Abstract
Objectives:
To determine incidence rates and risk factors of remote seizure after perinatal arterial ischemic stroke.
Methods:
We retrospectively identified a population-based cohort of children with perinatal arterial ischemic stroke (presenting acutely or in a delayed fashion) from a large Northern Californian integrated health care system. We determined incidence and predictors of a remote seizure (unprovoked seizure after neonatal period, defined as 28 days of life) by survival analyses, and measured epilepsy severity in those with active epilepsy (≥1 remote seizure and maintenance anticonvulsant treatment) at last follow-up.
Results:
Among 87 children with perinatal stroke, 40 (46%) had a seizure in the neonatal period. During a median follow-up of 7.1 years (interquartile range 3.2–10.5), 37 children had ≥1 remote seizure. Remote seizure risk was highest during the first year of life, with a 20% (95% confidence interval [CI] 13%–30%) cumulative incidence by 1 year of age, 46% (CI 35%–58%) by 5 years, and 54% (CI 41%–67%) by 10 years. Neonatal seizures increased the risk of a remote seizure (hazard ratio 2.8, CI 1.3–5.8). Children with neonatal seizures had a 69% (CI 48%–87%) cumulative incidence of remote seizure by age 10 years. Among the 24 children with active epilepsy at last follow-up, 8 (33%) were having monthly seizures despite an anticonvulsant and 7 (29%) were on more than one anticonvulsant.
Conclusions:
Remote seizures and epilepsy, including medically refractory epilepsy, are common after perinatal stroke. Neonatal seizures are associated with nearly 3-fold increased remote seizure risk.
Perinatal arterial ischemic stroke (AIS) occurs in 1 in 4,000 term births and is an important cause of childhood epilepsy.1 In some newborns, an ischemic brain injury is initially unrecognized and only diagnosed later during development when a hemiparesis becomes clinically apparent or a remote symptomatic seizure occurs. This subset often receives a diagnosis of presumed perinatal ischemic stroke (PPIS), which refers to a stroke presumed to have occurred in the perinatal period because of a chronic focal infarction on neuroimaging.2 Stroke that is clinically apparent in the neonatal period may differ from PPIS. Seizures during the neonatal period are perhaps the most notable factor differentiating these groups, and are reported in about 75%–90% of infants diagnosed with stroke during the perinatal period.3–5 Neonatal seizures of any cause may confer a long-term risk of postnatal epilepsy. An estimated 10%–20% of neonates with seizures from any cause will go on to have further seizures in childhood.6
Our objective was to measure the incidence rate and risk factors for a first remote seizure after perinatal AIS utilizing a previously identified population-based pediatric stroke cohort that included neonates presenting acutely with stroke and patients with PPIS. We hypothesized that seizures during the neonatal period were associated with a higher risk of remote seizure and epilepsy. We also describe the range of epilepsy severity after perinatal AIS by measuring medical encounters for seizures and the frequency of seizures and anticonvulsant usage at last follow-up.
METHODS
We performed a retrospective study of seizures after perinatal AIS within a population of 2.5 million children (<20 years of age) enrolled in Kaiser Permanente Northern California (KPNC), 1993–2007, with follow-up through 2011. From this population, a cohort of children diagnosed with symptomatic stroke was identified and confirmed through chart review for the Kaiser Pediatric Stroke Study as previously described.7–10 Briefly, stroke criteria were as follows: (1) clinical presentation consistent with stroke such as hemiparesis, encephalopathy, or seizures and (2) CT or MRI showing a focal ischemic infarct or hemorrhage in a location and of a maturity consistent with the neurologic signs and symptoms. For the purpose of this study, analyses were limited to cases of perinatal AIS (those that occurred or were presumed to have occurred between 28 weeks gestational age and 28 days of life). These included cases with stroke presentation in the neonatal period (≤28 days of life) and delayed presentation of PPIS (clinical presentation of stroke after 28 days of life).
Standard protocol approvals, registrations, and patient consents.
Institutional review boards at the University of California, San Francisco, and KPNC approved study procedures. Both institutional review boards approved waiver of informed consent for minimal risk research that could not practicably be carried out without the waiver.
Ascertainment and confirmation of outcomes.
Within the cohort, we electronically searched for ICD-9 codes related to seizure and epilepsy and prescriptions for anticonvulsant medications in KPNC inpatient, outpatient, and pharmacy databases to identify potential patients with seizures. KPNC electronic medical records include all outpatient and inpatient visits within KPNC and encounters at outside facilities, and all medications prescribed and filled are recorded in the KPNC electronic pharmacy database. Two child neurologists independently reviewed charts of all potential cases to confirm outcomes, with a third neurologist adjudicating in case of disagreement. Our primary outcome was a first unprovoked remote seizure, defined as an epileptic seizure occurring >28 days after birth not attributed to another provocation such as fever, acute systemic, metabolic, ischemic, or toxic insult, and excluding nonepileptic spells. A secondary outcome was active epilepsy, defined as at least one unprovoked remote seizure and ongoing anticonvulsant treatment at the time of the last follow-up.11,12 A child neurologist reviewed the available records to categorize epilepsy at last follow-up as focal epilepsy, generalized epilepsy, or epilepsy that could not be classified by the available documentation.
Data abstraction.
A single pediatric nurse professional medical record analyst abstracted demographic and clinical data from electronic and traditional medical records onto standardized forms. Race/ethnicity was self-described by parents. Infarct characteristics and location were determined by review of radiology reports. Neonatal seizures were either clinically suspected or EEG-confirmed seizures that occurred within the first 28 days of life.13 Risk factors for perinatal AIS were defined as the following, when documented in the medical record. Operational vaginal delivery included history of forceps delivery or vacuum-assisted delivery. Primiparity included the stroke patients who were the first live birth for the mother. Hypoxic-ischemic encephalopathy included hypoxic ischemic encephalopathy or birth asphyxia. Preeclampsia included history of preeclampsia or pregnancy-induced hypertension. Chorioamnionitis included chorioamnionitis, endometritis, or maternal fever (>101°F or 38.5°C) in the 24 hours prior to delivery. Prolonged rupture of membranes was rupture of membranes >24 hours prior to delivery. Prolonged second stage of labor was a second stage of labor >2 hours. Measures of epilepsy severity included frequency of seizures and number of maintenance anticonvulsants prescribed in the month prior to last available follow-up and total number of emergency medical encounters for seizure. A neurologist reviewed all abstracted data for accuracy. Follow-up time was calculated from stroke (assumed as the time of birth) for the patients with PPIS and the acutely recognized neonatal stroke groups, and is equivalent to the age at last follow-up for both groups.
Statistical analyses.
Statistical analyses were performed using Stata 14 (College Station, TX). We used descriptive statistics to compare baseline characteristics of children presenting with stroke in the neonatal period to those with PPIS. EEG results and neurodevelopmental outcomes were not available for many of the cohort; missingness for these variables was noted and the available data were described. To determine incidence rates, cumulative risk, and predictors of remote seizure and active epilepsy, we used survival analysis with time at risk beginning at 28 days after birth. The first unprovoked remote seizure was the failure event. Children without a remote seizure were right censored at death or the last follow-up available in the medical record. Cox proportional hazards models were compared to determine prespecified baseline univariable predictors associated with remote seizure, and used to address potential confounders in multivariable analysis. In our multivariable analysis, we first included all univariable predictors with p < 0.2 (using the log-rank test for statistical significance) then used backward selection to retain variables with p < 0.1. To evaluate the possibility of ascertainment bias, we performed sensitivity analyses excluding the 5 patients who were diagnosed with stroke because of a remote seizure. To determine the association of neonatal seizures with our secondary outcome, active epilepsy, we performed survival analyses in which the failure event was defined as the time of the first remote seizure among children with active epilepsy at last follow-up.11
RESULTS
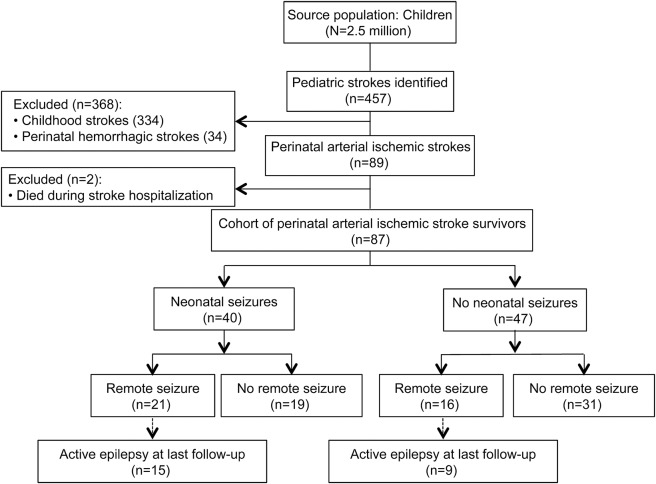
From an initial population of 2.5 million children, we identified 89 children with perinatal AIS (figure 1). Of these, 2 died during the acute stroke hospitalization, leaving a cohort of 87 stroke survivors for our analysis: 48 presenting in the neonatal period (median age at stroke presentation first day of life, interquartile range [IQR] 0–1 day) and 39 with delayed stroke presentation (median age at first presenting symptom of stroke 8 months, IQR 4–13 months). During the neonatal period, 40 had an acute symptomatic seizure (46% of the cohort, 83% of patients presenting with stroke in the neonatal period) (table 1). EEG reports were available for 22 patients, including 9 patients with electrographic seizures (table e-1 on the Neurology® Web site at Neurology.org).
Figure 1. Flow diagram of perinatal stroke cohort.
From a population of 2.5 million children enrolled in Kaiser Permanente Northern California, 1993–2007, survivors of perinatal arterial ischemic stroke were identified (n = 87). Over a median follow-up of 7.1 years, 37 of the children had ≥1 remote seizure.
Table 1.
Demographics and clinical characteristics of 87 patients with perinatal arterial ischemic stroke enrolled in Kaiser Permanente Northern California, 1993–2007
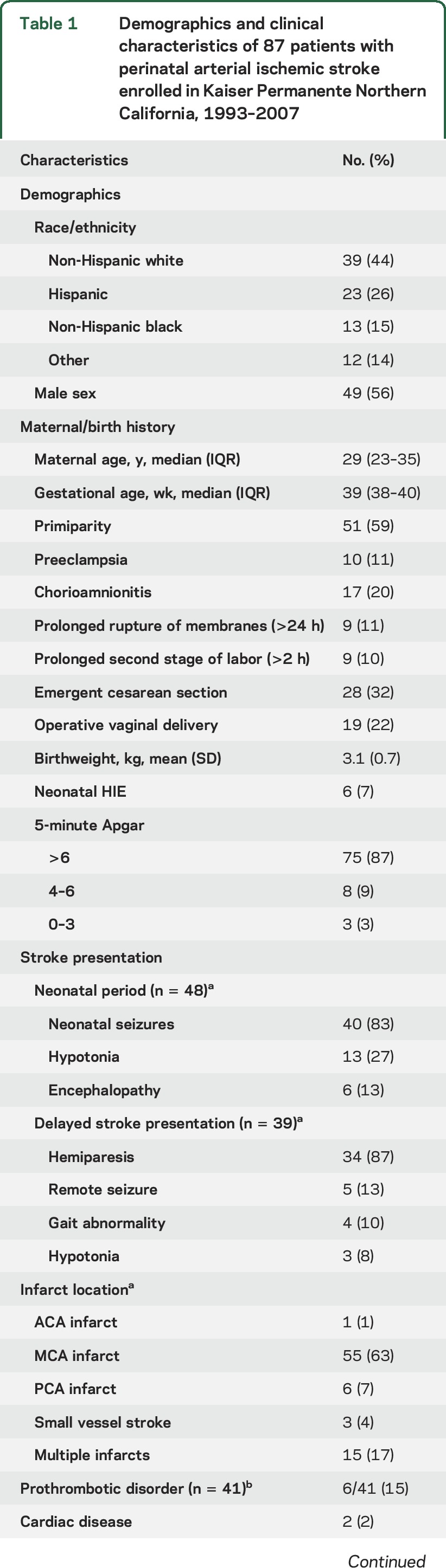
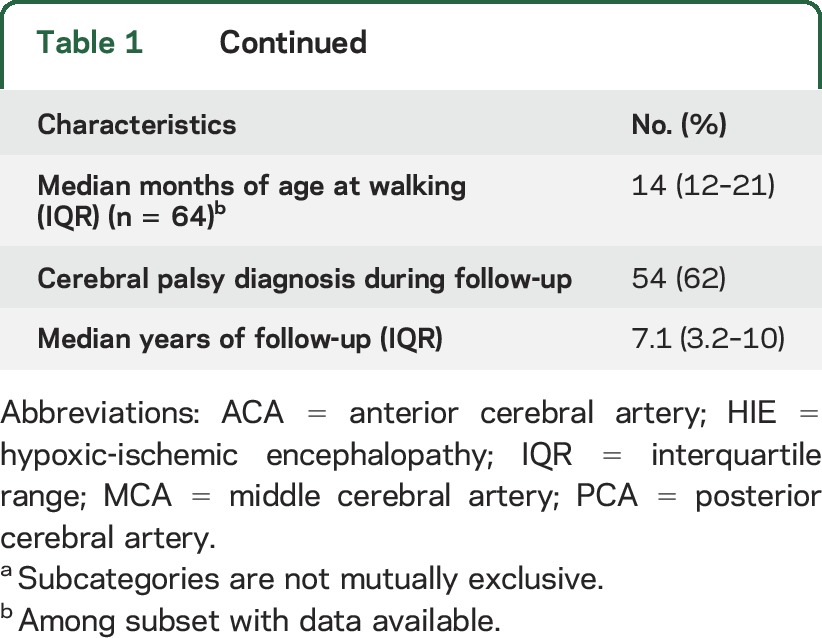
The stroke resulted in neurodevelopmental deficits in some children. Age at walking was available for 64 children (74%); among these the median was 14 months (IQR 12–21 months). In 54 children (62%), a physician diagnosis of cerebral palsy was recorded in the medical record. Children who presented with stroke during the neonatal period were more likely to have multiple infarcts (29% vs 3%, p = 0.001), were younger when they began walking (median age of 13 [IQR 10–17] vs 16 [IQR 13–22] months, p = 0.04), and were less likely to have a cerebral palsy diagnosis recorded (50% vs 85%, p = 0.001) (table e-2). Otherwise, demographics, birth history, and years of follow-up were similar between the 2 groups.
Incidence of remote seizures.
Median age at last follow-up was 7.1 years (IQR 3.2–10 years), with a total follow-up time of 333 person-years. During follow-up, 37 of the 87 children had at least one remote seizure (including 5 children with delayed stroke presentation who presented with a remote seizure). The annual incidence rate of a first unprovoked remote seizure was highest during the first year of life, with a cumulative incidence of 20% (95% confidence interval [CI] 13%–30%) by 12 months of age. The 5-year cumulative incidence of a first remote seizure was 46% (CI 35%–58%) and the 10-year cumulative incidence was 54% (CI 41%–67%) (figure 2).
Figure 2. Remote seizures among 87 children after perinatal arterial ischemic stroke.
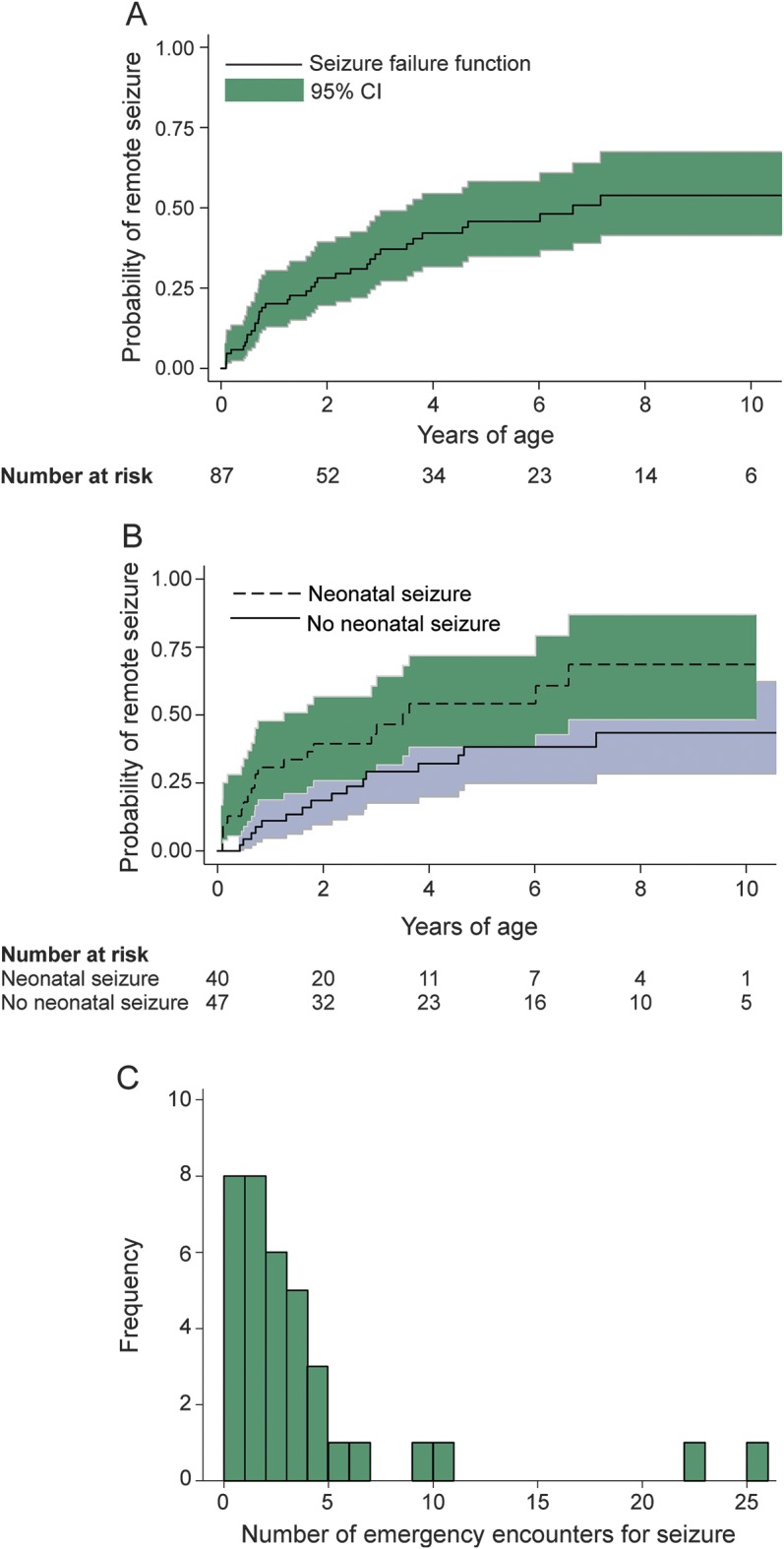
Kaplan-Meier failure plots demonstrate (A) the overall cumulative incidence of a first remote seizure (solid line represents the failure function) and (B) a 3-fold increased risk of remote seizure in children with a history of neonatal seizures (dashed line) compared to children with no neonatal seizures (solid line) (hazard ratio 2.8, 95% confidence interval [CI] 1.3–5.8, p = 0.03 for log-rank test for equality). In plots A and B, gray shading represents 95% confidence intervals and the x-axis represents time at risk beginning at 29 days of age. (C) During a median follow-up of 7.1 years, frequency of emergency medical encounters for seizure among the 37 children in the perinatal stroke cohort who had at least one remote seizure.
In sensitivity analyses that excluded the 5 children who were diagnosed with their stroke because of a remote seizure, delayed stroke presentation was associated with a decreased risk of remote seizure as a univariable predictor but not after multivariable adjustment (data not shown). Otherwise, the magnitude or point estimates of remote seizure predictors did not substantially change whether these children were included in or excluded from analyses.
Incidence of active epilepsy and measures of epilepsy severity.
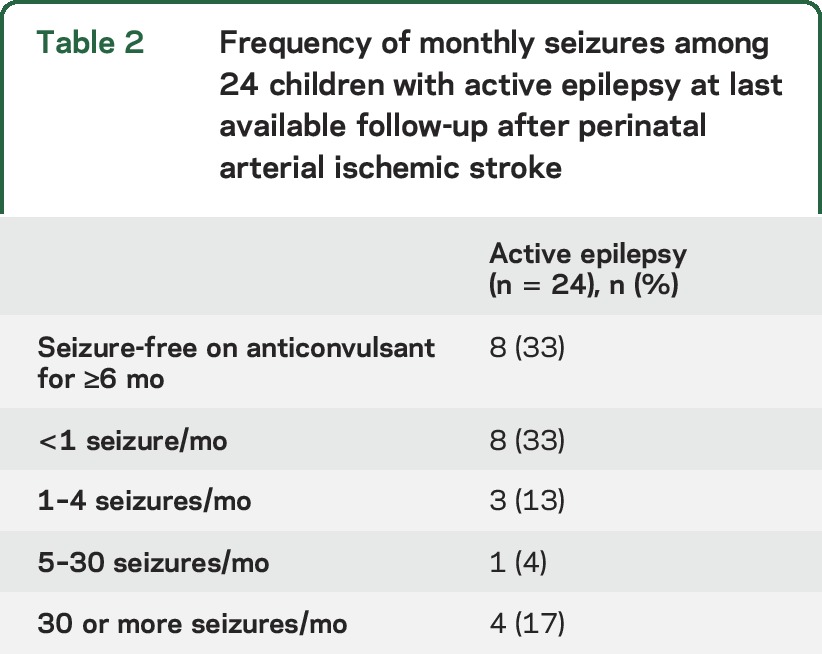
At the time of last follow-up, 24 children were on treatment with a maintenance anticonvulsant for active epilepsy, for a 10-year cumulative incidence of 40% (CI 27%–55%). Among the 24 patients with active epilepsy, 10 had focal epilepsy, 5 had generalized epilepsy syndrome, and the remaining 9 could not be classified. At the time of last follow-up, seizures were poorly controlled in 8 children (33% of those with epilepsy) who were having monthly seizures despite anticonvulsant medications (table 2). In 7 children (29% of those with epilepsy), seizures were treated with more than one concurrent maintenance anticonvulsant. Medical care for seizures was common among the stroke cohort. Emergency encounters for remote seizures were frequent (figure 2), 13 children were admitted to the hospital for a seizure at least once, and 4 children were admitted to the intensive care unit for status epilepticus. One child had status epilepticus with intubation on 3 separate encounters.
Table 2.
Frequency of monthly seizures among 24 children with active epilepsy at last available follow-up after perinatal arterial ischemic stroke
Predictors of remote seizure.
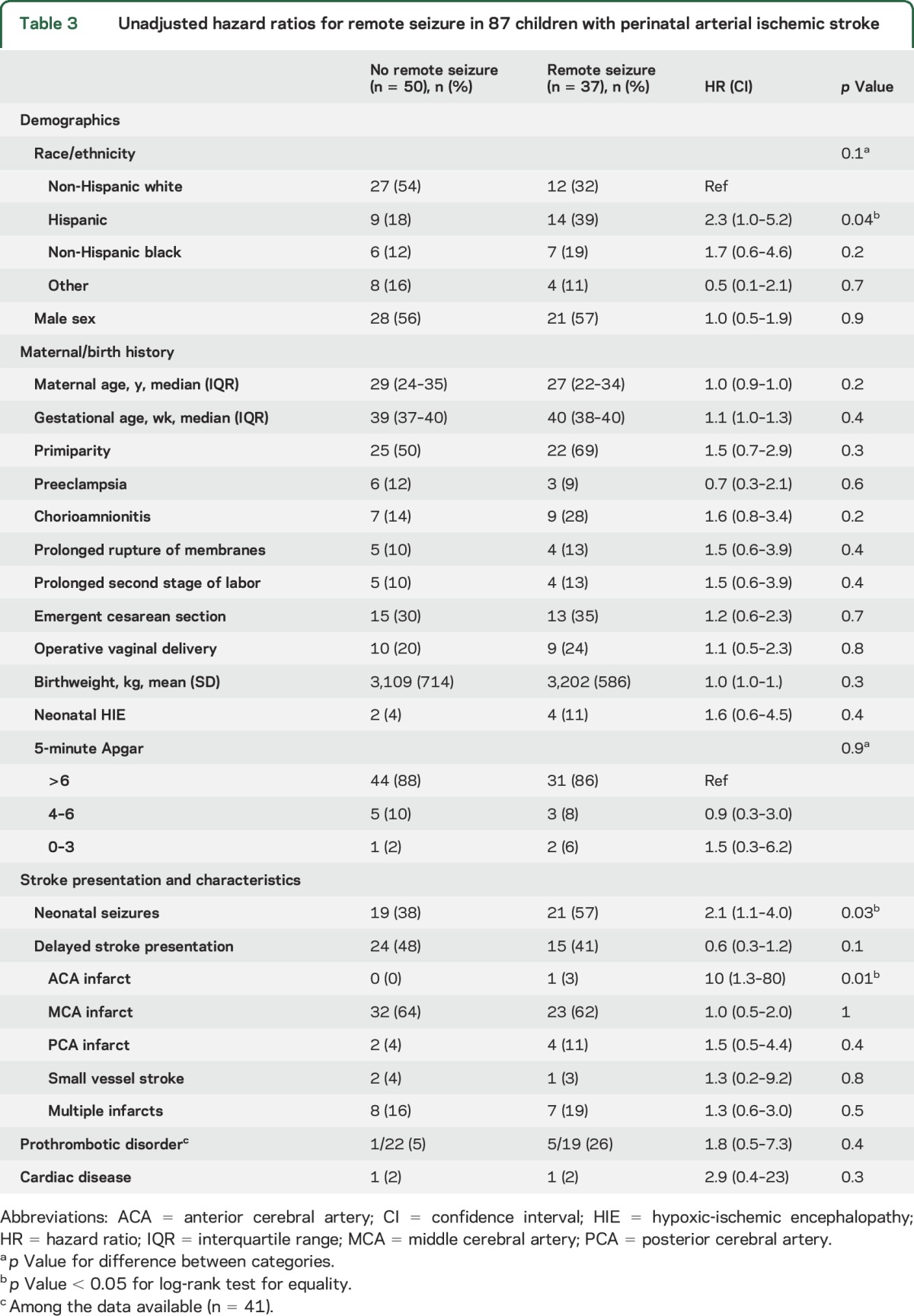
In univariable analyses, the risk of a first remote seizure was predicted by Hispanic ethnicity, neonatal seizures, and anterior cerebral artery infarcts (table 3). In multivariable analyses including race/ethnicity, chorioamnionitis, delayed stroke presentation, neonatal seizures, and anterior cerebral artery infarct distribution, only neonatal seizures (hazard ratio [HR] 2.0, CI 1.03–3.9) remained predictive of remote seizures. Neonatal seizures also predicted active epilepsy (HR 2.6, 95% CI 1.1–6.1, p = 0.02). Among children with a history of neonatal seizures, the 10-year cumulative incidence of a first remote seizure was 69% (CI 48%–87%) (figure 2) and of active epilepsy 54% (CI 32%–79%).
Table 3.
Unadjusted hazard ratios for remote seizure in 87 children with perinatal arterial ischemic stroke
DISCUSSION
In this population-based study, we found that remote seizures and epilepsy were common outcomes of perinatal stroke. By the end of the first decade of life, 54% of the overall perinatal stroke cohort had at least one remote seizure and 40% had developed active epilepsy. We included children with perinatal stroke whether they presented acutely in the neonatal period or in a delayed fashion with PPIS. Children with PPIS were less likely to have multiple infarcts and none had neonatal seizures. Consistent with prior studies, children with PPIS also tended to have worse motor outcomes, possibly related to ascertainment bias.14,15 Our epilepsy incidence rates are at the upper end of the range reported in prior studies. Among patients presenting with stroke during the neonatal period, studies have reported 9%–40% of children develop epilepsy by 2–4 years of age.4,5,16,17 Among children with delayed presentation of presumed perinatal ischemic stroke, estimates of epilepsy range from 38% to 55%.18,19 The broad ranges reported across prior studies may be related to modest sample sizes, differences in study setting, varying definitions for epilepsy, or methods of outcome ascertainment.
We found that neonatal seizures nearly tripled the risk of a remote seizure. Children with seizures during the neonatal period related to stroke may be at higher ongoing seizure risk than previously recognized: two-thirds had at least one remote seizure and over half developed active epilepsy during the first decade of life. Presumably, some of the infants with neonatal seizures were discharged home on an anticonvulsant, delaying onset of the first remote seizure. This would attenuate the difference in remote seizure incidence rate between the infants with neonatal seizures and those without neonatal seizures during early infancy, but would be unlikely to have ongoing effect over a 7-year follow-up. In contrast to our findings, a prior study of perinatal AIS did not find an association of acute seizures at stroke ictus with seizures later in childhood, but may have been underpowered with 46 children and a shorter average follow-up (mean 31 months).5 Seizures in the neonatal period previously have been associated with epilepsy in clinical studies of children with cerebral palsy20 and term newborns with encephalopathy, even after controlling for severity of initial brain injury.21 Animal models of neonatal brain injury also support an association between neonatal seizures and epilepsy.22–24
Whether seizures in the setting of an acute perinatal stroke have a deleterious effect on developing brain or are simply a marker for greater underlying brain injury is unknown, but the association with childhood epilepsy is worrisome. An active seizure focus in the setting of acute stroke could increase metabolic demand in ischemic penumbra and worsen brain injury, or the presence of neonatal seizures may contribute to epileptogenesis during a crucial developmental window.22,23 In animal models of pediatric stroke, early poststroke seizures are associated with disturbed brain metabolism, secondary excitotoxicity, and increasing size of infarcts.25,26 In adults, early poststroke seizures are a risk factor for increasing midline shift after hemorrhagic stroke27 and mortality after ischemic stroke.28 Therapeutic hypothermia for treatment of neonatal hypoxic ischemic encephalopathy may reduce metabolic demand and also reduce neonatal seizures,29 but whether this changes epilepsy risk is unknown. We did not assess for therapeutic hypothermia, but it is unlikely that this treatment played a large role in preventing neonatal seizures for our cohort. Only 6 of the 87 children with stroke also had neonatal hypoxic ischemic encephalopathy, and 5 of these children had neonatal seizures.
In our cohort, remote seizures were not benign. Children with perinatal stroke often had multiple emergency visits and hospital admissions for remote seizures, and more than a quarter of the children were on a maintenance anticonvulsant at their last observed follow-up. Although the majority of children with poststroke epilepsy in our cohort had relatively good seizure control, a third of the children with poststroke epilepsy were having at least monthly seizures despite anticonvulsant treatment, suggesting medically refractory epilepsy. Even a single seizure in children has been associated with a negative psychological effects and a decreased health-related quality of life.30 Medically refractory epilepsy, multiple anticonvulsants, and comorbid neurologic impairments can further worsen quality of life.31 It is possible that the seizures and epilepsy were not related to the stroke itself but rather an underlying genetic disorder or other brain injury such as neonatal hypoxic ischemic encephalopathy. We could not assess the concordance among neonatal seizure semiology, remote seizure semiology, neurodevelopment, clinical examination findings, EEG findings, and MRI findings because of the retrospective nature of our study. However, these data emphasize the need for further research in this area and may be valuable for accurately counseling families.
Our study has several strengths that contribute to more accurate measures of remote seizure incidence and greater generalizability than previously available: a large, population-based cohort, a long median follow-up time of 7 years, and searchable electronic health records including electronic pharmacy records for anticonvulsant prescriptions. Studies with a convenience sample of children identified from a tertiary care base might be biased towards inclusion of sicker children with more severe strokes who in turn might be more likely to develop epilepsy. In our study, incidence rate for a first remote seizure did not level off until the end of the first decade, so prior studies with a limited duration of follow-up might underestimate the frequency of remote seizures. In addition, the simple proportions reported in some studies may not account for variable lengths of follow-up.
Our study has limitations. First, infarct characteristics were determined by radiology report rather than source neuroimaging. Larger stroke size has been associated with an increased risk of remote seizures after perinatal AIS.5 We could not measure and adjust for infarct volume, which may influence both neonatal and remote seizure risk. Second, it is possible that some neonatal seizures in our cohort may have been missed. In neonates with acute AIS, an estimated 80% of seizures identified on continuous EEG may be clinically unsuspected.32 Misclassification of exposure to neonatal seizures may result in an underestimate of the magnitude of epilepsy risk associated with neonatal seizures. Third, our measures of epilepsy severity reflect the clinical care that was provided. We could not accurately determine anticonvulsant adherence or the appropriateness of specific anticonvulsant treatment. Our measures of epilepsy severity may not be applicable for individual prognostication, especially as epilepsy treatment options continue to improve.
Childhood seizures and epilepsy are common among children who have had a perinatal AIS. Although the risk of an unprovoked remote seizure is highest during the first year of life, the cumulative incidence continues to rise over the first decade. Acute seizures related to stroke in the neonatal period nearly triple the risk of remote seizures. Currently, the risk vs benefit of aggressive medical treatment of neonatal seizures is controversial, in part because the long-term clinical significance of these acute seizures is not clear. Ultimately, understanding the significance of acute symptomatic seizures in neonates will help clinicians to better their tailor management. Families of children with perinatal stroke should be counseled about the risk of future seizures, especially if the child has a history of neonatal seizures.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Barbara Rowe for medical record data abstraction.
GLOSSARY
- AIS
arterial ischemic stroke
- CI
confidence interval
- HR
hazard ratio
- ICD-9
International Classification of Diseases–9
- IQR
interquartile range
- KPNC
Kaiser Permanente Northern California
- PPIS
presumed perinatal ischemic stroke
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Christine Fox: contributed to the design and conceptualization of the study, analyses and interpretation of the data, and drafting and revising the manuscript. Hannah Glass: contributed to the acquisition, analyses and interpretation of data, and revision of the manuscript. Stephen Sidney: contributed to the conception and design of the study, interpretation of the data, and revision of the manuscript. Sabrina Smith: contributed to interpretation of the data and revision of the manuscript. Heather Fullerton: contributed to the design and conceptualization of the study, interpretation of the data, and revision of the manuscript.
STUDY FUNDING
Supported by NIH (grants K02 NS053883, 2K12NS001692-11, K23NS066137, KL2TR000143) and the Pediatric Epilepsy Research Foundation and the Neonatal Brain Research Institute at UCSF. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
DISCLOSURE
C. Fox is funded by NIH grants (2K12NS001692-11 and KL2TR000143) and receives royalties from published UpToDate pediatric stroke topics. H. Glass is funded by the NIH (grant K23NS066137), the Pediatric Epilepsy Research Foundation, and the Neonatal Brain Research Institute at UCSF. S. Sidney and S. Smith report no disclosures relevant to the manuscript. H. Fullerton is funded by NIH grants (K02 NS053883 and 1RO1NS062820). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lynch JK, Nelson KB. Epidemiology of perinatal stroke. Curr Opin Pediatr 2001;13:499–505. [DOI] [PubMed] [Google Scholar]
- 2.Raju TN, Nelson KB, Ferriero D, Lynch JK. Ischemic perinatal stroke: summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics 2007;120:609–616. [DOI] [PubMed] [Google Scholar]
- 3.Kirton A, Armstrong-Wells J, Chang T, et al. Symptomatic neonatal arterial ischemic stroke: the International Pediatric Stroke Study. Pediatrics 2011;128:e1402–1410. [DOI] [PubMed] [Google Scholar]
- 4.Golomb MR, Garg BP, Carvalho KS, Johnson CS, Williams LS. Perinatal stroke and the risk of developing childhood epilepsy. J Pediatr 2007;151:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wusthoff CJ, Kessler SK, Vossough A, et al. Risk of later seizure after perinatal arterial ischemic stroke: a prospective cohort study. Pediatrics 2011;127:e1550–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uria-Avellanal C, Marlow N, Rennie JM. Outcome following neonatal seizures. Semin Fetal Neonatal Med 2013;18:224–232. [DOI] [PubMed] [Google Scholar]
- 7.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics 2007;119:495–501. [DOI] [PubMed] [Google Scholar]
- 8.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Recurrent hemorrhagic stroke in children: a population-based cohort study. Stroke 2007;38:2658–2662. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong-Wells J, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Prevalence and predictors of perinatal hemorrhagic stroke: results from the Kaiser Pediatric Stroke Study. Pediatrics 2009;123:823–828. [DOI] [PubMed] [Google Scholar]
- 10.Jordan LC, Johnston SC, Wu YW, Sidney S, Fullerton HJ. The importance of cerebral aneurysms in childhood hemorrhagic stroke: a population-based study. Stroke 2009;40:400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox CK, Glass HC, Sidney S, Lowenstein DH, Fullerton HJ. Acute seizures predict epilepsy after childhood stroke. Ann Neurol 2013;74:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thurman DJ, Beghi E, Begley CE, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia 2011;52:2–26. [DOI] [PubMed] [Google Scholar]
- 13.Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. J Pediatr 1999;134:71–75. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Croen LA, Lindan C, et al. Predictors of outcome in perinatal arterial stroke: a population-based study. Ann Neurol 2005;58:303–308. [DOI] [PubMed] [Google Scholar]
- 15.Wu YW, March WM, Croen LA, Grether JK, Escobar GJ, Newman TB. Perinatal stroke in children with motor impairment: a population-based study. Pediatrics 2004;114:612–619. [DOI] [PubMed] [Google Scholar]
- 16.Suppiej A, Mastrangelo M, Mastella L, et al. Pediatric epilepsy following neonatal seizures symptomatic of stroke. Brain Dev 2016;38:27–31. [DOI] [PubMed] [Google Scholar]
- 17.Grunt S, Mazenauer L, Buerki SE, et al. Incidence and outcomes of symptomatic neonatal arterial ischemic stroke. Pediatrics 2015;135:e1220–e1228. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald KC, Williams LS, Garg BP, Golomb MR. Epilepsy in children with delayed presentation of perinatal stroke. J Child Neurol 2007;22:1274–1280. [DOI] [PubMed] [Google Scholar]
- 19.Wanigasinghe J, Reid SM, Mackay MT, Reddihough DS, Harvey AS, Freeman JL. Epilepsy in hemiplegic cerebral palsy due to perinatal arterial ischaemic stroke. Dev Med Child Neurol 2010;52:1021–1027. [DOI] [PubMed] [Google Scholar]
- 20.Bruck I, Antoniuk SA, Spessatto A, Bem RS, Hausberger R, Pacheco CG. Epilepsy in children with cerebral palsy. Arq Neuropsiquiatr 2001;59:35–39. [DOI] [PubMed] [Google Scholar]
- 21.Glass HC, Hong KJ, Rogers EE, et al. Risk factors for epilepsy in children with neonatal encephalopathy. Pediatr Res 2011;70:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter BE. Neurogenesis and epilepsy in the developing brain. Epilepsia 2008;49(suppl 5):50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Ari Y, Holmes GL. Effects of seizures on developmental processes in the immature brain. Lancet Neurol 2006;5:1055–1063. [DOI] [PubMed] [Google Scholar]
- 24.Dudek FE, Pouliot WA, Rossi CA, Staley KJ. The effect of the cannabinoid-receptor antagonist, SR141716, on the early stage of kainate-induced epileptogenesis in the adult rat. Epilepsia 2010;51:126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comi AM, Trescher WH, Abi-Raad R, Johnston MV, Wilson MA. Impact of age and strain on ischemic brain injury and seizures after carotid ligation in immature mice. Int J Dev Neurosci 2009;27:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Traa BS, Mulholland JD, Kadam SD, Johnston MV, Comi AM. Gabapentin neuroprotection and seizure suppression in immature mouse brain ischemia. Pediatr Res 2008;64:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vespa PM, O'Phelan K, Shah M, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology 2003;60:1441–1446. [DOI] [PubMed] [Google Scholar]
- 28.Goswami RP, Karmakar PS, Ghosh A. Early seizures in first-ever acute stroke patients in India: incidence, predictive factors and impact on early outcome. Eur J Neurol 2012;19:1361–1366. [DOI] [PubMed] [Google Scholar]
- 29.Harbert MJ, Tam EW, Glass HC, et al. Hypothermia is correlated with seizure absence in perinatal stroke. J Child Neurol 2011;26:1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modi AC, King AS, Monahan SR, Koumoutsos JE, Morita DA, Glauser TA. Even a single seizure negatively impacts pediatric health-related quality of life. Epilepsia 2009;50:2110–2116. [DOI] [PubMed] [Google Scholar]
- 31.Miller V, Palermo TM, Grewe SD. Quality of life in pediatric epilepsy: demographic and disease-related predictors and comparison with healthy controls. Epilepsy Behav 2003;4:36–42. [DOI] [PubMed] [Google Scholar]
- 32.Low E, Mathieson SR, Stevenson NJ, et al. Early postnatal EEG features of perinatal arterial ischaemic stroke with seizures. PLoS One 2014;9:e100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.