Abstract
Background
The aim of this study was to investigate the expression and clinical implications of proteins related to serine/glycine metabolism in different subtypes of thyroid cancer.
Methods
Tissue microarray (TMA) was constructed with tissues from 557 thyroid cancers, consisting of 244 papillary thyroid carcinomas (PTC), 112 follicular carcinomas (FC), 70 medullary carcinomas (MC), 23 poorly differentiated carcinomas (PDC), and 8 anaplastic carcinomas (AC). Immunohistochemical staining of the serine/glycine metabolism-related molecules phosphoglycerate dehydrogenase (PHGDH), phosphoserine aminotransferase, (PSAT), phosphoserine phosphatase (PSPH), serine hydromethyl transferase (SHMT), and glycine decarboxylase (GLDC) was performed with the TMA blocks and the results were analyzed together with clinicopathologic parameters.
Results
The expression of serine/glycine metabolism-related proteins differed among thyroid cancer subtypes. The expression rate of PHGDH (p < 0.001), PSAT1 (p = 0.001), PSPH (p = 0.008), and tumoral SHMT1 (p < 0.001) was higher in PDC and PTC (78.3, 21.7, 21.7, 30.4 and 63.4, 18.6, 12.8, 31.4 %, respectively), and lowest in MC (15.7, 1.4, 0.0, 10.0 %). Stromal SHMT1 expression was highest in AC (62.5 %) and absent in all FC (p < 0.001). In PTC, positivity for PSPH (p = 0.041), tumoral SHMT1 (p = 0.018), and stromal SHMT1 (p < 0.001) expression was higher in the conventional type compared to follicular type (14.1 versus 2.5 %, 33.6 versus 15.0 %, 42.1 versus 10.0 %, respectively). BRAF V600E mutation was associated with a higher rate of PHGDH (p < 0.001), PSAT1 (p = 0.001), PSPH (p < 0.001), tumoral SHMT1 (p = 0.001), stromal SHMT1 (p < 0.001), and GLDC (p < 0.001) expression compared to non-mutant cases (73.5 versus 40.6 %, 23.1 versus 8.5 %, 17.6 versus 1.9 %, 37.0 versus 18.9 %, 45.8 versus 21.7 %, 21.8 versus 6.6 %, respectively). In univariate analysis, stromal SHMT1 expression was associated with shorter disease-free survival (p = 0.015) in follicular variant PTC, and GLDC positivity was associated with shorter overall survival (OS) in sclerotic stromal type (p = 0.002). In FC, minimally invasive type, PSPH positivity correlated with shorter OS (p = 0.045) and in MC, PHGDH positivity correlated with shorter OS (p = 0.034).
Conclusion
The expression of serine/glycine metabolism-related proteins differs among different thyroid cancer types, with a higher rate of expression in PDC and PTC, and lower rate of expression in MC. In PTC, the rate of expression is lower in the follicular variant and higher in cases with BRAF V600E mutation.
Electronic supplementary material
The online version of this article (doi:10.1186/s12967-016-0915-8) contains supplementary material, which is available to authorized users.
Keywords: Glycine, Serine, Metabolism, Thyroid cancer
Background
In malignant neoplasms a metabolic shift from oxidative phosphorylation in mitochondria to glycolysis occurs, a phenomenon known as the Warburg effect [1]. Neoplastic cells exhibiting increased glycolysis show increased levels of glycolytic intermediates. Recent studies reported that the metabolism of glycolytic intermediates is involved in tumorigenesis, for example through the glycine and serine metabolic pathways [2–5]. In the serine biosynthesis pathway, the 3-phosphoglycerate (3PG) that is generated in the glycolysis process is oxidized to 3-phosphohydroxypyruvate (pPYR) by phosphoglycerate dehydrogenase (PHGDH) [6]. pPYR is transaminated into phosphoserine (pSER) by phosphoserine aminotransferase (PSAT) [7], and pSER is dephosphorylated by phosphoserine phosphatase (PSPH) to produce serine [8]. In glycine metabolism, methylene-tetrahydrofolate is generated by glycine decarboxylase (GLDC), using glycine and tetrahydrofolate as substrates [9]. Serine metabolism and glycine metabolism are linked by serine hydromethyl transferase (SHMT), which catalyzes the reversible conversion of serine and glycine [10]. In previous studies, expression of PHGDH was reported to be increased in breast cancer and melanoma [3, 4], and expression of GLDC was reported to be increased in lung cancer [5], revealing a role of proteins related to serine/glycine metabolism in tumorigenesis.
Thyroid cancer is a common malignant neoplasm that occurs in 1 % of the population [11]. The most common subtype is papillary thyroid carcinoma (PTC), although there are several other subtypes, including follicular carcinoma (FC), medullary carcinoma (MC), poorly differentiated carcinoma (PDC), and anaplastic carcinoma (AC). These subtypes differ in cell origin, clinical manifestation, metastatic pattern, and clinical prognosis [12].
A representative mutation gene in thyroid cancer is the BRAF mutation gene. BRAF is a 75–100 kDa protein in the Raf kinase family that is the most potent activator of MEK kinase in the Ras-Raf-MEK-ERK pathway [2, 5]. The Ras-Raf-MEK-ERK pathway is abnormally activated in tumors and this aberrant ERK signaling is due to the BRAF mutation [4]. The BRAF mutation first reported in 2002 is a mutation in nucleotide 1796 in about 90 % that converts valine to glutamic acid in codon 599 (V599E; After NOMENCLATURE CHANGE, it was renamed to V600E) [13]. The BRAF V600E mutation appears in a variety of tumors found in cancers including thyroid papillary carcinoma (36–53 %), malignant melanoma (40–70 %), colorectal carcinoma (5–22 %), glioma (11 %), and ovary serous carcinoma (30 %) [3]. The BRAF mutation in PTC is associated with extra-thyroidal extension, advanced TNM stage, lymph node metastasis, multifocality, and recurrence [14].
It is thought that tumor metabolism, including serine and glycine metabolism, may differ among these subtypes, however there are few reports on this subject. The aim of this study was to investigate the expression and clinical implications of proteins related to serine/glycine metabolism according to thyroid cancer subtype.
Methods
Patient selection
Patients who were diagnosed with PTC and had surgical resection from January 2012 to December 2013 were enrolled in this study. Patients who were diagnosed with other subtypes of thyroid cancer from January 2000 to December 2014 were also enrolled. Patients who received preoperative chemotherapy were excluded. A thyroid pathologist (Koo JS) reviewed hematoxylin/eosin (H&E)-stained slides for all cases. Clinicopathologic data were obtained from the patients’ medical records and included age at diagnosis, disease recurrence, metastasis, current status, and length of follow-up. Tumor size, location (right or left lobe), extent (confined to the thyroid parenchyme or with extrathyroidal spread), and number of metastatic lymph nodes were also noted from review of the slides and the surgical pathology reports.
Tissue microarray (TMA)
Representative areas were selected on H&E-stained slides and corresponding spots were marked on the surface of the matching paraffin blocks. Five-mm core biopsies were taken from selected areas and placed into a 5 × 4 recipient block. More than two tissue cores were extracted from each case to minimize extraction bias. Each tissue core was assigned a unique tissue microarray location number that was linked to a database containing other clinicopathologic data.
Immunohistochemistry
Antibodies used for immunohistochemistry are listed in Additional file 1: Table S1. All immunohistochemistry was performed with formalin-fixed paraffin-embedded tissue sections using an automatic immunohistochemistry staining device (Benchmark XT, Ventana Medical System, Tucson, AZ, USA). Briefly, 5-µm thick formaldehyde-fixed paraffin-embedded tissue sections were transferred onto adhesive slides and dried at 62 °C for 30 min. Standard heat epitope retrieval was performed for 30 min in ethylene diaminetetraacetic acid, pH 8.0, in the autostainer. The samples were then incubated sequentially with primary antibodies, biotinylated anti-mouse immunoglobulin, peroxidase-labeled streptavidin (LSAB kit, DakoCytomation), and 3,30-diaminobenzidine. Negative control samples were processed without the primary antibody. Slides were counterstained with Harris hematoxylin. Positive control tissue was used as recommended by the manufacturer.
Analysis of immunohistochemical staining
This study used the semi-quantitative ordinal scoring system based on visual inspection by pathologists. Immunohistochemical markers were assessed by light microscopy. The expression of serine/glycine metabolism-related proteins was evaluated semi-quantitatively as previously reported [15]. Tumor and stromal cell staining was scored as follows: 0, negative or weak immunostaining in <1 % of the tumor/stroma; 1, focal expression in 1–10 % of tumor/stroma; 2, positive in 11–50 % of tumor/stroma; 3, positive in 51–100 % of tumor/stroma. The evaluation was performed throughout the whole area of the tumor, and a score of 2 or higher was defined as positive. For the evaluation of BRAF V600E, tissue samples that had scores of 20 % or higher were regarded as positive [16]. Two pathologists (KJS and KHM) independently evaluated the expression according the scoring system and any mismatched result was further evaluated by a third pathologist (JW).
Statistical analysis
Data were analyzed using SPSS for Windows, Version 12.0 (SPSS Inc., Chicago, IL, USA). For determination of statistical significance, Student’s t and Fisher’s exact tests were used for continuous and categorical variables, respectively. In the case of multiple comparisons, a corrected p value with the application of the Bonferroni multiple comparison procedure was used. Statistical significance was set to p < 0.05. Kaplan–Meier survival curves and log-rank statistics were employed to evaluate time to tumor recurrence and overall survival. Multivariate regression analysis was performed using the Cox proportional hazards model.
Results
Basal characteristics of thyroid cancer
Among 557 thyroid cancers, 344 (61.8 %) were PTC, 112 (20.1 %) were FC, 70 (12.6 %) were MC, 23 (4.1 %) were PDC, and 8 (1.4 %) were AC. Basal characteristics of PTC are summarized in Additional file 1: Table S2. PTC cases consisted of 304 (88.4 %) cases of conventional type and 40 (11.6 %) cases of follicular variant. Follicular variant PTC had a higher rate of expanding tumor margin (p = 0.002) than conventional type (32.5 versus 13.8 %). BRAF V600E mutation was present in 238 of PTCs (69.2 %) and was associated with an infiltrative tumor margin (87.8 %, p = 0.004) and conventional type (93.7 %, p < 0.001, Additional file 1: Table S2). The rate of follicular variant was only 6.3 % among cases with BRAF V600E. Follicular carcinoma consisted of 99 (88.4 %) cases of minimally invasive type and 13 (11.6 %) cases of widely invasive type, and the widely invasive type was associated with larger tumor size (>2.0 cm 100 versus 65.7 %, p = 0.040), vascular invasion (69.2 versus 37.4 %, p = 0.028), extrathyroidal involvement (53.8 versus 10.1 %, p < 0.001), and distant metastasis (38.5 versus 6.1 %, p = 0.003) compared to minimally invasive type (Additional file 1: Table S3). Basal characteristics of MC, PDC, and AC are summarized in Additional file 1: Table S4.
Expression of serine/glycine metabolism-related proteins in thyroid cancer subtypes
The expression of serine/glycine metabolism-related proteins was investigated in all thyroid cancers. For the serine/glycine metabolism-related proteins, PHGDH had the highest expression rate (54.8 %) compared to lower expression rates of stromal SHMT1 (25.9 %), tumoral SHMT1 (25.5 %), GLDC (15.6 %), PSAT1 (14.4 %), and PSPH (10.8 %). Therefore, serine/glycine metabolism-related protein expression rate was low in most cases except for PHGDH (Table 1). The expression of PHGDH (p < 0.001), PSAT1 (p = 0.001), PSPH (p = 0.008), tumoral SHMT1 (p < 0.001), and stromal SHMT1 (p < 0.001) was different according to cancer subtype (Fig. 1a). The expression of PHGDH, PSAT1, PSPH, and tumoral SHMT1 was higher in PDC and PTC (78.3, 21.7, 21.7, 30.4 and 63.4, 18.6, 12.8, 31.4 %, respectively), and lowest in MC (15.7, 1.4, 0.0, 10.0 %). The expression of stromal SHMT1 was highest in AC (62.5 %) and lowest in FC (0.0 %) (Table 1; Fig. 2).
Table 1.
Expression of serine/glycine metabolism-related proteins according to the subtype of thyroid cancer
| Parameters | Total n = 557 (%) | PTC n = 344 (%) | FC n = 112 (%) | MC n = 70 (%) | PDC n = 23 (%) | AC n = 8 (%) | P value |
|---|---|---|---|---|---|---|---|
| PHGDH in tumor | <0.001 | ||||||
| Negative | 252 (45.2) | 126 (36.6) | 58 (51.8) | 59 (84.3) | 5 (21.7) | 4 (50.0) | |
| Positive | 305 (54.8) | 218 (63.4) | 54 (48.2) | 11 (15.7) | 18 (78.3) | 4 (50.0) | |
| PSAT1 in tumor | 0.001 | ||||||
| Negative | 477 (85.6) | 280 (81.4) | 103 (92.0) | 69 (98.6) | 18 (78.3) | 7 (87.5) | |
| Positive | 80 (14.4) | 64 (18.6) | 9 (8.0) | 1 (1.4) | 5 (21.7) | 1 (12.5) | |
| PSPH in tumor | 0.008 | ||||||
| Negative | 497 (89.2) | 300 (87.2) | 101 (90.2) | 70 (100.0) | 18 (78.3) | 8 (100.0) | |
| Positive | 60 (10.8) | 44 (12.8) | 11 (9.8) | 0 (0.0) | 5 (21.7) | 0 (0.0) | |
| SHMT1 in tumor | <0.001 | ||||||
| Negative | 415 (74.5) | 236 (68.6) | 94 (83.9) | 63 (90.0) | 16 (69.6) | 6 (75.0) | |
| Positive | 142 (25.5) | 108 (31.4) | 18 (16.1) | 7 (10.0) | 7 (30.4) | 2 (25.0) | |
| SHMT1 in stroma | <0.001 | ||||||
| Negative | 413 (74.1) | 212 (61.6) | 112 (100.0) | 65 (92.9) | 21 (91.3) | 3 (37.5) | |
| Positive | 144 (25.9) | 132 (38.4) | 0 (0.0) | 5 (7.1) | 2 (8.7) | 5 (62.5) | |
| GLDC in tumor | 0.529 | ||||||
| Negative | 470 (84.4) | 285 (82.8) | 96 (85.7) | 60 (85.7) | 21 (91.3) | 8 (100.0) | |
| Positive | 87 (15.6) | 59 (17.2) | 16 (14.3) | 10 (14.3) | 2 (8.7) | 0 (0.0) | |
PTC papillary thyroid carcinoma; FC follicular carcinoma; MC medullary carcinoma; PDC poorly differentiated carcinoma; AC anaplastic carcinoma; PHGDH phosphoglycerate dehydrogenase; PSAT phosphoserine aminotransferase; PSPH phosphoserine phosphatase; SHMT serine hydromethyl transferase; GLDC glycine decarboxylase
Italic values represent p < 0.05
Fig. 1.
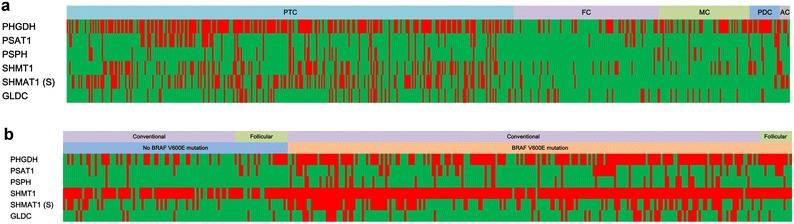
Heat map of expression of serine/glycine metabolism-related proteins in thyroid cancer (a) and papillary thyroid cancer (b). Red, positive; green, negative, PTC papillary thyroid carcinoma; FC follicular carcinoma; MC medullary carcinoma; PDC poorly differentiated carcinoma; AC anaplastic carcinoma; PHGDH phosphoglycerate dehydrogenase; PSAT phosphoserine aminotransferase; PSPH phosphoserine phosphatase; SHMT serine hydromethyl transferase; GLDC glycine decarboxylase; S stroma
Fig. 2.
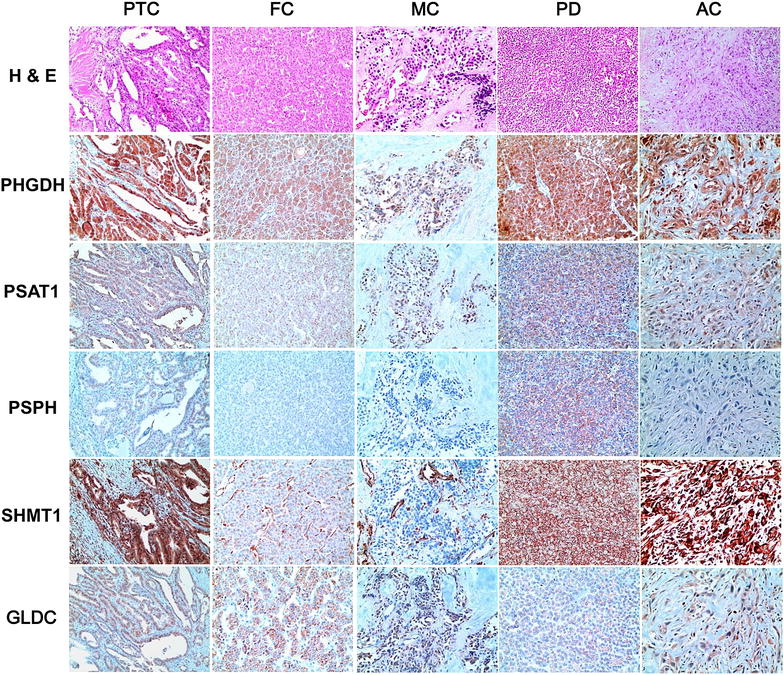
Expression of serine/glycine metabolism-related proteins in thyroid cancer. The expression of PHGDH, PSAT1, PSPH, tumoral SHMT1, and stromal SHMT1 is different according to cancer subtype. The expression of PHGDH, PSAT1, PSPH, and tumoral SHMT1 is higher in PDC and PTC, and lowest in MC. The expression of stromal SHMT1 is highest in AC and lowest in FC
Among PTCs, the expression of PSPH (p = 0.041), tumoral SHMT1 (p = 0.018), and stromal SHMT1 (p < 0.001) differed according to histologic subtypes, with a higher rate of expression in the conventional type compared to follicular type (14.1 versus 2.5 %, 33.6 versus 15.0 %, 42.1 versus 10.0 %, respectively) (Table 2; Fig. 1b). BRAF V600E mutation status was associated with the expression of PHGDH (p < 0.001), PSAT1 (p = 0.001), PSPH (p < 0.001), tumoral SHMT1 (p = 0.001), stromal SHMT1 (p < 0.001), and GLDC (p < 0.001), with higher expression of serine/glycine metabolism-related proteins in BRAF V600E mutation-positive cases compared to non-mutant cases (73.5 versus 40.6 %, 23.1 versus 8.5 %, 17.6 versus 1.9 %, 37.0 versus 18.9 %, 45.8 versus 21.7 %, 21.8 versus 6.6 %, respectively) (Table 2; Fig. 3).
Table 2.
Expression of glutamine metabolism-related proteins according to the histologic subtype of PTC
| Parameters | Total n = 344 (%) | Histologic subtype | p value | BRAF V600E mutation status | p value | ||
|---|---|---|---|---|---|---|---|
| Conventional type n = 304 (%) | Follicular variant n = 40 (%) | No mutation n = 106 (%) | Mutation n = 238 (%) | ||||
| PHGDH in tumor | 0.564 | <0.001 | |||||
| Negative | 126 (36.6) | 113 (37.2) | 13 (32.5) | 63 (59.4) | 63 (26.5) | ||
| Positive | 218 (63.4) | 191 (62.8) | 27 (67.5) | 43 (40.6) | 175 (73.5) | ||
| PSAT1 in tumor | 0.501 | 0.001 | |||||
| Negative | 280 (81.4) | 249 (81.9) | 31 (77.5) | 97 (91.5) | 183 (76.9) | ||
| Positive | 64 (18.6) | 55 (18.1) | 9 (22.5) | 9 (8.5) | 55 (23.1) | ||
| PSPH in tumor | 0.041 | <0.001 | |||||
| Negative | 300 (87.2) | 261 (85.9) | 39 (97.5) | 104 (98.1) | 196 (82.4) | ||
| Positive | 44 (12.8) | 43 (14.1) | 1 (2.5) | 2 (1.9) | 42 (17.6) | ||
| SHMT1 in tumor | 0.018 | 0.001 | |||||
| Negative | 236 (68.6) | 202 (66.4) | 34 (85.0) | 86 (81.1) | 150 (63.0) | ||
| Positive | 108 (31.4) | 102 (33.6) | 6 (15.0) | 20 (18.9) | 88 (37.0) | ||
| SHMT1 in stroma | <0.001 | <0.001 | |||||
| Negative | 212 (61.6) | 176 (57.9) | 36 (90.0) | 83 (78.3) | 129 (54.2) | ||
| Positive | 132 (38.4) | 128 (42.1) | 4 (10.0) | 23 (21.7) | 109 (45.8) | ||
| GLDC in tumor | 0.116 | <0.001 | |||||
| Negative | 285 (82.8) | 248 (81.6) | 37 (92.5) | 99 (93.4) | 186 (78.2) | ||
| Positive | 59 (17.2) | 56 (18.4) | 3 (7.5) | 7 (6.6) | 52 (21.8) | ||
PTC papillary thyroid carcinoma; PHGDH phosphoglycerate dehydrogenase; PSAT phosphoserine aminotransferase; PSPH phosphoserine phosphatase; SHMT serine hydromethyl transferase; GLDC glycine decarboxylase
Italic values represent p < 0.05
Fig. 3.
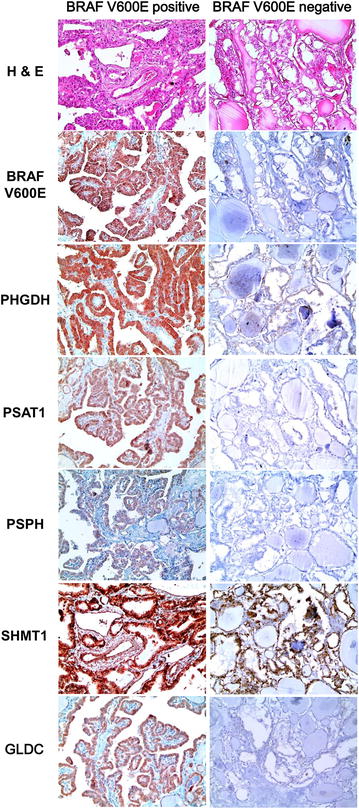
Expression of serine/glycine metabolism-related proteins according to the status of BRAF V600E mutation in papillary carcinoma. BRAF V600E mutation status was associated with the expression of PHGDH, PSAT1, PSPH, tumoral SHMT1, stromal SHMT1, and GLDC, with higher expression of serine/glycine metabolism-related proteins in BRAF V600E mutation-positive cases
Among follicular neoplasms, the expression of PSAT1 was higher in follicular adenoma compared to FC (24.2 versus 8.0 %, p = 0.001, Table 3). There was no difference in serine/glycine metabolism-related protein expression between the minimally invasive and widely invasive type of FC (Table 4).
Table 3.
Expression of serine/glycine metabolism-related proteins in follicular neoplasm
| Parameters | Total n = 264 (%) | Follicular adenoma n = 152 (%) | Follicular carcinoma n = 112 (%) | p value |
|---|---|---|---|---|
| PHGDH in tumor | 0.274 | |||
| Negative | 147 (55.7) | 89 (58.6) | 58 (51.8) | |
| Positive | 117 (44.3) | 63 (41.4) | 54 (48.2) | |
| PSAT1 in tumor | 0.001 | |||
| Negative | 219 (82.6) | 116 (75.8) | 103 (92.0) | |
| Positive | 46 (17.4) | 37 (24.2) | 9 (8.0) | |
| PSPH in tumor | 0.328 | |||
| Negative | 244 (92.1) | 143 (93.5) | 101 (90.2) | |
| Positive | 21 (7.9) | 10 (6.5) | 11 (9.8) | |
| SHMT1 in tumor | 0.137 | |||
| Negative | 211 (79.6) | 117 (76.5) | 94 (83.9) | |
| Positive | 54 (20.4) | 36 (23.5) | 18 (16.1) | |
| GLDC in tumor | 0.262 | |||
| Negative | 234 (88.3) | 138 (90.2) | 96 (85.7) | |
| Positive | 31 (11.7) | 15 (9.8) | 16 (14.3) | |
PHGDH phosphoglycerate dehydrogenase; PSAT phosphoserine aminotransferase; PSPH phosphoserine phosphatase; SHMT serine hydromethyl transferase; GLDC glycine decarboxylase
Italic value represents p < 0.05
Table 4.
Expression of serine/glycine metabolism-related proteins according to the histologic subtype of follicular carcinoma
| Parameters | Total n = 112 (%) | FC, minimally invasive type n = 99 (%) | FC, widely invasive type n = 13 (%) | p value |
|---|---|---|---|---|
| PHGDH in tumor | 0.454 | |||
| Negative | 58 (51.8) | 50 (50.5) | 8 (61.5) | |
| Positive | 54 (48.2) | 49 (49.5) | 5 (38.5) | |
| PSAT1 in tumor | 1.000 | |||
| Negative | 103 (92.0) | 91 (91.9) | 12 (92.3) | |
| Positive | 9 (8.0) | 8 (8.1) | 1 (7.7) | |
| PSPH in tumor | 0.357 | |||
| Negative | 101 (90.2) | 88 (88.9) | 13 (100.0) | |
| Positive | 11 (9.8) | 11 (11.1) | 0 (0.0) | |
| SHMT1 in tumor | 0.689 | |||
| Negative | 94 (83.9) | 82 (82.8) | 12 (92.3) | |
| Positive | 18 (16.1) | 17 (17.2) | 1 (7.7) | |
| GLDC in tumor | 1.000 | |||
| Negative | 96 (85.7) | 85 (85.9) | 11 (84.6) | |
| Positive | 16 (14.3) | 14 (14.1) | 2 (15.4) | |
PHGDH phosphoglycerate dehydrogenase; PSAT phosphoserine aminotransferase; PSPH phosphoserine phosphatase; SHMT serine hydromethyl transferase; GLDC glycine decarboxylase
Correlation between the expression of serine/glycine metabolism-related proteins and clinicopathologic factors
Correlations between the expression of serine/glycine metabolism-related proteins and clinicopathologic factors of thyroid cancers were analyzed. In PTC, PHGDH expression was associated with tumor size (p = 0.003), with an increased tumor size in PHGDH-positive cases. In addition, stromal histologic type of PTC was associated with PHGDH (p = 0.006), tumoral SHMT1 (p = 0.005), and stromal SHMT1 (p < 0.001) expression. Inflammatory type showed a lower rate of PHGDH expression compared to other subtypes, and desmoplastic type and inflammatory type had a higher rate of tumoral and stromal SHMT1 expression compared to pauci type and sclerotic type (Fig. 4).
Fig. 4.
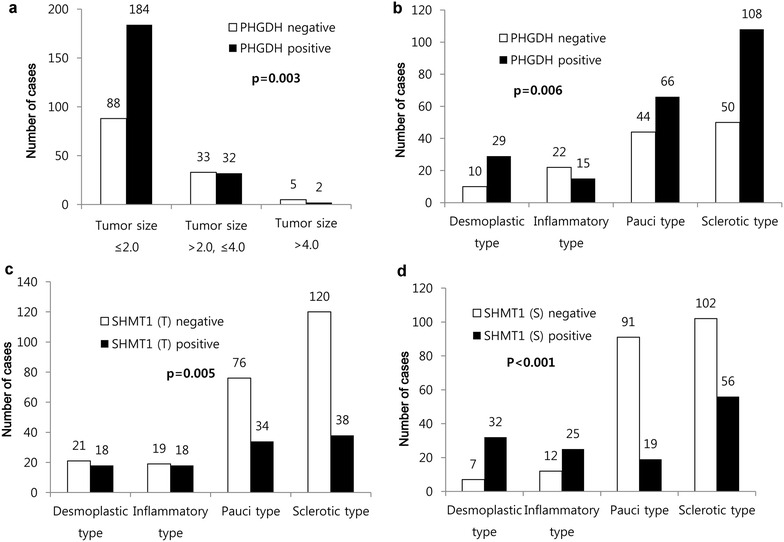
Correlation between the expression of serine/glycine metabolism-related proteins and clinicopathologic factors in papillary carcinoma. a In PTC, PHGDH expression is associated with tumor size, with an increased tumor size in PHGDH-positive cases. b Inflammatory type shows a lower rate of PHGDH expression compared to other subtypes, and desmoplastic type and inflammatory type have a higher rate of tumoral (c) and stromal d SHMT1 expression compared to pauci type and sclerotic type
Impact of the expression of serine/glycine metabolism-related proteins on patient prognosis
In analysis of the impact of the expression of serine/glycine metabolism-related proteins on patient prognosis, the expression of serine/glycine metabolism-related proteins did not have an association with prognosis for overall PTC (Table 5). However, for follicular variant PTC the expression of stromal SHMT1 was associated with shorter disease-free survival (DFS; p = 0.015), and in the sclerotic stromal type, GLDC positivity was associated with shorter overall survival (OS; p = 0.002). PSPH positivity was associated with shorter OS in minimally invasive type FC (p = 0.045) and PHGDH positivity correlated with shorter OS in MC (p = 0.034, and Fig. 5).
Table 5.
Univariate analysis of the impact of serine/glycine-related protein expression in papillary thyroid carcinoma on disease-free and overall survival by the log-rank test
| Parameter | Number of patientsa/recurrence/death | Disease-free survival | Overall survival | ||
|---|---|---|---|---|---|
| Mean survival (95 % CI) months | p value | Mean survival (95 % CI) months | p value | ||
| PHGDH in tumor | 0.790 | 0.806 | |||
| Negative | 126/6/6 | 105 (101–108) | 106 (103–109) | ||
| Positive | 218/12/12 | 106 (104–109) | 107 (105–110) | ||
| PSAT1 in tumor | 0.159 | 0.231 | |||
| Negative | 280/17/13 | 106 (103–109) | 108 (106–110) | ||
| Positive | 64/1/5 | 108 (105–111) | 103 (97–108) | ||
| PSPH in tumor | 0.606 | 0.221 | |||
| Negative | 300/15/14 | 107 (105–109) | 108 (106–110) | ||
| Positive | 44/3/4 | 103 (96–110) | 103 (96–109) | ||
| SHMT1 in tumor | 0.498 | 0.248 | |||
| Negative | 236/11/10 | 107 (105–110) | 108 (106–110) | ||
| Positive | 108/7/8 | 104 (100–109) | 105 (101–109) | ||
| SHMT1 in stroma | 0.655 | 0.684 | |||
| Negative | 212/12/10 | 106 (103–109) | 108 (106–110) | ||
| Positive | 132/6/8 | 107 (104–111) | 107 (103–110) | ||
| GLDC in tumor | 0.516 | 0.059 | |||
| Negative | 285/14/12 | 107 (105–109) | 108 (106–110) | ||
| Positive | 59/4/6 | 104 (97–110) | 103 (97–109) | ||
CI confidence interval; PHGDH phosphoglycerate dehydrogenase; PSAT phosphoserine aminotransferase; PSPH phosphoserine phosphatase; SHMT serine hydromethyl transferase; GLDC glycine decarboxylase
aCases of anaplastic carcinoma were excluded
Fig. 5.
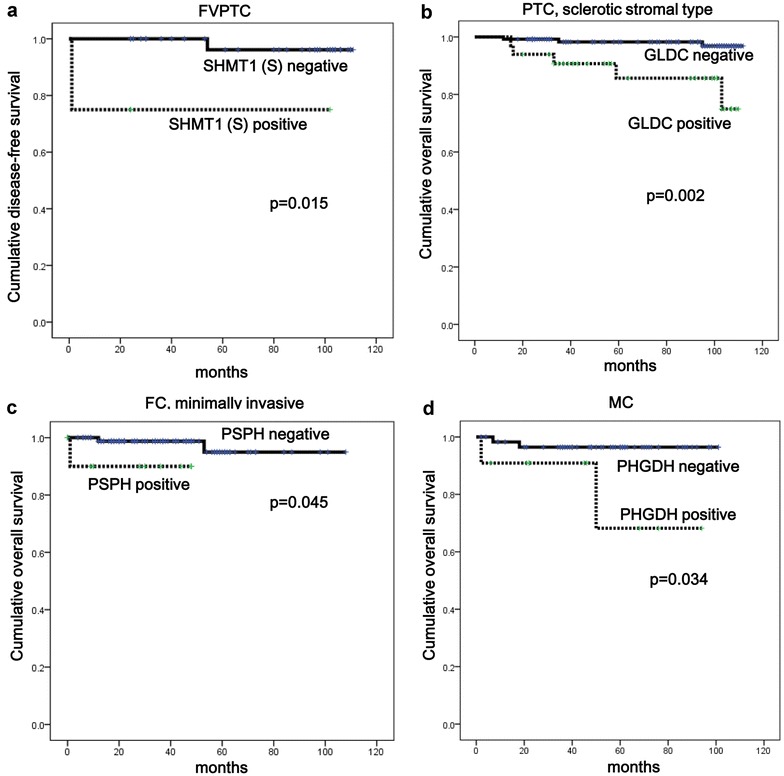
Impact of the expression of serine/glycine metabolism-related proteins on patient prognosis. a For follicular variant PTC, the expression of stromal SHMT1 is associated with shorter disease-free survival. b In the sclerotic stromal type, GLDC positivity is associated with shorter overall survival. c PSPH positivity is associated with shorter OS in minimally invasive type FC. d PHGDH positivity correlated with shorter OS in MC
Discussion
In this study, we investigated the expression of proteins related to serine/glycine metabolism in thyroid cancers and found differences in expression according to the different types of thyroid cancer. In general, the rate of serine/glycine metabolism-related protein expression was higher in PDC and PTC, and lower in MC. As there are no previous studies regarding serine/glycine metabolism in thyroid cancer a direct comparison cannot be made, however MC is a tumor that originates from C-cells whereas other thyroid neoplasms originate from follicular cells, so they might be expected to have different metabolic features. The results of our study suggest that C-cell origin MC may have lower serine/glycine metabolic activity, although further study is required to examine this finding. The present study showed that stromal SHMT1 expression was highest in AC. In previous studies of breast cancer, it was reported that serine/glycine metabolism-related proteins were expressed not only in tumor cells but also in stromal cells [17, 18]. Moreover, glycolysis [19–21] and glutamine metabolism were reported to mediate metabolic interactions between cancer cells and stromal cells such as cancer-associated fibroblasts (CAFs) [20, 22–25]. Metabolic interactions between cancer cells and CAFs promote tumor cell proliferation and growth, suggesting that serine/glycine metabolic activity in both tumor and stroma contributes to tumor aggressiveness in AC.
We observed a higher rate of serine/glycine metabolism-related protein expression in cases of PTC with the BRAF V600E mutation. In meta-analysis studies, the BRAF V600E mutation is reported to be associated with extra-thyroidal extension, advanced TNM stage, lymph node metastasis, multifocality, and recurrence [14], suggesting that PTC with a BRAF V600E mutation has more aggressive tumor biology. In general, higher metabolic activity is related to increased tumor aggressiveness [26–28], and higher expression levels of proteins related to serine/glycine metabolism might be expected in BRAF V600E-positive cases. In addition, PTC with the BRAF V600E mutation is reported to have enhanced glucose metabolism [29]. Since serine metabolism initiates from 3PG, which is generated in the glycolysis pathway, increased serine metabolism might be expected when glycolysis is increased.
A clinical implication of this study is the potential application of targeted therapy against the serine/glycine metabolism pathway. Preclinical studies are ongoing to identify drugs that target multiple sites of one-carbon metabolism, such as GLDC, PSAT, PSPH, and PHGDH, as a novel therapeutic approach [30, 31].
Conclusion
The expression of serine/glycine metabolism-related proteins differs among different thyroid cancer types, with a higher rate of expression in PDC and PTC, and a lower rate of expression in MC. In PTC, the rate of serine/glycine metabolism-related protein expression is lower in the follicular variant, and higher in cases with the BRAF V600E mutation.
Authors’ contributions
WYS participated in the design of the study and performed the statistical analysis. HMK did immunoassays. WHJ participated in the study design. JSK conceived the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board (IRB) of Yonsei University Severance Hospital. Informed consent from patients was exempted by IRB.
Funding
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1420080). This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2015R1A1A1A05001209).
Abbreviations
- 3PG
3-phosphoglycerate
- pPYR
phosphohydroxypyruvate
- PHGDH
phosphoglycerate dehydrogenase
- pSER
phosphoserine
- PSAT
phosphoserine aminotransferase
- PSPH
phosphoserine phosphatase
- GLDC
glycine decarboxylase
- SHMT
serine hydromethyl transferase
- PTC
papillary thyroid carcinoma
- FC
follicular carcinoma
- MC
medullary carcinoma
- PDC
poorly differentiated carcinoma
- AC
anaplastic carcinoma
- H&E
hematoxylin and eosin
- CAF
cancer-associated fibroblast
Additional file
10.1186/s12967-016-0915-8 Source, clone, and dilution of antibodies used in this study. Table S2. Basal characteristics of thyroid papillary carcinoma. Table S3. Basal characteristics of thyroid follicular carcinoma. Table S4. Basal characteristics of thyroid medullary carcinoma, poorly differentiated carcinoma, and anaplastic carcinoma.
Contributor Information
Woo Young Sun, Email: sun2729@naver.com.
Hye Min Kim, Email: pinkmin15@yuhs.ac.
Woo-Hee Jung, Email: jungwh96@yuhs.ac.
Ja Seung Koo, Phone: 82-2-2228-1772, Email: kjs1976@yuhs.ac.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, Kafri R, Kirschner MW, Clish CB, Mootha VK. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336:1040–1044. doi: 10.1126/science.1218595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullarky E, Mattaini KR, Vander Heiden MG, Cantley LC, Locasale JW. PHGDH amplification and altered glucose metabolism in human melanoma. Pigment Cell Melanoma Res. 2011;24:1112–1115. doi: 10.1111/j.1755-148X.2011.00919.x. [DOI] [PubMed] [Google Scholar]
- 4.Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, Chen WW, Barrett FG, Stransky N, Tsun ZY, Cowley GS, Barretina J, Kalaany NY, Hsu PP, Ottina K, Chan AM, Yuan B, Garraway LA, Root DE, Mino-Kenudson M, Brachtel EF, Driggers EM, Sabatini DM. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, Bhakoo KK, Jayapal SR, Nichane M, Yu Q, Ahmed DA, Tan C, Sing WP, Tam J, Thirugananam A, Noghabi MS, Pang YH, Ang HS, Mitchell W, Robson P, Kaldis P, Soo RA, Swarup S, Lim EH, Lim B. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 2012;148:259–272. doi: 10.1016/j.cell.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 6.Grant GA, Kim SJ, Xu XL, Hu Z. The contribution of adjacent subunits to the active sites of D-3-phosphoglycerate dehydrogenase. J Biol Chem. 1999;274:5357–5361. doi: 10.1074/jbc.274.9.5357. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch H, Greenberg DM. Studies on phosphoserine aminotransferase of sheep brain. J Biol Chem. 1967;242:2283–2287. [PubMed] [Google Scholar]
- 8.Borkenhagen LF, Kennedy EP. The enzymatic exchange of l-serine with O-phospho-l-serine catalyzed by a specific phosphatase. J Biol Chem. 1959;234:849–853. [PubMed] [Google Scholar]
- 9.Kikuchi G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol Cell Biochem. 1973;1:169–187. doi: 10.1007/BF01659328. [DOI] [PubMed] [Google Scholar]
- 10.Appaji Rao N, Ambili M, Jala VR, Subramanya HS, Savithri HS. Structure-function relationship in serine hydroxymethyltransferase. Biochim Biophys Acta. 2003;1647:24–29. doi: 10.1016/S1570-9639(03)00043-8. [DOI] [PubMed] [Google Scholar]
- 11.BW Stewart, P Kleihues. World cancer report. Lyon: IACR press; 2003.
- 12.Sherman SI. Thyroid carcinoma. Lancet. 2003;361:501–511. doi: 10.1016/S0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 13.Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, Heffron G, Metallo CM, Muranen T, Sharfi H, Sasaki AT, Anastasiou D, Mullarky E, Vokes NI, Sasaki M, Beroukhim R, Stephanopoulos G, Ligon AH, Meyerson M, Richardson AL, Chin L, Wagner G, Asara JM, Brugge JS, Cantley LC, Vander Heiden MG. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Yan K, Lin X, Zhao L, An W, Wang C, Liu X. The association between BRAF (V600E) mutation and pathological features in PTC. Eur Arch Otorhinolaryngol. 2014;271:3041–3052. doi: 10.1007/s00405-013-2872-7. [DOI] [PubMed] [Google Scholar]
- 15.Henry LR, Lee HO, Lee JS, Klein-Szanto A, Watts P, Ross EA, Chen WT, Cheng JD. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res. 2007;13:1736–1741. doi: 10.1158/1078-0432.CCR-06-1746. [DOI] [PubMed] [Google Scholar]
- 16.Bullock M, O’Neill C, Chou A, Clarkson A, Dodds T, Toon C, Sywak M, Sidhu SB, Delbridge LW, Robinson BG, Learoyd DL, Capper D, von Deimling A, Clifton-Bligh RJ, Gill AJ. Utilization of a MAB for BRAF(V600E) detection in papillary thyroid carcinoma. Endocr Relat Cancer. 2012;19:779–784. doi: 10.1530/ERC-12-0239. [DOI] [PubMed] [Google Scholar]
- 17.Kim SK, Jung WH, Koo JS. Differential expression of enzymes associated with serine/glycine metabolism in different breast cancer subtypes. PLoS One. 2014;9:e101004. doi: 10.1371/journal.pone.0101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noh S, Kim do H, Jung WH, Koo JS. Expression levels of serine/glycine metabolism-related proteins in triple negative breast cancer tissues. Tumour Biol. 2014;35:4457–4468. doi: 10.1007/s13277-013-1588-z. [DOI] [PubMed] [Google Scholar]
- 19.Choi J, Kim do H, Jung WH, Koo JS. Metabolic interaction between cancer cells and stromal cells according to breast cancer molecular subtype. Breast Cancer Res. 2013;15:R78. doi: 10.1186/bcr3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Outschoorn UE, Pavlides S, Howell A, Pestell RG, Tanowitz HB, Sotgia F, Lisanti MP. Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment. Int J Biochem Cell Biol. 2011;43:1045–1051. doi: 10.1016/j.biocel.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 22.Marino G, Kroemer G. Ammonia: a diffusible factor released by proliferating cells that induces autophagy. Sci Signal. 2010;3(124):pe19. doi: 10.1126/scisignal.3124pe19. [DOI] [PubMed] [Google Scholar]
- 23.Eng CH, Abraham RT. Glutaminolysis yields a metabolic by-product that stimulates autophagy. Autophagy. 2010;6:968–970. doi: 10.4161/auto.6.7.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavlides S, Tsirigos A, Migneco G, Whitaker-Menezes D, Chiavarina B, Flomenberg N, Frank PG, Casimiro MC, Wang C, Pestell RG, Martinez-Outschoorn UE, Howell A, Sotgia F, Lisanti MP. The autophagic tumor stroma model of cancer: role of oxidative stress and ketone production in fueling tumor cell metabolism. Cell Cycle. 2010;9:3485–3505. doi: 10.4161/cc.9.17.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko YH, Lin Z, Flomenberg N, Pestell RG, Howell A, Sotgia F, Lisanti MP, Martinez-Outschoorn UE. Glutamine fuels a vicious cycle of autophagy in the tumor stroma and oxidative mitochondrial metabolism in epithelial cancer cells: implications for preventing chemotherapy resistance. Cancer Biol Ther. 2011;12(12):1085–1097. doi: 10.4161/cbt.12.12.18671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang F, Zhang Q, Ma H, Lv Q, Zhang T. Expression of glutaminase is upregulated in colorectal cancer and of clinical significance. Int J Clin Exp Pathol. 2014;7:1093–1100. [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Jung WH, Koo JS. The expression of glutamine-metabolism-related proteins in breast phyllodes tumors. Tumour Biol. 2013;34:2683–2689. doi: 10.1007/s13277-013-0819-7. [DOI] [PubMed] [Google Scholar]
- 28.Kim S, Kim do H, Jung WH, Koo JS. Expression of glutamine metabolism-related proteins according to molecular subtype of breast cancer. Endocr Relat Cancer. 2013;20:339–348. doi: 10.1530/ERC-12-0398. [DOI] [PubMed] [Google Scholar]
- 29.Nagarajah J, Ho AL, Tuttle RM, Weber WA, Grewal RK. Correlation of BRAFV600E mutation and glucose metabolism in thyroid cancer patients: an (1)(8)F-FDG PET study. J Nucl Med. 2015;56:662–667. doi: 10.2967/jnumed.114.150607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeBerardinis RJ. Serine metabolism: some tumors take the road less traveled. Cell Metab. 2011;14:285–286. doi: 10.1016/j.cmet.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dann SG, Abraham RT. Serine biosynthesis: fuel for the melanoma cell growth engine. Pigment Cell Melanoma Res. 2011;24:875–877. doi: 10.1111/j.1755-148X.2011.00894.x. [DOI] [PubMed] [Google Scholar]


