Abstract
Background
A recently introduced high-sensitivity assay can measure troponin T (hsT) at low levels with greater precision than the fourth generation troponin T assay. As most patients with end-stage renal failure (ESRF) may have elevated hsT levels, data on biological variability and the impact of haemodialysis are needed for clinical interpretation of results.
Methods
This is a prospective observational cohort study aiming to identify baseline levels of hsT in stable haemodialysis patients in addition to examining variation in levels over time. Cardiovascular (CV) mortality was analysed at 6 months after the baseline hsT measurement. hsT was measured prior to the haemodialysis using the high-sensitivity Roche troponin T assay in 239 prevalent haemodialysis patients. In a subset of 78 patients, repeat measurements were made 1 month later, both before and after haemodialysis.
Results
hsT was above the 99th centile for the normal healthy population (14 ng/mL) in 98% of patients with a median level of 63 ng/L [Interquartile range (IQR) 37–108]. Higher hsT levels were associated with diabetes and left ventricular ejection fraction <50%. hsT was higher in patients who died from CV causes (median 418, IQR 109–776) compared with alive patients (median 59.5, IQR 36–96 P = 0.0027), and this association remained significant after adjustment for other predictors of mortality. In 95% of stable patients, variation in hsT over 1 month was <54%. In three patients with unstable coronary artery disease, hsT varied by >100% and >100 ng/L. Haemodialysis reduced hsT by a median of 24% (IQR 6–22, P = 0.0001).
Conclusions
hsT levels are elevated in almost all patients with ESRF. Variation in hsT over 1 month was <50% in most patients. Greater variation may indicate an acute coronary syndrome or worsening cardiac disease.
Keywords: cardiovascular risk, end-stage renal failure, haemodialysis, high-sensitivity troponin T
Introduction
Cardiac troponins are sensitive and specific biochemical markers of myocardial injury and necrosis. The recently introduced high-sensitivity troponin T (hsT) assay allows detection of ∼10-fold lower levels with less analytical variation compared with previous assays [1]. These higher-sensitivity assays have improved the accuracy of troponin testing for early diagnosis of acute myocardial infarction in patients presenting with chest pain but are also elevated in clinically stable persons in the community, especially in the elderly [1, 2] and in patients with diabetes or hypertension [3].
Patients with end-stage renal failure (ESRF) have a high prevalence of cardiovascular (CV) disease and an increased risk of acute myocardial infarction [4]. With previous generation troponin assays, 18–82% of dialysis patients will have a baseline troponin T level above the 99th centile in the healthy population [5–7]. A lesser proportion has an elevated troponin I level, although heterogeneity in assays is considerable [8, 9]. In these patients, the diagnosis of acute myocardial infarction relies on the detection of a rise and/or fall in troponin T or I level, with or without serial electrocardiographic changes. The National Academy of Clinical Biochemistry has defined a clear change in troponin values in patients with renal failure, based on analytical considerations, of 20% to define a rising pattern and therefore acute cardiac injury [10, 11].
For the clinical interpretation of troponin testing in patients with ESRF, it is important to know the expected range of troponin levels, how much they vary over time and the impact of haemodialysis. Data on normal biological variability are important as the sensitivity of the test increases and smaller values and changes are detected. Knowledge of clinical variables associated with higher troponin T levels and the prognostic significance of modest elevations in troponin T detected using the higher-sensitivity assays are also relevant.
This study evaluates a recently introduced high-sensitivity troponin T assay in a large cohort of patients on haemodialysis aiming to determine expected values and to measure variation over time and variation over a dialysis session. The relationships between troponin levels and other disease markers and mortality were also evaluated.
Materials and methods
Study sample
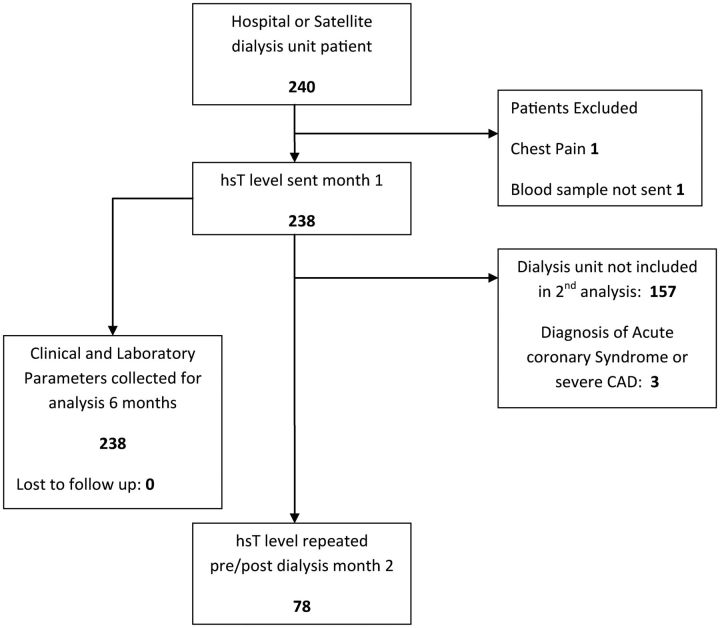
This was a prospective cohort study in a maintenance haemodialysis population. Two hundred and thirty-nine patients underwent baseline hsT level testing prior to dialysis and a subset of patients had repeat testing both prior to and at the end of a haemodialysis session a month later. Demographic and patient diagnostic data were collected at baseline and mortality and cardiac event data were captured prospectively. The study was approved by the Northern X Ethics Committee, Auckland, New Zealand (NTX10/EXP240).
All prevalent haemodialysis patients undergoing dialysis at the Auckland hospital centre and satellite dialysis units were eligible for inclusion for the first group of testing (Figure 1). One patient was excluded due to a recent admission with a cardiac event. One patient had incomplete troponin collection on the first month, so the total number in that analysis was 238. The second group of testing a month later was performed in one dialysis unit only, comprising 78 patients. Dialysis was by haemodiafiltration on Fresenius 5008 machines using high-flux membranes with a three times per week schedule.
Fig. 1.
Inclusion/exclusion cascade of study participants.
Demographic data were collected at baseline on the following variables: age, gender, ethnicity, body mass index, cause of end stage renal disease, duration of dialysis, presence of ischaemic heart disease (defined by previous myocardial infarction, angina/angina equivalent with confirmed coronary disease on angiogram or positive stress imaging study), previous coronary revascularisation, presence of diabetes mellitus, echocardiographic assessment of left ventricular (LV) ejection fraction where available, smoking status and presence of atrial fibrillation. In all subjects, mortality and cardiac event data were captured prospectively from electronic medical records over 6 months following the initial troponin testing, with a cut-off date of 19 October 2011.
Clinically relevant cut-offs were used for the continuous variables of haemoglobin, C-reactive protein, albumin level and serum phosphate. Cut-offs were undertaken at arbitrary but clinically relevant levels.
Measurement of troponin T
Bloods for hsT testing were drawn at baseline at the start of a mid-week haemodialysis session in all patients, along with their routine monthly blood tests. All initial testing was performed over a 1-week period across the dialysis units. The 78 patients who were in the longitudinally tested dialysis unit had repeat troponin testing a month later with levels drawn at the start and end of dialysis.
All hsT results were clinically available so assessment of patients could be undertaken if levels were elevated. All patients with high levels were reviewed clinically within 24 h if possible and echocardiograms and further investigations performed if indicated.
hsT levels were measured by an automated chemiluminescent immunoassay on a Roche Modular analyser using the Roche high-sensitivity troponin T assay (Roche Diagnostics, Mannheim, Germany). This assay has a detection limit of 5 ng/L and has a total imprecision of better than 10% at a level of 13 ng/L. In a sample of 616 healthy volunteers, the 99th centile was 13.5 ng/L [12].
Statistical methods
Continuous data are summarised as mean (standard deviation) or median (interquartile range) as appropriate while categorical data are reported as frequency (percentage). The Mann–Whitney U test was used to compare the differences in hsT levels between patients grouped according to different clinical and biochemical measurements and for type of deaths.
Univariate linear regression was used to assess the associations between clinical and biochemical measurements on hsT. Factors found to be statistically significant in the univariate models were included together in the multiple linear regression to assess if these clinical and biochemical measurements still have a statistically significant relationship with hsT after adjusting for each other. Diagnostic plots and Shapiro–Wilk test of the residuals indicated that it was not normally distributed and natural log transformation was applied. The regression coefficients are expressed as a percentage change in hsT levels. hsT levels were log-transformed due to non-Gaussian distribution.
Univariate logistic regression was used to assess the associations between clinical and biochemical measurements including hsT and CV mortality. Factors found to be statistically significant in the univariate models were included together in the multiple logistic regression models.
hsT levels were distributed/categorized into three tertiles: low, medium and high. The averages and measures of dispersion for each group and the overall troponin levels were calculated. The variation and percentage variation in hsT levels made pre- and post-dialysis and measurements made 1 month apart were calculated for all the three groups.
Bland–Altman plots were used to depict the agreement between measurements made pre- and post-dialysis and measurements made 1 month apart. Percentage change in troponin T levels between the different measurements was also depicted in plots.
Statistical analyses were performed with SAS software version 9.3 (SAS Institute, Cary, NC). All P-values resulted from two-sided tests and a P-value of <0.05 was considered statistically significant.
Results
Baseline troponin T levels
Baseline clinical and laboratory data for all 239 patients are described in Table 1. The mean age was 63 years and the mean duration of dialysis was 38.5 months. A third of patients had known ischaemic heart disease, with the majority (67%) of these having had a prior myocardial infarction. Of the 160 (66.6%) patients with an echocardiogram, 27% had an ejection fraction of <50%. Diabetes mellitus was present in 64% of the patients and in 95% of these diabetes was the cause of ESRF. The majority of patients were Maori or Pacific Islanders in keeping with the demographics of our dialysis population.
Table 1.
Baseline patient demographics
| Median (interquartile range) or number of patients (%) | |
|---|---|
| Age (years) | 63 (54–71) |
| Male | 122 (51%) |
| Ethnicity | |
| Pacific Islander | 116 (48.5%) |
| European | 66 (28%) |
| Maori | 35 (15%) |
| Asian | 21 (8%) |
| African | 1 (0.5%) |
| Body mass index (m2 kg) | 29 (25–34) |
| Dialysis duration (months) | 38.5 (17.2–70) |
| Coronary artery disease | 79 (33%) |
| Previous coronary revascularisation | 17 (7%) |
| Diabetes mellitus | 154 (64%) |
| Cause of ESRF | |
| Diabetes | 139 (58.2%) |
| Glomerulonephritis | 49 (20.5%) |
| Hypertension | 19 (7.9%) |
| Unknown | 9 (3.8%) |
| Obstructive uropathy | 5 (2%) |
| Adult polycystic kidney disease | 4 (1.7%) |
| Cardiorenal syndrome | 3 (1.3%) |
| Reflux nephropathy | 3 (1.3%) |
| Other | 8 (3.3%) |
| Ejection fraction <50%a | 45 (27% of 160) |
| Ejection fraction <35%a | 17 (10% of 160) |
| Previous smoker | 38 (16%) |
| Current smoker | 26 (11%) |
| Atrial Fibrillation | 26 (11%) |
| Albumin, g/L | 41 (39–43) |
| Calcium, mmol/L | 2.32 (2.21–2.43) |
| Phosphate, mmol/L | 1.65 (1.4–2.1) |
| Parathyroid hormone, pmol/L | 29.5 (13.2–56) |
| Haemoglobin, g/L | 113 (102–122) |
| C-reactive protein, mg/L | 5.8 (2.4–13) |
| HbA1c in diabetics, mg/mmol | 55 (45.5–65.5) |
One patient excluded for incomplete troponin data (n = 238).
aEchocardiogram available in 160 patients.
The median hsT level of all dialysis patients was 63 ng/L (IQR 37–108). In 234 patients (98%), the hsT level was elevated >14 ng/L, the 99th percentile range for a normal population [13].
Troponin T levels are categorized by selected baseline clinical and laboratory variables as shown in Table 2. This showed higher hsT levels in patients with older age, male sex, diabetes mellitus, hypoalbuminaemia and impaired LV ejection fraction on echocardiography.
Table 2.
High-sensitivity troponin T levels by selected clinical and biochemical variables at baseline
| Number (%) | Median troponin ng/L (IQR) | P-value | |
|---|---|---|---|
| Age | |||
| >65 years | 96 (40%) | 76 (46–114) | <0.01 |
| ≤65 years | 142(60%) | 55 (31–93) | |
| Gender | |||
| Male | 123 (51%) | 77.5 (45–115) | <0.01 |
| Female | 115 (49%) | 51 (31–88) | |
| Ethnicity | |||
| Maori or Pacific | 151 (63%) | 69 (41–122) | 0.05 |
| Other | 87 (37%) | 56 (32–90) | |
| Body mass index | |||
| ≤30 kg/m2 | 132 (55%) | 69 (41–110) | 0.20 |
| >30 kg/m2 | 106 (45%) | 57 (31–106) | |
| Dialysis duration | |||
| >36 months | 122 (51%) | 61 (37–107) | 0.55 |
| ≤36 months | 116 (49%) | 66 (37–108) | |
| Coronary artery disease | |||
| Yes | 79 (33%) | 66 (44–112) | 0.06 |
| No | 159 (67%) | 62 (32–106) | |
| Prior coronary revascularisation | |||
| Yes | 17 (7%) | 56 (43–103) | 0.84 |
| No | 221 (93%) | 63 (37–107) | |
| Diabetes | |||
| Diabetic | 153 (64%) | 75 (46–115) | <0.01 |
| Non-diabetic | 85 (36%) | 46 (26–84) | |
| Ejection fraction compared with >50% | |||
| EF ≥50% | 176 (74%) | 59 (37–108) | |
| EF <50% | 45 (19%) | 89 (66–148) | 0.01 |
| EF <35% | 17 (7%) | 107 (69–188) | 0.03 |
| Smoking status compared with never | |||
| Never | 178 (74%) | 64.5 (40–107) | |
| Previous | 36 (15%) | 68.5 (38–155) | 0.42 |
| Current | 24 (11%) | 55 (32–85) | 0.28 |
| Atrial fibrillation | |||
| Present | 27 (11%) | 79 (43–93) | 0.35 |
| Absent | 211 (89%) | 61 (36–109 | |
| Albumin | |||
| ≥38 g/L | 196 (88%) | 59 (35–102) | 0.03 |
| <38 g/L | 42 (18%) | 84 (49–121) | |
| C-reactive protein | |||
| ≤10 units | 166 (70%) | 62 (39–99) | 0.53 |
| >10 units | 71 (30%) | 74 (34–113) | |
| Haemoglobin | |||
| ≥100 g/L | 189 (80%) | 63 (40–109) | 0.55 |
| <100 g/L | 49 (20%) | 63 (31–96) | |
| Phosphate | |||
| ≥2 mmol/L | 170 (72%) | 60 (37–93) | 0.06 |
| <2 mmol/L | 68 (28%) | 81 (40–139) | |
In a multiple variable model, diabetes (65% increase in troponin, 95% CI 37–93, P < 0.0001), ejection fraction <50% (50% increase in troponin, 95% CI 20–50 P = 0.0015) and serum albumin <38 g/L (46% increase in troponin, 95% CI 13–80, P = 0.0069) were independently associated with higher troponin levels. Associations with age and sex were statistically significant on univariate but not multivariate analysis.
Troponin T levels and cardiovascular death
In the 6 months following the initial testing, 27 patients died. Ten deaths were from cardiac causes including five sudden deaths in the community. Of the 17 non-cardiac deaths, 8 were from dialysis withdrawal, 7 from sepsis and 2 from other causes. Patients who died had higher hsT levels (median 109, IQR 60–158) compared with those alive at 6 months (median 59.5, IQR 35–96, P = 0.0007). hsT was higher in patients who died from CV causes (median 418, IQR 109–776) versus alive (median 59.5, IQR 36–96, P = 0.0027), but was modestly and not significantly increased in patients who died from non-CV causes of death (median 76, IQR 48.5–113 versus alive 59.5, IQR 36–96, P = 0.24).
In a univariate analysis with CV death as the primary outcome, the most significant risk factor was an elevation in the baseline troponin T level (Table 3), while a low ejection fraction and elevated C-reactive protein were also significant predictors of CV deaths. An increase in baseline troponin levels by 100 units increased the odds of CV deaths (OR 1.5, 95% CI 1.182–1.918; P = 0.0009). This remained significant in multivariate analysis after adjustment for ejection fraction and C-reactive protein (OR 1.32, 95% CI 1.066–1.657; P = 0.011).
Table 3.
Associations with CV death: univariate analysis
| Variable | Odds ratio | 95% Confidence interval | P-value |
|---|---|---|---|
| Baseline hsT (continuous) 100 unit increase | 1.506 | 1.182–1.918 | <0.01 |
| Age | |||
| >65 versus ≤65 years | 2.33 | 0.64–8.50 | 0.20 |
| Age (continuous) | 1.01 | 0.96–1.06 | 0.66 |
| Gender | |||
| Female versus Male | 0.69 | 0.19–2.51 | 0.57 |
| Body mass index | |||
| BMI ≥30 versus <30 | 1.24 | 0.35–4.36 | 0.74 |
| Ethnic Group | |||
| Maori & Pacific versus all others | 2.41 | 0.50–11.59 | 0.27 |
| Dialysis duration | |||
| >36 versus ≤36 months | 1.47 | 0.41–5.36 | 0.56 |
| Coronary revascularization | |||
| Yes versus No | 1.49 | 0.18–12.47 | 0.72 |
| Diabetes | |||
| Yes versus No | 2.36 | 0.49–11.30 | 0.29 |
| Echo | |||
| EF <50% versus ≥50% | 4.42 | 1.186–16.50 | 0.03 |
| Smoking status | |||
| Previous smoker versus non-smokers | 2.39 | 0.59–9.71 | 0.22 |
| Current smoker versus non-smokers | 0.94 | 0.114–7.74 | 0.95 |
| Atrial fibrillation | |||
| Yes versus No | 0.86 | 0.11–7.09 | 0.89 |
| Albumin | |||
| Albumin <38 versus ≥38 | 3.37 | 0.907–12.51 | 0.07 |
| C-reactive protein | |||
| CRP >10 versus ≤10 | 6.05 | 1.52–24.13 | 0.01 |
| Haemoglobin | |||
| Hb <100 versus ≥100 | 1.714 | 0.43–6.89 | 0.45 |
| Phosphate | |||
| PO4 >2 versus ≥2 | 2.65 | 0.74–9.47 | 0.13 |
| HbA1c | |||
| HbA1c >53 versus ≤53 | 1.49 | 0.36–6.22 | 0.58 |
Baseline categories are in italics.
Variation in troponin T levels
Seventy-eight patients underwent testing of hsT prior to a routine dialysis session 1 month after the initial hsT testing. Three subjects were not included in the variation analysis because they had clinical evidence of severe unstable cardiac disease or an acute coronary syndrome. In these patients, hsT varied by >100% and >100 ng/L (Table 4).
Table 4.
Characteristics of patients with variation of high-sensitivity troponin of >100 ng/L
| Patient Characteristic | Troponin level 1 | Troponin level 2 | Clinical event |
|---|---|---|---|
| 48-year-old diabetic | 48 ng/L | 1820 ng/L | New echocardiogram changes |
| 69-year-old diabetic | 152 ng/L | 379 ng/L | Severe triple vessel disease on angiography |
| 53-year-old diabetic | 839 ng/L | 154 ng/L | Severe coronary artery disease, died from STEMI 1 month after second troponin. |
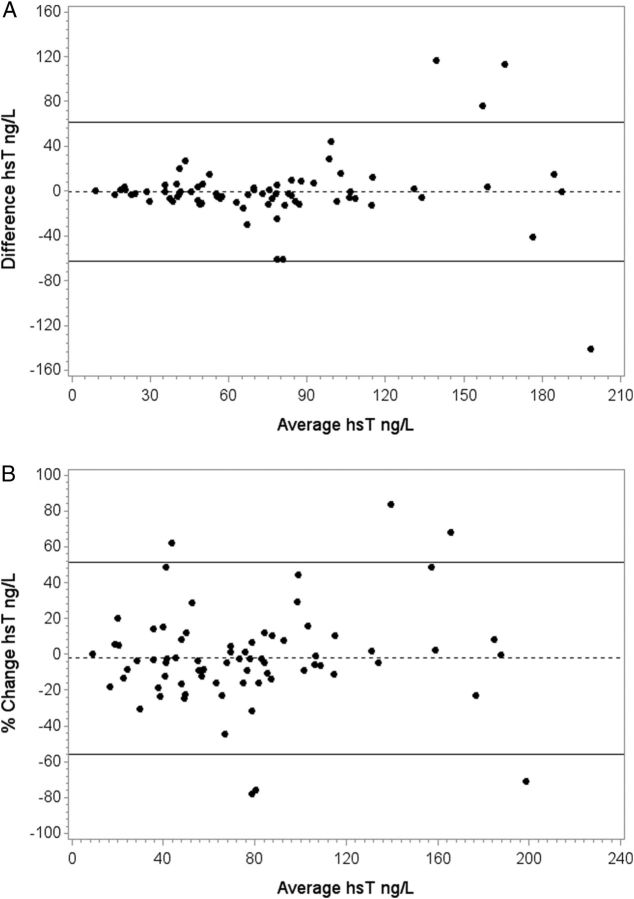
For the remaining 75 subjects, the average pre-dialysis hsT did not change over the month (76.1 IQR 43–93 versus 76.0 IQR 43–103). In 95% of all patients (2SD), variation over 1 month was <62 ng/L. Variation was lower in patients with lower troponin levels at baseline. When the variation was expressed as a percentage change, it was <54% in 95% of subjects and was similar for lower and higher troponin levels (Table 5 and Figure 2A and B).
Table 5.
Variation in high-sensitivity troponin T measured before and after dialysis and over 1 month
| All subjects | Lowest 1/3 hsT | Middle 1/3 hsT | Highest 1/3 hsT | |
|---|---|---|---|---|
| Number of subjectsa | 74 | 25 | 24 | 25 |
| Mean hsT (SD), ng/L+ | 76 (47) | 34 (12) | 67 (11) | 128 (42) |
| Median (IQR) | 66 (43–103) | 38 (23–43) | 63 (53.5–72.5) | 113 (97–156) |
| Range, ng/L | 9–198 | 9–49 | 50–83 | 84–198 |
| Variation (2SD) | ||||
| Over 1 month, ng/L | 62 | 17 | 37 | 97 |
| Over dialysis, ng/L | 22 | 8.6 | 13 | 28 |
| Over 1 month (%) | 54% | 43% | 49% | 61% |
| Over dialysis (%) | 24% | 28% | 23% | 20% |
hsT, high-sensitivity troponin T.
aOne value missing for pre-dialysis value.
Fig. 2.
(A) Agreement between hsT T levels (ng/L) measured 1 month apart (absolute change). Dotted and solid lines indicate median difference and 2SD (95%). (B) Percentage change in hsT T levels between the two measurements 1 month apart. Dotted and solid lines indicate median percentage change and 2SD (95%).
Decrease in troponin T over dialysis
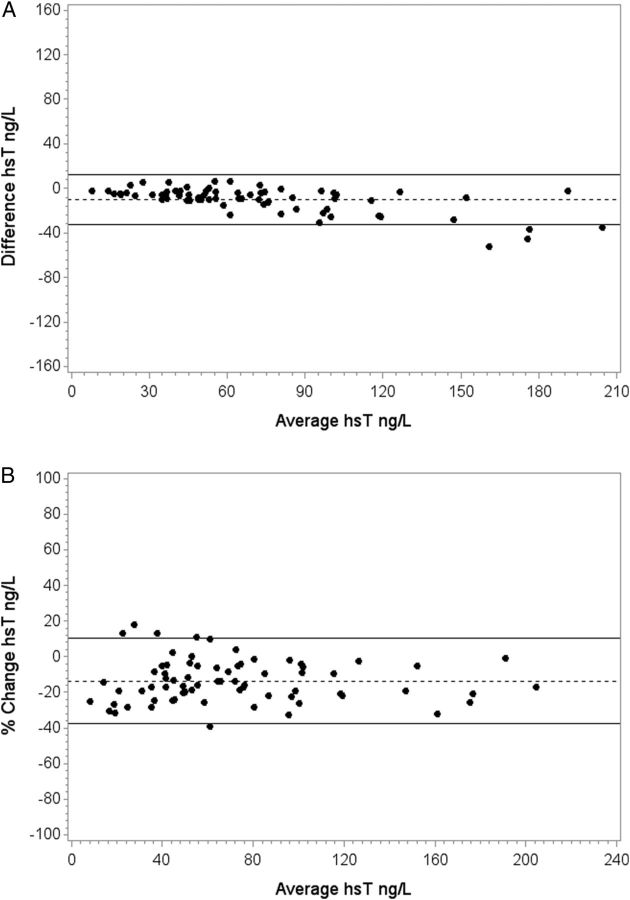
In the second month, hsT levels were evaluated at the start and end of a dialysis session in 75 subjects. The average level before dialysis was 76 (IQR 43–103) ng/L and at the end of the dialysis session, 66 (39–86) ng/L (P < 0.0001). Variation over dialysis in 95% of patients (2SD) was <22.4 ng/L and <24%. The variation in troponin levels increased slightly across increasing tertiles of baseline troponin, but the percentage change in variation did not. Variation was greater over 1 month than over a single dialysis session (Table 4, Figure 3A and B).
Fig. 3.
(A) Absolute change between pre- and post-dialysis hsT T measurements (ng/L). Dotted and solid lines indicate median difference and 2SD (95%). (B) Percentage change between pre- and post-dialysis hsT T measurements. Dotted and solid lines indicate median percentage change and 2SD (95%).
Discussion
This prospective study makes a number of novel observations relevant to the clinical interpretation of troponin T measured using the new high-sensitivity troponin T assay in patients with ESRF on haemodialysis. Unlike previous generation assays for troponin T, hsT was detectable in all subjects and was greater than the 99th centile for normal in 98% of the study cohort. The variation in hsT over 1 month was <54% in 95% of patients. Additionally, we demonstrated that hsT decreased by an average of 16% and <24% in 95% of patients over a haemodialysis session. Finally, higher troponin T levels were strongly associated with the presence of diabetes and predicted CV mortality during 6 months follow-up.
Both the relative change and absolute level of troponin T are important for the diagnosis of acute myocardial infarction [3, 11, 13]. This study shows that almost all haemodialysis patients have a hsT level that is above the 99th percentile for the healthy population. Therefore, a single measurement of hsT is not sufficient to diagnose acute myocardial infarction. In addition to the absolute level, measuring a change in hsT which is greater than variation for other reasons would be needed to diagnose acute myocardial infarction [3, 11, 13]. In the current study, the variation in hsT increased with the baseline level, but when expressed as a percentage, change was relatively constant over the range of hsT levels at <54% in 95% of subjects over 1 month. This level is consistent with the cut-off >50% change proposed for the universal definition of myocardial infarction. In previous studies of healthy individuals, hsT levels varied from 47 to 85% over 4 h [14–17] and from 85 to 315% over 4 weeks [14, 15].
Previous studies have evaluated the change in troponin levels in haemodialysis patients over longer time periods using older assays. A study was performed by Mongeon et al. evaluating a Troponin T assay with a limit of detection of 10 ng/L and a cut-off point for myocardial necrosis of 100 ng/L in 100 dialysis patients. More than 25% of patients had levels below the limit of detection, with an overall mean troponin of 60 ng/L. Ninety-five per cent of patients varied by <50 ng/L or 83% over 1 year [18]. Another small study evaluated a pre-commercial high-sensitivity troponin T and an older assay with a limit of detection of 10 ng/L in 32 stable dialysis patients over 6 months. They found a median hsT level of 53 ng/L. Over 6 months the median change in troponin in individual patients for the high-sensitivity assay was 44% compared with 150% for the older assay [19]. Our study showed a variability of <54% over a month in 95% of patients, concordant with the latter study using hsT.
This is the first study to report a reduction in high-sensitivity troponin T levels over a dialysis session. One previous study using an older assay showed an increase over dialysis, which was corrected after adjusting for haematocrit [20]. In another study, there was no significant change [18] and in a further small study, there was a small reduction in troponin T with high-flux membranes [20]. A study of a new high-sensitivity troponin I also showed no difference over haemodialysis [8]. It is possible that the modest decrease observed in the current study could be detected because of greater precision of the hsT assay. The molecular weight of troponin T is 37 kDa, which is larger than the pores in the dialysis membrane [21, 22]. Haemodiafiltration using high-flux membranes was used in the majority of patients in this study. This method is associated with a small amount of albumin leakage (albumin molecular weight 65 kDa) [23, 24], though this is not usually clinically apparent due to redistribution from other compartments and other compensatory mechanisms. It is possible that a similar mechanism may account for the reduction in hsT. Another potential mechanism is adsorption of degradation products of troponin by the dialysis membrane [25, 26].
In this study, a large change in hsT occurred in three patients with a definite acute coronary syndrome or newly diagnosed severe coronary artery disease. However, stable moderate elevations in troponin were not associated with the presence of known coronary artery disease after accounting for diabetes and reduced LV function on echocardiography in the multivariate analysis [27]. In patients on haemodialysis, associations between elevated troponin levels and coronary disease have been variable. Two studies found that coronary disease was associated with an increased troponin T on univariate analysis, but these studies did not control for LV function [18, 20]. Iliou et al. reported an association on univariate but not multivariate analysis when controlled for LV hypertrophy, [6] consistent with the current study.
The association between elevated hsT and cardiac mortality is consistent with previous studies in dialysis populations which used earlier generation troponin assays [7, 28, 29]. A similar association has also been observed in general population studies, with increasing troponin T levels being associated with higher mortality [30].
The association between diabetes and increased hsT suggests that troponin T may be a sensitive measure of diabetic cardiomyopathy. In the Women's Health Study, troponin T was also higher in subjects with diabetes, but the absolute differences in troponin T levels between diabetic and non-diabetic subjects were small compared with the current study [31]. Most previous studies in haemodialysis patients have also found strong associations between diabetes and increased troponin levels. In the Atherosclerosis risk in communities study, HbA1C levels correlated with levels of hsT [32].
The association that we found between increased hsT and low serum albumin, to our knowledge, has not been reported previously, and the mechanism is uncertain. Hypoalbuminaemia is a powerful predictor of all cause and cardiac mortality in dialysis patients [33–35] and is associated with inflammation. However, in this study neither C-reactive protein nor haemoglobin was associated with hsT. LV hypertrophy is common in patients with renal failure, but this was not systematically evaluated in this study [36]. In addition, haemodialysis may predispose to LV dysfunction, with emerging evidence that transient myocardial ischaemia during haemodialysis is common and associated with persistent LV dysfunction [37, 38].
This study has a number of limitations. First, short-term variability in hsT unrelated to dialysis was not assessed. The sample size was relatively small, but was larger than most previous studies of troponins in haemodialysis cohorts [6, 8, 18, 20, 28], and an unbiased sample of all haemodialysis patients at a single centre was evaluated. A larger study is required to reliably evaluate the prognostic significance of hsT cut-off points for outcomes other than CV mortality.
In conclusion, hsT levels correlate strongly with CV mortality and are above the 99th centile value for normal subjects in almost all stable haemodialysis patients. In 95% of clinically stable patients, hsT varied by <54% over 1 month by <24% over dialysis. These observations are relevant to the use of hsT for diagnosis of acute myocardial infarction, and for identifying patients at highest risk for CV death.
Conflict of interest statement
None declared.
References
- 1.Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 2.Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868–877. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 3.White HD. Higher sensitivity troponin levels in the community: what do they mean and how will the diagnosis of myocardial infarction be made? Am Heart J. 2010;159:933–936. doi: 10.1016/j.ahj.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Roberts MA, Polkinghorne KR, McDonald SP, et al. Secular trends in cardiovascular mortality rates of patients receiving dialysis compared with the general population. Am J Kidney Dis. 2011;58:64–72. doi: 10.1053/j.ajkd.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Hickman PE. Biomarkers and cardiac disease in patients with end-stage renal disease on dialysis. Clin Biochem Rev. 2011;32:115–119. [PMC free article] [PubMed] [Google Scholar]
- 6.Iliou MC, Fumeron C, Benoit MO, et al. Factors associated with increased serum levels of cardiac troponins T and I in chronic haemodialysis patients: pchronic Haemodialysis And New Cardiac Markers Evaluation (CHANCE) study. Nephrol Dial Transplant. 2001;16:1452–1458. doi: 10.1093/ndt/16.7.1452. [DOI] [PubMed] [Google Scholar]
- 7.Apple FS, Murakami MM, Pearce LA, et al. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation. 2002;106:2941–2945. doi: 10.1161/01.cir.0000041254.30637.34. [DOI] [PubMed] [Google Scholar]
- 8.Kumar N, Michelis MF, DeVita MV, et al. Troponin I levels in asymptomatic patients on haemodialysis using a high-sensitivity assay. Nephrol Dial Transplant. 2011;26:665–670. doi: 10.1093/ndt/gfq442. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Mair J, Katus H, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J. 2010;31:2197–2204. doi: 10.1093/eurheartj/ehq251. [DOI] [PubMed] [Google Scholar]
- 10.Wu AH, Jaffe AS, Apple FS, et al. National Academy of clinical biochemistry laboratory medicine practice guidelines: use of cardiac troponinand B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem. 2007;53:2086–2096. doi: 10.1373/clinchem.2007.095679. [DOI] [PubMed] [Google Scholar]
- 11.Thygesen K, Alpert JS, White HD. Joint ESC/ACCF/AHA/WHF task force for the redefinition of myocardial infarction. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Giannitsis E, Kurz K, Hallermayer K, et al. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 13.Reichlin T, Irfan A, Twerenbold R, et al. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation. 2011;124:136–145. doi: 10.1161/CIRCULATIONAHA.111.023937. [DOI] [PubMed] [Google Scholar]
- 14.Frankenstein L, Wu AH, Hallermayer K, et al. Biological variation and reference change value of high-sensitivity troponin T in healthy individuals during short and intermediate follow-up periods. Clin Chem. 2011;57:1068–1071. doi: 10.1373/clinchem.2010.158964. [DOI] [PubMed] [Google Scholar]
- 15.Vasile VC, Saenger AK, Kroning JM, et al. Biologic variation of a novel cardiac troponinI assay. Clin Chem. 2011;57:1080–1081. doi: 10.1373/clinchem.2011.162545. [DOI] [PubMed] [Google Scholar]
- 16.Vasile VC, Saenger AK, Kroning JM, et al. Biological and analytical variability of a novel high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:1086–1090. doi: 10.1373/clinchem.2009.140616. [DOI] [PubMed] [Google Scholar]
- 17.Wu AH, Lu QA, Todd J, et al. Short- and long-term biological variation in cardiac troponin I measured with a high-sensitivity assay: implications for clinical practice. Clin Chem. 2009;55:52–58. doi: 10.1373/clinchem.2008.107391. [DOI] [PubMed] [Google Scholar]
- 18.Mongeon FP, Dorais M, Lorier JL, et al. Effect of hemodialysis, coronary artery disease and diabetes on cardiac troponin T: a prospective survey over one year. Open Cardiovasc Med J. 2009;3:69–77. doi: 10.2174/1874192400903010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs LH, van de Kerkhof J, Mingles AM, et al. Haemodialysis patients longitudinally assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and cardiac troponin I assays. Ann Clin Biochem. 2009;46(Pt 4):283–290. doi: 10.1258/acb.2009.008197. [DOI] [PubMed] [Google Scholar]
- 20.Conway B, McLaughlin M, Sharpe P, et al. Use of cardiac troponin T in diagnosis and prognosis of cardiac events in patients on chronic haemodialysis. Nephrol Dial Transplant. 2005;20:2759–2764. doi: 10.1093/ndt/gfi125. [DOI] [PubMed] [Google Scholar]
- 21.Lippi G, Tessitore N, Montagnana M, et al. Influence of sampling time and ultrafiltration coefficient of the dialysis membrane on cardiac troponin I and T. Arch Pathol Lab Med. 2008;132:72–76. doi: 10.5858/2008-132-72-IOSTAU. [DOI] [PubMed] [Google Scholar]
- 22.Ahrenholz PG, Winkler RE, Michelsen A, et al. Dialysis membrane-dependent removal of middle molecules during hemodiafiltration: the beta2-microglobulin/albumin relationship. Clin Nephrol. 2004;62:21–28. doi: 10.5414/cnp62021. [DOI] [PubMed] [Google Scholar]
- 23.Wu AH. Cardiac troponin: friend of the cardiac physician foe to the cardiac patient? Circulation. 2006;114:1673–1675. doi: 10.1161/CIRCULATIONAHA.106.652123. [DOI] [PubMed] [Google Scholar]
- 24.Santoro A, Canova C, Mancini E, et al. Protein loss in on-line hemofiltration. Blood Purif. 2004;22:261–268. doi: 10.1159/000078495. [DOI] [PubMed] [Google Scholar]
- 25.Ooi DS, Zimmerman D, Graham J, et al. Cardiac troponin T predicts long-term outcomes in hemodialysis patients. Clin Chem. 2001;47:412–441. [PubMed] [Google Scholar]
- 26.Masson S, Anand I, Favero C, et al. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from two large randomized clinical trials. Circulation. 2011;125:280–288. doi: 10.1161/CIRCULATIONAHA.111.044149. [DOI] [PubMed] [Google Scholar]
- 27.Khan NA, Hemmelgarn BR, Tonelli M, et al. Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: a meta-analysis. Circulation. 2005;112:3088–3096. doi: 10.1161/CIRCULATIONAHA.105.560128. [DOI] [PubMed] [Google Scholar]
- 28.de Filippi C, Wasserman S, Rosanio S, et al. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA. 2003;290:353–359. doi: 10.1001/jama.290.3.353. [DOI] [PubMed] [Google Scholar]
- 29.de Flippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;124:136–145. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Everett BM, Cook NR, Magnone MC, et al. Sensitive cardiac troponin T assay and the risk of incident cardiovascular disease in women with and without diabetes mellitus: the Women's Health Study. Circulation. 2011;123:2811–2818. doi: 10.1161/CIRCULATIONAHA.110.009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubin J, Matsushita K, Ballantyne CM, et al. Chronic hyperglycemia and subclinical myocardial injury. JACC. 2012;59:484–489. doi: 10.1016/j.jacc.2011.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuevas X, Garcia F, Martin-Malo A, et al. Risk factors associated with cardiovascular morbidity and mortality in Spanish incident hemodialysis patients: two-year results from the ANSWER study. Blood Purif. 2011;33:21–29. doi: 10.1159/000332395. [DOI] [PubMed] [Google Scholar]
- 34.Honda H, Qureshi AR, Heimburger O, et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis. 2006;47:139–148. doi: 10.1053/j.ajkd.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Zoccali C, Tripepi G, Mallamaci F. Predictors of cardiovascular death in ESRD. Semin Nephrol. 2005;25:358–362. doi: 10.1016/j.semnephrol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Sharma R, Gaze DC, Pellerin D, et al. Cardiac structural and functional abnormalities in end stage renal disease patients with elevated cardiac troponin T. Heart. 2006;92:804–809. doi: 10.1136/hrt.2005.069666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McIntyre CW. Haemodialysis-induced myocardial stunning in chronic kidney disease–a new aspect of cardiovascular disease. Blood Purif. 2010;29:105–110. doi: 10.1159/000245634. [DOI] [PubMed] [Google Scholar]
- 38.de Filippi CR, Thorn EM, Aggarwal M, et al. Frequency and cause of cardiac troponin T elevation in chronic hemodialysis patients from study of cardiovascular magnetic resonance. Am J Cardiol. 2007;100:885–889. doi: 10.1016/j.amjcard.2007.04.028. [DOI] [PubMed] [Google Scholar]