Abstract
Accessory spleens are common, usually asymptomatic, incidentally discovered congenital foci of splenic tissue. They occur most commonly near the splenic hilum, with almost 20% in or near the pancreatic tail. On contrast-enhanced computed tomography (CT), differentiation of an intrapancreatic accessory splenule (IPAS) from other pancreatic tail lesions such as islet cell tumors and metastatic disease can present a diagnostic challenge. A high index of suspicion on the part of the radiologist, based on the classic location with typical imaging features and a combination of cross-sectional imaging studies such as ultrasound, computed tomograph (CT), or magnetic resonance imaging (MRI) with nuclear medicine examinations, can confirm the diagnosis of intrapancreatic accessory splenule and prevent unnecessary biopsy and/or surgery.
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; IPAS, intrapancreatic accessory splenule
Case report
A 50-year-old, otherwise healthy man presented to an outside institution with a several-month history of intermittent abdominal pain. The initial single venous-phase abdomen and pelvis CT scan obtained demonstrated an incidental 7-mm enhancing pancreatic tail mass. No other imaging findings that could explain his symptoms were identified. A repeat pancreatic protocol CT study performed at our institution 3 months later revealed a mild increase in the size of the enhancing mass lesion to 9 mm. The patient denied constitutional symptoms, and laboratory workup revealed no abnormalities. Given the interval and the mild enlargement, we suspected a nonfunctioning islet cell tumor of the pancreatic tail or a solid pseudopapillary tumor of the pancreatic tail. We did not consider the diagnosis of IPAS in the differential diagnosis, and we did not perform further imaging workup to characterize the lesion and/or biopsy. We performed open distal pancreatectomy and splenectomy via a left subcostal approach. The patient subsequently developed a subphrenic abscess and an enterocutaneous fistula. The abscess was drained, and the enterocutaneous fistula healed on conservative management. In retrospect, the lesion had similar attenuation values as the spleen on the arterial and portal venous phases (Figure 1A, Figure 1B, Figure 1C, Figure 1D). The diagnosis of an IPAS was made on histopathological examination of the surgical specimen (Fig. 2).
Figure 1A.

50-year-old man with intrapancreatic accessory spleen. Axial CT scan of the abdomen in the arterial phase shows a hypervascular mass (arrow) in the pancreatic tail with similar attenuation values as the spleen.
Figure 1B.
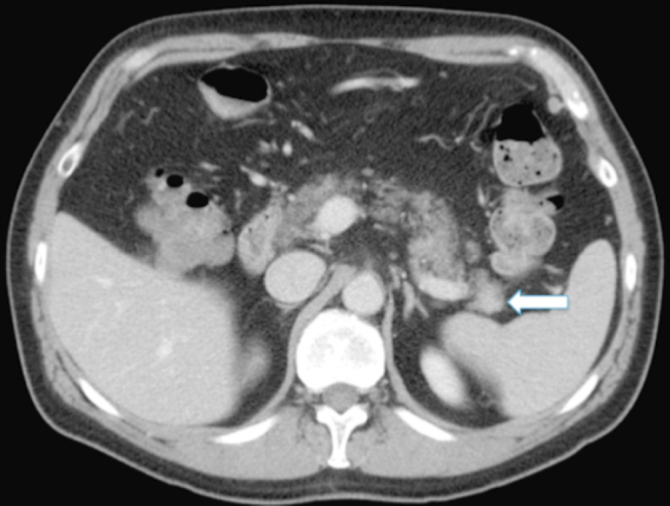
50-year-old man with intrapancreatic accessory spleen. Axial CT scan of the abdomen in the portal venous phase shows the same mass (arrow) in the pancreatic tail with similar attenuation values as the spleen.
Figure 1C.

50-year-old man with intrapancreatic accessory spleen. Coronal CT reconstruction in the arterial phase shows a hypervascular mass (arrow) in the pancreatic tail with similar attenuation values as the spleen.
Figure 1D.

50-year-old man with intrapancreatic accessory spleen. Sagittal CT reconstruction in the arterial phase shows a hypervascular mass (arrow) in the pancreatic tail with similar attenuation values as the spleen.
Figure 2.

50-year-old man with intrapancreatic accessory spleen. Hematoxylin and eosin stain performed on the surgical specimen shows an accessory spleen with a fibrotic capsule (arrows) surrounded by normal pancreatic tissue.
Discussion
Accessory spleens are common, usually asymptomatic, incidentally discovered congenital foci of splenic tissue, which are located at variable locations separate from the main spleen. They occur most commonly near the splenic hilum, with almost 20% in or near the pancreatic tail (1). These congenital normal variants carry with them a branch of the splenic artery and drain into the splenic vein (2). Accessory spleens can range in size from a few millimeters to several centimeters and have been reported to enlarge following splenectomy (1). They have been reported to be present in 16% of patients undergoing contrast-enhanced abdominal CT scan (3), and as many as 30% of patients at autopsy (4).
Most accessory spleens are readily identified on cross-sectional imaging by CT or MRI. However, because of their relatively hypervascular appearance on contrast-enhanced CT, differentiation of an IPAS from other pancreatic-tail lesions (islet-cell tumors and metastatic disease) can present a diagnostic challenge. A high index of suspicion by the radiologist, based on the classic location near the splenic hilum with typical imaging features and a combination of cross-sectional imaging studies such as ultrasound, CT, or MRI with nuclear medicine examinations, can confirm the diagnosis of IPAS and prevent unnecessary biopsy and/or surgery (5).
On ultrasound, accessory spleens are seen as round or oval structures with echogenecity similar to that of the main spleen; they show posterior acoustic enhancement. A high-amplitude interface is observed, due to a fibrotic capsule on histopathology. The presence of a vascular hilum entering the lesion on Doppler ultrasound is a sensitive diagnostic feature (2, 6). Contrast-enhanced ultrasound using Levovist (Schering, Berlin, Germany) demonstrates enhancement patterns similar to the spleen, with dense persistent enhancement for as long as 3 to 5 minutes. This persistent enhancement is related to the entrapment of Levovist by the reticuloendothelial system (RES) cells in the splenic tissue, a mechanism similar to that of Tc-99m heat-damaged red-blood-cell (HDRBC) scintigraphy and superparamagnetic iron-oxide (SPIO)-enhanced MRI (2).
The attenuation of IPAS on all dynamic CT phases is usually similar to that of the spleen and higher than that of the pancreas. Rarely, an IPAS may have attenuation lower than that of the surrounding pancreas on arterial and portal venous phases when splenic enhancement is delayed, such as in cirrhosis (2). A small IPAS can cause a difference in the attenuation values relative to the spleen due to partial volume effects; using 5-mm or thinner slices may help to accurately measure CT attenuation values (3).
As expected, signal intensities of IPAS on MRI are identical to that of the spleen on multiple pulse sequences with dynamic gadolinium enhancement similar to that of the normal spleen. Rarely, an IPAS may be brighter than the spleen on T2-weighted images due to higher white-to-red pulp ratio. SPIO-enhanced MRI can help confirm the diagnosis of IPAS by loss of signal intensity, similar to the spleen and negative contrast enhancement. It has the advantage of better spatial resolution than scintigraphy (2).
Technetium-99m HDRBC scintigraphy is a highly specific method for detecting splenic tissue, as up to 90% of injected HDRBCs are trapped by the splenic tissue. The diagnostic criterion for IPAS is the presence of a marked increase in uptake that exceeds that of the cardiac blood pool and the major vessels at the site of suspected accessory spleen. The drawback of this technique is poor anatomic resolution (even when single-photon-emission computed tomography (SPECT) is employed) when compared to CT and MRI (2). Alternatively, a technetium-99m sulphur colloid scan may be performed. No uptake is seen in neuroendocrine tumors or metastases using either of the two techniques (5).
It is sometimes difficult to distinguish an IPAS from a nonfunctioning islet-cell tumor or hypervascular metastasis based on imaging findings alone. On CT and MRI, islet-cell tumors often demonstrate uniform or ring-like enhancement, especially during the early arterial phase. Furthermore, the characteristic arciform or zebra-patterned enhancement pattern of the spleen is not seen in islet-cell tumors. On SPIO-enhanced MRI, islet tumors do not show loss of signal intensity or negative enhancement, due to the absence of RES cells. Hematogenous hypervascular metastases to the pancreas are rare and usually manifest in advanced disease. Melanoma, lung cancer, and breast carcinoma are the most common origins of pancreatic metastases. On CT and MRI, metastases usually demonstrate rapid enhancement on the arterial phase, but there is decreased lesion conspicuity on the portal venous phase, which is diminished even further on the delayed phase. Sometimes the distinction between metastasis and IPAS is difficult on imaging, and the clinical history is helpful in those cases. However, a hypervascular pancreatic tumor in the setting of renal-cell carcinoma should be considered a metastasis from renal cancer unless proven otherwise (2).
In conclusion, IPAS is becoming a more easily detectable lesion with ever-improving imaging capability. Radiologists should be aware of this entity and have a high index of suspicion if a subtle enhancing pancreatic tail mass is detected on CT. Knowledge of specific multimodality imaging characteristics using ultrasound, CT, MRI, and nuclear medicine examinations can prevent unnecessary biopsy and/or surgery.
Footnotes
Published: April 29, 2010
References
- 1.Sica GT, Reed MF. Case 27: intrapancreatic accessory spleen. Radiology. 2000;217(1):134–137. doi: 10.1148/radiology.217.1.r00oc30134. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Kim SH, Lee JM, Han JK, Lee JY, Kim KW, Cho KC. Intrapancreatic accessory spleen: findings on MR Imaging, CT, US and scintigraphy, and the pathologic analysis. Korean J Radiol. 2008;9(2):162–174. doi: 10.3348/kjr.2008.9.2.162. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mortele KJ, Mortele B, Silverman SG. CT features of the accessory spleen. AJR Am J Roentgenol. 2004;183(6):1653–1657. doi: 10.2214/ajr.183.6.01831653. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Halpert B, Gyorkey F. Lesions observed in accessory spleens of 311 patients. Am J Clin Pathol. 1959;32(2):165–168. doi: 10.1093/ajcp/32.2.165. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Spencer LA, Spizarny DL, Williams TR. Imaging features of intrapancreatic accessory spleen. Br J Radiol. 2009 Aug 18 doi: 10.1259/bjr/20308976. [PubMed] [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subramanyam BR, Balthazar EJ, Horii SC. Sonography of the accessory spleen. AJR Am J Roentgenol. 1984;143(1):47–49. doi: 10.2214/ajr.143.1.47. [PubMed] [DOI] [PubMed] [Google Scholar]


