Abstract
Objective:
Our prespecified dose-response analyses of A Very Early Rehabilitation Trial (AVERT) aim to provide practical guidance for clinicians on the timing, frequency, and amount of mobilization following acute stroke.
Methods:
Eligible patients were aged ≥18 years, had confirmed first (or recurrent) stroke, and were admitted to a stroke unit within 24 hours of stroke onset. Patients were randomized to receive very early and frequent mobilization, commencing within 24 hours, or usual care. We used regression analyses and Classification and Regression Trees (CART) to investigate the effect of timing and dose of mobilization on efficacy and safety outcomes, irrespective of assigned treatment group.
Results:
A total of 2,104 patients were enrolled, of whom 2,083 (99.0%) were followed up at 3 months. We found a consistent pattern of improved odds of favorable outcome in efficacy and safety outcomes with increased daily frequency of out-of-bed sessions (odds ratio [OR] 1.13, 95% confidence interval [CI] 1.09 to 1.18, p < 0.001), keeping time to first mobilization and mobilization amount constant. Increased amount (minutes per day) of mobilization reduced the odds of a good outcome (OR 0.94, 95% CI 0.91 to 0.97, p < 0.001). Session frequency was the most important variable in the CART analysis, after prognostic variables age and baseline stroke severity.
Conclusion:
These data suggest that shorter, more frequent mobilization early after acute stroke is associated with greater odds of favorable outcome at 3 months when controlling for age and stroke severity.
Classification of evidence:
This study provides Class III evidence that shorter, more frequent early mobilization improves the chance of regaining independence after stroke.
In our primary intention-to-treat analysis for A Very Early Rehabilitation Trial (AVERT), we reported that patients randomized to usual care had greater odds of a favorable outcome (modified Rankin Scale [mRS] score 0–2) at 3 months compared to those receiving the very early mobilization (VEM) protocol.1 However, a recommendation of “usual care” is of limited value to clinicians seeking guidance, as usual care was not standardized and mobilization often commenced within 24 hours of stroke. Importantly, “early” does not encapsulate all aspects of the interventions delivered in this trial. The VEM protocol was earlier, more frequent, and higher amounts of out-of-bed activity,2 a “complex intervention,”3 which was consistent with practices associated with better outcome in Norway.4 Greater amounts of physical training poststroke is associated with improved outcomes (without harm) in a number of meta-analyses,5,6 and many guidelines recommend increasing training dose. Increased frequency, a critical intervention characteristic, was supported by 2 lines of evidence. Exercise benefits in sedentary adults, accumulated in multiple, short bouts, appear equivalent to a single, longer bout,7,8 and bursts of training appear highly suited to acute stroke patients. Further, frequent repetition of training (distributed practice) is associated with improved motor learning after stroke.9,10 In AVERT, all interventions were carefully recorded, thus allowing a detailed investigation of dose response. Our aim for these prespecified dose-response analyses11 is to provide practical guidance for clinicians.
The trial is registered with the Australia and New Zealand Clinical Trial Registry (ACTRN12606000185561).
METHODS
Detailed methods are described elsewhere.1,11 In brief, AVERT, conducted in 56 stroke units in 5 countries, is a pragmatic, parallel-group, randomized controlled trial. Patients with ischemic or hemorrhagic stroke, admitted within 24 hours of onset, were eligible. Patients with early deterioration, another serious illness, unstable coronary conditions, not rousable to voice, or failing physiologic screening criteria were excluded. Patients or their nominated representative provided written consent. Randomization was blocked, balanced by site, and stratified by stroke severity. All trial personnel were masked to group, except the intervention monitor.
Standard protocol approvals, registrations, and patient consents.
Ethical approval was obtained from relevant site institutional boards.
Procedures.
Patients were randomized to receive either usual care or frequent out-of-bed activity (mobilization) in addition to usual care (VEM).1,11 VEM patients commenced mobilization within 24 hours of stroke and trained physiotherapy and nursing staff helped them continue task-specific out-of-bed activity, targeting recovery of active sitting, standing, and walking activity, at a frequency and intensity (amount) guided by an intervention protocol. Functional ability at baseline, monitored daily and adjusted with recovery, guided the intervention dose with 4 titrations specified. For example, in low arousal, dependent patients (level 1), active sitting with assistance was the mobilization target, with each session lasting a minimum of 10 and maximum of 30 minutes. With higher-functioning patients (level 4), standing and walking were likely targets, each session again lasting a minimum of 10 minutes with no restricted maximum (patient-dependent). The frequency of sessions per day also varied according to functional level. Importantly, passive sitting (resting in a chair) was not classified as a VEM mobilization activity and sitting for more than 50 minutes at one time was discouraged. Intervention lasted 14 days or until discharge, whichever was sooner. Physiotherapists and nurses, with separate intervention targets, worked together to deliver the intervention dose. All mobilization activities were recorded online.
Outcome measures.
Our primary outcome was a favorable outcome on the modified Rankin Scale (mRS 0–2) at 3 months poststroke.1 Secondary outcomes were time (days) to achieve unassisted walking over 50 meters, the proportion of patients achieving unassisted walking by 3 months, death, and the number of serious adverse events (SAEs) at 3 months. Immobility-related SAEs (deep venous thrombosis, pulmonary emboli, pressure sores, chest infections, urinary tract infections) and neurologic SAEs (stroke progression, recurrent stroke) were examined separately.
Dose measures.
The dose-response analyses examine the 3 main characteristics of dose: (1) time from stroke onset to first mobilization out of bed (TTFM, hours), (2) median number of out-of-bed sessions per patient per day (frequency), and (3) median minutes of out-of-bed activity per patient per day (daily amount). Total minutes of out-of-bed activity over the intervention period (total amount) accounts for varying lengths of hospital stay.
Nurses recorded type of activity and time of the day each activity began, but not minutes, as this was not routine practice. Physiotherapists recorded activity type, time the activity began, and total out-of-bed activity time (minutes), consistent with their routine practice. Consequently, daily amount (minutes) and total amount (minutes) of out-of-bed activity reflect physiotherapy data alone, while TTFM and frequency of mobilizations is derived from both nurse and physiotherapist data. Episodes of sitting, standing, or walking activity separated from another episode of activity by >5 minutes of rest (e.g., in a chair) constituted 2 separate mobilizations. Active time (minutes), e.g., practicing sit-to-stand from the chair, was recorded by the physiotherapist.
Statistical analysis.
These dose-response analyses repeat our major primary and secondary analyses1 with dose characteristics (TTFM, frequency, daily amount, total amount) as independent variables, and were prespecified in our statistical analysis plan.11 To avoid excessive collinearity between daily amount and total amount, we tested 2 separate models, adjusted for age and baseline stroke severity (NIH Stroke Scale [NIHSS]), for all analyses, as follows:
Model 1: TTFM, median daily number of out-of-bed sessions (frequency), median daily out-of-bed session time (in 5-minute increments).
Model 2: TTFM, median daily number of out-of-bed sessions (frequency), total minutes in out-of-bed activity over the intervention period (in 5-minute increments).
The primary analysis, with favorable outcome (mRS 0–2) at 3 months as the dependent variable, was conducted using binary logistic regression models.
The dose effect on the odds of achieving unassisted walking by 3 months was investigated using binary logistic regression analyses (effect sizes: adjusted odds ratios [ORs] and 95% confidence intervals [CIs]) while the time (days) to achieve unassisted walking (censored at 3 months) was assessed using Cox regression analyses (adjusted hazard ratios with 95% CIs).
We analyzed mortality outcomes using binary logistic regression with death at 3 months (mRS 6) as the dependent variable (effect sizes: adjusted ORs with 95% CIs). We investigated dose effect on counts of SAEs using negative binomial regression (effect sizes: adjusted incidence rate ratios with 95% CIs). Immobility-related and neurologic SAEs were analyzed separately.
We used Classification and Regression Tree (CART) advanced analysis (Salford Predictive Modeler Software Suite version 7, Salford Systems, San Diego, CA) to further investigate the complex interactions between patient and dose characteristics and favorable outcome. CART is a binary partitioning statistical method that starts with the total sample and, in a stepwise manner, splits the sample into subsamples that are homogenous with respect to a defined outcome.12 The input variable that achieves the most effective split is dichotomized by automated analysis at an optimal threshold, maximizing the homogeneity within, and separation between, resulting subgroups. To maximize model performance (assessed by area under the receiver operating characteristic [ROC] curve), a 10-fold internal cross-validation, where data are randomly divided into 10 groups with 9 used to build the model (training) and 1 used to validate (testing), is performed. In addition to the classification tree, CART numerically ranks each input used to build the tree by relative importance.
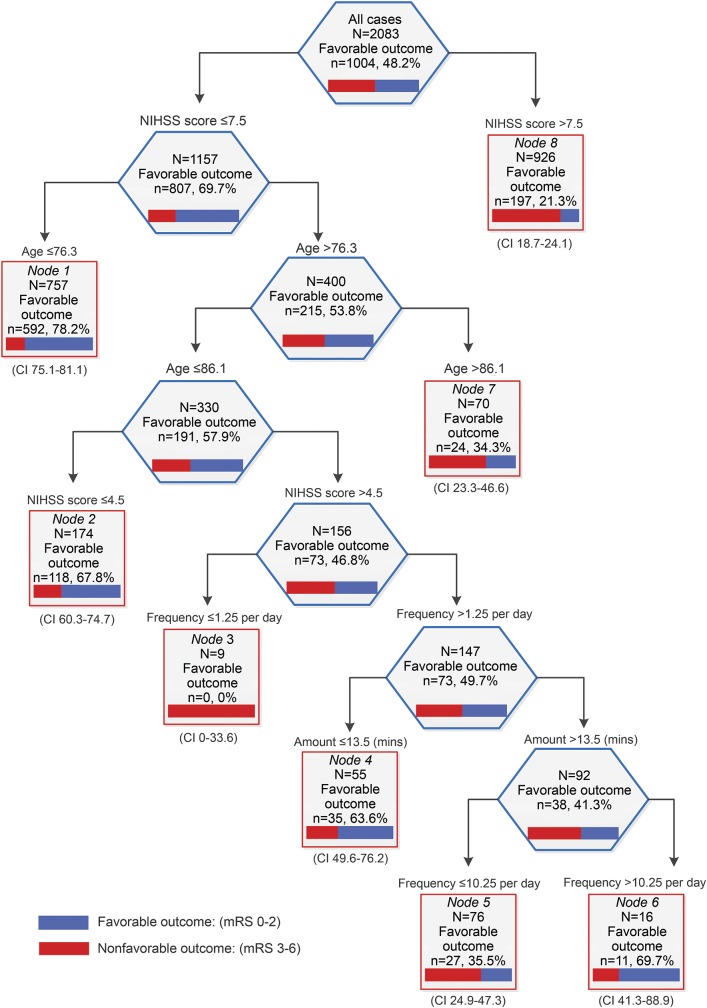
Our CART1 (figure 1) analysis included all prespecified subgroup variables1 (age, NIHSS, stroke type, recombinant tissue plasminogen activator [rtPA] treatment), group allocation, and the 3 dose characteristics (TTFM, frequency, and daily amount). We explored the relative importance of each variable to achieving a favorable outcome (mRS 0–2). CART2 (figure e-1 on the Neurology® Web site at Neurology.org) was used to investigate multidimensional relationships between dose characteristics alone and favorable outcome.
Figure 1. Classification and Regression Tree (CART) advanced analysis investigating interactions between dose and patient characteristics and odds of a favorable outcome (modified Rankin Scale [mRS] 0–2).
Time to first mobilization, median daily number of out-of-bed sessions per day (frequency), median daily out-of-bed activity session time (amount), age (in years), and stroke severity (NIH Stroke Scale [NIHSS]). Frequency is derived from nursing and physiotherapist data. Amount (minutes) is derived from physiotherapist data only. CI = confidence interval.
RESULTS
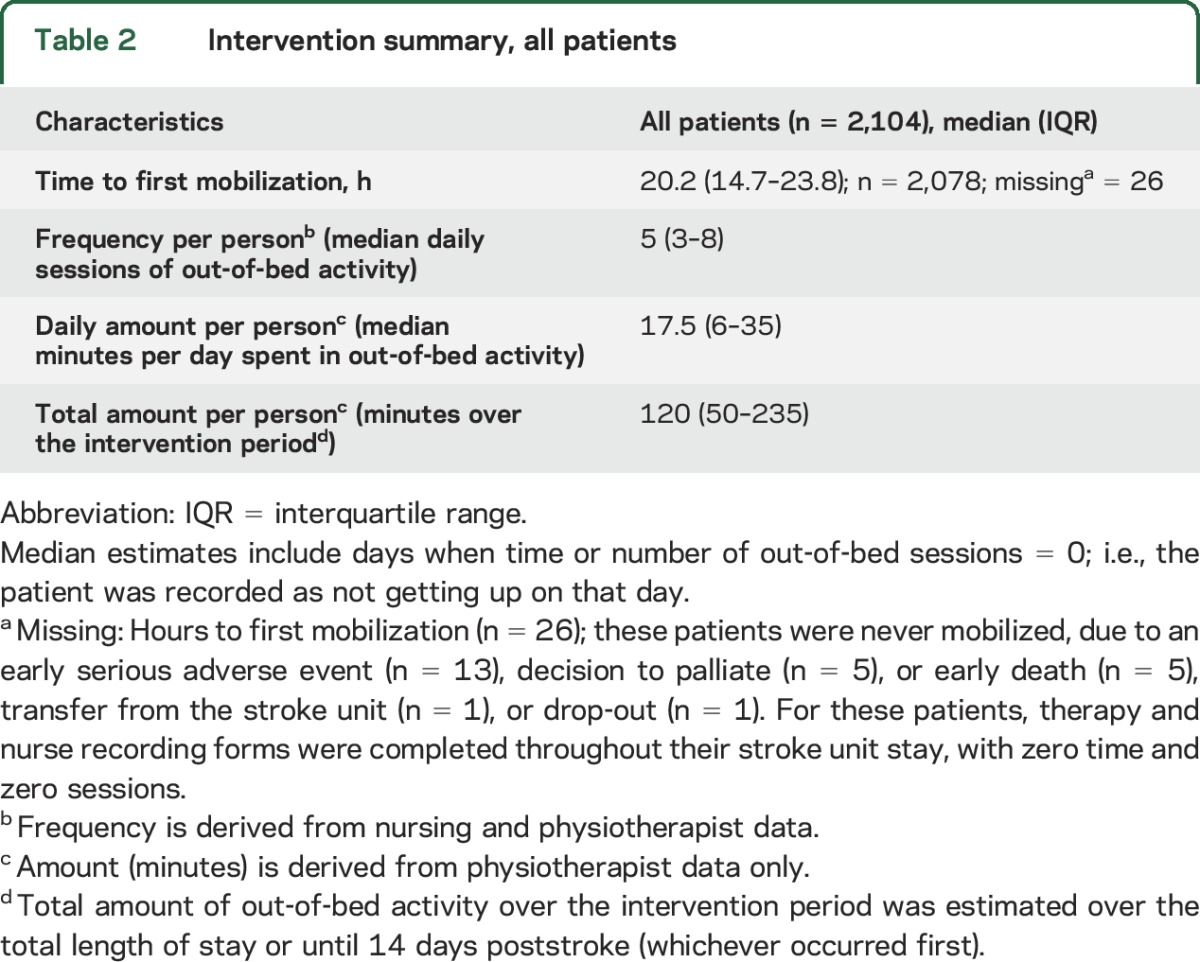
Between July 18, 2006, and October 16, 2014, we randomly assigned 2,104 patients to VEM (n = 1,054) and usual care (n = 1,050), with 2,083 (99%) patients followed to the primary 3-month endpoint. This group constitutes the dataset for all analyses in this article. In total, 25% were over 80 years of age, few were disabled prior to stroke, more than 43% of patients experienced a moderate to severe stroke (NIHSS > 7), and 12% were diagnosed with intracerebral hemorrhage (table 1). A total of 1,584 patients (75%) had no disability (premorbid mRS 0) prior to stroke, a further 519 (24%) had slight disability (mRS 1–2), and 1,833 (87%) could walk without aids. The median (interquartile range [IQR]) time to first mobilization was 20.2 hours (14.7–23.8), while 1,588 (75%) participants commenced out-of-bed activity within 24 hours of stroke (table 2).
Table 1.
Baseline characteristics of all patients
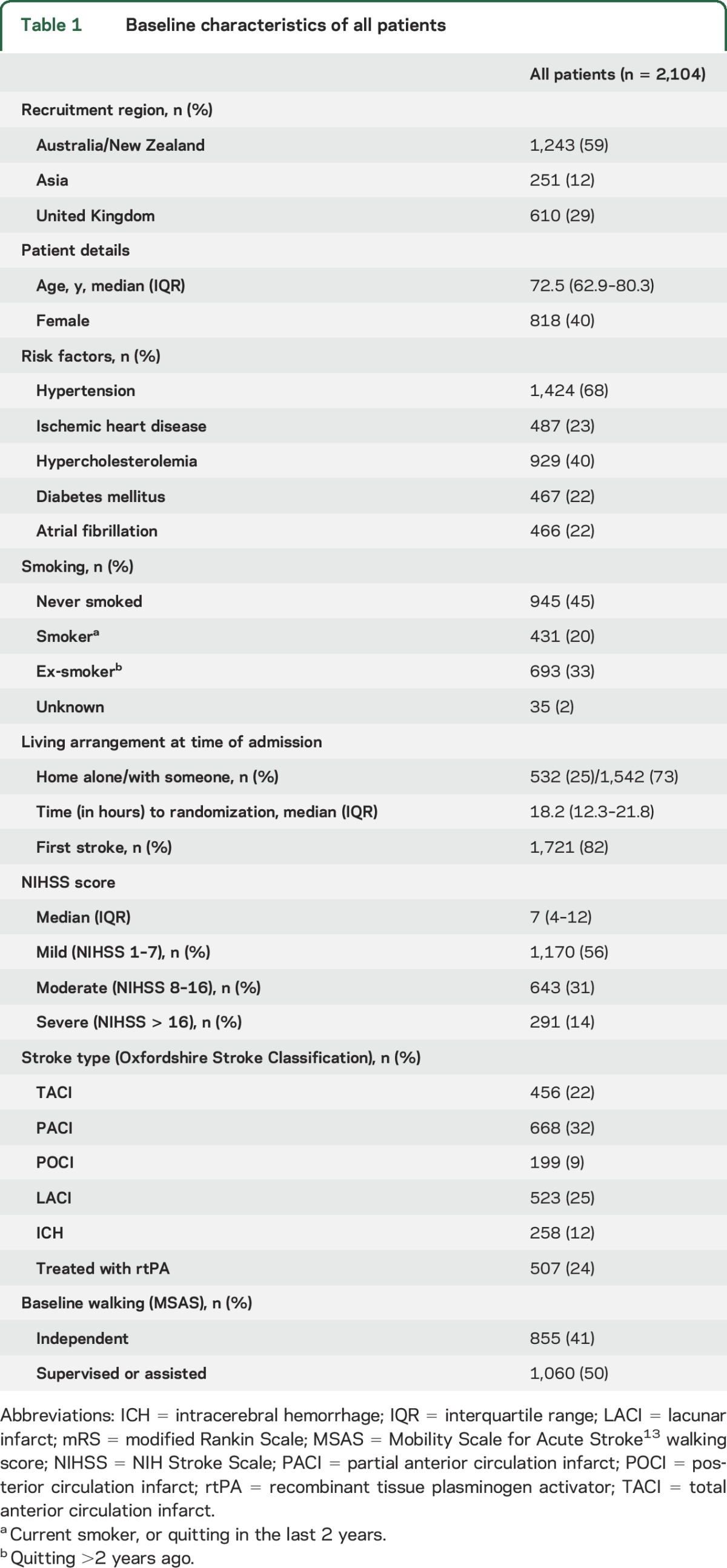
Table 2.
Intervention summary, all patients
Greater TTFM was associated with a reduced odds of favorable outcome (0.99, 0.98–1.00, p = 0.036; table 3). In model 1 (favorable outcome), the effect of TTFM was adjusted for median daily number of sessions (frequency), median daily minutes (daily amount), age, and baseline NIHSS. In this example, the significant effect for TTFM on the odds of a favorable outcome should be interpreted as follows: for 2 patients of similar age and stroke severity, receiving a similar frequency and daily amount of out-of-bed activity, the patient who starts mobilization earlier has improved odds of a favorable outcome.
Table 3.
Effect of intervention characteristics on favorable outcome (mRS 0–2) and unassisted walking
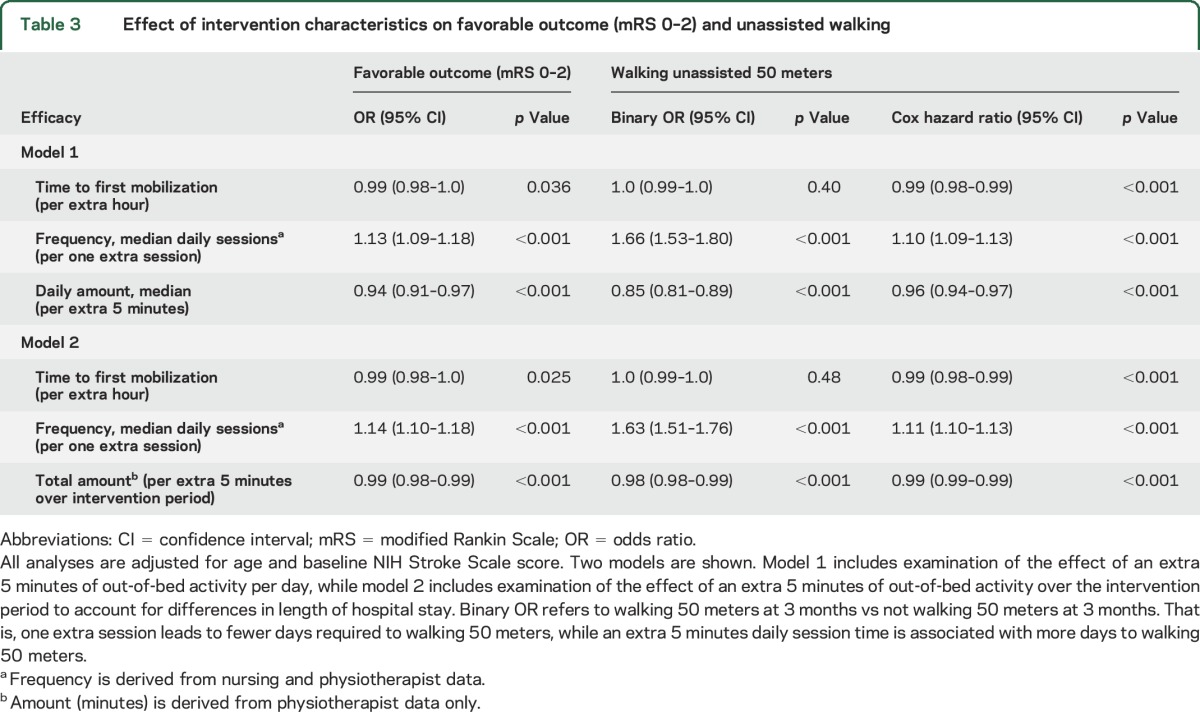
For efficacy outcomes, favorable outcome (mRS 0–2) and walking by 3 months, we found a similar pattern of association with each of the dose characteristics, and all associations were significant (table 3). In model 1, TTFM, frequency, and daily amount all significantly influenced the odds of a favorable outcome. Keeping TTFM and frequency constant, every extra 5 minutes of out-of-bed activity per day reduced the odds of a favorable outcome. Increasing the frequency of sessions improved the odds of favorable outcome by 13% (95% CI 9–18 p < 0.001) and improved the odds of walking 50 meters unassisted by 66% (95% CI 53–80, p < 0.001) when TTFM and daily amount were kept constant. This pattern was similar in model 2.
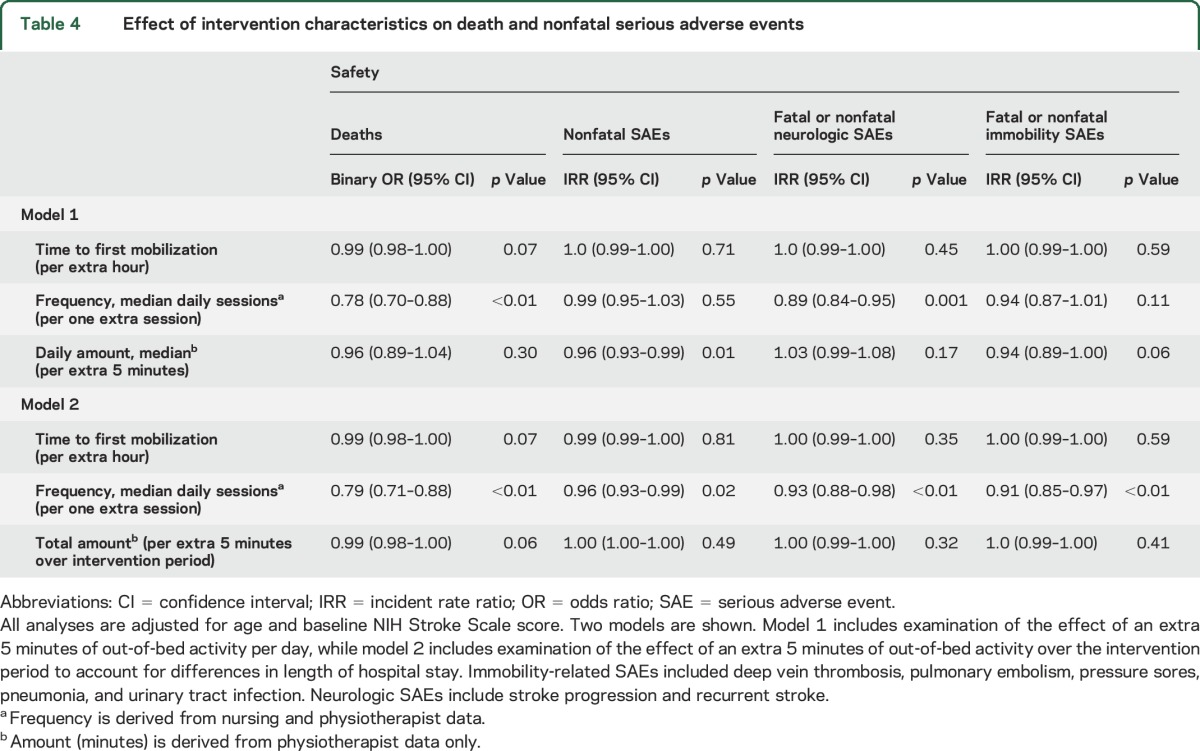
When examining associations with intervention characteristics and death, increasing session frequency was the only characteristic that reduced the odds of death by approximately 20% (table 4). Nonfatal SAEs showed less consistent associations between dose characteristics and outcome. TTFM was not significant in any model, but frequency and amount were (table 4). Given the relatively few immobility and neurologic SAEs, these results should be viewed with caution.
Table 4.
Effect of intervention characteristics on death and nonfatal serious adverse events
In CART1 (figure 1), including TTFM, frequency and daily amount, age, NIHSS, stroke subtype, rtPA treatment, and randomization group, we found good to excellent performance, with a training ROC of 0.78 and a testing ROC of 0.77. The relative contribution of variables to the model showed initial stroke severity (NIHSS), a known predictor of recovery, was most important (100%), followed by session frequency (39.2%), age (32.4%), TTFM (10.5%), and daily amount (2.7%). Treatment group was not an important discriminator.
Younger patients (≤76.3 years) and those with low NIHSS score (≤7.5) had high probability of a favorable outcome (78.2%). Those with NIHSS score >7.5 showed low (21.3%) probability of achieving little to no disability (mRS 0–2). The influence of intervention characteristics becomes evident as we move farther down the tree. For example, at terminal node 4 (figure 1), patients between 76.3 and 86.1 years, with an NIHSS score >4.5 (but ≤7.5), showed greater probability of a favorable outcome (63.6%) if they received no more than 13.5 minutes per day distributed across frequent (short) sessions. Frequency again split the tree for terminal nodes 5 and 6, indicating that more frequent sessions to achieve a higher dose (>13.5 minutes per day) was associated with greater odds of a good outcome. CART2 (figure e-1) explores the influence of dose variables on outcome. Further exploration of CART1 large terminal nodes 1 (younger age) and 8 (more severe stroke) are shown in figures e-2 and e-3. TTFM, frequency, amount, and group are all influential splitters in these models.
DISCUSSION
We found a consistent pattern of association between the odds of experiencing little or no disability (mRS 0–2) at 3 months and the intervention characteristics irrespective of treatment group. In particular, we saw 13% improvement in the odds of a favorable outcome with each additional session of out-of-bed activity per day (keeping the time to first mobilization and daily amount constant). Conversely, increasing the amount of time spent in out-of-bed activity, keeping the frequency and time to first mobilization constant, reduced the odds of a favorable outcome. The potentially beneficial effect of increasing the frequency of out-of-bed activity (but not the amount) was consistent across most of the efficacy and safety analyses.
These findings begin to unpack the primary results, where we reported that VEM (very early, frequent, and higher-dose out-of-bed activity) reduced the odds of a favorable outcome at 3 months.1 This dose-response analysis suggests that increased frequency of mobilization (keeping other intervention characteristics constant) helps reduce disability and increases the odds of walking by 3 months and reduces the odds of death. However, increasing the minutes of out-of-bed activity was more likely to result in worse outcomes. In other words, these findings indicate that short, frequent sessions may be preferable for many patients in the first weeks after stroke.
Addition of the CART analyses provided further support for the important influence of session frequency on outcome. Even with the inclusion of patient characteristics considered strongly predictive of outcome after stroke (such as stroke severity and age), intervention characteristics played an explicit and important role, defining homogenous groups of patients based on their chances of achieving the favorable outcome. Indeed, in patients with more severe stroke (NIHSS > 13.5), a more favorable outcome was evident in those with more rather than less sessions (figure e-3). In humans and animals, there is limited discussion about the potential effect of frequency of intervention on stroke outcome. Recently, Bell et al.14 studied skilled reaching in stroke-affected mice and found that twice daily, higher-dose training accelerated recovery and improved final outcome compared to a once-a-day, lower-dose regimen. In humans, a number of systematic reviews and meta-analyses suggest a dose-response relationship, with more intensive therapy resulting in improved functional outcome after stroke.5,6,15 Definitions for therapy intensity vary, but to date the focus has been on amount (minutes) rather than frequency (repetitions of a task, or sessions per day).16 Generally, inadequate reporting of therapy interventions, together with significant heterogeneity in the timing, amount, frequency, and intensity of training provided, complicates messaging of poststroke therapy, particularly in the first few weeks, where few studies exist.17 Interestingly, a 3-arm early rehabilitation trial testing upper limb constraint regimens, although small (n = 52), also found that a higher amount of training was inferior to lower-dose usual care.18 A critical challenge in rehabilitation is determining who should be targeted and when and what is the optimal intervention.19
The influence of time to first mobilization was less clear, partly due to a compact distribution pattern with the median time (IQR) less than 24 hours (14.7–23.8). The optimal time to commence out-of-bed activity remains unknown. While early animal studies showed that very-high-dose training within the first days poststroke increased brain lesion volume,20 our recent animal meta-analysis21 found that a shorter interval between stroke and exercise start reduced infarct volume (effect size −0.24, 95% CI −0.36 to −0.06, p < 0.004), without significantly influencing behavioral outcomes.21 A further animal systematic review showed that early initiated (24–48 hours poststroke) moderate exercise reduced lesion volume and protected perilesional tissue against oxidative damage and inflammation.22 Given that animal research to date suggests that activity within 24–48 hours of ischemic stroke onset may be helpful, an obvious translation gap exists.
Study strengths include that the dose-response analysis was prespecified11 to help understand our complex intervention, and we had a strong focus on the quality of the nurse and physiotherapy data collected in the trial.11 Our main limitation is that this exploratory analysis is not an RCT testing each of the intervention components separately (time, amount, frequency). Our results will need to be confirmed in further RCTs. Because we recorded physiotherapist-assisted out-of-bed therapy time only (not nursing), these data underestimate the actual minutes each day that a patient spent undertaking out-of-bed activity.
One could argue that the intervention protocol influenced our findings because intervention dose was greater when participants had a less severe stroke. However, given that we found the same relationship between time to first mobilization, frequency, and amount of time in the usual care group alone as found for the whole group, this seems unlikely. That is, more frequent sessions (keeping mobilization time and median minutes of out-of-bed activity per day constant) improved the odds of a good outcome by 12% (OR 1.12, 95% CI 1.04–1.21, p < 0.004). Interestingly, less time to first mobilization was also associated with improved odds of a good outcome (OR 0.98, 95% CI 0.97–0.99, p = 0.002), while more minutes of out-of-bed activity was not (table e-1). Therefore, while the intervention protocol itself may have confounded some of this association, the results provide us with important clues on how the components of early and intensive rehabilitation affect outcome.
These results provide insights into the drivers of outcome and provide clinicians with a guide to early rehabilitation practices. There are 3 important messages from our results. The first is that physiotherapist- and nurse-facilitated mobility interventions delivered in the acute phase of care can change a patient's long-term outcomes, so it is critical that trialists carefully define and measure these interventions. Second, these results suggest that the frequency of intervention may be a more important driver of outcome. This has received little attention to date and requires further evaluation in future trials. The final message is that the currently accepted philosophy of “more practice is always better” needs to be reconsidered, particularly within the first days after stroke. The issue of timing, frequency, and amount of therapy is more complex that previously realized. This represents fertile ground for future research.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the participants and their families; the AVERT Collaboration investigators; and Tara Purvis, the independent intervention monitor throughout the trial.
GLOSSARY
- AVERT
A Very Early Rehabilitation Trial
- CART
Classification and Regression Tree
- CI
confidence interval
- IQR
interquartile range
- mRS
modified Rankin Scale
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- ROC
receiver operating characteristic
- rtPA
recombinant tissue plasminogen activator
- SAE
serious adverse event
- TTFM
time from stroke onset to first mobilization out of bed
- VEM
very early mobilization
Footnotes
Supplemental data at Neurology.org
Editorial, page 2120
Contributor Information
Collaborators: AVERT Collaboration Group, Julie Bernhardt, Leonid Churilov, Janice Collier, Helen Dewey, Geoffrey Donnan, Fiona Ellery, Peter Langhorne, Richard Lindley, Marjory Moodie, Brooke Parsons, Amanda Thrift, Geoffrey Donnan, Helen Dewey, Julie Bernhardt, Peter Langhorne, Marjory Moodie, Brooke Parsons, Main Investigators MIs, Bent Indredavik, Torunn Askim, Phillip Bath, Christopher Bladin, Christopher Reid, Stephen Read, Cathy Said, Sandy Middleton, Judith Frayne, Velandai Srikanth, Australia: Julie Bernhardt, Helen Dewey, Julie Bernhardt, Geoffrey Donnan, Amanda Thrift, Robert Carter, Richard Lindley, Julie Bernhardt, Geoff Donnan, Richard Lindley, Amanda Thrift, Peter Langhorne, Marjory Moodie, Helen Dewey, Leonid Churilov, Peter Langhorne, Peter Langhorne, Olivia Wu, Julie Bernhardt, Matthew Walters Claire Ritchie, Lorraine Smith, Peter Langhorne, Olivia Wu, Anne Ashburn, Helen Rodgers, Julie Bernhardt, Peter Langhorne, Anne Ashburn, Julie Bernhardt, Helen Rogers, Olivia Wu, Sheila Lennon, Sheila Lennon, Michael Power, Julie Bernhardt, Shahul Hameed, Shahul Hameed, Ratnagopal Pavanni, Peter Lim, Julie Bernhardt, Dawn Tan, Leonid Churilov, Tim Brewer, Janice Collier, Nick Haritos, Edwin Leong, Cecilia Li, Caesar NayWin, Marcus Nicol, Liudmyla Olenka, Li Chun Quang, Marjory Moodie, Robert Carter, Silvia Hope, Lauren Sheppard, Kiusiang Tay- Teo, Olivia Wu, Toby Cumming, Thomas Linden, Karen Borschmann, Jan Chamberlain, Janice Collier, Toby Cumming, Fiona Ellery, Teresa Occhiodoro, Helen Palfreeman, Tara Purvis, Bernadette Sirgo, Nick Tiliacos, John Van Holsteyn, Henry Zhao, Beverly Armstrong, Louise Craig, Fiona Graham, Lynn Legg, Rosemary Morrison, Heather Moorhead, Lorraine O’Donohue, Susan Rogers, Myra Smith, Denise Forshaw, Jane Fitzgerald, E Hibbert, R Melling, S Petrolo, T Purvis, H Williamson MIs, P Adams, L Augoustakis, S Batcheler, S Berney, V Cobani, B Cohen, H Dewey, S Gangi, N Giofre, C Gordon, L Hegarty, M Hindson, F Horvath, S Kalinowski, A Kleine, S Kramer, J Lawrence, S Lindquist, N Logan, A Macdonell, J Matlioski, N McDonough, S McLennan, M McNamee, L Miller, C Nall, E Nelson, K Ng, Z Nicholas, C Nunn, K Owen, E Plant, L Proud, D Quah, K Rodway, S Sertori, V Sheldon, L Sherry, S Speare, K Stansfeld, N Studden, Z Teoh, L Twist, G Velupillai, L Walker, K Wall, A Warwick, R Wharrie, J Wilson, H Worboys, D Young, J Ancliffe MI, M Bryant, B Doran, M Field, P Fogliani, A Haber, G Hankey, D Hendrie, V Jackaman, A Jacobsen, S Jose, R Lim, R Louis, S Nanthakumar, S Pain, A Power, B Rappeport, J Reynolds, L Smith, S Tombe, A Wesseldine, T West, K Clarke, H Maccanti, L Marr, S Plumb, J Quiney, L Werner MIs, E Abeykoon, W Apirutvorrachod, L Attard, S Behanan, D Brown, K Buchanan, D Butler, M Camac, S Davis, D Diocera, N Gan, C Gendre, J Germaine, P Hand, L Maurenbrecher, J McCulloch, S Mcritchie, M Ong, R Pachett, L Pesavento, H Power, R Reilly, M Sawers, G Silva, C Stevens, L Taylor, T Timms, M Ugalde, A Vardy, J Wallace, S Walsh, E Whatley, E Winter, M Baxter, M Davis, L Sundararajan MIs, E Butler, K Caspers, E Coulter, S Shaw, F Kent, H Lack, F Leavold, J Lord, J Martin-Francisco, R Mohanraj, R Nelson, T O'Neill, R Otto, J Parker, V Rees, B Stevens, R Chen MI, RI Lindley, J Bindra, R Dongre, N Downey, M Ferris, L Gibson, R Gonzalez, M Kinniburgh, M Lazaridou, D McCormack, R Singh, A Stepney, Y Tria, K Bainbridge, B Killey, R Sheedy MIs, O Aitchison, L Bray, K Clatworthy, S Coghill, M Collins, L Cornwall, J Dow, P Gates, S Gillett, N Johnson, S Joseph, K Kopelke, R Lam, R Levy, N Lloyd, S Logan, G McPherson, M Newth, C Parsons, K Powles, M Rebis, T Samakowidic, L Sanders, S Savickas, J Shrimpton, H Smith, L Smith, J Spehr, J Summers, G Taylor, M Thackeray, B Wilkinson, K Richardson MI, J Frayne, E Barber, L Bode, A Brakey, K Chand, P Christin, G Crook, D Delrosario-Kelly, R Descallar, A Deutsch, S Easo, M Farquhar, P Fergus, J Ford, E Hamson, M Hlaing, E Hope, J Lacivita, J Laurenson, K Lock, N Ly, K McKay, C Mill, K Moloney, L Price, T Terry, A Tyers, S Willems, R Woolstencroft, N Crawshaw, J Luker, C Wood, S Choat MIs, C Archer, D Benham, M Billinger, M Bronca, S Curchin, C Dickie, M Dixon, D Douglass, M Enomoto, K Ernst, L Fries, S George, E Green, L Hamilton, Z Harris, T Heard, G Hunt, N Jamieson, M Mackenzie, H McKearney, B Oermann, C O'Reilly, T Pearson, N Reid, L Rodda, D Scutcheon, C Simons, R Smith, L Tait, J Troake, D Usher, L Mackey, T Wijeratne MIs, C Abela, S Ashoka, C Chen, T Cheng, V Chong, S Cooke, A Fok, L Galang, C Grant, S Karageorge, K Kat, L Keo, B Lee, A Luscombe, J Mackay, M Minett, J Mizen, P Nim, N Nunlist, V Patel, M Pathirage, A Paton, M Pombuena, N Rathnayake, L Rhodes, M Sequeira, S Smart, S Somaratne, N Sta Maria, L Talbot, R Tecle, M Shannon, R Gerraty MIs, S Allen, R Boyle, N Fatchen, N Hendley, A Hyde, M Inal, P Kalubowilage, M Laverde, K Lawless, A McFadyen, K Peters, C Pugh, C Qin, J Robertson, S Smee, R Tomlinson, V Wang, F Williams, D Woolley, R Yawieriin, N Austin, S Pomfret, M Tinsley MIs, L Allport, C Ang, L Armitage, E Blundell, A Courtney, M Dela Costa, T Devi Thapa, P Diwakar, M Dulleh, J Francis, P Cic, G Gellie, C Gill, D James, S Lee, T Mai, K Majcher, C Mawson, G Newton, N Qiu, E Ragonton, L Roberts, H Saitamis, L Stanwell, L Ting, P Xu, L Yin, V Crosby MI, K Broadhead, J Church, R Collins, K Everitt, M Fisher, K Hochmuth, N Jones, A Lieschke, E McCarthy, C McGlone, D Morey, D Neilson, S Spry, M Vile, R Grimley, D Rowley MIs, I Rosbergen, E Ahern, L Anderson, J Boreham, R Devin, R Doolan, M Dyke, L Griffiths, K Guest, D Hecita, N Kendal, J Koltermann, M Lacy, S Lebeter, D Lloyd, M Matthews, C McAuley, A Pollock, M Pyke, T Rogers, S Street, G Styles, A Tampiyappa, J Trinder, T Verral, K Walker, C White, T Beckwith, L Cormack MIs, J Arriagada, C Babenschneider, D Blacker, S Bennett, S Connor, J Cowmeadow, N Daniel, G Edmonds, M Faulkner, M Garcia-Vega, K Kruger, B Martial, P McGinley, H Mountford, V Riley, N Smith, F Stepan, S Tilley, S Whisson, P Groot MI, J Bailey, K Ballinger, C Bell, B Camilleri, C Charnley, D Crabbe, S Crossland, N Edirimanna, C Fitzgerald, C Gibbins, J Gibbs, K Hirst, A Kennedy, E Klose, K McDowall, S Miller, R Morgan, A Noonan, M North, M Oliver, K Richards, T Russell, N Scott, A Shlanski, A Traynor, S Smith MI, R Adams, C Banks, K Burke, S Hewat, B McKenna, M McKimmie, L Polmear, M Traumanis, S Whiteman, V Bramah, R Errey, M Halpin, V Molan, D Wheelwright, N Wilson, W Zhang MIs, M Bakshi, S Bracher, M Bryant, W Byrnes, T Denton, N DeVries, P Fay, P Galbraith, T Gallaher, O Haidar, K Holgate, K Hozack, N Jackson, S Kipps, S Lerner, R Markus, R Merheb, C Naismith, G Nolan, R Odelli, K Page, P Sangvatanakul, T Simpson, P van Vliet, K Walch, S Walker, T Yasue, G Auld MI, R Baker, K Cousins, M Fairbrother, K Hutchinson, M Maclean, E Maher, D Mills, S Ohlback, J Sturm, M Tooth, J Watkins, J Cramb MI, P Atkinson, J Conrad, D Fichera, S Follent, C Gilbert, M Herzig, S Kohler, S McCracken, L Nunan, S Roberts, J Shelley, S Varendorff, A Wills, A Moore, A Robertson MIs, J Britton, A Burgess, T Coates, J Croft, E Greening, J Holland, P O'Brien, R Strong, L Tighe MI, S Bilston, J Black, K De Rivera, I Dwyer, S Gissane, K Heckenberg, S Jackson, A Maclagan, L O'Hare, H Patel, J Pearce, C Scanlan, K Seymour, M Symington, A Tyers, A Waite, K Wiesner, O Katalinic, M Spear MIs, P Brown, E Difuntorum, S Gilbert, J Henderson, D James, H Janssen, E Lane, S Lowndes, D Smith, S Thompson, D Weaver, S Weston, S Wright, S Cox, C Tse MIs, J Adrian, M Doughty, J Kok, R McGrath, T Morris, A Pickup, E Ray, R Richardson, M Sims, C Thompson, K Trinh, N Walton, F Whittaker, P Andersen MI, J Burrows, M Dawson, D Griffiths, G Harris, P Kavalieros, B O'Brien, K Roberts, J Watkins, C Whyatt, A McRae, G Wavish MIs, F Anos, J Armstrong, E Au, A Barber, C Bates, M Bertulfo, A Boggs, F Burgess, K Cassels-Brown, M Chiu, S Dass, N Duff, J Farrell, W Foster, D Fuertez, C Gadhvi, S George, A Green, L Harvey-Fitzgerald, L Hau, L Hayward, D Holman, K Huggins, M Jacobs, A John, H Kaur, T Lagerstedt, J Lee, R Llenes, L Lyons, S Magandi, M Martin, S Mathew, T Mathew, D McKellar, E Moss, KL Nand, K Nicol, F Peterson, A Prasad, K Quick, E Revell, S Roy, J Ryan, N Samadi, B Scrivener, J Slow, S Tharakan, J Torrens, E van Bysterveldt, C Villaluz, S Yang, MA Katijjahbe, G Ai Sing, A Azlina, MI Azmi, MH Efri, AZ Fadilah, H Fathuddeen, H Haryani, H Hussien, M Izuani, ZC Man, C Man Ying, KM Mashitoh, A Noor Azah, MI Norlinah, I Norliza, KB Ravinder, R Rohaizah, K Rosnita, A Rozita, J Safwan, R Sahathevan, I Shahrul, SM Sharifah, HJ Tan, WY Wan Nafisah, YL Yee, MA Zaharah, AS Zunaidah, D Tan, MT Ahmad, S Hameed MIs, MFB Bakari, J Britto, JJ Chen, S Choo, M Faizal, FK Fong, S Hong, J Ja'afar, Z Ke, G Koh, CK Lee, YF Lee, P Lim, GM Lim, SH Ninhadi, G Ong, T Pei Pei, V Penero, N Rahim, P Ratnagopal, K Saleh, HC Seow, E Sim, CK Tan, PY Tay, I Teo, S Thilarajah, PHJ Wong, WP Wong, S Yeap, M Macleod MI, A Anderson, K Armstrong, K Baird, D Balfour, M Boyd, J Cameron, C Carswell, C Clanachan, L Cuthill, I Devoy, S Forsyth, J Gavin, M Hughes, E Marr, S McAuley, E McCagherty, K McCallum, N McDonald, C McGhee, TA McIntyre, L Noonan, A Smart, R Walshe, D Neal MI, J Allison, G Ball, S Board, H Brunt, C Buckley, C Carroll, D Hayward, T Hutchinson, E Jones, E Keeling, E Marsh, N Mead, H Smith, C Vickers, B Williams-Yesson, D Wood, J Coyle, M Keeling MIs, L Ackroyd, C Brown, K Donnan, N Dyer, H Green, G Kilbride, C Nicholson, M Porteous, S Louw MI, A Annamalai, A Barkat, S Crawford, M Fawcett, D Harvey, V Hogg, A Hughes, J Kemp, J Morrison, K Storey, T Thompson, J Furnace, MJ Macleod MIs, J Bell, K Bennett, M Bruce, R Clarke, H Cowie, H Gow, J Irvine, A Joyson, S MacDonald, A Macvicar, N Murphy, J Robertson, C Gordon, J Kwan, L Redpath, K Saunders MIs, J Bell, R Burrow, C Clarke, C Dickson, G Hann, M Heath, S Heath, A Hewett, R Humphrey, B Longland, A Orpen, C Ovington, J Page, E Rogers, K Toombs, R Howes, A Lacey, P Meakin MIs, D Ames, S Banerjee, E Beranova, S Berry, MJ Burke, V Cassama, K Collins, J Crow, A Dunne, C Gomez, A Hawkins, K Hellier, SA Howard, A Kar, E Lambert, H Lee, C Mandri, J Moye, E Murtagh, J Pushpa-Rajah, J Richardson, TSachs, J Stilwell, V Tilley, P Wilding, N Wilson, E Feely, S Kirk MIs, P Cassidy, A Chalmers, C Duguid, N Hughes, J Hutton, K Lapsley, J Lee, A Murray, L Weir, M Whitelaw, M Barber, D Esson MIs, H Armit, C Devlin, R Duncan, C Forman, K Frame, L Hogg, P McLeod, R McWhinney, J Porter, M Purves, L Snowball, B Wroath MI, L Ferson, M Gibson, S Gillespie, N Ignatius, T Kane, J Kwant, M Matthews, C McCallion, C McConville, M McDowell, C McNally, L Moore, P Murphy, A Nesbitt, J Newell, M Power, E Reid, K Robinson, C Charnley, M James MIs, S Bacon, N Booth, A Bowring, L Boxall, J Burt, J Cageao, N Green, K Gupwell, S Keenan, H Kingwell, M Kryszkowska, J Mortimore, B Peace, C Roughan, G Gunathilagan, J Sampson, G Thomas MIs, T Allen, G Dane, K Harris, S Hart, SA Jones, M Reader, P Browne, C McGoldrick, D Mullan MIs, P Adair, J Armstrong, E Beggs, I Bell, C Edwards, L Gilligan, C Kelly, M Kennedy, J Kurian, L Leal, A McAtamney, E McKay, E Rogan, M Smyth, E Wiseman, J Vahidassr, C Price, V Riddell MIs, E Bendix, K Craig, R Davison, A Harrison, A Smith, V Green MI, K Ashton, W Barkhuizen, A Daniel, C Dickinson, H Durdu, D Eastwood, H Goddard, R Hodkin, J Howard, C Jeffs, S Joyce, C Kelly, G Kerr, J Lanes, B Magnall, M McMahon, M Moody, S Patton, R Taylor, A Watson, K Mitchelson, L Mokoena MIs, L Aird, R Lakey, J Murdy, K Nelson, G Storey, R McGeown, S Tauro MIs, R Brady, D Holland, M Kinnaird, L Maltman, D Martin, K McCord, S McKenna, C Morgan, C Shannon, A Steele, I Wiggam, S Appleby, S Brotheridge, M Prescott MIs, P Bagot, D Baston, C Bennett, J Featherstone, C Hare, A McCluskey, S Wade, R Worton, E Hakim, J Herman, T Norman MIs, L Beale, E Buckley, K Byrne, M Gasior, B Robles, C Smallwood, S Stevens, M Thomas, V Williams, K Buck MI, S Armstrong, V Brice, A Edwards, S Gething, A Griffiths, T Hills, D Howells, S Langdon, S Moseley, G Powell, G Reynolds, B Richard, E Scott, R White, J Zebedee, M Walters MI, J Alexander, L Brand, E Colquhoun, A Hill, D Macartney, H MacDonald, B Manak, H Morgan, C Ritchie, H Hunter MI, T Blair, M Duffy, J Graham, J Scott, T Vu, P Yorston, A Nair MI, I Shakir, C Button, M Friend, J Greig, B Hairsine, S Wade, S Williamson, G Cloud MI, T Adedoyin, N Dayal, S Gawned, R Ghatala, N Jeyaraj, L Kerin, L Montague, C Orefo, J O'Reilly, J Styles, S Trippier, C Watchurst, F Watson, J Hunt, R Latif MIs, C Barrett, J Cox, F Hammonds, K Quick, K Robinson, A Skinner, C Vernon, S Burgess, T Elder-Gracie MIs, C Browne, W Cameron, V Coleman, C Fulgencio, L Gibson, P Halliday, D Heaney, L Main, K McGavin, G Mead, F Proudfoot, A Redpath, C Rodger, S Scott, K. Robinson, K Mason MI, L Baxter, A Bryce, M Halkett, J Halliday, A McAllister, M McGuiness, M Munro, A Robb, A Thompson, B Tougher, J Weadon, J Young, C Douglas MI, M McParland, S Boyle, B Byrne, L Comiskey, J Gilpin, S Gilpin, A Harris, S Harshaw, J Haughey, F McArdle, L McConnell, E McEneaney, M Millar, M Murphy, and J Tilley
AUTHOR CONTRIBUTIONS
J. Bernhardt conceived and designed the study, wrote the first draft of the manuscript, and approved the final version. L. Churilov was the study statistician who prepared the analyses, reviewed the manuscript, and provided input. F. Ellery prepared the CART figures, reviewed the manuscript, and provided input. J. Collier reviewed the manuscript and provided input. J. Chamberlain reviewed the manuscript and provided input. P. Langhorne reviewed the manuscript and provided input. R.I. Lindley reviewed the manuscript and provided input. M. Moodie reviewed the manuscript and provided input. H. Dewey reviewed the manuscript and provided input. A.G. Thrift reviewed the manuscript and provided input. G. Donnan reviewed the manuscript and provided input.
STUDY FUNDING
The trial was supported by the National Health and Medical Research Council (NHMRC) of Australia (project grant nos.: 386201, 1041401). Additional funding was received from Chest Heart and Stroke Scotland (Res08/A114), Northern Ireland Chest Heart and Stroke, Singapore Health (SHF/FG401P/2008), The Stroke Association, UK (TSA2009/09), and the National Institute of Health Research, UK (grant no.: HTA Project 12/01/16). The Florey Institute of Neuroscience and Mental Health received support from the Victorian government via the Operational Infrastructure Support Scheme. The content of the publication is solely the responsibility of the authors and does not reflect the views of the funders.
DISCLOSURE
J. Bernhardt reports fellowship funding from the NHMRC (105863), the Australia Research Council (0991086), and the National Heart Foundation; NHMRC and Stroke Association UK project grants; support to provide an overview of early mobilization research at a European Stroke Conference 2013 seminar sponsored by Ever Pharma Neuro Pharma GmbH; and travel support to attend the European Stroke Conference and World Stroke Conference. L. Churilov reports membership on 5 editorial boards. F. Ellery reports salary from NHMRC grants (386201, 1041401). J. Collier reports salary from NHMRC grants (386201, 1041401). J. Chamberlain reports salary from NHMRC grants (386201, 1041401). P. Langhorne reports funding from Chest Heart and Stroke Scotland (Res08/A114), The Stroke Association, UK (TSA 2009/09), and the National Institute of Health Research, UK (Grant No: HTA Project 12/01/16). R. Lindley reports receiving compensation from Boehringer Ingelheim for chairing a scientific committee, lecturing honoraria from Covidien and Pfizer, is Associate Editor for the Australasian Journal on Ageing, receives publishing royalties from 2 books, and has received various project grants from NHMRC. M. Moodie reports no disclosures. H. Dewey reports fellowship funding from the NHMRC (336102). A. Thrift reports fellowship funding from the NHMRC (1042600). G. Donnan reports membership on Boehringer Ingelheim, Seriver, Sanofi Aventis, and Bristol Myer Squibb scientific advisory boards and serves as Editor-in-Chief of International Journal of Stroke, as an advisory board member for Stroke, Journal of Neuroimaging, and Lancet Neurology, and is an editorial board member of Stroke Research and Treatment. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bernhardt J, Langhorne P, Lindley R, et al. Efficacy and safety of very early mobilisation within 24 hours of stroke onset (AVERT): a randomised controlled trial. Lancet 2015;386:46–55. [DOI] [PubMed] [Google Scholar]
- 2.van Wijk R, Cumming T, Churilov L, Donnan G, Bernhardt J. An early mobilization protocol successfully delivers more and earlier therapy to acute stroke patients: further results from phase II of AVERT. Neurorehabil Neural Repair 2012;26:20–26. [DOI] [PubMed] [Google Scholar]
- 3.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Indredavik B, Bakke RPT, Slordahl SA, Rokseth R, Haheim LL. Treatment in a combined acute and rehabilitation stroke unit: which aspects are most important? Stroke 1999;30:917–923. [DOI] [PubMed] [Google Scholar]
- 5.Veerbeek J, van Wegen E, Van Peppen RPS, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One 2014;9:e87987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwakkel G, van Peppen RPS, Wagenaar R, et al. Effects of augmented exercise therapy time after stroke: a meta-analysis. Stroke 2004;35:2529–2536. [DOI] [PubMed] [Google Scholar]
- 7.Macfarlane DJ, Taylor LH, Cuddihy TF. Very short intermittent vs continuous bouts of activity in sedentary adults. Prev Med 2006;43:332–336. [DOI] [PubMed] [Google Scholar]
- 8.Murphy MH, Blair SN, Murtagh EM. Accumulated versus continuous exercise for health benefit: a review of empirical studies. Sports Med 2009;39:29–43. [DOI] [PubMed] [Google Scholar]
- 9.Kitago T, Krakauer JW. Motor learning principles for neurorehabilitation. In: Barnes MP, Good DC, eds. Neurological Rehabilitation. New York: Elsevier; 2013. [DOI] [PubMed] [Google Scholar]
- 10.Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil Neural Repair 2012;26:923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernhardt J, Churilov L, Dewey H, et al. Statistical analysis plan (SAP) for a very early rehabilitation trial (AVERT): an international trial to determine the efficacy and safety of commencing out of bed standing and walking training (very early mobilisation) within 24 h of stroke onset vs usual stroke unit care. Int J Stroke 2015;10:23–24. [DOI] [PubMed] [Google Scholar]
- 12.James G, Witten D, Hastie T, Tibshirani R. An Introduction to Statistical Learning with Applications in R. 1st ed New York: Springer Science + Business Media; 2013:303. [Google Scholar]
- 13.Simondson J, Goldie P, Brock K, Nosworthy J. The mobility scale for acute stroke patients: intra-rater and inter-rater reliability. Clin Rehab 1996;10:295–300. [Google Scholar]
- 14.Bell JA, Wolke ML, Ortez RC, Jones TA, Kerr AL. Training intensity affects motor rehabilitation efficacy following unilateral ischemic insult of the sensorimotor cortex in C57BL/6 mice. Neurorehabil Neural Repair 2015;29:590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollock A, Baer G, Campbell P, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst Rev 2014;4:CD001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwakkel G. Impact of intensity of practice after stroke: issues for consideration. Disabil Rehabil 2006;28:823–830. [DOI] [PubMed] [Google Scholar]
- 17.Billinger SA, Arena R, Bernhardt J, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:1–22. [DOI] [PubMed] [Google Scholar]
- 18.Dromerick AW, Lang CE, Birkenmeirer RL, et al. Very early constraint-induced movement during stroke rehabilitation (VECTORS): a single-centre RCT. Neurology 2009;73:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernhardt J, Indredavik B, Langhorne P. When should rehabilitation begin after stroke? Int J Stroke 2013;8:5–7. [DOI] [PubMed] [Google Scholar]
- 20.Humm JL, Kozlowski DA, James DC, Gotts JE, Schallert T. Use-dependent exacerbation of brain damage occurs during an early post-lesion vulnerable period. Brain Res 1998;783:286–292. [DOI] [PubMed] [Google Scholar]
- 21.Egan K, Janssen H, Sena E, et al. Exercise reduces infarct volume and facilitates neurobehavioural recovery: a systematic review and meta-analysis of exercise models in ischaemic stroke. Neurorehabil Neural Repair 2014;28:800–812. [DOI] [PubMed] [Google Scholar]
- 22.Austin MW, Ploughman M, Glynn L, Corbett D. Aerobic exercise effects on neuroprotection and brain repair following stroke: a systematic review and perspective. Neurosci Res 2014;87:8–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.