Abstract
Background
Urbanization in African cities has major impact on malaria risk. Niamey, the capital of the Republic of Niger, is situated in the West African Sahel zone. The short rainy season and human activities linked with the Niger River influence mosquito abundance. This study aimed at deciphering the factors of distribution of urban malaria vectors in Niamey.
Methods
The distribution of mosquito aquatic stages was investigated monthly from December 2002 to November 2003, at up to 84 breeding sites, throughout Niamey. An exploratory analysis of association between mosquito abundance and environmental factors was performed by a Principal Component Analysis and confirmed by Kruskall–Wallis non-parametric test. To assess the relative importance of significant factors, models were built for Anopheles and Culicinae. In a second capture session, adult mosquitoes were collected weekly with pyrethrum sprays and CDC light-traps from June 2008 to June 2009 in two differentiated urban areas chosen after the study’s first step. Members of the Anopheles gambiae complex were genotyped and Anopheles females were tested for the presence of Plasmodium falciparum circumsporozoite antigens using ELISA.
Results
In 2003, 29 % of 8420 mosquitoes collected as aquatic stages were Anopheles. They were significantly more likely to be found upstream, relatively close to the river and highly productive in ponds. These factors remained significant in regression and generalized linear models. The Culicinae were found significantly more likely close to the river, and in the main temporary affluent stream. In 2009, Anopheles specimens, including Anopheles gambiae s.l. (95 %), but also Anopheles funestus (0.6 %) accounted for 18 % of the adult mosquito fauna, with a large difference between the two sampled zones. Three members of the An. gambiae complex were found: Anopheles arabiensis, Anopheles coluzzii, and An. gambiae. Nineteen (1.3 %) out of 1467 females tested for P. falciparum antigen were found positive.
Conclusion
The study provides valuable update knowledge on malaria vector ecology and distribution in Niamey. The identification of spatial and environmental risk factors could pave the way to larval source management strategy and allow malaria vector control to focus on key zones for the benefit of the community.
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-016-1352-0) contains supplementary material, which is available to authorized users.
Keywords: Malaria, Niamey, Anopheles, Anopheles gambiae, Vector ecology, Urban, Sahel, Niger
Background
There is a crucial need for a better understanding of the malaria vector ecology in Niamey and quantification of transmission. Knowledge about malaria transmission and vector ecology in this capital city is outdated and may be insufficient to implement adapted vector control measures. Urban human populations increase rapidly, especially in West Africa. In this region, the population growth is twice as much the general population growth [1]. This has major implications for malaria risk and control [2]. The epidemiology of malaria in African urban areas is characterized by a declining gradient of transmission from the periphery to the centre [2, 3]. This is due to the limited flight capacity and spread of Anopheles in urban environment and to the scarcity and pollution level of potential breeding sites. In urban environments, lower densities of larval habitats are found relative to rural environments [2, 4]. This presumably fosters greater interactions, including competition, between the different taxa of Anopheles gambiae sensu lato and other mosquito species, often in a context of polluted water bodies.
Niamey is located within the Sahel zone. The population is about two million people. The rainy season is short and intense from June to October. Locally, people use to manage their water needs in coherence with the scarcity of water during the dry season and, for some of them, to adopt agricultural and farming practices in a sustainable manner. Historically, Niamey was settled on the left bank of the Niger River, but now it extends largely on both banks and a large portion of the city is notably distant from the river. The periphery of the city reaches and integrates several rural villages. The Niger River has allowed the development of irrigation schemes for rice cultivation and many rice fields are at short distance from the city centre. Beside these modern practices, traditional agricultural activities as gardening for fruits, vegetables and flowers are carried out in town. Malaria in rural Sahel is strongly seasonal [5, 6]. It peaks during and shortly after the rainy season. However transmission could occur at very low level during the dry seasons. In specific edaphic conditions, where the water table is close to the surface or due to permanent or semi-permanent water bodies, breeding sites for malaria vectors rely less upon rainfall and could last well beyond the rainy season and induce a longer transmission period [7]. Entomological data about malaria vectors in Niamey come from studies [8, 9] led before the drought period beginning 40 years ago. The authors described Anopheles gambiae s.l. and Anopheles funestus as the major potential vectors to be present. In 1973, Chauvet and Dyemkouma [8] focused their attention on the kanaris, the domestic adobe vases used for daily water storage and did not find any Anopheles in them. In 1997, Julvez et al. [3] published prevalence data on malaria parasites in humans and defined the period of malaria transmission in Niamey as the 5 months of the rainy season (June to October), with more intense transmission in neighborhoods close to the Niger River and less intense transmission in districts further away the river, differing to the classical schema of lower level of malaria transmission in urban centres [2]. Such a situation has already been described in Libreville, Gabon [10]. However the authors emphasize the role of socio-cultural factors rather than environmental ones. In a wide range of transmission magnitude, and considering this relative low density of breeding sites available for malaria vectors and the high density of human population susceptible to malaria infections, urban environments could present a high benefit-cost ratio for vector control, and especially larval control [11]. In comparison, rural zones could offer a wider range of breeding sites for malaria vectors, dispersed on larger areas, making the vector control more critical. The human population is less dense than in urban areas and a bigger effort, in logistics and funding, is needed to protect a comparable population. The World Health Organization (WHO) maintains its strong advice for use of long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) as the backbone for malaria vector control. However, the WHO issued in 2012 a position statement on larviciding [12]. The method was recommended as a supplement in situations “where vectors tend to breed in permanent or semi-permanent water bodies that can be readily identified and accessed, i.e. breeding sites which are ‘few, fixed and findable’, and where the density of the human population to be protected is sufficiently high to justify the necessary resources” [12]. The experience of Dar-es-Salaam, Tanzania, in term of larval control [13] has proven it to be cost-effective in urban area [11]. The Niger has implemented a nation-wide impregnated bed net distribution [6]. Time has come to reinforce the malaria control. The larval source management, including larviciding method, could be combined to LLINs and IRS to decrease the malaria burden.
The study aims at characterizing the ecology and the distribution of pre-imago stages of Anopheles vectors in view of the potential importance of the Niger River and new agricultural practices. In a second step the study confirms the pre-imago ecology results with quantification of adult mosquito abundance and infection in two differentiated zones of Niamey.
Methods
Study site
The area is located in Niamey (Fig. 1) in the southwestern region of Niger, Africa, (13°31′N, 2°06′E). Niamey has a semi-arid climate, with a rainy season lasting about 5 months (June to October) during which 99 % of annual rainfalls occur. The rainy season peaks in August, but local dry spells of up to 10 days are possible even during this month, the temporal distribution of rainfall being extremely irregular [14]. The coolest months are November to February, with the Harmattan (an arid trade wind particularly prevalent from December to February). The mean monthly maximum temperature reaches 40 °C in April, during the dry and hot season from March to May, and a low mark of 19 °C in December. The Niger River is the principal permanent body of water in the city, but there are widespread hollows that are transformed into temporary pools during the rainy season, some of which may persist during the cold and hot periods of the dry season, thereby constituting potential breeding sites for mosquitoes. The conurbation extends over an area of more than 255 km2, still unevenly distributed on either side of the Niger River (Fig. 1).
Fig. 1.
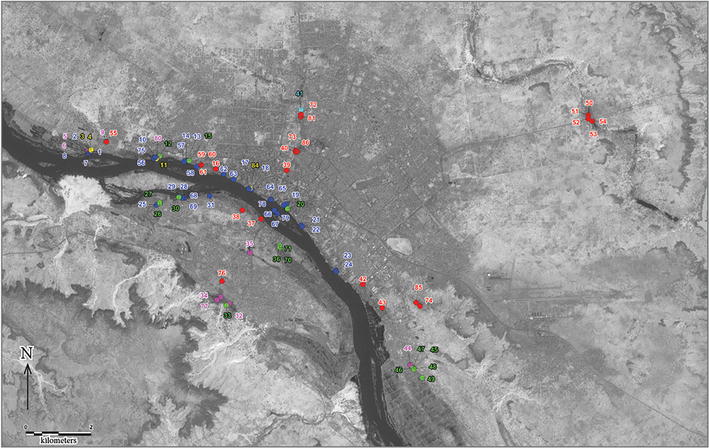
Distribution of the 84 studied larval sites. The sites have been grouped into six main classes according to their typology (see text): Human made (red), Ricefield (green), Niger River (blue), Kori (light blue), Pond (pink) and very small sites (yellow). The background is extracted from a satellite image of LANDSAT ETM+ band 8, acquired on 2nd December 1999. The river is at a high water level
Aquatic stages collection sites
The survey first focused on the vicinity of the Niger River and its affluent the Gountou Yena kori, which crosses the town, but many other sites were sampled: ponds, drains, gardens, flooded areas, puddles, irrigation systems, with channels and wells. For a better framing of the rainy season, the number of sampled sites increased from 33 at starting in November 2002, to a total of 84 water bodies until the end of the larval and pupae survey period (Figs. 1, 2).
Fig. 2.
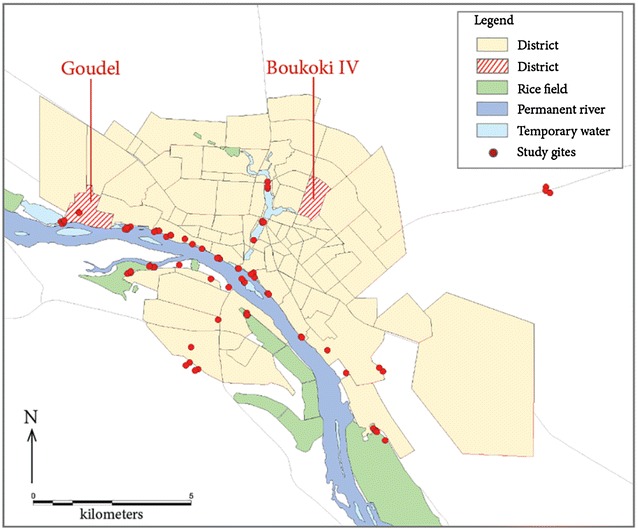
Situation of the two districts where mosquito adults were collected. Situation of the two districts where mosquito adults were collected in 2009, in red-striped surfaces. Red circles indicate the study sites for aquatic stages. The administrative limits of urban districts are in pale yellow; the main Niger Riverbed is deep blue; significantly large but temporary water bodies (Kori, flooded zones) are pale blue; rice fields are green
Aquatic stages collection and specimen processing
Larvae and pupae were sampled by a standard dipping method: a total of 10 dips per aquatic habitat were carried out, or less if the site water volume was not enough. The total of the larvae and pupae collected with these dips were quickly sorted on site in a white tray according to the sub-family level by eye as Anopheles, the only genus of the subfamily in the zoneoras Culicinae. Then they were counted according to their stage of development: first, second, third or fourth instars and grouped together in breeding containers for each positive site. The larvae were kept and fed with finely ground dog food until the adult stage. Once emerged, the adults were identified morphologically under a stereomicroscope, as described below. A subset of An.gambiae s.l. specimens were genotyped (see below).
Geographic information system
Geographical coordinates of study sites were collected with a GPS device (Garmin12) and recorded in a Geographic Information System built with MapInfo Professional (Version 8.5.1 © 2006 MapInfo Corporation) and Quantum GIS (version 1.7.4-Worclaw) [15]. The sites were characterized by their orthogonal distance to the main stream (median of the riverbed line), ordered as following: “1”, <500 m, “2”, 500–1500 m, “3” >1500 m; and by their relative situation along the river stream, beginning by the most upstream site at 0 m: upstream (<2500 m, “1”) or downstream (>6000 m, “3”) or at the city level (2500–6000 m, “2”). The dataset was built up with mosquito aquatic stages and absolute numbers for Anopheles and Culicinae by site and by month. The breeding sites were characterized by their typology and the estimated surface of the water body. The typology of each breeding sites was described in six modalities: ‘river’, ‘pond’, ‘human made’ gathering wells, channels and drains, ‘rice field’ and ‘small breeding sites’ (recipients, footprints) and ‘kori’. The total surface of the sampled site was assessed by eye, as a whole and coded as follows: “0” if dry, “1” <0.1 m2, “2” <1 m2, “3” <10 m2, “4” <100 m2, “5” <1000 m2, “6” ≥1,000 m2, “7” in relation with the river bed and “8” if no access was available. The sampling and coding were performed by a team composed of several persons who shared their estimations.
Adult mosquito collection and specimen processing
From June 2008 to May 2009, a longitudinal entomological survey was carried out in two districts, selected on the basis of the results of the 2003 study: (1) Boukoki, in the city centre and distant to the Niger River and (2) Goudel, a semi-urban district relatively close to the River Niger, upstream from the city center and next to a pond (Fig. 2).
In each study site, both traditional and modern houses were randomly selected and sampled for mosquito collection, after agreement of householders.
Adult mosquitoes were collected weekly by two sampling methods:
Indoor pyrethrum spray catches: two houses (one room per house) per district were sprayed with insecticide early in the morning (between 6:00 and 9:00 am) for the collection of the resting female mosquitoes. Knocked-down female mosquitoes were quickly collected from white sheets laid on the floor and the furniture of sprayed rooms.
Indoor CDC light-traps (CDC miniature light trap, Model 512, John W. Hock Co., Gainesville, FL): Three other houses (one room per house) close to the indoor pyrethrum sprayed houses were sampled. Light traps were set up at 7:00 pm and mosquitoes were collected at 7:00 am.
The mosquitoes were identified at the genus or species level for Anopheles on the basis of morphological criteria, with the identification keys of Gillies and De Meillon [16] and Gillies and Coetzee [17]. Each Anopheles specimen was stored in an individual well of a 96-well plate and stored at −20 °C until processing for molecular genotyping and ELISA-CSP testing.
PCR identification of the Anopheles gambiae complex
For the two sampling periods, subsets of the An. gambiae complex individuals were identified by the polymerase chain reaction (PCR), as described by Scott et al. [18] targeting An. gambiae s.s, and Anopheles arabiensis. Further, An. gambiae specimens were identified on the basis of their ribosomal RNA genes as previously belonging to the Mopti/M molecular form or Savannah/S molecular form, as described by Favia et al. [19] and Santolamazza et al. [20]. However, due to the recent erection of the M form and the Mopti chromosomal form to Anopheles coluzzii [21] and the nomination of the S form and the Savannah chromosomal form as An. gambiae, results are expressed referring to this new taxonomic revision.
ELISA for the detection of parasite infections in Anopheles gambiae s.l.
Specimens of Anopheles caught as adults during the second study were tested for the presence of the Plasmodium falciparum circumsporozoite protein (CSP) in the head and the anterior third of thorax. ELISA was carried out as described by Burkot et al. [22] and Wirtz et al. [23]. The heads of uninfected laboratory-reared mosquitoes were used as negative controls and a 10 μg/ml CSP antigen solution was used as a positive control. Samples were considered positive if absorbance values were more than twice the mean value for the four negative controls. Sporozoite indices (i.e. salivary gland infection rates) were estimated from the ratio of positive specimens on head/thorax on tested specimens.
Analysis and statistics of the aquatic stage ecology
In a first step, exploratory analysis of the association between abundances of mosquito aquatic stages and environmental factors was performed with the ade4 [24] package for R-CRAN. A principal component analysis (PCA) was drawn out for both Culicinae and Anopheles followed by a between-groups analysis with environmental factors [25]. The statistical significance was assessed with permutation tests (Monte-Carlo procedure with 1000 permutations). Then bilateral associations were tested by a Kruskall–Wallis non-parametric test with the Rcmdr [26] package on each of the Culicinae and Anopheles datasets for each variable. Two variables were temporal: Season (Rainy: June to October, Cold Dry: November to February and Hot Dry: March to May), and Month of collection (=12). The variable Sites including 84 modalities, was stored as information as supplementary variable in the dataset and used in the PCA but not for model analysis. In addition to the variables describing the typology and the surface of the water bodies, two other variables addressed the spatial dimension of the data set, both with three ordered modalities: the distance to the river bed DistFlFAC (<500 m, 500–1500 m, and >1500 m) and the situation upstream or downstream to the city UpDown (‘upstream’, ‘city’, and ‘downstream’). To assess the hierarchy of the effects of the variables and to estimate the interaction between variables, two models were built: logistic regression (LG) with binomial distribution and the response variable (mosquito) set as presence/absence (0/1), and generalized linear model (GLM) with Poisson distribution for the response variable set as counts. Interaction between variables was tested by the effect of the ‘Season’ on the typology of sites and the distance to the river. The data included high number of zeros (dry breeding sites, no access). To minimize this effect, a dataset excluding the sites which were either dry or not accessible (coded ‘0’ or ‘8’ in the surface size variable) was built up and used in modeling. The goodness of model was visually assessed by the distribution of the residuals which should follow a Gaussian or near-Gaussian distribution. The residual deviance was compared to the null deviance. The best model within each model family was chosen after the Akaike Information Criterion (AIC).
Results
Aquatic stages survey
A total of 5223 dips collected 8420 larva and pupae. Among them, 2434 Anopheles (29 %) and 5986 Culicinae (71 %) were counted. Among Anopheles aquatic stages, An. gambiae s.l. represented the major part (96 %), Anopheles rufipes, Anopheles pharoensis and Anopheles ziemanni being present in small numbers. The Culicinae mosquito aquatic stages were identified as Culex sp. (90 %), Mansonia sp. (6 %) and Aedes sp. (4 %). Eight species of Culex were identified, and Culex pipiens group (Culex quinquefasciatus), and Culex antennatus predominated.
Ecological study: anopheles ecological preferences
The PCA performed on the Anopheles and Culicinae datasets with the three last larval and pupal stages by collection site and by month showed a different distribution of the two groups of taxa according to two main axes (Fig. 3a). These two first axes held for 61 % of the total variance of the data. They were comparable (respective Eigen values of 2.61 and 2.26) and supported the variance of Culicinae (x-axis) and Anopheles specimens (y-axis) (Fig. 3a).
Fig. 3.
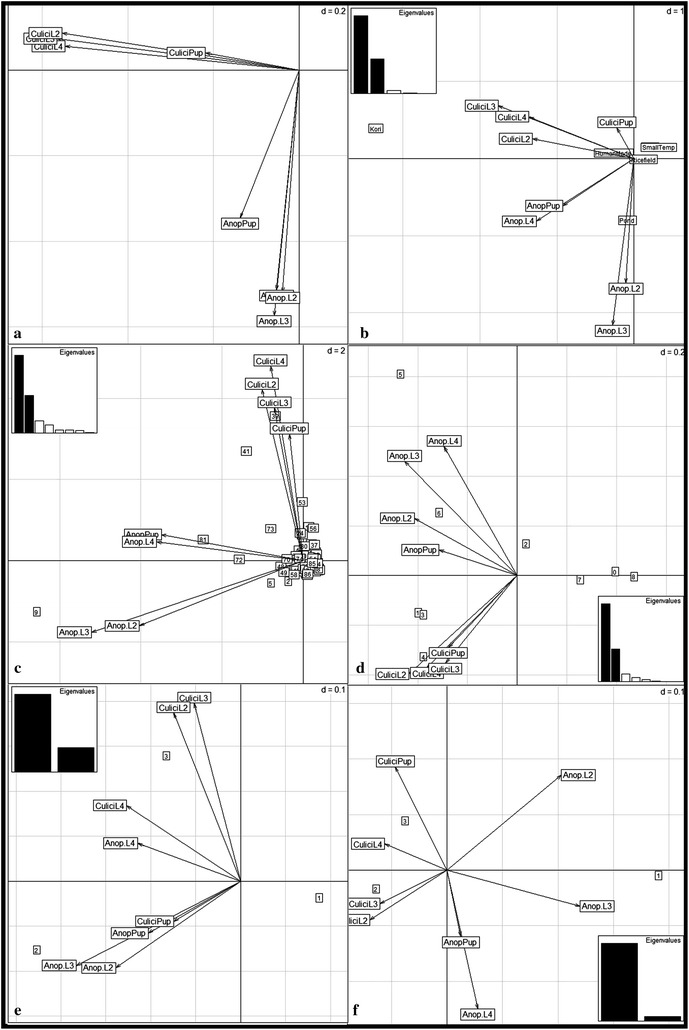
Principal component analysis (PCA) and between classes analysis (BCA) of Anopheles and Culicinae aquatic stages. ‘CuliciL2-3-4’ means Culicinae larval stage 2-3-4, ‘CuliciPup’ = Culicinae Pupa, ‘Anop.L2-3-4’ = Anopheles Larval stage 2-3-4, ‘AnopPup’ = Anopheles pupae. a Principal component Analysis. The horizontal axis is mainly defined by the Culicinae stages and the vertical axis by Anopheles stages. b BCA according to the typology of sampled sites. The ‘River’ tag is hidden behind the ‘Small Temp’ tag. The ‘Pond’ tag is within the space of Anopheles vectors. The ‘Kori’ tag aligns with the Culicinae vectors. c BCA according to the sites, the site ‘9’ is the Goudel Pond; the 41 site is the Kori (Fig. 1). d BCA according to the surface of water bodies. The three values ‘0’, ‘7’ and ‘8’ correspond respectively to sites which are dry, or in relation to the Niger River (>10,000 m2), or not accessible. They are at the opposite of the mosquito space of vector projection. e BCA according to the distance to the NigerRiver. ‘1’ at <500 m to the median line of the river bed, ‘2’ between 500 and 1500 m and ‘3’ beyond 1500 m from the river median line, meaning in the city centre. f BCA according to the up or down stream situation of sampled sites; ‘1’ is upstream, ‘2’ is at the city level, ‘3’ is downstream the city. Please note that the ‘Anopheles’ vectors all expand within the right part of the space (~X-axis, given the very high Eigenvalue of this axis, as shown in the bottom right legend)
To assess the effect of each environmental factor on the mosquito distribution, the ‘between-classes analysis’ option was performed on this PCA against the variables: ‘typology’ (Fig. 3b), ‘site’ (Fig. 3c), ‘season’, ‘surface’ (Fig. 3d) of breeding sites, the orthogonal distance to the Niger River ‘distf’ (Fig. 3e) and the up-downstream situation (Fig. 3f). All these associations, tested by a Monte Carlo procedure with 1000 permutations, gave significant values except for the ‘season’ factor (p = 0.194). More specifically, according to the typology of breeding sites (p = 0.002), the Anopheles stages seemed more associated with the ‘Pond’ value when the Culicinae tend to be linked to the ‘Kori’ and ‘Human made’ breeding sites (Fig. 3b). If the analysis targeted the sites themselves, the Anopheles showed a strong association (p = 0.001) with ponds or wells in gardens (Figs. 1, 3c the sites 9, 72, 81 and 5) when the Culicinae are linked to the kori (Figs. 1, 3c the sites 39, 41), and to wells in gardens (Figs. 1, 3c the sites 53 and 73), but different from those associated with the Anopheles. If the surface of the water bodies is tested (p = 0.004), the mosquito vectors were on the opposite side to the values 0 (dry), 8 (no access) and 7 (Niger River). Anopheles were more likely to be found in larger water bodies (5 and 6) than the Culicinae (1, 3 and 4) (Fig. 3d). The negative effect of the river was confirmed if the distance to the Niger River is assessed (Fig. 3e) and Anopheles were more often found between 500 and 1500 m to the river (like the Goudel pond). The Culicinae tended to be more abundant distantly from the river (p = 0.001). The situation of the sites along the stream showed (p = 0.004) that the Anopheles tend to breed in sites situated upstream and the Culicinae at the level of, or downstream of the city (Fig. 3f).
Ecological study: non parametric test
As the mosquito data are not normally distributed, the Kruskall–Wallis non-parametric test was used to test the associations between environmental factors and mosquito data. Significant associations were confirmed between Anopheles and the upstream/downstream situation (p = 0.011) with higher values upstream (Fig. 4a; Table 1). Anopheles were less abundant close to the river with the distance to the Niger River factor (p < 0.0001) (Fig. 4b; Table 2). The ‘Kori and ‘Pond’ classes of typology (Fig. 4c; Table 3) presented higher values (p = 0.0005) of Anopheles abundances. The larger water bodies (p < 0.0001) were found with more Anopheles (Fig. 4d). The relationship between Anopheles and season remained not significant (p = 0.077) (Table 4).
Fig. 4.
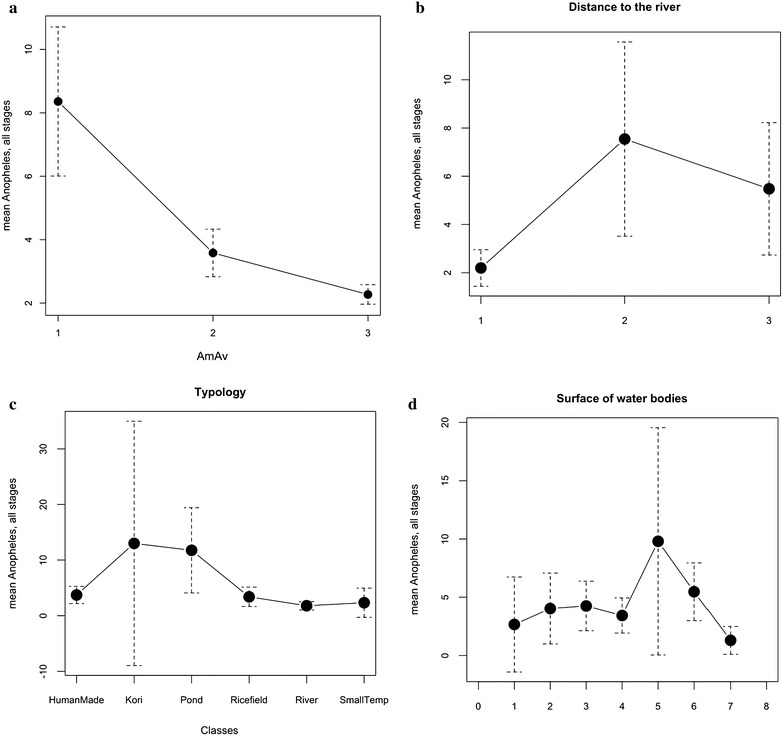
Means of Anopheles aquatic stages according to environment factors. a Upstream (‘1’), at the city level (‘2’) and downstream (‘3’). b Distance to the river: <500 m from the river median line (‘1’), between 500 and 1500 m (‘2’) and beyond 1500 m (‘3’). c Typology of sampled breeding sites. d: According to the surface of water bodies equals decimal log from <0.1 m2 (=1) to <1000 m2 (=5), >1000 m3 (=6), in contact with the river (=7). The ‘8’ (no access) or ‘0’ (dry) values have not been sampled
Table 1.
Upstream to downstream
| Anopheles | Culicinae | |||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Upstream | 936 | 8.43 | 24.99 | 423 | 3.81 | 15.93 |
| City | 971 | 3.71 | 12.54 | 3723 | 14.21 | 62.60 |
| Downstream | 527 | 2.25 | 4.68 | 1837 | 7.85 | 23.16 |
Anopheles and Culicinae aquatic stages collected according to the situation of sites along the river stream. Data show mean, standard deviation and number of collection events (one per site and sampling time)
Table 2.
Distance to the river
| Anopheles | Culicinae | |||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| <500 m | 802 | 2.247 | 7.429 | 2281 | 6.389 | 23.942 |
| 500–1500 m | 958 | 7.484 | 22.853 | 1591 | 12.430 | 53.171 |
| >1500 m | 674 | 5.525 | 15.427 | 2111 | 17.303 | 71.041 |
Anopheles and Culicinae aquatic stages collected according to the situation of sites from the riverbed. Data show mean, standard deviation and number of collection events (one per site and sampling time)
Table 3.
Site classes
| Site class | Anopheles | Culicinae | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Human made | 711 | 3.76 | 10.96 | 3030 | 16.03 | 49.71 |
| Kori | 130 | 13.00 | 30.71 | 786 | 78.60 | 234.22 |
| Pond | 776 | 11.76 | 31.16 | 233 | 3.53 | 13.59 |
| Ricefield | 400 | 3.36 | 9.48 | 692 | 5.82 | 15.80 |
| River | 396 | 1.85 | 5.72 | 1196 | 5.59 | 23.93 |
| Small temperature | 21 | 2.33 | 3.43 | 46 | 5.11 | 9.69 |
Anopheles and Culicinae aquatic stages collected by typology classes. Data show mean, standard deviation and number of collection events (one per site and sampling time)
Table 4.
Seasons
| Anopheles | Culicinae | |||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Dry cool | 737 | 3.96 | 15.69 | 2736 | 14.71 | 71.64 |
| Dry hot | 590 | 3.99 | 13.34 | 1121 | 7.57 | 24.98 |
| Rainy | 1107 | 4.06 | 13.01 | 2126 | 7.79 | 22.54 |
Anopheles and Culicinae aquatic stages collected by season. Data show mean, standard deviation and number of collection events (one per site and sampling time)
For Culicinae, significant associations were still confirmed for the distance to the Niger River (p < 0.0001) with more Culicinae when the distance to the river increases (Fig. 5b; Table 2), the typology (p < 0.0001) with higher values with the ‘Kori’ and, to a lesser degree with ‘Human made’ breeding sites (Fig. 5c; Table 3), and the water surface (p < 0.0001) with a wide range of size of the water bodies (Fig. 5d), but smaller than the Anopheles (Fig. 3). The relationship with the situation along the stream (up/down) (Fig. 5a; Table 1) was not confirmed (p = 0.056), neither the seasonal effect (p = 0.4949) (Table 4).
Fig. 5.
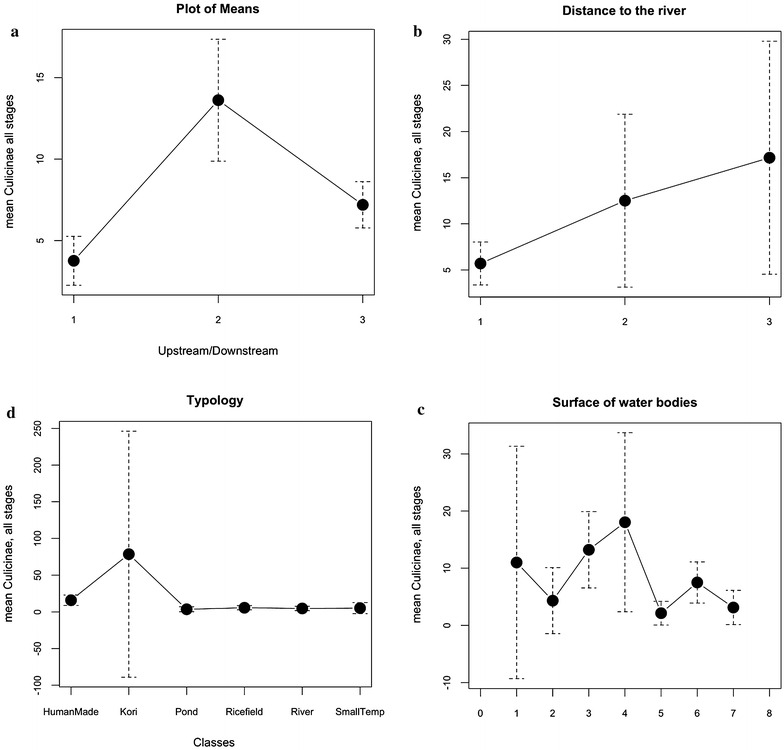
Means of Culicinae aquatic stages according to environment factors. a Upstream (‘1’), at the city level (‘2’) and downstream (‘3’). b Distance to the river: <500 m from the river median line (‘1’), between 500 and 1500 m (‘2’) and beyond 1500 m (‘3’). c Typology of sampled breeding sites. d According to the surface of water bodies equals decimal log from 0.1 m2 (=1) to <1000 m2 (=5), >1000 m2 (=6), in contact with the river (=7). The ‘8’ (no access) or ‘0’ (dry) values have not been sampled
Ecological study: models
To test the relative importance of the different factors, two different models were built with the significant factors for each of the Anopheles and Culicinae data (Table 5) (see Additional file 1).
Table 5.
Summary of model parameters and goodness indices
| Model parameters | Goodness indices | |||||
|---|---|---|---|---|---|---|
| Variable type: presence/absence (YN) or count) | Model type | Interaction with season (N = Add/Y = Interact) | Residuals deviance | Null residual | Degrees of freedom | AIC |
| Anopheles | ||||||
| YN | logreg–binom (Ano1) | N | 740.81 | 782.92 | 596 | 762.81 |
| YN | logreg–binom | Y | 710.35 | 782.92 | 580 | 764.35 |
| Count | glmPoisson | N | 7437.9 | 9536.4 | 596 | 8189.1 |
| Count | glmPoisson (Ano2) | Y | 6831 | 9536.4 | 580 | 7614.1 |
| Culex | ||||||
| YN | logreg–binom (Culex1) | N | 719.22 | 773.65 | 598 | 737.22 |
| YN | logreg–binom | Y | 698.48 | 773.65 | 582 | 748.48 |
| count | glmPoisson | N | 22.691 | 27.036 | 598 | 23.528 |
| count | glmPoisson (Culex2) | Y | 18,694 | 27,036 | 582 | 19,564 |
It shows the residual and Null deviances, degrees of freedom and the Akaike Information Criterion (AIC). The selected models are in italic with names in brackets
For Anopheles the two following formulae were used
Anopheles ~ Typology + DistRiver + Up/Down + Water surface, with no interaction between factors
Anopheles ~ (Typology + DistRiver)*Season + Up/Down + Water surface including the Season interaction with both the typology and Distance to the river.
The logistic regression (LR) model improved only slightly the data and the model without interaction with Season gave only by few a better AIC (Table 5) (see Additional file 1). The GLM-Poisson model decreased the number of residuals compared to the null model. The AIC was better with the model with interaction between Season and Typology and Distance to the river. The histogram of residuals showed a skewed distribution (see Additional file 2) and the residual vs fit plot show a divergence in the higher values of abundances (see Additional file 3).
The predictor factors ‘Typology’, ‘Distance to the river’, UpDown stream’ were kept in all the models (Table 6). The ‘water surface’ factor was significant only with GLM-Poisson. The predictor factors influenced the models in the same way (Table 6). The ‘Pond’ as typology modality was found significantly and positively associated with the abundance of Anopheles (p < 0.0015). A distance higher than 500 m to the river was negatively associated with Anopheles abundances (p < 0.035–p < 2e−16) in all models. The ‘up/down stream’ factor gave negative estimates when being at the city or downstream (p < 0.0002), but not significantly in the case of LR. The water surface was negatively associated (less Anopheles if greater size of sites) only in GLM model.
Table 6.
Summary of model results
| Significant variable in the model: Yes/No | Interactions | ||||||
|---|---|---|---|---|---|---|---|
| Typology (reference “human made”) | Distance to river (reference “<500 m”) | Up/down stream (reference “upstream”) | Water surface | Season (reference “Dry cool”) | Typology * season | Distance river * season | |
| Ano1 | Kori (p = 0.0524) | Yes (Q—p = 0.0281) | (p = 0.0977) | No | NA | NA | NA |
| Ano2 | Pond (p < 2e−16), River (p = 0.0217) | Yes (Q—p < 2e−16) | Yes (L—p < 2e−16) | Yes (p = 2e-6) | Rainy (p = 0.0003) | Yes | Yes |
| Culex1 | Pond (p = 0.0028), River (p = 0.0027) | Yes (L—p = 0.0139) | NA | No | NA | NA | NA |
| Culex2 | Kori (p < 2e−16), Pond (p < 2e−16), Ricefield(p < 2e−16), River (p = 8.7e−11), Small temp(p = 2e-11) | Yes (Q—p < 2e−16) | NA | Yes p < 2e−16 | Dryhot (p < 2e−16), Rainy (p < 2e−16) | Yes | Yes |
The interaction between factors improved the GLM model, but not the logistic regression model (Table 5). The Rainy season impacted negatively on the abundances of Anopheles for ‘Pond’, ‘River’ and ‘Small Temp’ and positively for ‘Ricefield’ (Table 7). The Dry and Hot Season had a negative impact in the cases of ‘Kori’ and ‘Pond’, but a positive impact for the ‘River’. Both the Rainy Season and the Hotand Dry Season had positive interactions with the distance to the river (p < 0.01).
Table 7.
Significant interactions between factors in GLM models
| Typology (reference “Human made”) | Anopheles | Culicinae | ||
|---|---|---|---|---|
| Rainy season | Hot and dry season | Rainy season | Hot and dry season | |
| Kori | Ano2*** | Culex2*** | ||
| Pond | Ano2*** | Ano2*** | Culex2*** | |
| Ricefield | Ano2** | Culex2*** | Culex2*** | |
| River | Ano2*** | Culex2*** | Culex2* | |
| Small Temp | Ano2*** | Culex2** | ||
| Distance river(L-Linear, Q-Quadratic) | Ano2*** (Q + L) | Ano2***(L) | Culex2*** (Q) | Culex2*** (Q) |
The significant interactions upon mosquito aquatic stages abundances are shown between modalities (classes) of the Season Factor and Typology and Distance to the river. The reference modality is indicated in column or row heads. In bolditalic, positive interactions; italicized, negative interactions; the p values are coded as: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘·’ 0.1 ‘ ' 1
For the Culicinae, the two following formulae were used:
Culicinae ~ Typology + DistRiver + Water surface
Culicinae ~ (Typology + DistRiver)*Season + Water surface, including the Season interaction with both the typology and Distance to the river.
As for Anopheles, the LR model improved the data and decreased the residual deviance with also a very small difference between the AIC with or without interactions between variables (Table 5) (see Additional file 1). The GLM Poisson model was better if including the interactions of the Season upon Typology and Distance to the river (Table 5) (see Additional file 1). The histogram of distribution of residuals was better than for Anopheles but still deviant from normality (see Additional file 2). As for Anopheles the plot of residuals vs fitted deviated in the higher values (see Additional file 3). All the predictors were kept: The ‘Pond’ (p < 0.0045) and ‘River’ (p < 0.025) modalities of the Typology are negatively associated with Culicinae in the two models. The ‘Kori’ modality is very significantly and positively associated with the abundances of Culicinae in GLM, but not with their presence in case of LR model (Table 6).
The water surface showed a negative impact in the GLM-Poisson (p < 2e−16). When included in the GLM model, the Season predictor showed a negative impact on Culicinae abundances (p < 2e−16) for both the ‘Rainy and Dry and Hot’ modalities compared to the Dry cool reference modality (Table 7). The interactions between season and the typology classes showed a positive impact of the Rainy Season on the Abundances of Culicinae sampled the ‘RiceField’, ‘Small Temp’ and with the ‘River’ in the GLM-Poisson model (Table 7). The Rainy season had a negative impact on the abundances of Culicinae sampled in the ‘Kori’ for the GLM integrating the interactions. The Hot and Dry Season influenced positively the Culicinae abundances in ‘Ricefield’ and ‘Pond’ and ‘River’. The distance to the river has a positive interaction with the two ‘Rainy’ and ‘Hot and Dry’ seasons (Table 7).
Adult mosquito study
Composition of the population and abundance
Between June 2008 and June 2009, 196 rooms were sprayed with insecticide and 294 light traps set at night giving a total of 11,957 female mosquitoes (Table 8). The total mosquito counts were comparable in the two sites: 6381 for Goudel and 5576 for Boukoki (Fig. 2). Among them, 2139 Anopheles females were collected, with 95 % of An. gambiae s.l., 1.7 % An. rufipes, 1.7 % An. ziemanni, 1.4 % An. pharoensis and 0.6 % An. funestus. However An. gambiae s.l. accounted for 33 % of the mosquitoes captured in the Goudel (peri-urban) area and only 0.7 % in the Boukoki (urban) area. Anopheles funestus was found only in the peri-urban area. Culex sp. predominated in both districts, accounting for 62 % of the mosquitoes in the peri-urban area and 99 % in the urban area.
Table 8.
Composition of mosquito fauna
| Districts of Niamey | ||||||||
|---|---|---|---|---|---|---|---|---|
| Species | Goudel (semi-urban area) | Boukoki 4 (urban area) | Total | |||||
| PS | LT | Total | PS | LT | Total | |||
| An. gambiae s.l. | 715 | 1273 | 1988 | 10 | 27 | 37 | 2025 | |
| An. funestus | 2 | 10 | 12 | 0 | 0 | 0 | 12 | |
| An. rufipes | 12 | 21 | 33 | 0 | 3 | 3 | 36 | |
| An. ziemanni | 0 | 36 | 36 | 0 | 0 | 0 | 36 | |
| An. pharoensis | 1 | 29 | 30 | 0 | 0 | 0 | 30 | |
| Culex sp. | 1110 | 2849 | 3959 | 1031 | 4483 | 5514 | 9473 | |
| Mansonia sp. | 1 | 218 | 219 | 1 | 1 | 2 | 221 | |
| Aedes sp. | 1 | 13 | 14 | 0 | 6 | 6 | 20 | |
| Phlebotomine | 74 | 16 | 90 | 1 | 13 | 14 | 104 | |
| 1916 | 4465 | 6381 | 1043 | 4533 | 5576 | 11,957 | ||
Composition and counts of female mosquito collected in 98 rooms after morning spraying and in 147 light traps per locality in two districts of Niamey during 1 year (2008–2009); PS means pyrethrum spray and LT means light trap
Variation of adult mosquito abundances
Mean mosquito density per district varied over time (Fig. 6). An. gambiae s.l. showed two different seasonal patterns: unimodal, peaking during the rainy season at Boukoki, and bimodal at Goudel, with one high peak during the rainy season, and one wide and smoothed peak during the dry seasons. At Goudel, the maximum capture event for An. gambiae s.l. was in September with 64 females in one sprayed room and 59 females in one light trap. The second peak showed a maximum of 39 females for residual spraying in December and36 for light trap, in February. At Boukoki 4, the highest captures of An. gambiae s.l. were in August, September and October, peaking only at three females per sampling event. The highest captures of Culex mosquitoes were done with light traps from November to February (>150 females per event) in Boukoki, in several occasions. Such a level of Culex adult capture was reached only once at Goudel, in February.
Fig. 6.
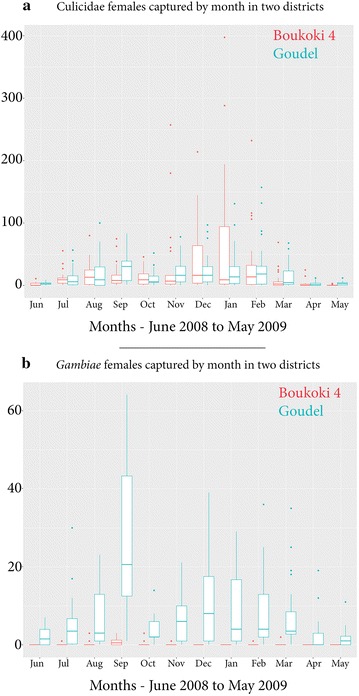
Monthly variation of mosquito abundance. Monthly variation of mosquito abundance showing different temporal dynamics for Anopheles gambiae (b) compared to the overall Culicidae (a) for the peri-urban district close to the river (Goudel) and the urban district close to the kori (Boukoki 4). Note the different y axis scales
Circumsporozoite protein detection
In Boukoki 4 (urban area), none of the 34 females of An. gambiae s.l., tested by ELISA for the presence of the circumsporozoite protein of P. falciparum was found positive. In Goudel, nineteen (1.3 %) among 1433 Anopheles gambiae s.l. females were found positive by ELISA. CSP-positive An. gambiae s.l. Specimens were found from July 2008 up to February 2009 with maximum value in August (Fig. 7).
Fig. 7.
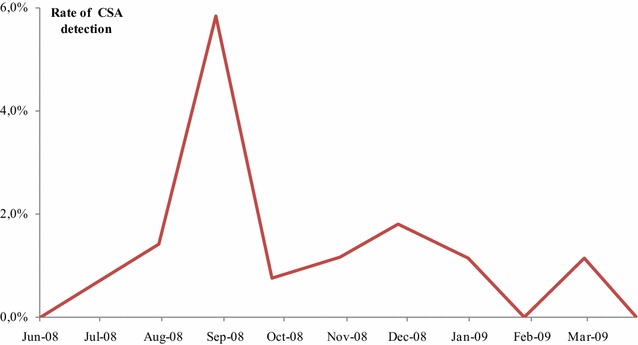
Temporal pattern of change in CSA levels in An. gambiae s.l. in Goudel. Temporal pattern of change in CSA levels in An. gambiae s.l. in Goudel, a semi-urban area of Niamey
Distribution of An. gambiae s.l. complex
The distribution by Anopheles gambiae complex species of adults caught in pyrethrum spray catches, CDC light-traps and reared from larval collections is shown in Fig. 8. In 2003, 255 out the 2434 An. gambiae s.l. emerging adults were genotyped by PCR. The population of adult An. gambiae complex mosquitoes bred from larval collections consisted of 56 % An. gambiae s.s. (An. gambiae + An.coluzzii) and 44 % An. arabiensis. The relative frequency of the larvae of An. gambiae s.s. was particularly high in the rainy season (100 %) and then the cold season, whereas An. arabiensis was more frequent in the dry and hotter season (February to May). Anopheles gambiae s.s. was found in 34 out of 35 sites whereas An. arabiensis was found in only 12 sites. The Goudel pond was the most prevalent site for An. arabiensis (79 % of the specimens identified as An. arabiensis). Further molecular identification of 103 specimens from 2003 showed 61 % An. coluzzii (61 %) and 39 % of An. gambiae (=S molecular form). Out of 27 sites, An. coluzzii was found in 23 sites whereas An. gambiae was found in 11 sites. In 2009, the molecular identification of 431 adult An. gambiae s.l. showed 90 % An. coluzzii (M form) and 10 % An. arabiensis, without any An. gambiae (S form) identified. The difference of proportions of gambiae/arabiensis between 2003 and 2009 is significant (χ2 = 105, p < 0.0001).
Fig. 8.
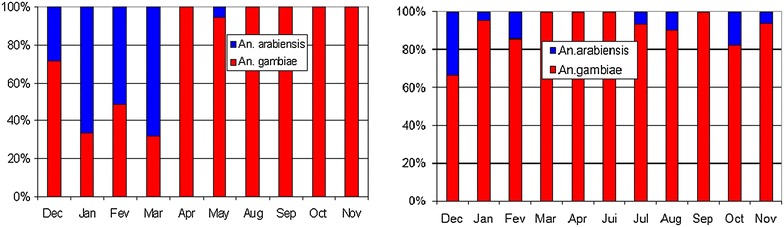
Monthly distribution of An. gambiae complex species. Monthly distribution of An. gambiae complex species for adults reared from larvae in 2003 (left) and for adults collected in 2009 (right) from urban areas of Niamey
Discussion
The study aimed at describing and analysing the ecology of urban malaria vectors in a Sahelian environment with high constraints on water sources. The mosquito species that were found during the study are already known from this region [6, 27]. In African urban environments, the most commonly found malaria vectors are: An. gambiae and An. arabiensis [1]. Anopheles funestus has been found in urban environments of Gabon and Mozambique [1], at low abundance in Nigeria [28] and very rarely in Antananarivo (Madagascar) [29]. The case of An. funestus is noteworthy, as this species was described reappearing in the Sahel zone and particularly in Niger [30] after being absent since the 1970s [3]. The capture of An. funestus during the 2009 collection is the first mention in Niamey city. The absence of Anopheles funestus from the aquatic stage survey could be linked to its true absence or to its ability to escape the dipping method. The aquatic net would be probably more appropriate for its collection [31] but this vector was not expected at that time. However, given the vector capacity of this vector, the survey of its potential expansion is of prime importance.
New insights for An. gambiae complex distribution in the Sahel are provided. According to genotyping, An. coluzzii is the most prevalent species found both in 2003, as aquatic stage, and in 2009, as adult. An. arabiensis is found associated with the hot and dry season as in neighbouring rural areas of Niger [6] and confirmed its affinities for arid and dry climate, as in the northern parts of Cameroon [32]. The significant difference of relative proportion of An. arabiensis and An. gambiae between 2003 and 2009 may be related to differences in the sampled population stages (aquatic and adult) but also to changes in the true distribution of these species. Such a change has been observed in rural areas 60 km distant from Niamey [6]. In the same region, the S form (An. gambiae) decreased significantly from 2005 to 2007, after a large distribution of impregnated bed nets [6]. In the present study, the disappearance of the S form during the 2009 collection, despite a notable (>400) number of genotyped samples is puzzling and could reveal a general trend for this species. The difference of total rainfall between the two sampling periods is relatively important (−32 % in 2008–2009) but remains within the range of the year-to-year recorded variability within the total period of the study. Therefore, it is difficult to conclude between a short-term, year-to-year, difference or a longer term trend.
The sampling effort and the number of collected pre-imaginal stages are very comparable to a survey led in garden wells of Dakar, Senegal by Robert et al. [33]. However the proportion of Anopheles aquatic stages is higher in Niamey than in Dakar (29 vs 12 %). The results show a clear difference in the distribution of aquatic stages by sites and by month (Fig. 3a) between the Anopheles and the Culicinae. By different approaches of data analysis, we found environmental factors which seem important for abundances of malaria vectors. The models were not perfectly fit to the aquatic stage collection data. However the Poisson distribution GLM improved the residual deviance by one-third compared to the null deviance. The modelling of the pre-imago malaria vector ecology confirmed the PCA and Kruskall–Wallis test results; it showed the importance of the situation upstream to the city and the importance of the ponds and wells in gardens, as well as the distance to the Niger River. Models allowed to look at the interactions between seasonality and the typology of the breeding sites, as well as the effect of the season on the river, by flooding both in rainy and in the cool and dry seasons, or by the residual breeding sites in the riverbed during the hot and dry season. In Niamey, as in Dakar, An. gambiae could be found in relation to garden wells. The Gountou Yena kori is an important site for Anopheles breeding. Its situation in continuity with garden drains increases the exposure of Anopheles aquatic stages to insecticide. In 2007, the resistant allele frequency of the knock-down resistance gene was found higher in this area than in other areas of the city [34]. In the sub-region, urban agriculture has been shown increasing the abundances of Anopheles and Culex in Kumasi [35] and Accra [36], Ghana and Dakar, Senegal [33]. In the two last situations, authors discussed not only the role of larval breeding sites but also agriculture sites as potential resting sites for adult mosquitoes.
In a first analysis, the seasonality was not found a significant factor for the distribution of the pre-imago stages of Anopheles. Indeed, malaria vectors are found all along the year with different and alternative breeding sites in the zone. In Ouagadougou (Burkina Faso), Fournet et al. [37] also found malaria vectors during the whole year. However, the adult collection conducted in Niamey in 2009 showed a clear temporal signal, particularly in the city centre, with maximal values during the rainy season. This impact was confirmed when modelling the abundance of Anopheles larvae and pupae included the interaction of the season with the typology of breeding sites. The impact of the season could be different according to the types of breeding sites: a positive impact of the rainy season in the rice field and a negative for the breeding sites associated with the river and the small and temporary sites. The influence of the rains flushing out the small breeding sites, included the ones created in the river bed by the previous dry season could be locally important. The ‘Pond’ type is negatively associated by both the ‘rainy’ and the ‘hot and dry’ seasons (Table 7) for influencing the Anopheles aquatic stage abundances. Indeed, these types of breeding sites will favour higher abundances of Anopheles during the dry and cold seasons, after the rainy season, as shown by the adults dynamics near the Goudel pond: here, the abundance temporal peak of An. gambiae adults is much wider (Fig. 6) than in the city centre and widens the season of abundance up to the drier seasons. The sporozoite index estimated in the Goudel zone, near the pond is 1.3 % and is close to the index found in Zindarou at 1.4 % in 2007, after the insecticide impregnated bed net distribution [6]. No infected Anopheles was found in Boukoki in the city centre. As the number of sites for adult collection is limited, we cannot extrapolate for the whole city. However we have shown that notable malaria transmission still occurs in the peri-urban area. The Goudel area is at high risk for malaria and after local health centre data, people do experience the malaria burden. In the centre of the city, the Gountou Yena kori is also a hot spot for malaria vectors.
The city has changed during the 7 years separating the beginning and the end of the study. However, most of the changes in urban structure have occurred in the Northern and Eastern boundaries of the city, far from the heart of the city and the river, which was the main focus of the study. The ecological factors identified here are not outdated and provide important features of the ecology of malaria vectors in Niamey. These data represent the first steps for the potential larval source management [38] as control method. The Goudel Pond, and the Gountou Yena kori and its proximity are identified as permanent or semi-permanent sources of Anopheles. If models could be considered as relatively accurate, the proximity of the river is confirmed as a risk factor for Anopheles. The upstream location of the breeding sites is a newly identified risk factor for Anopheles in Niamey. However many sites are transitory and may make larval control difficult [36]. However the quantification of the parasite transmission by the measure of sporozoite index within the city is an important step forward. In complement to the LLINs strategy already implemented, stakeholders should ask if the larval source management could be a cost-effective approach [10] to improve the malaria control for the community of Niamey.
Conclusion
Malaria transmission is occurring in Niamey. In the peripheral area, the sporozoite index is comparable to local rural villages. However, aquatic stages are not distributed randomly and distribution factors have been identified. Encouraged by the Dar-es-Salaam experience [12], the results of the study show that the vector malaria control could focus on key-areas and provide seeds for future works on larval and pupae ecology in Niamey.
Authors’ contributions
RbL, JBD, IJ and TF: Conception of study, data collection, analysis and interpretation, drafted and critically revised the manuscript. IJ and CC: contributed to data analysis. JBD, IJ and EW: Contributed substantially to conception and critically reading the manuscript. RbL, IA, AS and RmL: performed field and laboratory work. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank the populations of Boukoki 4 and Goudel for participating in the project, and the medical team (Health Director of Niamey, manager of the CSI for Goudel and Boukoki 4) for performing some of the field work. We would also like to thank Dr Jocelyne Rocourt, Dr Odile Ouwe Missi Oukem, Dr François Rivière and Sani Ousmane for critical reading of the manuscript, and the laboratory staff of the “Unité de Parasitologie du CERMES”, including Aboubacar Mahamadou, Mahamadou Izamné, Sani Haladou and Saadou Kadri in particular, for technical assistance.
Competing interests
The authors have declared that they have no competing interests.
Funding
This work was supported by Pasteur Institute of Paris, WHO (RBM-Sahel and APW from WHO-Afro), NASA Hydrology (Jared Entin) and Massachusetts Institute of Technology (MIT-USA).
Additional files
10.1186/s12936-016-1352-0 Model parameters and main results. Models using Anopheles data and Culex data are detailed with formulas and main results.
10.1186/s12936-016-1352-0 Residuals histograms. Residuals histogram with normal distribution curve for 4 models.
10.1186/s12936-016-1352-0 Residuals vs fit plots. Plots of the residuals against the fitted values side by side for GLM Poisson models for Anopheles and Culicinae counts.
Contributor Information
Rabiou Labbo, Phone: +227 20752045, Email: rabiou@cermes.org.
Thierry Fandeur, Email: thierryfandeur@yahoo.fr.
Isabelle Jeanne, Email: ijeanne@free.fr.
Cyril Czeher, Email: cyril_czeher@yahoo.fr.
Earle Williams, Email: earlew@ll.mit.edu.
Ibrahim Arzika, Email: iarzika@cermes.org.
Amadou Soumana, Email: soumana@cermes.org.
Ramatoulaye Lazoumar, Email: lramatoulaye@yahoo.fr.
Jean-Bernard Duchemin, Email: Jean-Bernard.Duchemin@csiro.au.
References
- 1.Donnelly MJ, McCall PJ, Lengeler C, Bates I, D’Alessandro U, Barnish G, et al. Malaria and urbanization in sub-Saharan Africa. Malar J. 2005;4:12. doi: 10.1186/1475-2875-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert V, Macintyre K, Keating J, Trape JF, Duchemin JB, Warren M, et al. Malaria transmission in urban subsaharan Africa. Am J Trop Med Hyg. 2003;68:169–176. [PubMed] [Google Scholar]
- 3.Julvez J, Mouchet J, Michault A, Fouta A, Hamidine M. [Eco-epidemiology of malaria in Niamey and in the river valley, the Republic of Niger, 1992–1995](in French) Bull Soc Pathol Exot. 1990;1996(90):94–100. [PubMed] [Google Scholar]
- 4.Walker K, Lynch M. Contributions of Anopheleslarval control to malaria suppression in tropical Africa: review of achievements and potential. Med Vet Entomol. 2007;21:2–21. doi: 10.1111/j.1365-2915.2007.00674.x. [DOI] [PubMed] [Google Scholar]
- 5.Guillebaud J, Mahamadou A, Zamanka H, Katzelma M, Arzika I, Ibrahim ML, et al. Epidemiology of malaria in an area of seasonal transmission in Niger and implications for the design of a seasonal malaria chemoprevention strategy. Malar J. 2013;12:379. doi: 10.1186/1475-2875-12-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labbo R, Czeher C, Djibrila A, Arzika I, Jeanne I, Duchemin J-B. Longitudinal follow-up of malaria transmission dynamics in two villages in a Sahelian area of Niger during a nationwide insecticide-treated bednet distribution programme. Med Vet Entomol. 2012;26:386–395. doi: 10.1111/j.1365-2915.2012.01011.x. [DOI] [PubMed] [Google Scholar]
- 7.Bomblies A, Duchemin J-B, Eltahir EA. A mechanistic approach for accurate simulation of village scale malaria transmission. Malar J. 2009;8:223. doi: 10.1186/1475-2875-8-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauvet G, Dyemkouma A. Enquête sur les insectes vecteurs de maladies ou de nuisances dans la ville de Niamey (Niger), enquête du 23 juillet au 6 août 1973. OCCGE Rapp. 1973; 29 pp.
- 9.Sales S, Ochoumare J. Etude des moustiques vecteurs de maladies ou constituant des nuisances dans la ville de Niamey, République du Niger (enquête effectuée du 19 au 23 avril 1971). 1971; 18 pp.
- 10.Mourou J-R, Coffinet T, Jarjaval F, Cotteaux C, Pradines E, Godefroy L, et al. Malaria transmission in Libreville: results of a one year survey. Malar J. 2012;11:40. doi: 10.1186/1475-2875-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maheu-Giroux M, Castro MC. Cost-effectiveness of larviciding for urban malaria control in Tanzania. Malar J. 2014;13:477. doi: 10.1186/1475-2875-13-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. Interim position statement–the role of larviciding for malaria control in sub-Saharan Africa. Geneva, World Health Organization Global Malaria Programme; 2012.
- 13.Fillinger U, Kannady K, William G, Vanek MJ, Dongus S, Nyika D, et al. A tool box for operational mosquito larval control: preliminary results and early lessons from the Urban Malaria Control Programme in Dar es Salaam, Tanzania. Malar J. 2008;7:20. doi: 10.1186/1475-2875-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Amato N, Lebel T. On the characteristics of the rainfall events in the Sahel with a view to the analysis of climatic variability. Int J Climatol. 1998;18:955–974. doi: 10.1002/(SICI)1097-0088(199807)18:9<955::AID-JOC236>3.3.CO;2-Y. [DOI] [Google Scholar]
- 15.Quantum GIS Development Team. QGIS geographic information system. Open Source Geospatial Foundation Project. 2012. http://qgis.osgeo.org
- 16.Gillies MT, Meillon BD. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region) Publ South Afr Inst Med Res. 1968;54:1–343. [Google Scholar]
- 17.Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. Publ South Afr Inst Med Res. 1987;55:1–48. [Google Scholar]
- 18.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 19.Favia G, Lanfrancotti A, Spanos L, Sidén-Kiamos I, Louis C. Molecular characterization of ribosomal DNA polymorphisms discriminating among chromosomal forms of Anopheles gambiae s.s. Insect Mol Biol. 2001;10:19–23. doi: 10.1046/j.1365-2583.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- 20.Santolamazza F, della Torre A, Caccone A. A new polymerase chain reaction-restriction fragment length polymorphism method to identify Anopheles arabiensis from An. gambiae and its two molecular forms from degraded DNA templates or museum samples. Am J Trop Med Hyg. 2004;70:604–606. [PubMed] [Google Scholar]
- 21.Coetzee M, Hunt RH, Wilkerson R, Della Torre A, Coulibaly MB, Besansky NJ. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa. 2013;3619:246–274. doi: 10.11646/zootaxa.3619.3.2. [DOI] [PubMed] [Google Scholar]
- 22.Burkot TR, Williams JL, Schneider I. Identification of Plasmodium falciparum-infected mosquitoes by a double antibody enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1984;33:783–788. doi: 10.4269/ajtmh.1984.33.783. [DOI] [PubMed] [Google Scholar]
- 23.Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Burkot TR, Schneider I, et al. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65:39–45. [PMC free article] [PubMed] [Google Scholar]
- 24.Dray S, Dufour A-B. The ade4 package: implementing the duality diagram for ecologists. J Stat Softw. 2007;22:1–20. doi: 10.18637/jss.v022.i04. [DOI] [Google Scholar]
- 25.Dolédec S, Chessel D. Rythmes saisonniers et composantes stationnelles en milieu aquatique. I: Description d’un plan d’observation complet par projection de variables. Acta Oecologica Oecologia Gen. 1987;8:403–426. [Google Scholar]
- 26.Fox J. Getting started with the R commander: a basic-statistics graphical user interface to R. J Stat Softw. 2005;14:1–42. [Google Scholar]
- 27.Julvez J, Mouchet J, Suzzoni J, Larrouy G, Fouta A, Fontenille D. [The anopheles of Niger](in French) Bull Soc Pathol Exot. 1998;91:321–326. [PubMed] [Google Scholar]
- 28.Oduola AO, Olojede JB, Oyewole IO, Otubanjo OA, Awolola TS. Abundance and diversity of Anopheles species (Diptera: Culicidae) associated with malaria transmission in human dwellings in rural and urban communities in Oyo State, Southwestern Nigeria. Parasitol Res. 2013;112:3433–3439. doi: 10.1007/s00436-013-3522-0. [DOI] [PubMed] [Google Scholar]
- 29.Rabarijaona LP, Ariey F, Matra R, Cot S, Raharimalala AL, Ranaivo LH, et al. Low autochtonous urban malaria in Antananarivo (Madagascar) Malar J. 2006;5:27. doi: 10.1186/1475-2875-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labbo R, Fouta A, Jeanne I, Ousmane I, Duchemin JB. Anopheles funestus in Sahel: new evidence from Niger. Lancet. 2004;363:660. doi: 10.1016/S0140-6736(04)15606-7. [DOI] [PubMed] [Google Scholar]
- 31.Robert V, Le Goff G, Ariey F, Duchemin J-B. A possible alternative method for collecting mosquito larvae in rice fields. Malar J. 2002;1:4. doi: 10.1186/1475-2875-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wondji C, Simard F, Petrarca V, Etang J, Santolamazza F, Della Torre A, et al. Species and populations of the Anopheles gambiae complex in Cameroon with special emphasis on chromosomal and molecular forms of Anopheles gambiae s.s. J Med Entomol. 2005;42:998–1005. doi: 10.1603/0022-2585(2005)042[0998:SAPOTA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Robert V, Awono-Ambene P, Thioulouse J. Ecology of larval mosquitoes, with special reference to Anopheles arabiensis (Diptera: Culcidae) in market-garden wells in urban Dakar, Senegal. J Med Entomol. 1998;35:948–955. doi: 10.1093/jmedent/35.6.948. [DOI] [PubMed] [Google Scholar]
- 34.Czeher C, Labbo R, Arzika I, Duchemin J-B. Evidence of increasing Leu-Phe knockdown resistance mutation in Anopheles gambiae from Niger following a nationwide long-lasting insecticide-treated nets implementation. Malar J. 2008;7:189. doi: 10.1186/1475-2875-7-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Afrane YA, Klinkenberg E, Drechsel P, Owusu-Daaku K, Garms R, Kruppa T. Does irrigated urban agriculture influence the transmission of malaria in the city of Kumasi, Ghana? Acta Trop. 2004;89:125–134. doi: 10.1016/j.actatropica.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Klinkenberg E, McCall P, Wilson MD, Amerasinghe FP, Donnelly MJ. Impact of urban agriculture on malaria vectors in Accra, Ghana. Malar J. 2008;7:151. doi: 10.1186/1475-2875-7-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fournet F, Cussac M, Ouari A, Meyer P-E, Toé HK, Gouagna L-C, et al. Diversity in anopheline larval habitats and adult composition during the dry and wet seasons in Ouagadougou (Burkina Faso) Malar J. 2010;9:78. doi: 10.1186/1475-2875-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO . Larval source management: a supplementary measure for malaria vector control. Geneva: World Health Organization; 2013. [Google Scholar]


