differences have been found between young and older adults regardless of whether or not the walking speeds were the same during the experimental protocol [12]. Therefore, we have attempted to obtain an accurate representation of the differences between controls and patients with PAD, and between the younger and older groups of subjects. The healthy older adult subjects recruited for this study were carefully screened and persons with conditions affecting the lower extremity musculoskeletal system were excluded in an attempt to truly determine the affect of aging in the absence of ambulatory co-morbidities. This approach may limit the generalizability of our findings since diseases of the musculoskeletal system are not uncommon in the older adult population. However, from a methodological standpoint, this approach is required to accurately differentiate the affect of PAD and aging on gait. Furthermore, aging itself may not lend itself to gait abnormalities and if movement pathologies can be prevented, gait can be preserved even into advanced age.
CONCLUSION
In conclusion, our study of joint kinematics and joint kinetics indicates patients with PAD experience significantly altered gait parameters even in a pain free state resulting in a significant ambulatory deficit definable by biomechanical analysis. The current study provides evidence that typical aging in the absence of ambulatory comorbidities may not result in a significant decline in gait function. The presence of PAD among sampled subjects may influence the data in a negative direction, leading to misguided conclusions. Therefore, previous studies must be examined in the context of potential PAD patients being present in the population and future ambulatory studies must include PAD as a confounding factor when assessing the gait function of elderly individuals.
ACKNOWLEDGEMENTS
This work was supported by the American Geriatrics Society's Hartford Foundation Dennis W. Jahnigen Award (1I01RX000604 to JMJ); from the Rehabilitation Research and Development Service of the Veterans Affairs Office of Research and Development (JMJ), from the National Institutes of Health (5R01AG034995 to IIP); the Veterans Affairs Cooperative Studies Project #498 (NCT 000 945 75 to IP); the Nebraska Research Initiative (to SAM); and the National Institute of General Medical Sciences of the National Institutes of Health (Award Number P20GM109090 to SAM)
This material is the result of work supported with resources and the use of facilities at the VA Nebraska-Western Iowa Health Care System. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Veterans Affairs Office of Research and Development.
The Institutional Review Boards of the Nebraska Western Iowa Veterans Affairs Medical Center and the University of Nebraska Medical Center approved the study. Written informed consent was obtained from all subjects before data collection.
Footnotes
Author Contributions:
Study Concept and Design: SM, IP, JJ
Acquisition of Subjects and/or Data: SM, BA, JH, IP, JJ
Analysis and Interpretation: SM, BA, JH, IP, JJ
Preparation of Manuscript: SM, BA, JH, IP, JJ
REFERENCES
- 1.Herman SD, Liu K, Tian L, Guralnik JM, Ferrucci L, Criqui MH, et al. Baseline lower extremity strength and subsequent decline in functional performance at 6-year follow-up in persons with lower extremity peripheral arterial disease. J AM GERIATR SOC. 2009;57(12):2246–52. doi: 10.1111/j.1532-5415.2009.02562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nehler MR, McDermott MM, Treat-Jacobson D, Chetter I, Regensteiner JG. Functional outcomes and quality of life in peripheral arterial disease: current status. VASC MED. 2003;8(2):115–26. doi: 10.1191/1358863x03vm483ra. [DOI] [PubMed] [Google Scholar]
- 3.Nicoloff AD, Taylor LM, Jr., McLafferty RB, Moneta GL, Porter JM. Patient recovery after infrainguinal bypass grafting for limb salvage. J Vasc Surg. 1998;27(2):256–63. doi: 10.1016/s0741-5214(98)70356-8. discussion 64-6. [DOI] [PubMed] [Google Scholar]
- 4.Toursarkissian B, Shireman PK, Harrison A, D'Ayala M, Schoolfield J, Sykes MT. Major lower-extremity amputation: contemporary experience in a single Veterans Affairs institution. The American surgeon. 2002;68(7):606–10. [PubMed] [Google Scholar]
- 5.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 6. [2008 August 28];2005-2007 American Community Survey 3-year estimates. 2008 [homepage on the internet]. Available from: http://factfinder.census.gov.
- 7.Byrne JE, Stergiou N, Blanke D, Houser JJ, Kurz MJ, Hageman PA. Comparison of gait patterns between young and elderly women: an examination of coordination. PERCEPT MOTOR SKILL. 2002;94(1):265–80. doi: 10.2466/pms.2002.94.1.265. [DOI] [PubMed] [Google Scholar]
- 8.Elble RJ, Thomas SS, Higgins C, Colliver J. Stride-dependent changes in gait of older people. J NEUROL. 1991;238(1):1–5. doi: 10.1007/BF00319700. [DOI] [PubMed] [Google Scholar]
- 9.McGibbon CA, Puniello MS, Krebs DE. Mechanical energy transfer during gait in relation to strength impairment and pathology in elderly women. Clin Biomech. 2001;16(4):324–33. doi: 10.1016/s0268-0033(01)00004-3. [DOI] [PubMed] [Google Scholar]
- 10.Whittle MW. Gait analysis: An introduction. Reed Educational and Professional; Oxford, England: 2002. [Google Scholar]
- 11.Winter DE, Kinetics P. Our window into the goals and strategies of the central nervous system. BEH BR RES. 1995;67(2):111–20. doi: 10.1016/0166-4328(94)00154-8. [DOI] [PubMed] [Google Scholar]
- 12.DeVita P, Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. J APPL PHYS. 2000;88(5):1804–11. doi: 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- 13.American Hearth Association ASA Heart disease and stroke statistics. 2007 [Google Scholar]
- 14.Celis R, Pipinos II, Scott-Pandorf MM, Myers SA, Stergiou N, Johanning JM. Peripheral arterial disease affects kinematics during walking. J VASC SURG. 2009;49(1):127–32. doi: 10.1016/j.jvs.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Chen S-J, Pipinos I, Johanning J, Radovic M, Huisinga JM, Myers SA, et al. Bilateral claudication results in alterations in the gait biomechanics at the hip and ankle joints. J BIOMECH. 2008;41(11):2506–14. doi: 10.1016/j.jbiomech.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 16.McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the women's health and aging study. Circulation. 2000;101(9):1007–12. doi: 10.1161/01.cir.101.9.1007. [DOI] [PubMed] [Google Scholar]
- 17.Gardner AW, Forrester L, Smith GV. Altered gait profile in subjects with peripheral arterial disease. VASC MED. 2001;6(1):31–4. [PubMed] [Google Scholar]
- 18.Scott-Pandorf MM, Stergiou N, Johanning JM, Robinson L, Lynch TG, Pipinos II. Peripheral arterial disease affects ground reaction forces during walking. J VASC SURG. 2007;46(3):491–9. doi: 10.1016/j.jvs.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Crowther RG, Spinks WL, Leicht AS, Quigley F, Golledge J. Relationship between temporal-spatial gait parameters, gait kinematics, walking performance, exercise capacity, and physical activity level in peripheral arterial disease. J VASC SURG. 2007;45(6):1172–8. doi: 10.1016/j.jvs.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 20.Judge JO, Ounpuu S, Davis RB., 3rd. Effects of age on the biomechanics and physiology of gait. Clin Geriatr Med. 1996;12(4):659–78. [PubMed] [Google Scholar]
- 21.Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ. Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments. Arch Phys Med Rehabil. 1998;79(3):317–22. doi: 10.1016/s0003-9993(98)90013-2. [DOI] [PubMed] [Google Scholar]
- 22.Koutakis P, Johanning JM, Haynatzki GR, Myers SA, Stergiou N, Longo GM, et al. Abnormal joint powers before and after the onset of claudication symptoms. J VASC SURG. 2010;52(2):340–7. doi: 10.1016/j.jvs.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koutakis P, Pipinos II, Myers SA, Stergiou N, Lynch TG, Johanning JM. Joint torques and powers are reduced during ambulation for both limbs in patients with unilateral claudication. J VASC SURG. 2010;51(1):80–8. doi: 10.1016/j.jvs.2009.07.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wurdeman SR, Koutakis P, Myers SA, Johanning JM, Pipinos, Stergiou N. Patients with peripheral arterial disease exhibit reduced joint powers compared to velocity-matched controls. Gait Posture. 2012;36(3):506–9. doi: 10.1016/j.gaitpost.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wurdeman SR, Myers SA, Johanning JM, Pipinos II, Stergiou N. External work is deficient in both limbs of patients with unilateral PAD. MED ENG PHYS. 2012;34(10):1421–6. doi: 10.1016/j.medengphy.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foundation . CfDCaPaTMC. The state of aging and health in America 2007. The Merck Company Foundation; Whitehouse Station, NJ: 2007. [Google Scholar]
- 27.Nigg BM, Cole GK, Nachbauer W. Effects of arch height of the foot on angular motion of the lower extremities in running. J Biomech. 1993;26(8):909–16. doi: 10.1016/0021-9290(93)90053-h. [DOI] [PubMed] [Google Scholar]
- 28.Vaughan CL, Davis BL, O'Connor JC. Dynamics of human gait. Human Kinetics; Champaign, IL: 1992. [Google Scholar]
- 29.Wurdeman SR, Koutakis P, Myers SA, Johanning JM, Pipinos II, Stergiou N. Patients with peripheral arterial disease exhibit reduced joint powers compared to velocity-matched controls. Gait Posture. 2012;36(3):506–9. doi: 10.1016/j.gaitpost.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner AW, Montgomery PS. The relationship between history of falling and physical function in subjects with peripheral arterial disease. VASC MED. 2001;6(4):223–7. doi: 10.1177/1358836x0100600404. [DOI] [PubMed] [Google Scholar]
- 31.Kerrigan DC, Lee LW, Nieto TJ, Markman JD, Collins JJ, Riley PO. Kinetic alterations independent of walking speed in elderly fallers. Arch Phys Med Rehabil. 2000;81(6):730–5. doi: 10.1016/s0003-9993(00)90101-1. [DOI] [PubMed] [Google Scholar]
- 32.Garg PK, Liu K, Tian L, Guralnik JM, Ferrucci L, Criqui MH, et al. Physical activity during daily life and functional decline in peripheral arterial disease. Circulation. 2009;119(2):251–60. doi: 10.1161/CIRCULATIONAHA.108.791491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDermott MM, Ferrucci L, Guralnik J, Tian L, Liu K, Hoff F, et al. Pathophysiological changes in calf muscle predict mobility loss at 2-year follow-up in men and women with peripheral arterial disease. Circulation. 2009;120(12):1048–55. doi: 10.1161/CIRCULATIONAHA.108.842328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDermott MM, Hoff F, Ferrucci L, Pearce WH, Guralnik JM, Tian L, et al. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J AM GERIATR SOC. 2007;55(3):400–6. doi: 10.1111/j.1532-5415.2007.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkins LM, Gardner AW. The relationship between lower extremity functional strength and severity of peripheral arterial disease. Angiology. 2004;55(4):347–55. doi: 10.1177/000331970405500401. [DOI] [PubMed] [Google Scholar]
- 36.McDermott MM, Ferrucci L, Guralnik JM, Tian L, Green D, Liu K, et al. Elevated levels of inflammation, d-dimer, and homocysteine are associated with adverse calf muscle characteristics and reduced calf strength in peripheral arterial disease. J Am Coll Cardiol. 2007;50(9):897–905. doi: 10.1016/j.jacc.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDermott MM, Tian L, Ferrucci L, Liu K, Guralnik JM, Liao Y, et al. Associations between lower extremity ischemia, upper and lower extremity strength, and functional impairment with peripheral arterial disease. J AM GERIATR SOC. 2008;56(4):724–9. doi: 10.1111/j.1532-5415.2008.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brass EP, Hiatt WR. Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral arterial disease. VASC MED. 2000;5(1):55–9. doi: 10.1177/1358836X0000500109. [DOI] [PubMed] [Google Scholar]
- 39.Weber F, Ziegler A. Axonal neuropathy in chronic peripheral arterial occlusive disease. Muscle Nerve. 2002;26(4):471–6. doi: 10.1002/mus.10235. [DOI] [PubMed] [Google Scholar]
- 40.Pipinos, Judge AR, Zhu Z, Selsby JT, Swanson SA, Johanning JM, et al. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radic Biol Med. 2006;41(2):262–9. doi: 10.1016/j.freeradbiomed.2006.04.003. [DOI] [PubMed] [Google Scholar]