Abstract
Powered robotic exoskeletons are a potential intervention for gait rehabilitation in stroke to enable repetitive walking practice to maximize neural recovery. As this is a relatively new technology for stroke, a scoping review can help guide current research and propose recommendations for advancing the research development. The aim of this scoping review was to map the current literature surrounding the use of robotic exoskeletons for gait rehabilitation in adults post-stroke. Five databases (Pubmed, OVID MEDLINE, CINAHL, Embase, Cochrane Central Register of Clinical Trials) were searched for articles from inception to October 2015. Reference lists of included articles were reviewed to identify additional studies. Articles were included if they utilized a robotic exoskeleton as a gait training intervention for adult stroke survivors and reported walking outcome measures. Of 441 records identified, 11 studies, all published within the last five years, involving 216 participants met the inclusion criteria. The study designs ranged from pre-post clinical studies (n = 7) to controlled trials (n = 4); five of the studies utilized a robotic exoskeleton device unilaterally, while six used a bilateral design. Participants ranged from sub-acute (<7 weeks) to chronic (>6 months) stroke. Training periods ranged from single-session to 8-week interventions. Main walking outcome measures were gait speed, Timed Up and Go, 6-min Walk Test, and the Functional Ambulation Category. Meaningful improvement with exoskeleton-based gait training was more apparent in sub-acute stroke compared to chronic stroke. Two of the four controlled trials showed no greater improvement in any walking outcomes compared to a control group in chronic stroke. In conclusion, clinical trials demonstrate that powered robotic exoskeletons can be used safely as a gait training intervention for stroke. Preliminary findings suggest that exoskeletal gait training is equivalent to traditional therapy for chronic stroke patients, while sub-acute patients may experience added benefit from exoskeletal gait training. Efforts should be invested in designing rigorous, appropriately powered controlled trials before powered exoskeletons can be translated into a clinical tool for gait rehabilitation post-stroke.
Keywords: Stroke, Cerebrovascular accident, Robotic exoskeleton, Gait rehabilitation, Scoping review
Background
Stroke is a leading cause of acquired disability in the world, with increasing survival rates as medical care and treatment techniques improve [1]. This equates to an increasing population with stroke-related disability [1, 2], who experience limitations in communication, activities of daily living, and mobility [3]. A majority of this population ranks recovering the ability to walk or improving walking ability among their top rehabilitation goals [4, 5]; furthermore, the ability to walk is a determining factor as to whether an individual is able to return home after their stroke [6]. However, 30 – 40 % of stroke survivors have limited or no walking ability even after rehabilitation [7, 8] and so there is an ongoing need to advance the efficacy of gait rehabilitation for stroke survivors.
Powered robotic exoskeletons are a recently developed technology that allows individuals with lower extremity weakness to walk [9]. These wearable robots strap to the legs and have electrically actuated motors that control joint motion to automate overground walking. Powered exoskeletons were originally designed to be used as an assistive device to allow individuals with complete spinal cord injury to walk [10]. However, because they allow for walking without overhead body weight support or a treadmill, they have gained attention as an alternate intervention for gait rehabilitation in other populations such as stroke where repetitive gait training has been shown to yield improvements in walking function [11, 12]. Several powered exoskeletons are already commercially available, such as the Ekso (Ekso Bionics, USA), Rewalk (Rewalk Robotics, Israel), and Indego (Parker Hannifin, USA) exoskeletons, with more being developed.
There have been many forms of gait retraining proposed for stroke survivors. Conventional physical therapy gait rehabilitation leads to improvements in speed and endurance [13], particularly when conducted early post-stroke [14]. However, conventional gait retraining using hands-on assistance can be taxing on therapists; the number of steps actually taken in a session reflects this and has been shown to be low in sub-acute hospital rehabilitation [15]. Many of the proposed technology-based gait intervention strategies have focused on reducing the physical strain to therapists while increasing the amount of walking repetition that individuals undergo. For example, body weight-supported treadmill training (BWSTT) allows therapists to manually move the hemiparetic limb in a cyclical motion while the patient’s trunk and weight are partially supported by an overhead harness system; this has shown improvements in stroke survivors’ gait speed and endurance compared to conventional gait training [16], yet still places a high physical demand on therapists. Advances in technology have led to treadmill-based robotics, such as the Lokomat (Hocoma, Switzerland), LOPES (University of Twente, Netherlands), and G-EO (Reha-Technology, Switzerland), which have bracing that attaches to the patient’s legs to take them through a walking motion on the treadmill. The appeal of this technology is that it can provide substantially higher repetitions for walking practice than BWSTT without placing strain on therapists; however, there is conflicting evidence regarding the efficacy of treadmill-based robotics for gait training compared to conventional therapy or BWSTT. Some studies have shown that treadmill robotics improve walking independence in stroke [17, 18] but do not improve speed or endurance [18, 19]. There has been some sentiment that such technology has not lived up to the expectations originally predicted based on theory and practice [20]. One argument is that these treadmill robotics with a pre-set belt speed, combined with body weight support, create an environment where the patient has less control over the initiation of each step [21]; another argument against treadmill-based gait training is the lack of variability in visuospatial flow, which is an essential challenge of overground walking [20]. Powered robotic exoskeletons, though similar in structure to treadmill-based robotics, differ in that they require active participation from the user for both swing initiation and foot placement; for example, some exoskeletons have control strategies which will only assist the stepping motion when it detects adequate lateral weight-shifting [9]. Furthermore, because the powered exoskeletons are used for overground walking, it requires the user to be responsible for maintaining trunk and balance control, as well as navigating their path over varying surfaces.
While these powered exoskeletons hold promise, the literature surrounding their use for gait training is only just beginning to gather, with the majority focusing on spinal cord injury [22–24]. Several [25–27] systematic reviews have shown safe usage, positive effects as an assistive device, and exercise benefits for individuals with spinal cord injury. Only one systematic review [28] specifically focusing on powered exoskeletons has included studies involving stroke participants, though studies in spinal cord injury and other conditions were also included. This review focused exclusively on the Hybrid Assistive Limb (HAL) exoskeleton (Cyberdyne, Japan), (which currently is not approved for clinical use outside of Japan), and found beneficial effects on gait function and walking independence; however, the results were combined generally across all included patient populations and not specifically for stroke.
Given that this is a relatively new intervention for stroke, the objective of this scoping review was to map the current literature surrounding the use of powered robotic exoskeletons for gait rehabilitation in post-stroke individuals and to identify gaps in the research. The second objective of this scoping review was to preliminarily explore the efficacy of exoskeleton-based gait rehabilitation in stroke. As this is a relatively new technology for stroke, a scoping review can help guide current research and propose recommendations for advancing the technology.
Review
Methods
This scoping review was conducted in accordance with the framework proposed by Arksey and O’Malley [29], and guided by the refined process highlighted by Levac et al. [30].
OVID MEDLINE, Embase, Cochrane Central Register of Controlled Trials, PubMed, and CINAHL databases were accessed and searched from inception on October 14, 2015. We combined the search terms (robot* OR exoskeleton OR “powered gait orthosis” OR PGO OR HAL OR “hybrid assistive limb” OR ReWalk OR Ekso OR Indego) AND (stroke OR CVA OR “cerebrovascular accident” OR “cerebral infarct” OR “cerebral hemorrhage” OR hemiplegia OR hemiparesis OR ABI OR “acquired brain injury”) AND (gait OR walk OR walking OR ambulation), with humans and English language as limits.
Inclusion criteria were full-text, peer-reviewed articles that used a powered robotic exoskeleton with adults post-stroke as an intervention for gait rehabilitation. Articles were included if they reported functional walking outcomes (e.g., speed, distance, independence). We defined a powered robotic exoskeleton as a wearable robotic device which actuates movement of at least one joint while walking, either unilaterally or bilaterally. We further defined powered robotic exoskeletons as stand-alone devices that can be used for overground walking, with programmable control. Articles were excluded if they: reported only technology development; reported only electromyography, physiological cost, or joint kinematic data; combined other interventions (e.g., functional electrical stimulation); included healthy participants or children; utilized a treadmill-based device (i.e., the exoskeleton and treadmill are a single device, where the exoskeleton cannot be used separately overground); included mixed diagnosis participants (<50 % stroke); or if only an abstract was available.
Titles and abstracts were screened for relevance by two reviewers (DRL, CC) according to the inclusion and exclusion criteria above. In the event of conflict, a third reviewer (JJE) was consulted for resolution. Full-texts were then screened and reference lists of all selected articles were searched for additional studies. Included articles were then examined to extract data regarding study design, exoskeleton device, participant characteristics, intervention, training period, outcome measures, adverse effects, and results. We examined the changes in functional walking outcomes relative to clinically meaningful change values published in the literature (Table 1).
Table 1.
Meaningful change values for functional walking outcomes in stroke
| Outcome measure | Sub-acute stroke | Chronic stroke |
|---|---|---|
| TUG | Not available | MDC = 2.9 s [44] |
| 6MWT | MDC = 61 m [43] | MCID = 34.4 m [42] |
| 10MWT/gait speed | MCID = 0.16 m/s [45] | MCID = 0.06 m/s (small) [43] MCID = 0.14 m/s (substantial) [43] |
| FAC | Not available | Not available |
6MWT six-minute walk test, 10MWT ten meter walk test, FAC functional ambulation category, MCID minimal clinically important difference, MDC minimal detectable change, TUG timed up and go
Results
As seen in Fig. 1, our electronic database search returned 440 unique titles. Only one additional article was identified through reference list searching. After screening titles, abstracts, and full-texts for eligibility, 11 articles were included [31–41]. All 11 articles were published in the last five years, with seven [31–33, 35–37, 39] published in the last two years. Five studies were conducted in the United States, five in Japan, and one in Sweden.
Fig. 1.
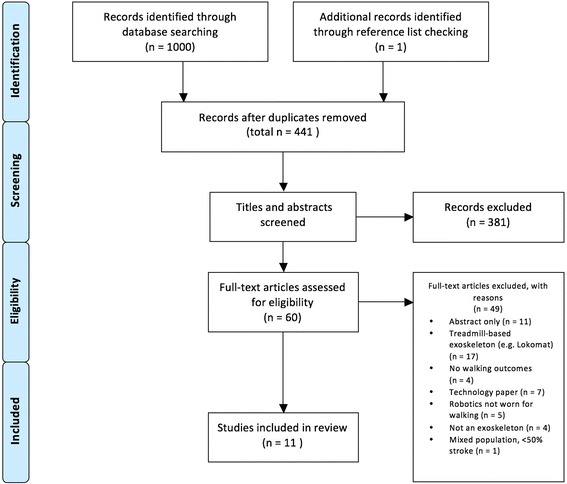
Study results: A flowchart of selection process based on inclusion/exclusion criteria
Study design
Of the included studies, three were randomized controlled trials (RCTs) [31, 35, 36], and one was a non-randomized controlled study [37]. The rest were a variety of single-group pre-post clinical trials as seen in Table 2. Of the three RCTs, two were smaller in size (n = 24 and n = 22) and considered pilot studies [31, 36].
Table 2.
Summary of studies included in the review
| Study & Design | Participants | Exoskeleton & Training Period | Training Protocol | Walking outcomes & Results |
|---|---|---|---|---|
| Subacute Stroke | ||||
| Watanabe et al. (2014) [31] Unblinded RCT |
Sub-acute stroke 1 – 2 person assist ambulation (HAL group n = 11, mean 58.9 days post-stroke Conventional group n = 11, mean 50.6 days post-stroke) |
HAL – Unilateral 12 sessions over 4 weeks 20 minute sessions |
HAL group – gait training while wearing HAL, facilitating improvements in walking ability, partial BWS if needed; progress as able from complete assistance by device to assist-as-needed through bioelectric signal detection Conventional group – facilitate improvements in walking ability, customized to functional level; speed and duration of walking gradually increased |
1) TUG – No significant difference in improvement between groups 2) 6MWT – No significant difference in improvement between groups 3) Gait speed – No significant difference in improvement between groups 4) FAC – HAL group improved significantly (p = 0.04) more than Conventional group (change of +1.1 for HAL group; change of +0.6 for Conventional group) |
| Nilsson et al. (2014) [32] Pre-post study |
Sub-acute stroke 1 – 2 person assist ambulation (n = 8, 6 – 46 days post-stroke) |
HAL – Bilateral 5 sessions/week, median 17 sessions 25 minutes training |
Progression from weight shift control to bioelectric signalling control, training with BWS on treadmill; progression of speed and BWS as tolerated | 1) 10MWT – median change of +0.24 m/s, 4 previously non-ambulatory progressed to ambulatory 2) FAC – median change of +1.5 (from 0 to 1.5) |
| Fukuda et al. (2015) [33] Pre-post study |
Sub-acute stroke (n = 53, 12 non-ambulatory, 41 ambulatory) | HAL – Uni/bilateral 2 sessions/week, mean 3.9 sessions |
Walking on treadmill in exoskeleton, progress from complete control to bioelectric signalling | 1) 10MWT – change of +0.1 m/s for Brunnstrom stage III (greater severity with lower stage) (n = 12); no change for Brunnstrom stage IV (n = 7); change of +0.1 m/s for Brunnstrom stage V (n = 12); change of +0.4 m/s for Brunnstrom stage VI (N = 10) |
| Maeshima et al. (2011) [34] Pre-post study |
Sub-acute stroke 1 – 2 person assist ambulation (n = 16, 27 – 116 days post-stroke) |
HAL – Bilateral Single session |
Walking and stair practice after standing practice in exoskeleton | 1) 10MWT – positive change for 14 of 16 patients (values not provided) |
| Chronic Stroke | ||||
| Buesing et al. (2015) [35] Single-blind RCT |
Chronic stroke Limited community ambulation (SMA group – n = 25, mean 7.1 years post-stroke Functional task specific training group – n = 25, mean 5.4 years post-stroke) |
SMA – Bilateral 18 sessions over 6 – 8 weeks 45 minute sessions |
SMA group – 30 minutes of high intensity overground walking with SMA (12-16 RPE or 75 % HR max) and 15 minutes of dynamic functional gait training with SMA (varied surfaces, multi-directional stepping, stair climbing, obstacles, community mobility) Functional task specific training group – 15 minutes of high intensity overground walking training and 30 minutes of functional goal-based mobility training |
1) Gait speed – No significant difference in improvement between groups |
| Stein et al. (2014) [36] Single-blind RCT |
Chronic stroke Independent ambulation (AlterG group n = 12, mean 49.1 months post-stroke Exercise group n = 12, mean 88.5 months post-stroke) |
AlterG – Unilateral 18 sessions over 6 weeks 60 minute sessions |
AlterG group – standardized overground functional tasks including transfers, stepping, turning, reaching, gait training, stairs and curbs while wearing exoskeleton Exercise group – group exercises including relaxation, meditation, self-stretching, active range of motion of upper and lower limbs, minimal gait training (5 min/session) |
1) TUG – No significant difference between groups 2) 6MWT – No significant difference in improvements between groups 3) 10MWT – No significant difference in improvement between groups |
| Yoshimoto et al. (2015) [37] Non-randomized controlled trial |
Chronic stroke Independent ambulation (HAL group n = 9, mean 92.4 months post-stroke Conventional PT group n = 9, mean 80.5 months post-stroke) |
HAL – Unilateral 8 sessions over 8 weeks 60 minute sessions |
HAL group – 20 minutes of HAL walking per session, with some BWS, walking at speed 1.5-1.7 times max walking speed without device Conventional PT group – exercise to improve walking ability including static and dynamic postural tasks, range of motion, and 20 minutes of overground walking training |
1) TUG – HAL group improved significantly compared to Conventional PT group (change of -11.5 s for HAL group; change of +0.1 s for Conventional PT group) 2) 10MWT – HAL group improved significantly compared to Conventional PT group (change of +0.21 m/s for HAL group; change of -0.02 m/s for Conventional PT group) |
| Kawamoto et al. (2013) [38] Pre-post study |
Chronic stroke (n = 16, 1 – 11 years post-stroke, 8 dependent ambulatory, 8 independent ambulatory) | HAL – Bilateral 16 sessions over 8 weeks 20 – 30 minutes training |
Overground walking with overhead harness for safety and partial BWS; gradual progression from sit-to-stand to walking (gradually increased intensity by changing speed, duration, BWS, and HAL control mechanism) | 1) TUG – mean change of -1.1 s 2) 10MWT – mean change of +0.04 m/s |
| Bortole et al. (2015) [39] Pre-post study |
Chronic stroke Independent ambulation (n = 3; 60, 6, 11 months post-stroke) |
H2 – Bilateral 12 sessions over 4 weeks 30 minute sessions |
Overground walking over a linear track Participants in charge of speed and encouraged to walk as much as possible, with breaks |
1) TUG – change of +1.7 s, -2.5 s, -2.5 s 2) 6MWT – change of -115 m, +16 m, +103 m |
| Byl et al. (2012) [40] Pre-post study |
Chronic stroke Independent ambulation (n = 3; 6, 1.3, 10 years post-stroke) |
AlterG – Unilateral 2 – 4 sessions/week over 4 weeks 90 minute sessions |
Walking practice, with sit-to-stand transfers, squatting, and stepping activities; obstacle clearance, uneven terrain, community ambulation, stair climbing | 1) TUG – change of -6.9 s, +1.9 s, -0.2 s 2) 6MWT – change of +37 m, +47 m, +29 m 3) 10MWT – change of +0.21 m/s, +0.14 m/s, +0.20 m/s |
| Wong et al. (2011) [41] Pre-post study |
Chronic stroke Independent ambulation (n = 3; 37, 26, 40 months post-stroke) |
AlterG – Unilateral 18 sessions over 6 weeks 60 minute sessions |
45 minutes while wearing device, standardized weight-bearing functional mobility activities, sit-to-stand transfers, balance exercises, gait practice at various speeds on different surfaces, functional task practice | 1) TUG – change of -11.7 s, -2.3 s, -4.2 s 2) 6MWT – change of +17 m, +14 m, +15 m 3) 10MWT – change of -0.01 m/s, +0.05 m/s, +0.13 m/s |
6MWT six-minute walk test, 10MWT ten meter walk test, BWS body weight support, FAC functional ambulation category, H2 H2 exoskeleton, HAL hybrid assistive limb, HR heart rate, SMA stride management assist system, PT physical therapy, RCT randomized controlled trial, RPE rate of perceived exertion, TUG timed up and go
Bold indicates value surpasses established meaningful change score detailed in Table 1
Participants
Across the 11 studies, there was a total of 216 (male/female:136/80) participants with stroke enrolled (Table 2), with variability in the inclusion criteria for participation. Seven studies [35–41] included participants with chronic stroke (at least six months post-stroke). Four studies [31–35] investigated the exoskeleton with sub-acute participants (less than six months post-stroke) during inpatient rehabilitation. The majority of participants were in the 50 – 70 age range. Six studies [35–37, 39–41] specifically enrolled participants with the ability to walk without physical assistance from a therapist, permitting walking devices such as a cane or walker, while three studies [31, 32, 34] specified a requirement of needing manual physical assistance to walk. The former studies aimed to improve mobility for ambulatory individuals with chronic stroke, whereas the latter sought to restore independent ambulation for sub-acute stroke participants. The other two studies [33, 38] enrolled participants with a mix of functional levels.
Exoskeletons
The included studies investigated a variety of exoskeletons, each having different set-ups and control mechanisms. Five studies [31, 36, 37, 40, 41] used a robotic exoskeleton unilaterally on the affected leg, while another five studies [32, 34, 35, 38, 39] used a bilateral set-up for gait training. One study [33] progressed participants, as they were able, from a bilateral design to a unilateral configuration. The most studied exoskeleton was the HAL, used in six studies [31–34, 37, 38]; in these studies, participants’ hip and knee joints were electrically actuated in a walking motion. In one study [39] the H2 exoskeleton (Technaid SL, Spain), assisted the hip, knee, and ankle joints. Four studies [35, 36, 40, 41] utilized an exoskeleton powering only one joint of the lower extremity (either hip or knee, uni- or bilaterally); no studies were found in which only the ankle was actuated during gait. Control of the exoskeletons ranged from remote-control button activation [39] to active movement control of stepping; the devices are able to detect movement intention through monitoring joint angles and limb torque [35, 36, 40, 41], or through bio-electric signalling of muscle activity [31–34, 37, 38]. All exoskeletons except the HAL provided supplementary gait assistance on an as-needed basis, in which the user generates as much of the walking movements as possible and the device provides extra torque or support to ensure step completion. The HAL has two modes, one that provides complete stepping assistance and one that adapts to user force generation. Table 3 further details the exoskeletons, their control strategies, and the level of assistance provided.
Table 3.
Details of powered exoskeletons in this review
| Exoskeleton | Joints actuated | Stepping initiation | Stepping assistance |
|---|---|---|---|
| H2 [39] | Hip, knee, ankle | Initiated by hand buttons on walker Pre-set speed |
Assist-as-needed for swing |
| SMA [35] | Hip | Initiated by movement Internal sensors detect hip joint angle to regulate walking |
Assist-as-needed for swing |
| HAL [31–34, 37, 38] | Hip, knee | Initiated by movement (2 modes) Internal sensors detect lateral weight shift Surface electrodes detect muscle activation via bioelectric signals |
Full-assistance for swing Assist-as-needed for swing |
| AlterG [36, 40, 41] | Knee | Initiated by movement Internal sensors detect movement intention via variable force threshold |
Assist-as-needed for stance, free swing |
AlterG AlterG Bionic Leg, formerly Tibion Bionic Leg; H2 H2 exoskeleton; HAL Hybrid Assistive Limb; SMA Stride Management Assist system (Honda R&D Corporation, Japan)
Training period
There was variability in the training period of the included studies, ranging from a single session [34] to several weeks [31–33, 39, 40] or months [35–38, 41] of training. Training duration lasted from 20 – 90 min per session, and frequency ranged from two to five sessions per week. Table 2 details the different training periods for each study.
Training protocol
The training protocol employed in each study differed, and varied depending on the study design, length of the training period, and exoskeleton used (Table 2). Generally, subjects were progressed as tolerated from weight-bearing functional tasks (sit-to-stand, standing balance, weight shifting) to walking practice while wearing the exoskeleton device. Two studies [32, 33] had participants train on a treadmill, which allowed therapists to adjust the walking speed externally. The most detailed training protocols were described in the controlled trials [31, 35–37], wherein individuals were progressed according to various intensity guidelines such as rate of perceived exertion (RPE) [35] and non-exoskeletal walking speed [37]. For example, Yoshimoto et al. [37] advanced the training speed to 1.5-1.7 times the maximal non-exoskeletal 10MWT walking speed before each session. Several studies [31, 32, 37, 38] allowed some body weight support using an overhead harness to improve walking mechanics.
Walking measures
Ten of the 11 studies included a measure of gait speed in their assessment of walking ability, either measuring it directly or via the 10-m Walk Test (10MWT). Five studies [31, 36, 39–41] assessed walking endurance by means of a 6-min Walk Test (6MWT), and seven studies [31, 36–41] assessed the Timed Up and Go (TUG) test, which is a measure of functional mobility as it includes sit-to-stand and turning. Two studies [31, 32] also included level of independence or assistance in their assessment of walking ability, using the Functional Ambulation Category (FAC). Participants were not wearing an exoskeleton device when assessed for the above measures in all studies, but gait aids such as canes and walkers were permitted.
Effectiveness of exoskeleton-based gait training
Ten studies reported varying degrees of improved walking ability after exoskeleton training (Table 2). Of the four sub-acute stroke studies, only one [31] was a randomized controlled trial (n = 22) which showed that participants using the HAL experienced a significant improvement in FAC scores compared to conventional gait rehabilitation matched for training time, no longer requiring manual assistance to walk after the training period (medium effect size). However, they found no significant difference between the HAL intervention and conventional therapy for walking speed or endurance. One small pre-post sub-acute study [32] (n = 8) also found an improvement in the median FAC score of their sub-acute participants from 0 (2-person assist to walk) to 1.5 (1-person assist to walk) after exoskeleton-based gait training. Participants in the two other pre-post studies [33, 34] in sub-acute stroke demonstrated improvements in walking speed with only a few sessions, though not all of their participants demonstrated a change greater than the established minimal clinically important difference (MCID) (Table 1).
Across the seven chronic stroke studies, improvements in walking ability were less apparent. In an RCT with 50 participants [35], there was no significant difference between the clinically meaningful improvements in gait speed made by participants in either the exoskeleton or functional training group matched for training time. Similarly, participants using the AlterG Bionic Leg (AlterG, USA) did not demonstrate significant improvements compared to the control group or to baseline after 18 training sessions in a small RCT with 24 participants [36]. In contrast, a nonrandomized controlled trial [37] found significant and clinically meaningful improvements in gait speed and TUG time after training using a HAL compared to conventional physical therapy; however, the control group did not receive the same number of exercise sessions. One larger pre-post study [38] (n = 16) did not find changes in gait speed that were beyond the established MCID (Table 1) while three small pre-post studies [39–41], each with three participants, found varying results. Clinical improvements in endurance were made by four participants in two of the pre-post studies [39, 40], using a minimal clinically important difference of 34.4 m in the 6MWT. [42] Three participants across the three smaller pre-post studies [39–41] made meaningful improvements in TUG scores. Four participants in two of the pre-post studies [40, 41] demonstrated a clinically meaningful improvement in walking speed, using an MCID of 0.06-0.14 m/s [43].
Adverse effects
Eight studies confirmed that no adverse events occurred during the course of the gait training intervention. One study [32] reported minor and temporary adverse effects such as skin irritation and pain from cuffs and bioelectric detection electrodes. Two studies [33, 34] did not report on adverse events. No studies reported adverse effects on the therapists.
Discussion
This scoping review was conducted to map the literature surrounding the use of powered robotic exoskeletons for gait retraining for individuals after stroke and to identify preliminary findings and areas where further research is required. This is a relatively new application of powered exoskeletons, as they have only recently become available for clinical use. As expected, there are only a small number of studies published relevant to this topic.
There were four different powered exoskeletons utilized amongst the included studies, ranging from unilateral, single joint devices to bilateral, multi-joint robotics with the capacity to detect volitional bioelectrical signals to initiate powered movement. Other exoskeletons exist on the commercial market for clinical application that have not yet been investigated for stroke such as the Ekso, Rewalk, and Indego (Parker Hannifin Corporation, USA). Research with these other exoskeletons is required to determine their clinical usefulness and would also strengthen the literature in general support of exoskeleton use for gait rehabilitation in stroke patients. Studies comparing unilateral to bilateral designs may also be another avenue for investigating the efficacy of exoskeletal gait retraining.
The majority of the included studies investigated exoskeleton-based gait training in chronic stroke participants. However, the greatest amount of functional and neurological recovery after stroke occurs in the first six weeks after stroke [3, 7]. In reflection of this, all four studies in the sub-acute phase of stroke reported positive effects of exoskeleton training. Two studies [31, 32] demonstrated improved walking independence with repeated exoskeletal gait training for more limited stroke participants, which is in line with findings using treadmill-based robotics [17]. In another study [33], there was significant improvement in walking speed (0.4 m/s) for stroke participants who had some voluntary motor control, but much less change (0.1 m/s) for those without voluntary control. The magnitude and parameter (ability, speed) of walking improvement may vary depending on the initial functional presentation of the exoskeleton user; furthermore, the spontaneous recovery following stroke is a confounding factor for the improvements reported that has yet to be rigorously controlled for in the current literature.
Study findings were not consistent for chronic stroke participants. All chronic stroke participants included were ambulatory, and so studies investigated changes in gait parameters rather than functional ability. While there were modest, but not consistent changes in the pre-post studies, the more rigorous RCTs [35, 36] did not show a difference from their respective control groups when groups were matched for exercise time and interaction with a physical therapist. Even in studies with longer training protocols [35, 36, 38, 41], there was not a trend for greater improvements. Despite receiving the repetitious practice that is required for motor learning [11, 12], chronic stroke participants do not respond as positively to exoskeletal gait training as sub-acute patients. This is consistent with findings in a systematic review [18] of treadmill-based exoskeleton devices for gait training in chronic, ambulatory individuals with stroke. A possible explanation for this is that once an individual is able to walk, they benefit more from unconstrained walking practice with greater variability and unpredictable challenges [14]. While powered exoskeletons do not require the participant to use a treadmill, they still constrain the user to a stereotyped movement pattern and may thus under-challenge them.
The majority of included studies had small sample sizes, which may have limited the power of their study findings and analysis. In addition to this, the majority of these studies were pilot feasibility or pre-post clinical studies; recruitment and lack of a control group may have introduced bias to their findings. For example, one study [37] used a non-randomized controlled design, where the control group was formed of participants who were less able to attend the study training protocol. These results inform the preliminary evidence in the field and more rigorous, appropriately powered randomized controlled trials will continue to advance the clinical application of powered exoskeletons.
Future directions for research and suggestions for clinical practice
From our data synthesis we have identified various considerations when using an exoskeleton for gait retraining and propose several questions for future research:
Do non-ambulatory chronic stroke participants experience the same improvement in walking ability as sub-acute stroke participants when using an exoskeleton device for gait retraining?
How does initial functional presentation impact the nature of improvement in walking ability when using an exoskeleton device for gait rehabilitation?
What is the impact of different exoskeletons (number of joints actuated, level of assistance and control of stepping) on gait rehabilitation in stroke?
What is the impact of using a bilateral design compared to a unilateral design for gait rehabilitation in hemiparetic stroke?
What is the optimal dose of exoskeletal gait training for stroke patients to regain the most walking ability?
How does overground exoskeletal gait training compare to body weight-supported treadmill training?
Can exoskeletons be used to safely ambulate 2-person assist patients early after stroke with minimal injury risk to therapists?
Additionally, larger sample sizes and rigorous methodology investigating the efficacy of powered exoskeletons in stroke will further strengthen findings for or against their utilization for gait rehabilitation.
At the moment there is insufficient evidence to advocate in favour or against use of powered exoskeletons in clinical practice. The patient’s acuity and functional presentation need to be considered and the extent of benefit has yet remain to be determined through high quality research. The devices, however, have been shown to be safe and feasible for use with stroke patients. They can be used to mobilize more impaired individuals without physically straining therapists. It thus remains up to therapists to use their own clinical judgement of whether to utilize powered exoskeletons with their patients for gait rehabilitation, considering its application for weight-bearing, standing, and automated walking.
Limitations
There are a few limitations with the present review. This review excluded non-English studies, which may have led to an incomplete synthesis of data, given that some exoskeletons are developed in non-English countries such as Japan, Germany, Iran, Israel, and Spain. There was heterogeneity in the studies, especially with variability in the training protocols and exoskeletons utilized (control mechanism, unilateral or bilateral application), which makes interpretation of the results challenging. In addition, type, side, and severity of stroke and comorbid conditions were not considered in this review because of the scarcity of studies in this area. As more research trials in stroke rehabilitation using powered exoskeletons are conducted, a systematic review will be able to address these additional considerations.
Conclusion
Currently, clinical trials demonstrate that powered robotic exoskeletons can be used safely as a gait training intervention for sub-acute and chronic stroke. Preliminary findings suggest that exoskeletal gait training is equivalent to traditional therapy for chronic stroke patients, while sub-acute patients may experience added benefit from exoskeletal gait training. Efforts should be invested in designing rigorous, appropriately powered controlled trials before it can be translated into a clinical tool for gait rehabilitation post-stroke.
Abbreviations
10MWT, 10-m walk test; 6MWT, 6-min walk test; BWSTT, body weight-supported treadmill training; FAC, functional ambulation category; HAL, hybrid assistive limb; MCID, minimal clinically important difference; RCT, randomized controlled trial; RPE, rate of perceived exertion; TUG, timed up and go
Acknowledgements
The authors acknowledge and thank Christina Cassady (CC), who acted as second reviewer in the search portion of this review.
Funding
The project was supported by funding from a Grant-in-Aid from the Heart and Stroke Foundation of Canada and the Canada Research Chairs Program.
Availability of data and materials
No original data is presented in this review.
Authors’ contributions
DRL formulated the idea, performed the search, and prepared the manuscript for this review. JJE participated in the writing process. Both authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This scoping review did not collect original data on human subjects; therefore ethics approval is not applicable. All studies included in this review reported receiving ethical approval and gaining consent from their participants.
References
- 1.Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Mensah GA, Bennett DA, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: the GBD 2013 study. Neuroepidemiology. 2015;45:161–76. doi: 10.1159/000441085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg. 2011;76:S85–90. doi: 10.1016/j.wneu.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Kwakkel G, Kollen BJ. Predicting activites after stroke: what is clinically relevant? Int J Stroke. 2013;8:25–32. doi: 10.1111/j.1747-4949.2012.00967.x. [DOI] [PubMed] [Google Scholar]
- 4.Bohannon RW, Andrews AW, Smith MB. Rehabilitation goals of patients with hemiplegia. Int J Rehabil Res. 1988;11:181–3. doi: 10.1097/00004356-198806000-00012. [DOI] [Google Scholar]
- 5.Harris JE, Eng JJ. Goal priorities identified by individuals with chronic stroke: Implications for rehabilitation professionals. Physiother Canada. 2004;56:171–6. doi: 10.2310/6640.2004.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portelli R, Lowe D, Irwin P, Pearson M, Rudd AG. Institutionalization after stroke. Clin Rehabil. 2005;19:97–108. doi: 10.1191/0269215505cr822oa. [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:27–32. doi: 10.1016/S0003-9993(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 8.Kollen BJ, Kwakkel G, Lindeman E. Longitudinal robustness of variables predicting independent gait following severe middle cerebral artery stroke: a prospective cohort study. Clin Rehabil. 2006;20:262–8. doi: 10.1191/0269215506cr910oa. [DOI] [PubMed] [Google Scholar]
- 9.Chen G, Chan CK, Guo Z, Yu H. A review of lower extremity assistive robotic exoskeletons in rehabilitation therapy. Crit Rev Biomed Eng. 2013;41:343–63. doi: 10.1615/CritRevBiomedEng.2014010453. [DOI] [PubMed] [Google Scholar]
- 10.Sale P, Franceschini M, Waldner A, Hesse S. Use of the robot assisted gait therapy in rehabilitation of patients with stroke and spinal cord injury. Eur J Phys Rehabil Med. 2012;48:111–21. [PubMed] [Google Scholar]
- 11.Scrivener K, Sherrington C, Schurr K. Exercise dose and mobility outcome in a comprehensive stroke unit: description and prediction from a prospective cohorot study. J Rehabil Med. 2012;44:824–9. doi: 10.2340/16501977-1028. [DOI] [PubMed] [Google Scholar]
- 12.French B, Thomas L, Leathley M, Sutton C, McAdam J, Forster A, et al. Does repetitive task training improve functional activity after stroke? A Cochrane systematic review and meta-analysis. J Rehabil Med. 2010;42:9–15. doi: 10.2340/16501977-0473. [DOI] [PubMed] [Google Scholar]
- 13.States RA, Salem Y, Pappas E. Overground gait training for individuals with chronic stroke: a Cochrane systematic review. J Neurol Phys Ther. 2009;33:179–86. doi: 10.1097/NPT.0b013e3181c29a8c. [DOI] [PubMed] [Google Scholar]
- 14.Hornby TG, Holleran CL, Hennessy PW, Leddy AL, Connolly M, Camardo J, et al. Variable intensity early walking poststroke (VIEWS): a randomized controlled trial. Neurorehabil Neural Repair. 2015 doi: 10.1177/1545968315604396. [DOI] [PubMed] [Google Scholar]
- 15.Rand D, Eng JJ. Disparity between functional recovery and daily use of the upper and lower extremities during subacute stroke rehabilitation. Neurorehabil Neural Repair. 2012;26:76–84. doi: 10.1177/1545968311408918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehrholz J, Pohl M, Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev. 2014;1 doi: 10.1002/14651858.CD002840.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ada L, Dean CM, Vargas J, Ennis S. Mechanically assisted walking with body weight support results in more independent walking than assisted overground walking in non-ambulatory patients early after stroke: a systematic review. J Physiother. 2010;56:153–61. doi: 10.1016/S1836-9553(10)70020-5. [DOI] [PubMed] [Google Scholar]
- 18.Mehrholz J, Elsner B, Werner C, Kugler J, Pohl M. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev. 2013;7 doi: 10.1002/14651858.CD006185.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hidler J, Nichols D, Pelliccio M, Brady K, Campbell DD, Kahn JH, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Repair. 2009;23:5–13. doi: 10.1177/1545968308326632. [DOI] [PubMed] [Google Scholar]
- 20.Dobkin BH, Duncan PW. Should body weight-supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate? Neurorehabil Neural Repair. 2012;26:308–17. doi: 10.1177/1545968312439687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turchetti G, Vitiello N, Trieste L, Romiti S, Geisler E, Micera S. Why effectiveness of robot-mediated neurorehabilitation does not necessarily influence its adoption. IEEE Rev Biomed Eng. 2014;7:143–53. doi: 10.1109/RBME.2014.2300234. [DOI] [PubMed] [Google Scholar]
- 22.Kolakowsky-Hayner SA, Crew J, Moran S, Shah A. Safety and feasibility of using the Ekso™ bionic exoskeleton to aid ambulation after spinal cord injury. J Spine. 2013 [Google Scholar]
- 23.Kressler J, Thomas CK, Field-Fote EC, Sanchez J, Widerström-Noga E, Cilien DC, et al. Understanding therapeutic benefits of overground bionic ambulation: Exploratory case series in persons with chronic, complete spinal cord injury. Arch Phys Med Rehabil. 2014;95:1878–87. doi: 10.1016/j.apmr.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Esquenazi A, Talaty M, Packel A, Saulino M. The ReWalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am J Phys Med Rehabil. 2012;91:911–21. doi: 10.1097/PHM.0b013e318269d9a3. [DOI] [PubMed] [Google Scholar]
- 25.Louie DR, Eng JJ, Lam T, SCIRE Research Team Gait speed using powered robotic exoskeletons after spinal cord injury: a systematic review and correlational study. J Neuroeng Rehabil. 2015;12:82. doi: 10.1186/s12984-015-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arazpour M, Samadian M, Bahramizadeh M, Joghtaei M, Maleki M, Bani MA, et al. The efficiency of orthotic interventions on energy consumption in paraplegic patients: a literature review. Spinal Cord. 2015;53:168–75. doi: 10.1038/sc.2014.227. [DOI] [PubMed] [Google Scholar]
- 27.Federici S, Meloni F, Bracalenti M, De Filippis ML. The effectiveness of powered, active lower limb exoskeletons in neurorehabilitation: a Systematic review. NeuroRehabilitation. 2015;37:321–40. doi: 10.3233/NRE-151265. [DOI] [PubMed] [Google Scholar]
- 28.Wall A, Borg J, Palmcrantz S. Clinical application of the Hybrid Assistive Limb (HAL) for gait training – a systematic review. Front Syst Neurosci. 2015;9:48. doi: 10.3389/fnsys.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 30.Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maeshima S, Osawa A, Nishio D, Hirano Y, Takeda K, Kigawa H, et al. Efficacy of a hybrid assistive limb in post-stroke hemiplegic patients: a preliminary report. BMC Neurol. 2011;11:116. doi: 10.1186/1471-2377-11-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byl NN. Mobility training using a bionic knee orthosis in patients in a post-stroke chronic state: a case series. J Med Case Rep. 2012;6:216. doi: 10.1186/1752-1947-6-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong CK, Bishop L, Stein J. A wearable robotic knee orthosis for gait training: a case-series of hemiparetic stroke survivors. Prosthet Orthot Int. 2012;36:113–20. doi: 10.1177/0309364611428235. [DOI] [PubMed] [Google Scholar]
- 34.Kawamoto H, Kamibayashi K, Nakata Y, Yamawaki K, Ariyasu R, Sankai Y, et al. Pilot study of locomotion improvement using hybrid assistive limb in chronic stroke patients. BMC Neurol. 2013;13:141. doi: 10.1186/1471-2377-13-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilsson A, Vreede KS, Haglund V, Kawamoto H, Sankai Y, Borg J. Gait training early after stroke with a new exoskeleton – the hybrid assistive limb: a study of safety and feasibility. J NeuroEng Rehabil. 2014;11:92. doi: 10.1186/1743-0003-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein J, Bishop L, Stein DJ, Wong CK. Gait training with a robotic leg brace after stroke: a randomized controlled pilot study. Am J Phys Med Rehabil. 2014;93:987–94. doi: 10.1097/PHM.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe H, Tanaka N, Inuta T, Saitou H, Yanagi H. Locomotion improvement using a hybrid assistive limb in recovery phase stroke patients: a randomized controlled pilot study. Arch Phys Med Rehabil. 2014;95:2006–12. doi: 10.1016/j.apmr.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Bortole M, Venkatakrishnan A, Zhu F, Moreno JC, Francisco GE, Pons JL, et al. The H2 robotic exoskeleton for gait rehabilitation after stroke: early findings from a clinical study. J NeuroEng Rehabil. 2015;12:54. doi: 10.1186/s12984-015-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buesing C, Fisch G, O’Donnell M, Shahidi I, Thomas L, Mummidisetty CK, et al. Effects of a wearable exoskeleton stride management assist system (SMA®) on spatiotemporal gait characteristics in individuals after stroke: a randomized controlled trial. J NeuroEng Rehabil. 2015;12:69. doi: 10.1186/s12984-015-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukuda H, Samura K, Hamada O, Saita K, Ogata T, Shiota E, et al. Effectiveness of acute phase hybrid assistive limb rehabilitation in stroke patients classified by paralysis severity. Neurol Med Chir (Tokyo) 2015;55:487–92. doi: 10.2176/nmc.oa.2014-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshimoto T, Shimizu I, Hiroi Y, Kawaki M, Sato D, Nagasawa M. Feasibility and efficacy of high-speed gait training with a voluntary driven exoskeleton robot for gait and balance dysfunction in patients with chronic stroke: nonrandomized pilot study with concurrent control. Int J Rehabil Res. 2015;38:338–43. doi: 10.1097/MRR.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 42.Tang A, Eng JJ, Rand D. Relationship between perceived and measured changes in walking after stroke. J Neurol Phys Ther. 2012;36:115–21. doi: 10.1097/NPT.0b013e318262dbd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–9. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 44.Flansbjer UB, Homback AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37:75–82. doi: 10.1080/16501970410017215. [DOI] [PubMed] [Google Scholar]
- 45.Tilson JK, Sullivan KJ, Cen SY, Rose DK, Koradia CH, Azen SP, et al. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther. 2010;90:196–208. doi: 10.2522/ptj.20090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No original data is presented in this review.


