Abstract
Information encoded in the DNA is interpreted, modified and propagated by chromatin. The diversity of inputs encountered by eukaryotic genomes demands a matching capacity for transcriptional outcomes provided by the combinatorial and dynamic nature of epigenetic processes. Advances in genome editing, visualization technology and genome-wide analyses have revealed unprecedented complexity of chromatin pathways, offering explanations to long-standing questions and presenting new challenges. Here, we review recent findings, exemplified by the emerging understanding of cross-regulatory interactions within chromatin, and emphasize the pathologic outcomes of epigenetic misregulation in cancer.
Introduction
The genetic information in eukaryotic cells is presented in the context of chromatin, a complex of nucleic acid and associated proteins. Genome functions are therefore linked inexorably with the epigenome – the combinatorial variance in localization and modifications of chromatin factors and underlying DNA. Epigenetic regulation impacts transcriptional readout, local and global chromatin compaction, and partitioning of genetic material during DNA damage responses and cell cycle progression. Five decades after the seminal work of Stedman, Allfrey, and colleagues, the field of epigenetics is growing at an unprecedented pace, fueled by recent advances in bioinformatics, structural biology, and genome-editing technologies.
The dynamic nature of the genome in the form of chromatin is a defining feature of epigenetic regulation. While the genetic material is only edited in few exceptional cases, the protein and RNA components of chromatin are extensively exchanged, modified and remodeled, affecting interpretation by a vast array of specific “readers” of epigenetic information. In this review, we emphasize the dynamically integrated nature of chromatin regulation. First, we briefly summarize the organization of information in chromatin, from DNA sequence and modifications, to nucleosome positioning, core and linker histone post-translational modifications, histone variant incorporation, and higher order folding of chromatin fiber. Second, we highlight recent advances that exemplify the prominence of cross-talk between distinct histone modifications, histone variants and chaperone systems, DNA and histone modifications, as well as the role of chromatin factors in global nuclear architecture. Third, we discuss paradigm-shifting examples of chromatin dysregulation in disease, highlighting the interconnected nature of epigenomic circuits.
Hierarchical organization of epigenetic information
Information encoded in the nucleic acid polymer is interpreted in a quantitatively and temporally controlled manner. Much of the genome function is exerted at the genetic level, wherein the regulatory output is dictated directly by the DNA sequence and transcription regulators that engage the DNA template to bring about functional consequences. Yet the identical genomes are interpreted in multiple ways, resulting in distinct transcriptional outcomes in diverse cell types and highlighting the existence of DNA sequence-independent, or epigenetic regulation.
The epigenetic phenomena are commonly defined as “alterations to chromatin template that establish and propagate different patterns of gene expression from the same genome”. These include a variety of processes, from chemical alterations to the bases in the DNA template, to post-translational modifications of core and linker histone proteins and incorporation of specific histone variants, to global nuclear topology. Epigenetic regulation is commonly represented as hierarchically organized process, with local effects of DNA base and histone modification on one end of the spectrum, and higher-order chromosome folding on another. While the emerging data suggest that individual epigenetic processes are deeply interconnected, such layered organization is intuitive, and represents a convenient organizational scheme to discuss recent key advances (Fig. 1, discussed below).
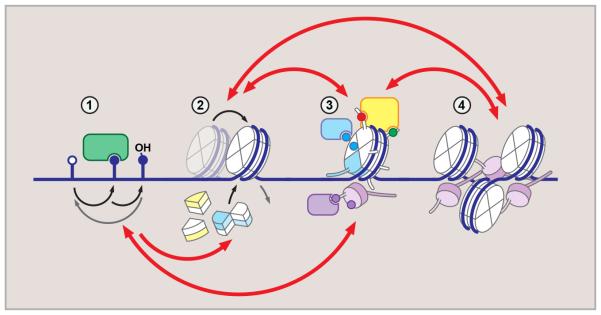
Figure 1. Hierarchical organization of epigenetic regulation.
Dynamic and reversible epigenetic processes generate diverse regulatory environment. DNA methylation (1) (mC, methylcytosine – closed circle) may result in eviction of DNA-binding proteins, or recruitment of methyl-binding factors. mC oxidation (shown is hydroxymethylcytosine, hmC) generates additional diversity. Core and linker histone exchange, including variant histone incorporation (2), regulate local DNA accessibility, and, together with histone modifications (3), introduce local variations to chromatin structure. These are interpreted by the “reader” machinery (3) and drive higher-order chromatin organization and nuclear topology (4). Red arrows above and below indicate crosstalk between regulatory layers.
DNA modifications
The general concept of “writers”, “readers” and “erasers” of epigenetic information, often applied to histone modifications, is fully relevant to DNA methylation. Several excellent reviews on the key players and regulation of DNA methylation machinery are available (Jeltsch and Jurkowska, 2014; Rose and Klose, 2014). Therefore, here we will only briefly emphasize the complexity of DNA modifications beyond cytosine methylation.
The ten-eleven translocation (TET) enzymes catalyze progressive oxidation of 5mC to 5-hydroxymethyl-, 5-formyl- and 5-carboxylcytosine, collectively known as oxi-mC. The results of these modifications are two-fold. First, they facilitate both active and passive demethylation (reviewed in (Kohli and Zhang, 2013)). Second, specific readers for oxi-mC marks expand the functional alphabet of DNA modifications (Spruijt et al., 2013). TET enzymes, together with Jumonji C (JmjC)-domain demethylases, rely on α-ketoglutarate (αKG), a metabolite of the TCA cycle, as cofactor in demethylation reactions (Kohli and Zhang, 2013). The TET and JmjC sensitivity to αKG levels was recently demonstrated in mouse embryonic stem cells, connecting glucose metabolism to maintenance of cell identity via epigenetic regulation (Carey et al., 2015). Moreover, αKG dependence makes these enzymes vulnerable to competitive inhibition by 2-hydroxyglutarate (2HG), an onco-metabolite accumulated in tumors carrying IDH1/2 mutations (Figueroa et al., 2010; Lu et al., 2012). While aberrant global increases in DNA and histone methylation are documented in these tumors, concomitant loss of oxi-mC may have additional, yet unknown effects. As aberrant DNA hypermethylation is widely observed in malignancy (Hansen et al., 2011), pharmacologic modulation of TET activity was suggested as broad therapeutic strategy in many cancers, necessitating better understanding of the factors and pathways involved in TET regulation, as well as consequences of oxi-mC loss in cancer biology (Huang and Rao, 2014).
Histone dynamics
The nucleosome is the basic unit of chromatin, with ~147 base pairs of DNA wrapped around a protein octamer, containing two copies each of “core” histones H2A, H2B, H3 and H4. A subset of nucleosomes associate with linker histone H1, which competes with high mobility group proteins to regulate linker DNA length and thus, DNA accessibility within compacted chromatin. The nucleosome placement is partly regulated by underlying DNA sequence, with poly-A/T tracts associated with nucleosome-free regions (Segal et al., 2006). Active repositioning of intact nucleosomes is mediated by ATP-dependent chromatin remodeling machinery, whereas nucleosome disassembly and exchange of core and linker histones is performed by histone chaperones (Burgess and Zhang, 2013).
During de novo nucleosome assembly, a pair of H3/H4 dimers are loaded first, rapidly followed by the addition of two H2A/H2B dimers to assemble the canonical histone octamer. The existence of functional subnucleosomal particles has been proposed, but remains controversial (Dalal et al., 2007; Miell et al., 2013). Using high-resolution chromatin immunoprecipitation-exonuclease assay (ChIP-exo), non-canonical nucleosomes were identified genome-wide (Rhee et al., 2014). These include asymmetrical structures, corresponding to hexasomes (lacking one H2A/H2B dimer) and half-nucleosomes (containing one of each H3/H4 and one H2A/H2B dimers). Additionally, these experiments identified extensive contacts between the H3 amino-terminal tail and the linker DNA, recently confirmed and extended by structural studies of reconstituted nucleosomal particles (Stutzer et al., 2016). These results demonstrate that regulatory effects of histone dynamics extend beyond the boundaries of core nucleosome particle.
Histone exchange is critical to regulation of specific transcriptional programs and responses. This requires removal and degradation of pre-existing histones, and incorporation of newly synthesized histones into chromatin. Downregulation of turnover by perturbation of histone synthesis or proteasome inhibition leads to distinct transcriptional defects across several cell types, suggesting universal role of these processes in maintenance of cell identity (Maze et al., 2015). Together, these results demonstrate the highly dynamic and extensively regulated nature of nucleosomes as the building blocks of chromatin
Histone modifications
An extensive catalog of covalent post-translational modifications (PTMs) of core and linker histones has been compiled to date. While many have attracted significant attention and are considered among the defining features of processes such as transcriptional activation (N-terminal tail acetylation), repression (H3 lysines 9 and 27 methylation), and DNA damage response (histone H2A.X phosphorylation), the vast majority await better characterization both on biochemical and genomic levels.
Despite the wealth of correlations linking specific histone PTMs to transcriptional states, establishment of causation in vivo remains challenging. However, advances in gene targeting enable locus-specific studies of chromatin modifications. The catalytic domain of acetyltransferase p300 is sufficient to activate transcription when targeted to either proximal promoter regions or distal enhancer elements using either nuclease-deficient Cas9, TALEN or zinc finger targeting technology (Hilton et al., 2015). Similarly, removal of enhancer-specific lysine 4 mono- and dimethylation of histone H3 (H3 K4me1/2) by targeting of histone demethylase LSD1 decreases enhancer activity and histone H3 acetylation, suggesting a hierarchical relationship between the two marks (Mendenhall et al., 2013). Likewise, transcriptional repression can be recapitulated by targeting H3 lysine 9 (H3 K9) methyltransferases SUV39H1 and G9A to endogenous loci (Snowden et al., 2002). Finally, a different approach using synthetic biology to achieve protein-specific trans-splicing of histones in situ, allows tracking the effects of “writer”-independent incorporation of chromatin modifications (David et al., 2015). These results demonstrate a causal relationship between epigenetic modifications and transcriptional activity, and hold promise in both locus-specific regulation of gene activity and large-scale studies of chromatin effectors (Keung et al., 2014). Together, these advances provide the means for controlled manipulation of epigenetic modifications in vivo, with broad implications for both fundamental and applied biology.
Histone variants
Variant isoforms have long been described for all core and linker histones and include diverse families of H1, H2A and H2B variants, more closely related H3 variants, and a Protozoa-specific H4 variant (Siegel et al., 2009). Deposited to discrete genomic loci by variant-specific chaperones, variant histones locally alter nucleosome structure and allow incorporation of additional PTMs into chromatin. The subject of histone variants and respective chaperones has recently garnered significant attention, with many extensive reviews available (Filipescu et al., 2014; Gurard-Levin et al., 2014; Skene and Henikoff, 2013). Here, we will briefly discuss the distinctions of the histone H2A and H3 variants that underscore the importance of small changes in histone amino acid residues in fundamental cellular functions.
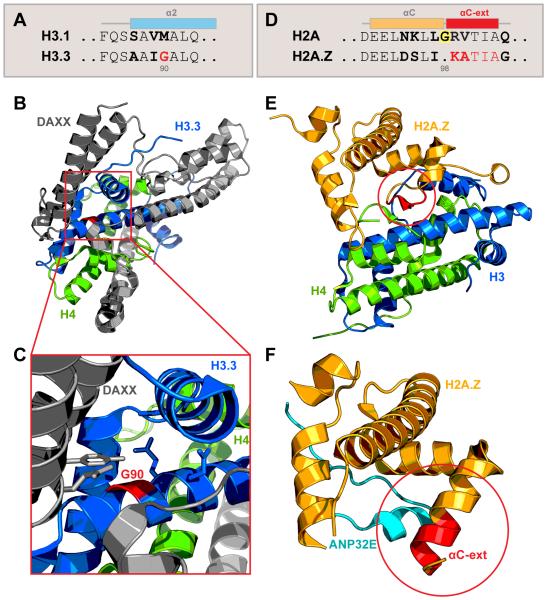
Incorporation of canonical histone H3 isoforms H3.1 and H3.2 into chromatin is strictly coupled to DNA replication and occurs in S-phase via stepwise action of anti-silencing function protein 1 (ASF1) and chromatin assembly factor-1 (CAF-1) chaperones, resulting in broad and non-specific genomic distribution. The variant histone H3.3, expressed and incorporated in a replication-independent manner, relies on two distinct chaperone systems. Histone regulator A (HIRA) complex deposits H3.3 at transcriptionally active genic regions, and death-domain associated protein/α-thalassemia and mental retardation syndrome X-linked protein (DAXX/ATRX) complex targets H3.3 to pericentromeric, telomeric, and repeat regions of the genome (Elsasser et al., 2015; Goldberg et al., 2010; Ray-Gallet et al., 2011). The molecular mechanism of H3.3 recognition by DAXX/ATRX has been elucidated. While H3.1 and H3.2 carry methionine at position 90, H3.3 contains a glycine, critical for extensive local hydrogen bond network formation between H3.3 and DAXX. Notably, point mutation Met90Gly, but not Met90Ala, confers H3.3-like recognition of H3.2 by DAXX, demonstrating the critical role of a single amino acid side chain in recognition by chaperone complex (Fig. 2A-C) (Elsasser et al., 2012). Interestingly, the mechanism for eviction of histone variant H2A.Z by its chaperone acidic nuclear phosphoprotein 32 family member E (ANP32E) also relies on a single amino acid difference within C-terminal alpha-helix (Cα) of the histone fold domain. The canonical H2A carries a glycine at position 98, which breaks the α-helix extension. In contrast, H2A.Z, missing Gly98, is able to extend the Cα-helix specifically when bound by ANP32E. This extension is incompatible with H2A.Z binding with the nucleosome and facilitates the ANP32E-mediated H2A.Z eviction (Fig.2D-E) (Mao et al., 2014; Obri et al., 2014). Together, these structural insights demonstrate how single amino acid differences result in profound effects on histone dynamics.
Figure 2. Structural basis of histone variant dynamics: implications of single amino acid differences.
(A-C) Glycine at position 90 is critical for H3.3 recognition by chaperone DAXX.
(A) Amino acid sequence of histone H3.1 and variant H3.3 α2 helix, with three distinct residues in bold. G90 is highlighted in red.
(B, C) Ribbon diagram of DAXX-H3.3-H4 complex structure, with DAXX shown in gray, H3.3 in blue and H4 in green [PDB 4H9N (Elsasser et al., 2012)]. Variant-specific interface is shown is expanded in (C). G90 is highlighted in red, nearby amino acid side chains are shown.
(D-F) Structural basis for H2A.Z eviction by chaperone ANP32E.
(D) Amino acid sequence of histone H2A and variant H2A.Z αC-helix. Glycine in position 98 (highlighted) prevents the helix extension in canonical H2A; five additional amino acids can extend the helix (αC-ext) in H2A.Z variant. Distinct amino acid residues are shown in bold.
(E) Within the nucleosome, H2A.Z αC-helix is short and accommodates contacts with C-terminus of H4 [PDB: 1F66 (Suto et al., 2000)].
(F) Bound by ANP32E (teal), H2A.Z αC-helix is extended (red) and is incompatible with the H4 interface, resulting in eviction from the nucleosome [PDB: 4CAY (Obri et al., 2014)].
Histone variant H3.3 differs from replication-dependent canonical H3.1 and H3.2 by five and four amino acids, respectively, only one of which is located in the heavily modified N-terminal tail. Specifically, while H3.1 and H3.2 carry alanine at position 31, H3.3 contains a serine. The substitution of serine for alanine was recently found to be critical for association of H3.3-specific lysine 36 trimethylation (K36me3) reader, zinc finger MYND domain-containing protein 11 (ZMYND11, also known as BS69), a candidate tumor suppressor protein (Guo et al., 2014; Wen et al., 2014). K36me3 recognition by the PWWP domain is stabilized when the polar side chain of Ser31 is enveloped in a pocket made by the bromo, ZnF, and PWWP domains of BS69, resulting in seven- to eight-fold preference for H3.3 K36me3 by the reader (Wen et al., 2014), further underscoring the combinatorial effect of multiple reader domains (Ruthenburg et al., 2007). The chromatin-associated BS69 colocalizes with trimethylated H3.3 K36 genome-wide, and associates with the splicing machinery, possibly suppressing spliceosome activation (Guo et al., 2014). Additionally, and not exclusive with the previous observation, BS69 depletion increases RNA Polymerase II occupancy in gene bodies, suggesting a function as a suppressor of elongation (Wen et al., 2014). Whereas the specific function of BS69 remains to be elucidated, phosphorylation of Ser31 in H3.3 provides a likely mechanism to regulate its chromatin association. In vitro, BS69 to a phospho-Ser31-containing peptide is dramatically reduced relative to an unmodified peptide (Guo et al., 2014). While H3.3 Ser31phos has been identified in vivo as a mitotic histone modification (Hake et al., 2005), additional studies are required to determine its biological function in the cell cycle or otherwise, with the regulation of BS69 recruitment providing important clues.
Similar to H3.3, histone variant H3.5, found exclusively in hominids and great apes, carries serine and glycine at positions corresponding to 31 and 90 of H3.3, suggesting that two replication-independent variants have overlapping functions (Schenk et al., 2011). However, while all other H3 isoforms carry a conserved phenylalanine at position 104, H3.5 contains a leucine at corresponding position 103. Unlike phenylalanine, Leu103 does not support extensive hydrophobic contacts with H4, significantly reducing the nucleosome stability (Urahama et al., 2016). In agreement with the biophysical and structural observations, H3.5 was found to localize predominantly around the transcription start sites, suggesting the possible role for an unstable H3.5-containing nucleosome in transcription (Urahama et al., 2016). Taken together, these studies highlight the key contribution of seemingly small, even single amino acid, differences within histone variants to many levels of epigenetic regulation. The recent identification of genetic mutations mapping to H3 variants at or near sites of PTMs in various human cancers underscores not only critical links between histone variants and the PTMs that they carry, but also the importance of both to human biology and disease (expanded upon below).
Higher-order chromatin organization
Epigenetic modifications shape the topology of the genome, with effects on gene expression and cell fate. Results from several recent studies support causative role of epigenetic processes in regulation of genome architecture, from local chromatin fiber compaction to global effects on gene localization within the three-dimensional nuclear space (Fig. 1G-I).
Continued development of super-resolution microscopy will likely have a profound impact on chromatin studies. While the existence of orderly compacted “30-nm” fiber in vivo has been debated (Grigoryev and Woodcock, 2012), analysis of nucleosome organization using stochastic optical reconstruction microscopy (STORM) identified organized domains of condensed nucleosomes, termed “nucleosome clutches” (Ricci et al., 2015). Varied in size and density, the clutches correlate positively with linker histone, and negatively with Pol II occupancy. Importantly, median number of nucleosomes per clutch is a stable property of cellular state, indicative of the pluripotency grade of stem cells (Ricci et al., 2015). In a complementary study, STORM imaging of chromosomal domains labeled by DNA in situ hybridization revealed distinct chromatin fiber organization for transcriptionally active, inactive and Polycomb-silenced domains in Drosophila (Boettiger et al., 2016). Results demonstrate that the longer domains are increasingly more compact, with a high degree of intra-domain interactions. These data support the model that Polycomb-dependent repression, enacted in part via chromatin compaction, is likely reinforced by a positive feedback loop (Boettiger et al., 2016). Together, these studies highlight the complexity of three-dimensional nature of chromatin in vivo, by visualizing individual components of chromatin fiber at nanoscale resolution.
Interactions between genomic loci are a critical component in regulation of gene expression. CCCTC-binding factor (CTCF) is a methylation-sensitive DNA-binding protein shaping the topology of vertebrate genomes. Recently, the CTCF binding profiles and alterations in DNA conformation were investigated in IDH mutant gliomas, a class of tumors demonstrating DNA hypermethylation phenotype (Flavahan et al., 2016). Remarkably, PDGFRA, a known glioma oncogene, was identified among the top targets inappropriately activated via long-distance interaction with a constitutive enhancer, due to loss of CTCF-dependent chromatin domain boundary. Treatment with demethylating agents restored CTCF binding profile and normalized PDGFRA expression, suggesting that alterations of chromatin topology are causative to transcriptional misregulation.
The unifying feature of the epigenetic phenomena described above is the extensive cross-talk between the different pathways. Cross-talk interactions provide regulatory inputs for signaling pathways, metabolic conditions, and developmental states to act upon, serving to reinforce and stabilize specific chromatin states. Understanding the checks and balances within this system, where no process occurs in isolation, is in our view key to deciphering the epigenetic code.
Cross-talk between epigenetic regulatory pathways
The combinatorial potential of epigenetic modifications is staggering. With dozens of modifiable amino acids in each histone, histone variants within the nucleosome, DNA and histone PTM reader combinations, nucleosome occupancy states, and higher-order chromatin structures, the challenge is to identify key nodes in the network which may serve as regulatory “command centers”. Here, we will focus on several recent findings refining the mechanistic underpinnings of epigenetic regulation.
DNA methylation and histone modifications
Ample evidence supports the combinatorial nature of the epigenetic regulation. The strong relationship between histone modifications and DNA methylation was observed in early genome-wide studies (Lister et al., 2009) and has since gained significant attention. The enzymatic system driving DNA methylation in mammals has two principal catalytically active components: de novo methyltransferases Dnmt3a and Dnmt3b (collectively referred to as Dnmt3), and maintenance methyltransferase Dnmt1. As their names imply, the Dnmt3 system targets unmethylated CpG dinucleotides, preferentially generating hemimethylated sites, which in turn are targeted by Dnmt1 to generate fully methylated CpGs (Smith and Meissner, 2013).
Further, while cooperation between de novo and maintenance methyltransferases elegantly coordinates how DNA methylation is stably inherited over many cycles of DNA replication (Chen et al., 2003; Fatemi et al., 2002) it does not feature in the where and when of DNA methylation in diverse cellular contexts. Accordingly, targeting of both de novo and maintenance methyltransferases relies on, and cooperates with, multiple chromatin factors (Fig. 3A).
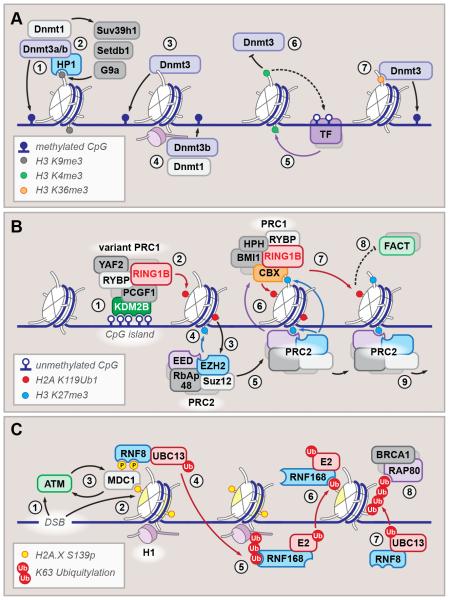
Figure 3. Diverse processes are defined by extensive cross-talk between epigenetic circuits.
(A) Regulation of DNA methylation. Heterochromatin protein 1 (HP1) binds H3 K9me2/3 (gray circle) and recruits de novo and maintenance DNA methyltransferases (1), which in turn associate with H3 K9-specific methyltransferases Suv39h1, Setdb1 and G9a (2). Other targeting mechanisms include unmodified H3 tail recognition by Dnmt3 ADD domain (3) and linker histone-dependent recruitment (4). Histone H3 K4 methylation (green circle) (5) prevents Dnmt3 association and protects transcription factor (TF) binding sites from methylation (6). Additionally, PWWP domain of Dnmt3 reads H3 K36 methylation (orange circle) and is required for Dnmt recruitment to the gene bodies (7).
(B) Hierarchical recruitment and spreading of Polycomb complexes PRC1 and PRC2. Unmethylated CpG islands are bound by KDM2B (1), which recruits variant PRC1 complex; the RING1B E3 ubiquitin ligase then monoubiquitylates H2A K119 (red circle) (2), which facilitates recruitment of PRC2 (3) and methylation of H3 K27 (blue circle) (4). Processive PRC2 spreading (5) expands the K27me domain, which in turn facilitates PRC1 recruitment, which recognizes K27me via CBX subunit (6) and in turn, expands the K119 ubiquitylation (7). This may counteract histone replacement by chaperone FACT (8) and result in chromatin compaction (8). For clarity, some labels are omitted from the schematic.
(C) DNA double-stranded break response relies on a cascade of dynamic core and linker histone modifications. Initiated by ATM recruitment to the DSB site (1) and phosphorylation of H2A.X variant at serine 139 (2), the cascade is amplified by ATM1-MDC1 positive feedback loop (3), which facilitates recruitment of ubiquitin ligases RNF8/UBC13 (4). K63-linked polyubiquitylation of linker histone then recruits RNF168 (5), which, together with yet unidentified E2 ligase, monoubiquitylates H2A (6). RNF8 then extends the H2A ubiquitylation placed by RNF168 (7), which serves as recruitment platform for many DNA damage response factors (BRCA1 and RAP80 are shown) (8).
The first compelling mechanistic evidence that connected DNA and histone methylation was obtained from genetic studies in Neurospora and Arabidopsis (Jackson et al., 2002; Tamaru and Selker, 2001). Loss of histone H3 lysine 9 methylation (H3K9me) disrupted the DNA methylation and phenocopied the DNA methyltransferase mutant phenotypes. Biochemical studies demonstrated that the Heterochromatin protein 1 (HP1), a canonical reader of H3K9me, directly interacts with DNA methyltransferases in both plants and animals, recruiting Dnmts to repressed regions (Jackson et al., 2002; Smallwood et al., 2007). In a positive regulatory loop, Dnmts recruit H3K9 methyltransferases, including Suv39H1, associated with heterochromatin formation, and G9a/GLP, associated with H3K9 methylation in euchromatic regions (Fuks et al., 2003; Smallwood et al., 2007). Thus, Dnmt-H3K9me relationship is bi-directional and mutually reinforcing. Further, structural studies solved an intriguing autoregulatory mechanism, mediated by interaction between the two protein domains connected by a flexible linker. The ATRX– DNMT3–DNMT3L (ADD) domain, present in all Dnmt3 isoforms, directly binds the H3 N-terminal tail; this interaction is abrogated by methylation of lysine 4, as well as phosphorylation of threonine 3, serine 10 or threonine 11 (Zhang et al., 2010). Importantly, ADD binding to H3 tail serves not only as a positioning mechanism for the Dnmt3 on chromatin, but induces a conformational change, whereby ADD domain releases the adjacent catalytic domain, which now is able to bind its DNA substrate (Guo et al., 2015). Thus, Dnmt3 is preferentially targeted to chromatin domains enriched for H3K9 methylation and contains a failsafe mechanism to avoid deposition of DNA methylation in the vicinity of H3K4me – a mark associated with active transcriptional processes. Proof of principle studies manipulating the Dnmt3a ADD domain to switch recognition from unmodified H3 tail to H3K4 methylation demonstrated that alteration of the ADD failsafe caused partial redistribution of Dnmt3 and DNA methylation, leading to aberrant repression of developmentally regulated genes (Noh et al., 2015). Expectedly, the redistribution was only partial, demonstrating that ADD-independent targeting mechanisms remain. In addition to HP1 interaction, Dnmts associate with linker histone H1d isoform; this association promotes establishment of DNA methylation at imprint control regions in mouse ES cells (Yang et al., 2013). Further, Dnmt3 is targeted to the gene bodies of actively transcribed genes via its PWWP domain recognizing H3 lysine 36 trimethylation (Baubec et al., 2015; Dhayalan et al., 2010). The consequences of genic DNA methylation may involve regulation of transcriptional fidelity and, indirectly, splicing outcomes (de Almeida and Carmo-Fonseca, 2014; Shukla et al., 2011). Additionally, the function of PWWP domain likely extends beyond targeting to genic regions, as PWWP truncation results in loss of methylation at H3K9me3-marked pericentric heterochromatin (Chen et al., 2004). Partially redundant, this system is both stable to perturbations, and at the same time amenable to many regulatory inputs. As additional mechanistic aspects are being refined, the “big picture” of processes involved in regulation of DNA methylation will undoubtedly become more clear.
Histone modifications by Polycomb complexes
The remarkable rich history of Polycomb mutants discovery and, later, Ed Lewis’ realization that “Pc+ in all likelihood is coding for a repressor [of Bithorax complex genes]” (Lewis, 1978) have deservedly been described in great detail as one of seminal breakthroughs in gene regulation (Duncan and Montgomery, 2002). Later studies, demonstrating the histone methyltransferase activity of SET domain (Rea et al., 2000) and identifying Enhancer of Zeste [E(Z)] subunit of Drosophila Polycomb complex as a functional histone H3 lysine 27 (H3K27) methyltransferase (Muller et al., 2002) laid mechanistic groundwork for understanding the developmentally regulated mechanism of repression. Subsequently, genetic and biochemical studies defined two core PcG complexes, PRC1 and PRC2, with distinct enzymatic activities and related targeting mechanisms; PRC1 is defined by monoubiquitylation activity towards H2A lysine 119 (H2A K119Ub), and PRC2 carries H3K27 methyltransferase subunit. Apart from the core enzymatic machinery, subunit composition of PcG complexes is dynamic and reflects the profound regulatory role PcG proteins play in development [reviewed in (Di Croce and Helin, 2013; Grossniklaus and Paro, 2014; Schwartz and Pirrotta, 2013; Simon and Kingston, 2013)].
Mechanisms controlling PcG targeting to specific loci have been under extensive investigation. In Drosophila – historically the model of choice for Polycomb biology - this is achieved primarily by specialized DNA modules, termed Polycomb Response Elements (PREs). PREs, containing multiple binding sites for PcG-recruiting transcription factors, act as autonomous and portable repressor elements, and have been described in many developmentally regulated loci (Kassis and Brown, 2013). In vertebrates, however, despite high conservation of core PcG machinery, different recruitment mechanisms appear to prevail. In search for the mammalian “PRE” analogs, genome-wide mapping experiments demonstrated that, in mouse ES cells, PcG proteins are enriched at large CpG islands (Ku et al., 2008). Elegant follow-up studies found that an artificial GC-rich element, depleted of activating transcription factor binding sites, is sufficient to target PRC2 (Mendenhall et al., 2010). Subsequently, KDM2B/Fbxl10 was shown to specifically bind unmethylated CpG islands and recruit variant PRC1 complex (Fig. 3B) (Farcas et al., 2012). Of note, KDM2B is a histone H3 K36me2-specific demethylase, with this activity likely playing a role in PcG retention (Yuan et al., 2011). PRC1 variant complex recruited by KDM2B contains RING1B subunit, which monoubiquitylates H2A K119. This modification in turn facilitates PRC2 binding and methylation of H3 K27 (Blackledge et al., 2014; Kalb et al., 2014). Further, the K27me3 is recognized by canonical PRC1 component CBX (vertebrate homolog of Drosophila Polycomb protein), facilitating further H2A K119 ubiquitylation. The results of this hierarchical and processive propagation of PRC1 and PRC2-dependent chromatin modifications are at least two-fold. First, H2A K119Ub was reported to directly interfere with nucleosome remodeling by FACT complex, thus blocking transcriptional elongation by RNA polymerase II (Zhou et al., 2008). Second, while the mechanistic implications of H3 K27me3 are not yet fully understood, recent imaging studies and structural insights suggest that PRC2 may be involved in chromatin compaction, similar to HP1 proteins which recognize and compact H3K9me2/3-decorated chromatin (Boettiger et al., 2016; Jiao and Liu, 2015). Of note, H3 K27me, but not H2A K119Ub, appears to be critical for PcG-mediated repression in Drosophila, suggesting that PRC2 may be the ultimate effector in the hierarchy (Pengelly et al., 2013; Pengelly et al., 2015). Interestingly, recent study in yeast C. neoformans demonstrated that PRC2 anchoring to the H3 K27me substrate prevents it from redistribution to the H3 K9me2 heterochromatin (Dumesic et al., 2015). Similarly, HP1 depletion in Neurospora resulted in PRC2 and H3K27me spreading into constitutive H3 K9me2 domains (Jamieson et al., 2016). While the H3 K27me and H3 K9me domains are distinct in eukaryotes, these studies highlight the close relationship between the two PTMs, underscoring the roles of protein partnerships and substrate recognition in targeting of chromatin modifying enzymes. Additionally, nucleosome turnover and deposition of H3.3 variant at PcG target loci by histone chaperone HIRA facilitates PRC2-dependent repression in mouse ES cells (Banaszynski et al., 2013). Taken together, these studies highlight how DNA methylation, histone modifications and respective readers, and nucleosome dynamics act together in a concerted fashion to bring about developmental gene regulation by PcG complex proteins.
Linker and core histone modifications in DNA damage response
Chromatin dynamics during DNA damage exemplifies a rapid and potent response involving a hierarchy of diverse factors (Fig. 3C). A key factor in DNA damage response (DDR) is the kinase Ataxia teleangiectasia mutated (ATM), which is recruited to DNA double stranded break (DSB) sites by Mre11-Rad50-Nbs1 (MRN) surveillance complex or via alternate, yet unknown mechanisms (Hartlerode et al., 2015). Immediately following the damage event, H2A/H2B turnover is increased at the DSB site (Dinant et al., 2013). Histone variant H2A.X, pre-existing or newly replaced at the DNA damage site (Seo et al., 2012), is then phosphorylated by ATM at serine 139; modification generally referred to as γH2A.X. It was noted early that induction of approximately 35 DSBs per haploid genome results in conversion of about 1% of total H2A.X to γH2A.X (Rogakou et al., 1998). These calculations, together with the subsequent microscopic studies demonstrate that H2A.X phosphorylation occurs not only in the immediate vicinity of the DSB, but broadly over megabase-scale domains (Rogakou et al., 1999). One mechanism by which this occurs involves the recognition of γH2A.X epitope by adaptor protein Mediator of DNA damage checkpoint 1 (MDC1), which first, recruits additional ATM to γH2A.X foci and, second, itself is a substrate of ATM-dependent phosphorylation (Lou et al., 2006). Phosphorylated MDC1 binds E3 ubiquitin ligase RNF8, which based on biochemical studies was proposed to first target extra-nucleosomal protein(s), in order to recruit and activate another E3 ligase RNF168 to target H2A and H2A.X at the DSB site (Mattiroli et al., 2012). This hypothesis was recently validated when RNF8 substrate was identified as the linker histones (Thorslund et al., 2015). This intermediate step allows efficient recruitment of RNF168 to the DSB, and cooperation between RNF168, which recognizes and monoubiquitylates H2A, and RNF8, which extends the H2A ubiquitylation via lysine 63 (K63) linkage, allows the propagation of the signal across the broad domain. Interestingly, RNF168 contains two Ub-recognizing motifs, UDM1 being specific for RNF8-generated K63-poly-Ub, and UDM2, binding RNF168-generated H2A-Ub. Therefore, two separate domains facilitate targeting to the DSB (UDM1) and propagation of the H2A ubiquitylation (UDM2) (Thorslund et al., 2015). Together, this cascade of modifications results in rapid and potent establishment of polyubiquitylation in chromatin surrounding the DSB, facilitating recruitment of checkpoint and repair complexes, including RAP80/BRCA1, RAD18 and 53BP1. While many mechanistic aspects of these processes yet need to be refined, chromatin dynamics in DDR highlight the extensive connections between histone exchange, PTMs, and as recently demonstrated, interplay between linker histones and core nucleosomal particles. Of note, recent identification of high prevalence of missense mutations in linker histones in hematologic malignancy raises an intriguing possibility that some early components of DNA damage response may be affected in such cases (Morin et al., 2013; Okosun et al., 2014; Pasqualucci et al., 2014). Additional studies will address this possibility.
Histone phosphorylation and chromatin activation
Histone tails contain regulatory hotspots – sites of abundant PTMs with important function. An intriguing feature of histone tails is a recurrent amino acid sequence, ARKS, at three of these regulatory hotspots. The H3 N-terminal tail features two of these, with one around K9 and another around K27, the third occurring on H1E at K26 (Fig. 4A). The recurrent use of this motif suggests that this sequence and its modification have optimal and rich regulatory potential. Here we will describe examples of histone modification “cross-talk” within these motifs during dynamic regulation of chromatin states with important biological outcomes. Beyond these examples, we anticipate that future work will reveal additional regulatory potency of these motifs.
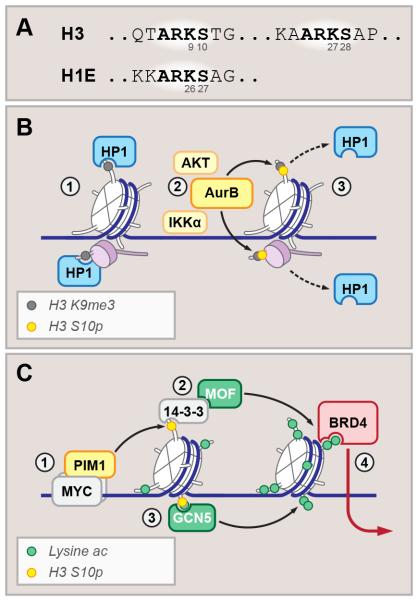
Figure 4. Binary switches and cooperative activators: roles of histone phosphorylation.
(A) Sequences of H3 and H1E N-terminal tails, with stereotypical ARKS motifs highlighted.
(B) H3K9me2/3 and H1E K26me2/3 (gray circles) recruit HP1 in interphase cells. phosphorylation of adjacent lysines by cell-cycle dependent kinase Aurora B (yellow circles) (2) results in HP1 eviction from mitotic chromosomes (3).
(C) MYC recruits PIM1 kinase to facilitate H3 S10 phosphorylation (1). Bound by 14-3-3 proteins (2) which in turn recruit histone acetyltransferase MOF, or by GCN5 acetyltransferase (3), S10 phosphorylation facilitates acetylation-dependent transcriptional activation, recruiting bromodomain-containing factors (BRD4 is shown) (4).
Featured in the ARKS motif are the adjacent lysine and serine residues, with potential for acetylation or methylation, and phosphorylation, respectively. These sequences contain K9 and K27, which have important regulatory functions (described above) raising the potential for serine phosphorylation to influence these activities. As a prominent example, phosphorylation of H3S10 (H3S10ph) by Aurora B (AurB), AKT, or IKKa kinases disrupts the HP-1 chromodomain interaction with methylated H3K9, and thereby reverses HP-1 chromatin binding and chromatin compaction (Fischle et al., 2005) with potential function in both mitotic chromatin compaction and interphase gene activation (Fig. 4B). Similarly, H3S28ph disrupts the interaction between PRC2 component Eed, and methylated H3K27, potentially interfering with a critical feedback pathway in PRC2-repressed chromatin (Gehani et al., 2010; Lau and Cheung, 2011). The H1E ARKS motif represents a third example of an ARKSph that acts to eject readers of the adjacent methyl-lysine. H1E lysine 26 is methylated by G9a/KMT1C, and is bound by HP-1 with contributions to formation of repressed heterochromatin. HP-1 is ejected from methylated H1E26 by phosphorylation of the neighboring S27 by AurB (and potentially other histone kinases) with potential to disrupt its function (Daujat et al., 2005; Trojer et al., 2009). Finally, the factor BAHD1, which acts as a repressor of interferon stimulated genes via interactions with HP1, MBD1, SETDB1, and HDAC5 (Bierne et al., 2009; Lebreton et al., 2011), has recently been reported as a reader for H3K27me3 via the BAH domain, with H3S28ph abrogating this H3 tail binding (Zhao et al., 2016). Remarkably, all known instances of methyl-lysine reader ejection by histone phosphorylation within ARKS motifs represent loss of repressive factor binding. Further studies are needed to assess the cellular context and functional effects of these events.
Beyond ejection of methyl-lysine readers by indirect switch-based mechanisms, ARKS motif phosphorylation may also feature generally as a direct substrate for histone readers, although few histone phospho-serine readers have been characterized. Beyond MDC1 binding to S139ph on H2A.X in the context of the DNA damage response, 14-3-3 proteins appear to be generic readers for ARKSph, binding to both H3S10ph and H3S28ph (Macdonald et al., 2005; Walter et al., 2008; Winter et al., 2008). 14-3-3 isoforms may activate gene expression by recruiting Brg1 (Drobic et al., 2010) or the histone acetyltransferase, MOF (Zippo et al., 2009). 14-3-3-mediated recruitment of MOF to chromatin featuring phosphorylated ARKS motifs results in subsequent acetylation of H4K16, a rate-limiting step in the recruitment of Brd4 and transcription elongation (Fig. 4C). Consistent with a role for histone phosphorylation and acetylation in chromatin activation, the combinatorial ARKacSph modification stabilizes interactions with positive transcriptional regulators including 14-3-3 and bromo-containing factors. Indeed, while histone phosphorylation universally ejects repressive methyl-lysine readers, the activating acetyl-lysine readers (BRDs), and phospho-serine readers (14-3-3) appear to tolerate or even prefer the dual modification ARKacSph (Filippakopoulos et al., 2012; Winter et al., 2008).
There is likely to be more to the ARKS sequence, if “every amino acid matters”. One more intriguing example is the potential for cross-talk between the arginine residue and phospho-serine. Crystal structures of histone peptides bound by readers (ATRX, 14-3-3) demonstrate that H3R8 and H3S10ph can engage in stabilizing intra-molecular ionic interactions that effectively present the H3K9 residue for reader recognition (Macdonald et al., 2005). Similar intramolecular dynamics are likely to result in increased accessibility of H3K27 in the context of H3S28ph. Beyond these specific intra-molecular interactions within the ARKS motifs, histone modifications appear to generally increase the dynamics of the histone tail sequence around the modified residues, thereby improving histone tail accessibility to regulatory factors. This is critical in the context of condensed chromatin, featuring extensive histone tail-DNA and histone tail-H1 interactions (Stutzer et al., 2016). Both of these mechanisms – R-Sph stabilization leading to presentation of the internal lysine residue and PTM-mediated increases in tail dynamics, – could be thought of as positive feedback, lowering the threshold for subsequent reader and writer activities within ARKS sequences. One example of this feature is the activation of the histone acetyltransferase (HAT) GCN5 by H3S10ph; GCN5 HAT activity is substantially increased on substrate pre-modified with S10ph (Clements et al., 2003). With the above mechanistic insights, we suggest that histone phosphorylation, within ARKS motifs, may feature generally to activate regional acetylation on the histone tails, as has been described for GCN5/PCAF.
The ARKS phosphorylation events feature ejection of repressive factors, recruitment of activating factors (14-3-3, BRD-containing factors), or activation of HATs (GCN5/PCAF) highlighting the general association of histone phosphorylation with active chromatin processes and transcription. Indeed, histone H3 phosphorylation is a common feature of cellular responses to stimulation (Mahadevan et al., 1991). Therefore, kinase pathways can potently and directly regulate chromatin via histone phosphorylation during cellular responses to environmental cues and this feature likely acts coordinately with the wide array of other kinase-regulated cellular processes, especially activation of DNA-binding transcription factors.
Hanging by the balance: misregulation of epigenetic circuits in disease
Emerging evidence implicates chromatin misregulation in human disease. The “readers”, “writers” and “erasers” of specific epigenetic marks have been long known among the most highly mutated in cancer and developmental disorders (Morgan and Shilatifard, 2015). No attempt will be made here to provide comprehensive coverage of this important topic. Instead, we will discuss examples illustrating the oncogenic chromatin circuits arising from alterations in cross-talk relationships (Fig.5). A surprising general observation from genome-wide comparisons of multiple tumor types that is not yet fully understood is that malfunctions of globally expressed and essential factors often result in lineage-specific defects, driving progression of highly specific tumor types. For example, either inactivating mutations or overexpression resulting in increased activity of PRC2 catalytic subunit EZH2 are prevalent in T-cell leukemia and B-cell lymphomas, respectively (Morin et al., 2010; Simon et al., 2012). This likely reflects the differences in cell of origin and indicates that PRC2 broadly regulates diverse transcriptional programs. Additionally, evidence discussed below suggest that in many cases the disease phenotype arises from complex interaction of multiple circuits, where loss or gain of one function triggers pathogenic misregulation of other factor(s), which in turn result in disease phenotype.
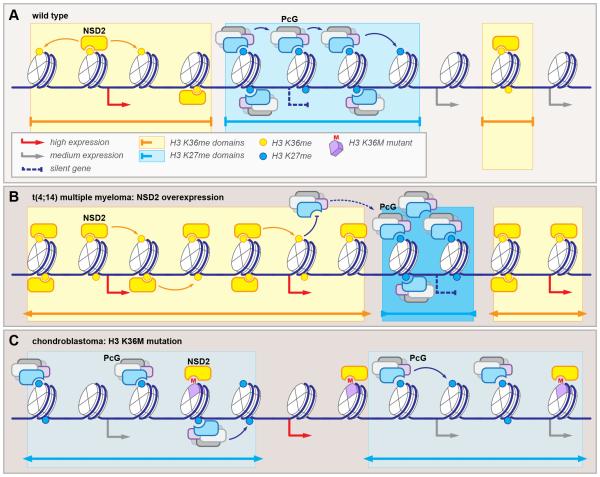
Figure 5. Reciprocal misregulation of Polycomb-dependent silencing by alterations of H3 K36 methyltransferase function: the yin and yang of histone methylations.
(A) In wild type cells, gene expression is correlated positively with histone H3 K36 methylation (yellow circles) and negatively with K27 methylation (blue circles). The domains demarcated by these two modifications (yellow and blue rectangles) are non-overlapping.
(B) NSD2 overexpression in t(4;14) multiple myeloma leads to expansion of H3 K36me, which counteracts PcG complex activity and causes PcG relocalization to few highly methylated ectopic domains (darker shade of blue), resulting in aberrant gene repression.
(C) Histone H3 K36M mutation in chondroblastoma dominantly inhibits NSD2 methyltransferase activity, resulting in global loss of H3 K36 methylation. Loss of counterbalancing signal causes spreading of PcG from well-demarcated repressed regions to intergenic “sinks” (lighter shade of blue), resulting in ectopic gene activation.
Ectopic acetylation in NUT midline carcinoma
A translocation t(15;19) was independently reported in several isolated cases of aggressive thymic and laryngeal carcinomas (French, 2012). Currently known as NUT midline carcinoma (NMC), this tumor is predominantly characterized by an oncogenic fusion between bromodomain-containing protein 4 (BRD4) and putative transcription factor nuclear protein in testis (NUT). As the patient cohort expanded, other NUT fusions, involving BRD4 paralog BRD3, and histone H3K36 methyltransferase NSD3 have been reported (Alekseyenko et al., 2015). BRD4 is a ubiquitous transcriptional coactivator that associates with acetylated histone tails via its double bromodomain and recruits Mediator and P-TEFb to the target promoters (Wu and Chiang, 2007). NUT encodes a gonad-specific transcriptional activator that recruits the histone acetyltransferase p300 and facilitates its enzymatic activity at a number of substrates, including autoacetylation and histone H3 lysines 9, 14, 18, 27, and 56 (Reynoird et al., 2010). Genome-wide and colocalization studies carried out in NMC lines and cells transduced with BRD4-NUT fusion demonstrated that ectopic expression of NUT fused to BRD4 resulted in recruitment of activated p300/CBP complexes directly to the BRD4-bound sites; propagated locally, these form approximately a hundred hyperacetylated "megadomains", on average 2 Mb in size, delimited by known topological domain boundaries (Alekseyenko et al., 2015; Reynoird et al., 2010). Therefore, BRD4-NUT fusion represents a classic positive feedback loop which, in absence of negative regulation, results in a dramatic regulatory malfunction. The consequences of this dysfunction are two-fold. First, the megadomains include and aberrantly activate several oncogenes, including c-MYC, PVT1, MED24, and TP63 (Alekseyenko et al., 2015). Second, extensive hyperacetylation driven by BRD4-NUT may result in genomic “sink” for many transcription factors, including p300/CBP, resulting in downregulation of genes outside of the megadomains (Reynoird et al., 2010). Support for the latter hypothesis comes from the seemingly counterintuitive observation that histone deacetylase inhibitors abrogate NMC growth in vitro and in vivo. Further, encouraging results were obtained in experimental treatment of NMC with histone deacetylase inhibitor vorinostat (Schwartz et al., 2011). Additionally, targeting BRD4 bromodomains in NMC model resulted in BRD4-NUT displacement from chromatin and cell differentiation in tissue culture and xenograft model (Filippakopoulos et al., 2010). These reassuring results exemplify how deciphering of an epigenetic regulatory circuit may improve treatment outcomes for a highly aggressive tumor. Fundamentally, insights into pathogenesis of NMC highlight how highly connected chromatin circuits may be thrown off-balance by the oncogene-hijacked positive feedback loop.
Redistribution of histone H3 methylation in multiple myeloma
Multiple myeloma with translocation t(4;14) is characterized by overexpression of “writer” MMSET (also known as WHSC1 and NSD2), which catalyzes histone H3 lysine 36 dimethylation (H3 K36me2). While the significance of this mark is not well understood, it is generally associated with increased transcription and mechanistically counteracts the PRC2-dependent H3 K27 methylation (Wagner and Carpenter, 2012). Genome-wide, NSD2 and H3 K36me2 domains are reciprocal to the PcG proteins and H3 K27me in wild type cells (Fig. 5A). Increased expression of NSD2 due to t(4;14) chromosomal abnormality results in expansion of H3 K36me-decorated chromatin, encroaching onto the PcG-dependent repressed domains. As PRC2 activity is inhibited by the K36-methylated nucleosomes (Yuan et al., 2011), and PcG repression relies on positive feedback-regulated spreading, the PcG complexes are ectopically redistributed to the diminishing H3 K36me-free loci, resulting in aberrant gene repression (Fig. 5B) (Popovic et al., 2014). These observations suggest potential synergistic “yin-yang” effects of NSD2 and EZH2 targeting. While future studies will determine the contribution of this mechanism to multiple myeloma phenotypes, it is intriguing that a point mutation E1099K in NSD2, resulting in enhanced methyltransferase activity was reported in hematologic malignancy (Jaffe et al., 2013; Oyer et al., 2014), and overexpression of NSD2 is associated with poor prognosis in many solid tumors (Hudlebusch et al., 2011). An investigation of reciprocal relationship between NSD2-and PRC2-dependent chromatin domains in these tumors would therefore be of significant interest.
Complex effects of oncohistone mutations
Recurrent somatic mutations in histone H3 genes encoding canonical H3.1 or replication-independent variant H3.3 were recently identified in a number of pediatric and young adult tumors, including astrocytoma, glioblastoma, chondroblastoma, and giant cell tumor of the bone (Behjati et al., 2013; Schwartzentruber et al., 2012; Wu et al., 2012). The dominant K27M and K36M mutations result in selective and potent inhibition of respective SET domain methyltransferases (Lewis et al., 2013). However, somatic inactivating mutations in EZH2, NSD2 or SETD2 are not characteristic of these tumor types, suggesting that the pathogenic mechanism is more complex and requires residual function of the enzyme (Behjati et al., 2013; Wu et al., 2014). This is supported by the observation that H3 K27me signal is redistributed to a few select loci in H3.3 K27M transformed neural progenitor cells and glioblastoma cell lines, indicating that redistribution of chromatin domains likely plays a role (Chan et al., 2013; Funato et al., 2014). Likewise, H3 K36M mutation in chondroblastoma results in redistribution of PcG complexes to intergenic “sinks”, with oncogenic transformation resulting from loss of PcG-mediated repression of developmentally-regulated genes (Fig. 5C) (Lu et al., 2016). Interestingly, the observation that PcG and NSD2/SETD2 complexes act in balanced and mutually antagonistic fashion was made in C. elegans during the studies of dosage compensation in mes-4 mutant. In nematode, both the PcG complex, represented by maternal effect sterile factors MES-2, MES-3 and MES-6, and NSD2 homolog MES-4, are required for dosage compensation via X-chromosome silencing. The seemingly counterintuitive finding was explained when, in absence of MES-4, MES2/3/6 complex was found to ectopically relocalize to the autosomes, resulting in loss of X chromosome silencing (Gaydos et al., 2012). While much remains to be understood about the role of epigenetic misregulation in disease, these findings emphasize the difficulty in translating carefully-dissected biochemical pathways to in vivo systems, where consequences from all interdependent processes need to be considered. We envision that in the coming years, with technological advances rapidly evolving, many other unexpected genetic and epigenetic links will be uncovered, and hopefully, translated into novel therapeutic approaches.
In conclusion, considerable insights have been gained over the past decades into a diverse collection of biological phenomena that cannot be explained by genetics alone. While much more progress will follow, it is becoming clear that a wide range of epigenetic pathways, affecting nucleic acids and histone proteins, are dynamic and reversible, offering considerable promise for a better understanding of human biology and the treatment of human disease. These insights have had a paradigm-shifting impact on our understanding of normal and perturbed development. Indeed, the dynamic epigenome adds an additional layer of complexity to the function of our genome, leading to a sum greater than its parts.
ACKNOWLEDGEMENTS
We thank members of the Allis lab and anonymous reviewers for valuable input, and apologize to the many colleagues whose important contributions could not be cited due to space constraints.
FUNDING
This work was funded by STARR Cancer Consortium (I9-A9-062), Quadrivium Foundation Award for Innovative Research in Epigenetics, and institutional support to from The Rockefeller University to C.D.A.. A.A.S. is HHMI Fellow of the Damon Runyon Cancer Research Foundation (DRG-2185-14). S.Z.J. is supported by NIH Pathway to Independence Award (1K99GM113019-01)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alekseyenko AA, Walsh EM, Wang X, Grayson AR, Hsi PT, Kharchenko PV, Kuroda MI, French CA. The oncogenic BRD4-NUT chromatin regulator drives aberrant transcription within large topological domains. Genes & development. 2015;29:1507–1523. doi: 10.1101/gad.267583.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banaszynski LA, Wen D, Dewell S, Whitcomb SJ, Lin M, Diaz N, Elsasser SJ, Chapgier A, Goldberg AD, Canaani E, et al. Hira-dependent histone H3.3 deposition facilitates PRC2 recruitment at developmental loci in ES cells. Cell. 2013;155:107–120. doi: 10.1016/j.cell.2013.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baubec T, Colombo DF, Wirbelauer C, Schmidt J, Burger L, Krebs AR, Akalin A, Schubeler D. Genomic profiling of DNA methyltransferases reveals a role for DNMT3B in genic methylation. Nature. 2015;520:243–247. doi: 10.1038/nature14176. [DOI] [PubMed] [Google Scholar]
- 4.Behjati S, Tarpey PS, Presneau N, Scheipl S, Pillay N, Van Loo P, Wedge DC, Cooke SL, Gundem G, Davies H, et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat Genet. 2013;45:1479–1482. doi: 10.1038/ng.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bierne H, Tham TN, Batsche E, Dumay A, Leguillou M, Kerneis-Golsteyn S, Regnault B, Seeler JS, Muchardt C, Feunteun J, et al. Human BAHD1 promotes heterochromatic gene silencing. Proc Natl Acad Sci U S A. 2009;106:13826–13831. doi: 10.1073/pnas.0901259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackledge NP, Farcas AM, Kondo T, King HW, McGouran JF, Hanssen LL, Ito S, Cooper S, Kondo K, Koseki Y, et al. Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell. 2014;157:1445–1459. doi: 10.1016/j.cell.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boettiger AN, Bintu B, Moffitt JR, Wang S, Beliveau BJ, Fudenberg G, Imakaev M, Mirny LA, Wu CT, Zhuang X. Super-resolution imaging reveals distinct chromatin folding for different epigenetic states. Nature. 2016;529:418–422. doi: 10.1038/nature16496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgess RJ, Zhang Z. Histone chaperones in nucleosome assembly and human disease. Nature structural & molecular biology. 2013;20:14–22. doi: 10.1038/nsmb.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518:413–416. doi: 10.1038/nature13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan KM, Fang D, Gan H, Hashizume R, Yu C, Schroeder M, Gupta N, Mueller S, James CD, Jenkins R, et al. The histone H3. K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes & development. 2013;27:985–990. doi: 10.1101/gad.217778.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen T, Tsujimoto N, Li E. The PWWP domain of Dnmt3a and Dnmt3b is required for directing DNA methylation to the major satellite repeats at pericentric heterochromatin. Mol Cell Biol. 2004;24:9048–9058. doi: 10.1128/MCB.24.20.9048-9058.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen T, Ueda Y, Dodge JE, Wang Z, Li E. Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. Mol Cell Biol. 2003;23:5594–5605. doi: 10.1128/MCB.23.16.5594-5605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clements A, Poux AN, Lo WS, Pillus L, Berger SL, Marmorstein R. Structural basis for histone and phosphohistone binding by the GCN5 histone acetyltransferase. Molecular cell. 2003;12:461–473. doi: 10.1016/s1097-2765(03)00288-0. [DOI] [PubMed] [Google Scholar]
- 14.Dalal Y, Wang H, Lindsay S, Henikoff S. Tetrameric structure of centromeric nucleosomes in interphase Drosophila cells. PLoS biology. 2007;5:e218. doi: 10.1371/journal.pbio.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daujat S, Zeissler U, Waldmann T, Happel N, Schneider R. HP1 binds specifically to Lys26-methylated histone H1 , whereas simultaneous Ser27 phosphorylation blocks HP1 binding. The Journal of biological chemistry. 2005;280:38090–38095. doi: 10.1074/jbc.C500229200. [DOI] [PubMed] [Google Scholar]
- 16.David Y, Vila-Perello M, Verma S, Muir TW. Chemical tagging and customizing of cellular chromatin states using ultrafast trans-splicing inteins. Nature chemistry. 2015;7:394–402. doi: 10.1038/nchem.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Almeida SF, Carmo-Fonseca M. Reciprocal regulatory links between cotranscriptional splicing and chromatin. Seminars in cell & developmental biology. 2014;32:2–10. doi: 10.1016/j.semcdb.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Dhayalan A, Rajavelu A, Rathert P, Tamas R, Jurkowska RZ, Ragozin S, Jeltsch A. The Dnmt3a PWWP domain reads histone 3 lysine 36 trimethylation and guides DNA methylation. The Journal of biological chemistry. 2010;285:26114–26120. doi: 10.1074/jbc.M109.089433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Croce L, Helin K. Transcriptional regulation by Polycomb group proteins. Nature structural & molecular biology. 2013;20:1147–1155. doi: 10.1038/nsmb.2669. [DOI] [PubMed] [Google Scholar]
- 20.Dinant C, Ampatziadis-Michailidis G, Lans H, Tresini M, Lagarou A, Grosbart M, Theil AF, van Cappellen WA, Kimura H, Bartek J, et al. Enhanced chromatin dynamics by FACT promotes transcriptional restart after UV-induced DNA damage. Molecular cell. 2013;51:469–479. doi: 10.1016/j.molcel.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Drobic B, Perez-Cadahia B, Yu J, Kung SK, Davie JR. Promoter chromatin remodeling of immediate-early genes is mediated through H3 phosphorylation at either serine 28 or 10 by the MSK1 multi-protein complex. Nucleic acids research. 2010;38:3196–3208. doi: 10.1093/nar/gkq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumesic PA, Homer CM, Moresco JJ, Pack LR, Shanle EK, Coyle SM, Strahl BD, Fujimori DG, Yates JR, 3rd, Madhani HD. Product binding enforces the genomic specificity of a yeast polycomb repressive complex. Cell. 2015;160:204–218. doi: 10.1016/j.cell.2014.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan I, Montgomery G. E. B. Lewis and the bithorax complex: part II. From cis-trans test to the genetic control of development. Genetics. 2002;161:1–10. doi: 10.1093/genetics/161.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elsasser SJ, Huang H, Lewis PW, Chin JW, Allis CD, Patel DJ. DAXX envelops a histone H3 -H4 dimer for H3.3-specific recognition. Nature. 2012;491:560–565. doi: 10.1038/nature11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elsasser SJ, Noh KM, Diaz N, Allis CD, Banaszynski LA. Histone H3. is required for endogenous retroviral element silencing in embryonic stem cells. Nature. 2015;522:240–244. doi: 10.1038/nature14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farcas AM, Blackledge NP, Sudbery I, Long HK, McGouran JF, Rose NR, Lee S, Sims D, Cerase A, Sheahan TW, et al. KDM2B links the Polycomb Repressive Complex 1 (PRC1) to recognition of CpG islands. eLife. 2012;1:e00205. doi: 10.7554/eLife.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fatemi M, Hermann A, Gowher H, Jeltsch A. Dnmt3a and Dnmt1 functionally cooperate during de novo methylation of DNA. European journal of biochemistry / FEBS. 2002;269:4981–4984. doi: 10.1046/j.1432-1033.2002.03198.x. [DOI] [PubMed] [Google Scholar]
- 28.Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, Li Y, Bhagwat N, Vasanthakumar A, Fernandez HF, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filipescu D, Muller S, Almouzni G. Histone H3 variants and their chaperones during development and disease: contributing to epigenetic control. Annual review of cell and developmental biology. 2014;30:615–646. doi: 10.1146/annurev-cellbio-100913-013311. [DOI] [PubMed] [Google Scholar]
- 30.Filippakopoulos P, Picaud S, Mangos M, Keates T, Lambert JP, Barsyte-Lovejoy D, Felletar I, Volkmer R, Muller S, Pawson T, et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149:214–231. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischle W, Tseng BS, Dormann HL, Ueberheide BM, Garcia BA, Shabanowitz J, Hunt DF, Funabiki H, Allis CD. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature. 2005;438:1116–1122. doi: 10.1038/nature04219. [DOI] [PubMed] [Google Scholar]
- 33.Flavahan WA, Drier Y, Liau BB, Gillespie SM, Venteicher AS, Stemmer-Rachamimov AO, Suva ML, Bernstein BE. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature. 2016;529:110–114. doi: 10.1038/nature16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.French CA. Pathogenesis of NUT midline carcinoma. Annual review of pathology. 2012;7:247–265. doi: 10.1146/annurev-pathol-011811-132438. [DOI] [PubMed] [Google Scholar]
- 35.Fuks F, Hurd PJ, Deplus R, Kouzarides T. The DNA methyltransferases associate with HP1 and the SUV39H1 histone methyltransferase. Nucleic acids research. 2003;31:2305–2312. doi: 10.1093/nar/gkg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funato K, Major T, Lewis PW, Allis CD, Tabar V. Use of human embryonic stem cells to model pediatric gliomas with H3. K27M histone mutation. Science. 2014;346:1529–1533. doi: 10.1126/science.1253799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaydos LJ, Rechtsteiner A, Egelhofer TA, Carroll CR, Strome S. Antagonism between MES-4 and Polycomb repressive complex 2 promotes appropriate gene expression in C. elegans germ cells. Cell reports. 2012;2:1169–1177. doi: 10.1016/j.celrep.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gehani SS, Agrawal-Singh S, Dietrich N, Christophersen NS, Helin K, Hansen K. Polycomb group protein displacement and gene activation through MSK-dependent H3K27me3S28 phosphorylation. Molecular cell. 2010;39:886–900. doi: 10.1016/j.molcel.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg AD, Banaszynski LA, Noh KM, Lewis PW, Elsaesser SJ, Stadler S, Dewell S, Law M, Guo X, Li X, et al. Distinct factors control histone variant H3. localization at specific genomic regions. Cell. 2010;140:678–691. doi: 10.1016/j.cell.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grigoryev SA, Woodcock CL. Chromatin organization - the 30 nm fiber. Experimental cell research. 2012;318:1448–1455. doi: 10.1016/j.yexcr.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Grossniklaus U, Paro R. Transcriptional silencing by polycomb-group proteins. Cold Spring Harbor perspectives in biology. 2014;6:a019331. doi: 10.1101/cshperspect.a019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo R, Zheng L, Park JW, Lv R, Chen H, Jiao F, Xu W, Mu S, Wen H, Qiu J, et al. BS69/ZMYND11 reads and connects histone H3.3 lysine 36 trimethylation-decorated chromatin to regulated pre-mRNA processing. Molecular cell. 2014;56:298–310. doi: 10.1016/j.molcel.2014.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo X, Wang L, Li J, Ding Z, Xiao J, Yin X, He S, Shi P, Dong L, Li G, et al. Structural insight into autoinhibition and histone H3-induced activation of DNMT3A. Nature. 2015;517:640–644. doi: 10.1038/nature13899. [DOI] [PubMed] [Google Scholar]
- 44.Gurard-Levin ZA, Quivy JP, Almouzni G. Histone chaperones: assisting histone traffic and nucleosome dynamics. Annual review of biochemistry. 2014;83:487–517. doi: 10.1146/annurev-biochem-060713-035536. [DOI] [PubMed] [Google Scholar]
- 45.Hake SB, Garcia BA, Kauer M, Baker SP, Shabanowitz J, Hunt DF, Allis CD. Serine 31 phosphorylation of histone variant H3. is specific to regions bordering centromeres in metaphase chromosomes. Proc Natl Acad Sci U S A. 2005;102:6344–6349. doi: 10.1073/pnas.0502413102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hansen KD, Timp W, Bravo HC, Sabunciyan S, Langmead B, McDonald OG, Wen B, Wu H, Liu Y, Diep D, et al. Increased methylation variation in epigenetic domains across cancer types. Nat Genet. 2011;43:768–775. doi: 10.1038/ng.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartlerode AJ, Morgan MJ, Wu Y, Buis J, Ferguson DO. Recruitment and activation of the ATM kinase in the absence of DNA-damage sensors. Nature structural & molecular biology. 2015;22:736–743. doi: 10.1038/nsmb.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hilton IB, D'Ippolito AM, Vockley CM, Thakore PI, Crawford GE, Reddy TE, Gersbach CA. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nature biotechnology. 2015;33:510–517. doi: 10.1038/nbt.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Y, Rao A. Connections between TET proteins and aberrant DNA modification in cancer. Trends in genetics : TIG. 2014;30:464–474. doi: 10.1016/j.tig.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hudlebusch HR, Santoni-Rugiu E, Simon R, Ralfkiaer E, Rossing HH, Johansen JV, Jorgensen M, Sauter G, Helin K. The histone methyltransferase and putative oncoprotein MMSET is overexpressed in a large variety of human tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:2919–2933. doi: 10.1158/1078-0432.CCR-10-1302. [DOI] [PubMed] [Google Scholar]
- 51.Jackson JP, Lindroth AM, Cao X, Jacobsen SE. Control of CpNpG DNA methylation by the KRYPTONITE histone H3 methyltransferase. Nature. 2002;416:556–560. doi: 10.1038/nature731. [DOI] [PubMed] [Google Scholar]
- 52.Jaffe JD, Wang Y, Chan HM, Zhang J, Huether R, Kryukov GV, Bhang HE, Taylor JE, Hu M, Englund NP, et al. Global chromatin profiling reveals NSD2 mutations in pediatric acute lymphoblastic leukemia. Nat Genet. 2013;45:1386–1391. doi: 10.1038/ng.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jamieson K, Wiles ET, McNaught KJ, Sidoli S, Leggett N, Shao Y, Garcia BA, Selker EU. Loss of HP1 causes depletion of H3K27me3 from facultative heterochromatin and gain of H3K27me2 at constitutive heterochromatin. Genome research. 2016;26:97–107. doi: 10.1101/gr.194555.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeltsch A, Jurkowska RZ. New concepts in DNA methylation. Trends in biochemical sciences. 2014;39:310–318. doi: 10.1016/j.tibs.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Jiao L, Liu X. Structural basis of histone H3K27 trimethylation by an active polycomb repressive complex 2. Science. 2015;350:aac4383. doi: 10.1126/science.aac4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalb R, Latwiel S, Baymaz HI, Jansen PW, Muller CW, Vermeulen M, Muller J. Histone H2A monoubiquitination promotes histone H3 methylation in Polycomb repression. Nature structural & molecular biology. 2014;21:569–571. doi: 10.1038/nsmb.2833. [DOI] [PubMed] [Google Scholar]
- 57.Kassis JA, Brown JL. Polycomb group response elements in Drosophila and vertebrates. Advances in genetics. 2013;81:83–118. doi: 10.1016/B978-0-12-407677-8.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keung AJ, Bashor CJ, Kiriakov S, Collins JJ, Khalil AS. Using targeted chromatin regulators to engineer combinatorial and spatial transcriptional regulation. Cell. 2014;158:110–120. doi: 10.1016/j.cell.2014.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. 2013;502:472–479. doi: 10.1038/nature12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ku M, Koche RP, Rheinbay E, Mendenhall EM, Endoh M, Mikkelsen TS, Presser A, Nusbaum C, Xie X, Chi AS, et al. Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. PLoS genetics. 2008;4:e1000242. doi: 10.1371/journal.pgen.1000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lau PN, Cheung P. Histone code pathway involving H3 S28 phosphorylation and K27 acetylation activates transcription and antagonizes polycomb silencing. Proc Natl Acad Sci U S A. 2011;108:2801–2806. doi: 10.1073/pnas.1012798108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lebreton A, Lakisic G, Job V, Fritsch L, Tham TN, Camejo A, Mattei PJ, Regnault B, Nahori MA, Cabanes D, et al. A bacterial protein targets the BAHD1 chromatin complex to stimulate type III interferon response. Science. 2011;331:1319–1321. doi: 10.1126/science.1200120. [DOI] [PubMed] [Google Scholar]
- 63.Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- 64.Lewis PW, Muller MM, Koletsky MS, Cordero F, Lin S, Banaszynski LA, Garcia BA, Muir TW, Becher OJ, Allis CD. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340:857–861. doi: 10.1126/science.1232245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lou Z, Minter-Dykhouse K, Franco S, Gostissa M, Rivera MA, Celeste A, Manis JP, van Deursen J, Nussenzweig A, Paull TT, et al. MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Molecular cell. 2006;21:187–200. doi: 10.1016/j.molcel.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 67.Lu C, Jain SU, Hoelper D, Bechet D, Molden RC, Ran L, Murphy D, Venneti S, Hameed M, Pawel BR, et al. Histone H3K36 mutations promote sarcomagenesis through altered histone methylation landscape. Science. 2016 doi: 10.1126/science.aac7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, Edwards CR, Khanin R, Figueroa ME, Melnick A, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Macdonald N, Welburn JP, Noble ME, Nguyen A, Yaffe MB, Clynes D, Moggs JG, Orphanides G, Thomson S, Edmunds JW, et al. Molecular basis for the recognition of phosphorylated and phosphoacetylated histone h3 by 14-3-3. Molecular cell. 2005;20:199–211. doi: 10.1016/j.molcel.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 70.Mahadevan LC, Willis AC, Barratt MJ. Rapid histone H3 phosphorylation in response to growth factors, phorbol esters, okadaic acid, and protein synthesis inhibitors. Cell. 1991;65:775–783. doi: 10.1016/0092-8674(91)90385-c. [DOI] [PubMed] [Google Scholar]
- 71.Mao Z, Pan L, Wang W, Sun J, Shan S, Dong Q, Liang X, Dai L, Ding X, Chen S, et al. Anp32e, a higher eukaryotic histone chaperone directs preferential recognition for H2A . Cell research. 2014;24:389–399. doi: 10.1038/cr.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mattiroli F, Vissers JH, van Dijk WJ, Ikpa P, Citterio E, Vermeulen W, Marteijn JA, Sixma TK. RNF168 ubiquitinates K13-15 on H2A/H2AX to drive DNA damage signaling. Cell. 2012;150:1182–1195. doi: 10.1016/j.cell.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 73.Maze I, Wenderski W, Noh KM, Bagot RC, Tzavaras N, Purushothaman I, Elsasser SJ, Guo Y, Ionete C, Hurd YL, et al. Critical Role of Histone Turnover in Neuronal Transcription and Plasticity. Neuron. 2015;87:77–94. doi: 10.1016/j.neuron.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mendenhall EM, Koche RP, Truong T, Zhou VW, Issac B, Chi AS, Ku M, Bernstein BE. GC-rich sequence elements recruit PRC2 in mammalian ES cells. PLoS genetics. 2010;6:e1001244. doi: 10.1371/journal.pgen.1001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mendenhall EM, Williamson KE, Reyon D, Zou JY, Ram O, Joung JK, Bernstein BE. Locus-specific editing of histone modifications at endogenous enhancers. Nature biotechnology. 2013;31:1133–1136. doi: 10.1038/nbt.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miell MD, Fuller CJ, Guse A, Barysz HM, Downes A, Owen-Hughes T, Rappsilber J, Straight AF, Allshire RC. CENP-A confers a reduction in height on octameric nucleosomes. Nature structural & molecular biology. 2013;20:763–765. doi: 10.1038/nsmb.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morgan MA, Shilatifard A. Chromatin signatures of cancer. Genes & development. 2015;29:238–249. doi: 10.1101/gad.255182.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morin RD, Johnson NA, Severson TM, Mungall AJ, An J, Goya R, Paul JE, Boyle M, Woolcock BW, Kuchenbauer F, et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010;42:181–185. doi: 10.1038/ng.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morin RD, Mungall K, Pleasance E, Mungall AJ, Goya R, Huff RD, Scott DW, Ding J, Roth A, Chiu R, et al. Mutational and structural analysis of diffuse large B-cell lymphoma using whole-genome sequencing. Blood. 2013;122:1256–1265. doi: 10.1182/blood-2013-02-483727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muller J, Hart CM, Francis NJ, Vargas ML, Sengupta A, Wild B, Miller EL, O'Connor MB, Kingston RE, Simon JA. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell. 2002;111:197–208. doi: 10.1016/s0092-8674(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 81.Noh KM, Wang H, Kim HR, Wenderski W, Fang F, Li CH, Dewell S, Hughes SH, Melnick AM, Patel DJ, et al. Engineering of a Histone-Recognition Domain in Dnmt3a Alters the Epigenetic Landscape and Phenotypic Features of Mouse ESCs. Molecular cell. 2015;59:89–103. doi: 10.1016/j.molcel.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Obri A, Ouararhni K, Papin C, Diebold ML, Padmanabhan K, Marek M, Stoll I, Roy L, Reilly PT, Mak TW, et al. ANP32E is a histone chaperone that removes H2A. from chromatin. Nature. 2014;505:648–653. doi: 10.1038/nature12922. [DOI] [PubMed] [Google Scholar]
- 83.Okosun J, Bodor C, Wang J, Araf S, Yang CY, Pan C, Boller S, Cittaro D, Bozek M, Iqbal S, et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet. 2014;46:176–181. doi: 10.1038/ng.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oyer JA, Huang X, Zheng Y, Shim J, Ezponda T, Carpenter Z, Allegretta M, Okot-Kotber CI, Patel JP, Melnick A, et al. Point mutation E1099K in MMSET/NSD2 enhances its methyltranferase activity and leads to altered global chromatin methylation in lymphoid malignancies. Leukemia. 2014;28:198–201. doi: 10.1038/leu.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pasqualucci L, Khiabanian H, Fangazio M, Vasishtha M, Messina M, Holmes AB, Ouillette P, Trifonov V, Rossi D, Tabbo F, et al. Genetics of follicular lymphoma transformation. Cell reports. 2014;6:130–140. doi: 10.1016/j.celrep.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pengelly AR, Copur O, Jackle H, Herzig A, Muller J. A histone mutant reproduces the phenotype caused by loss of histone-modifying factor Polycomb. Science. 2013;339:698–699. doi: 10.1126/science.1231382. [DOI] [PubMed] [Google Scholar]
- 87.Pengelly AR, Kalb R, Finkl K, Muller J. Transcriptional repression by PRC1 in the absence of H2A monoubiquitylation. Genes & development. 2015;29:1487–1492. doi: 10.1101/gad.265439.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Popovic R, Martinez-Garcia E, Giannopoulou EG, Zhang Q, Zhang Q, Ezponda T, Shah MY, Zheng Y, Will CM, Small EC, et al. Histone methyltransferase MMSET/NSD2 alters EZH2 binding and reprograms the myeloma epigenome through global and focal changes in H3K36 and H3K27 methylation. PLoS genetics. 2014;10:e1004566. doi: 10.1371/journal.pgen.1004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ray-Gallet D, Woolfe A, Vassias I, Pellentz C, Lacoste N, Puri A, Schultz DC, Pchelintsev NA, Adams PD, Jansen LE, et al. Dynamics of histone H3 deposition in vivo reveal a nucleosome gap-filling mechanism for H3. to maintain chromatin integrity. Molecular cell. 2011;44:928–941. doi: 10.1016/j.molcel.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 90.Rea S, Eisenhaber F, O'Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 91.Reynoird N, Schwartz BE, Delvecchio M, Sadoul K, Meyers D, Mukherjee C, Caron C, Kimura H, Rousseaux S, Cole PA, et al. Oncogenesis by sequestration of CBP/p300 in transcriptionally inactive hyperacetylated chromatin domains. The EMBO journal. 2010;29:2943–2952. doi: 10.1038/emboj.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rhee HS, Bataille AR, Zhang L, Pugh BF. Subnucleosomal structures and nucleosome asymmetry across a genome. Cell. 2014;159:1377–1388. doi: 10.1016/j.cell.2014.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ricci MA, Manzo C, Garcia-Parajo MF, Lakadamyali M, Cosma MP. Chromatin fibers are formed by heterogeneous groups of nucleosomes in vivo. Cell. 2015;160:1145–1158. doi: 10.1016/j.cell.2015.01.054. [DOI] [PubMed] [Google Scholar]
- 94.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. The Journal of cell biology. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. The Journal of biological chemistry. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 96.Rose NR, Klose RJ. Understanding the relationship between DNA methylation and histone lysine methylation. Biochimica et biophysica acta. 2014;1839:1362–1372. doi: 10.1016/j.bbagrm.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nature reviews. Molecular cell biology. 2007;8:983–994. doi: 10.1038/nrm2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schenk R, Jenke A, Zilbauer M, Wirth S, Postberg J. H3. is a novel hominid-specific histone H3 variant that is specifically expressed in the seminiferous tubules of human testes. Chromosoma. 2011;120:275–285. doi: 10.1007/s00412-011-0310-4. [DOI] [PubMed] [Google Scholar]
- 99.Schwartz BE, Hofer MD, Lemieux ME, Bauer DE, Cameron MJ, West NH, Agoston ES, Reynoird N, Khochbin S, Ince TA, et al. Differentiation of NUT midline carcinoma by epigenomic reprogramming. Cancer research. 2011;71:2686–2696. doi: 10.1158/0008-5472.CAN-10-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schwartz YB, Pirrotta V. A new world of Polycombs: unexpected partnerships and emerging functions. Nature reviews. Genetics. 2013;14:853–864. doi: 10.1038/nrg3603. [DOI] [PubMed] [Google Scholar]
- 101.Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tonjes M, et al. Driver mutations in histone H3. and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 102.Segal E, Fondufe-Mittendorf Y, Chen L, Thastrom A, Field Y, Moore IK, Wang JP, Widom J. A genomic code for nucleosome positioning. Nature. 2006;442:772–778. doi: 10.1038/nature04979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seo J, Kim SC, Lee HS, Kim JK, Shon HJ, Salleh NL, Desai KV, Lee JH, Kang ES, Kim JS, et al. Genome-wide profiles of H2AX and gamma-H2AX differentiate endogenous and exogenous DNA damage hotspots in human cells. Nucleic acids research. 2012;40:5965–5974. doi: 10.1093/nar/gks287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shukla S, Kavak E, Gregory M, Imashimizu M, Shutinoski B, Kashlev M, Oberdoerffer P, Sandberg R, Oberdoerffer S. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature. 2011;479:74–79. doi: 10.1038/nature10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Siegel TN, Hekstra DR, Kemp LE, Figueiredo LM, Lowell JE, Fenyo D, Wang X, Dewell S, Cross GA. Four histone variants mark the boundaries of polycistronic transcription units in Trypanosoma brucei. Genes & development. 2009;23:1063–1076. doi: 10.1101/gad.1790409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simon C, Chagraoui J, Krosl J, Gendron P, Wilhelm B, Lemieux S, Boucher G, Chagnon P, Drouin S, Lambert R, et al. A key role for EZH2 and associated genes in mouse and human adult T-cell acute leukemia. Genes & development. 2012;26:651–656. doi: 10.1101/gad.186411.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Simon JA, Kingston RE. Occupying chromatin: Polycomb mechanisms for getting to genomic targets, stopping transcriptional traffic, and staying put. Molecular cell. 2013;49:808–824. doi: 10.1016/j.molcel.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Skene PJ, Henikoff S. Histone variants in pluripotency and disease. Development. 2013;140:2513–2524. doi: 10.1242/dev.091439. [DOI] [PubMed] [Google Scholar]
- 109.Smallwood A, Esteve PO, Pradhan S, Carey M. Functional cooperation between HP1 and DNMT1 mediates gene silencing. Genes & development. 2007;21:1169–1178. doi: 10.1101/gad.1536807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nature reviews. Genetics. 2013;14:204–220. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- 111.Snowden AW, Gregory PD, Case CC, Pabo CO. Gene-specific targeting of H3K9 methylation is sufficient for initiating repression in vivo. Current biology : CB. 2002;12:2159–2166. doi: 10.1016/s0960-9822(02)01391-x. [DOI] [PubMed] [Google Scholar]
- 112.Spruijt CG, Gnerlich F, Smits AH, Pfaffeneder T, Jansen PW, Bauer C, Munzel M, Wagner M, Muller M, Khan F, et al. Dynamic readers for 5-(hydroxy)methylcytosine and its oxidized derivatives. Cell. 2013;152:1146–1159. doi: 10.1016/j.cell.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 113.Stutzer A, Liokatis S, Kiesel A, Schwarzer D, Sprangers R, Soding J, Selenko P, Fischle W. Modulations of DNA Contacts by Linker Histones and Post-translational Modifications Determine the Mobility and Modifiability of Nucleosomal H3 Tails. Molecular cell. 2016;61:247–259. doi: 10.1016/j.molcel.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 114.Suto RK, Clarkson MJ, Tremethick DJ, Luger K. Crystal structure of a nucleosome core particle containing the variant histone H2A . Nature structural biology. 2000;7:1121–1124. doi: 10.1038/81971. [DOI] [PubMed] [Google Scholar]
- 115.Tamaru H, Selker EU. A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature. 2001;414:277–283. doi: 10.1038/35104508. [DOI] [PubMed] [Google Scholar]
- 116.Thorslund T, Ripplinger A, Hoffmann S, Wild T, Uckelmann M, Villumsen B, Narita T, Sixma TK, Choudhary C, Bekker-Jensen S, et al. Histone H1 couples initiation and amplification of ubiquitin signalling after DNA damage. Nature. 2015;527:389–393. doi: 10.1038/nature15401. [DOI] [PubMed] [Google Scholar]
- 117.Trojer P, Zhang J, Yonezawa M, Schmidt A, Zheng H, Jenuwein T, Reinberg D. Dynamic Histone H1 Isotype 4 Methylation and Demethylation by Histone Lysine Methyltransferase G9a/KMT1C and the Jumonji Domain-containing JMJD2/KDM4 Proteins. The Journal of biological chemistry. 2009;284:8395–8405. doi: 10.1074/jbc.M807818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Urahama T, Harada A, Maehara K, Horikoshi N, Sato K, Sato Y, Shiraishi K, Sugino N, Osakabe A, Tachiwana H, et al. Histone H3. forms an unstable nucleosome and accumulates around transcription start sites in human testis. Epigenetics & chromatin. 2016;9:2. doi: 10.1186/s13072-016-0051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wagner EJ, Carpenter PB. Understanding the language of Lys36 methylation at histone H3. Nature reviews. Molecular cell biology. 2012;13:115–126. doi: 10.1038/nrm3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Walter W, Clynes D, Tang Y, Marmorstein R, Mellor J, Berger SL. 14-3-3 interaction with histone H3 involves a dual modification pattern of phosphoacetylation. Mol Cell Biol. 2008;28:2840–2849. doi: 10.1128/MCB.01457-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wen H, Li Y, Xi Y, Jiang S, Stratton S, Peng D, Tanaka K, Ren Y, Xia Z, Wu J, et al. ZMYND11 links histone H3. K36me3 to transcription elongation and tumour suppression. Nature. 2014;508:263–268. doi: 10.1038/nature13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Winter S, Simboeck E, Fischle W, Zupkovitz G, Dohnal I, Mechtler K, Ammerer G, Seiser C. 14-3-3 proteins recognize a histone code at histone H3 and are required for transcriptional activation. The EMBO journal. 2008;27:88–99. doi: 10.1038/sj.emboj.7601954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu G, Broniscer A, McEachron TA, Lu C, Paugh BS, Becksfort J, Qu C, Ding L, Huether R, Parker M, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44:251–253. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu G, Diaz AK, Paugh BS, Rankin SL, Ju B, Li Y, Zhu X, Qu C, Chen X, Zhang J, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46:444–450. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. The Journal of biological chemistry. 2007;282:13141–13145. doi: 10.1074/jbc.R700001200. [DOI] [PubMed] [Google Scholar]
- 126.Yang SM, Kim BJ, Norwood Toro L, Skoultchi AI. H1 linker histone promotes epigenetic silencing by regulating both DNA methylation and histone H3 methylation. Proc Natl Acad Sci U S A. 2013;110:1708–1713. doi: 10.1073/pnas.1213266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuan W, Xu M, Huang C, Liu N, Chen S, Zhu B. H3K36 methylation antagonizes PRC2-mediated H3K27 methylation. The Journal of biological chemistry. 2011;286:7983–7989. doi: 10.1074/jbc.M110.194027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang Y, Jurkowska R, Soeroes S, Rajavelu A, Dhayalan A, Bock I, Rathert P, Brandt O, Reinhardt R, Fischle W, et al. Chromatin methylation activity of Dnmt3a and Dnmt3a/3L is guided by interaction of the ADD domain with the histone H3 tail. Nucleic acids research. 2010;38:4246–4253. doi: 10.1093/nar/gkq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao D, Zhang X, Guan H, Xiong X, Shi X, Deng H, Li H. The BAH domain of BAHD1 is a histone H3K27me3 reader. Protein & cell. 2016 doi: 10.1007/s13238-016-0243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou W, Zhu P, Wang J, Pascual G, Ohgi KA, Lozach J, Glass CK, Rosenfeld MG. Histone H2A monoubiquitination represses transcription by inhibiting RNA polymerase II transcriptional elongation. Molecular cell. 2008;29:69–80. doi: 10.1016/j.molcel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zippo A, Serafini R, Rocchigiani M, Pennacchini S, Krepelova A, Oliviero S. Histone crosstalk between H3S10ph and H4K16ac generates a histone code that mediates transcription elongation. Cell. 2009;138:1122–1136. doi: 10.1016/j.cell.2009.07.031. [DOI] [PubMed] [Google Scholar]