Abstract
Currently, immunotherapy by blocking the immune checkpoint inhibitors, such as anti-PD-1, has been carried out in many clinical studies on recurrent glioma, and the preliminary results are satisfactory, which provides a rationale for the exploration of immune checkpoint inhibitors in glioma. B7-H6 is a newly discovered member of the B7 family, which triggers antitumor of natural killer cell cytotoxicity and cytokine secretion by binding the NKp30 receptor. B7-H6 mRNA and protein expressions, which are not detected in normal tissues, are expressed mainly on the cell surface of various primary tumors and cell lines. However, up until now, there is no data about the clinical significance of B7-H6 expression in astrocytoma patients. The present study provides an investigation on the relationship between prognostic and clinical value of B7-H6 protein in astrocytoma tissues. All the astrocytic glioma tissues were stained for B7-H6. Immunohistochemistry stain of 122 astrocytoma samples showed that immunoreactivity of B7-H6 was seen predominantly in the cytoplasm. The B7-H6 expression did not show significant relevance with patient age, sex distribution, Karnofsky performance status score, extent of resection, and tumor location in astrocytoma patients, but B7-H6 positive expression is significantly associated with World Health Organization grade (P=0.046). However, the survival rate after operation presented no significant difference of B7-H6 expression in astrocytoma patients. Kaplan–Meier analysis and the log-rank test revealed that B7-H6 expression cannot predict the overall survival. In all, it seems that the B7-H6 expression might be a marker to differentiate the World Health Organization grade level of astrocytoma, but the prognosis value of B7-H6 in astrocytoma should be studied in detail.
Keywords: B7-H6, astrocytoma, glioma, immunotherapy
Introduction
Astrocytic glioma is the most common type of primary malignant brain tumor.1 The 5-year survival rate in patients with glioma is among the lowest for all cancers.2 Conventional therapies, such as surgery, chemotherapy, and radiotherapy, play an important role in the treatment of malignant gliomas; however, the prognosis of malignant gliomas is still poor.3, 4 Since astrocytoma patients face a dismal prognosis and have limited therapeutic options, developing a new treatment modality is necessary. Immunotherapy with immune checkpoint inhibitors, such as ipilimumab and nivolumab, has provided relevant clinical improvements in other advanced tumors for which conventional therapies have had limited success, making immunotherapy an appealing strategy in glioma, which provides a rationale for the exploration of immune checkpoint inhibitors in glioma.
The B7 family members, which played critical roles in the control and fine tuning of antigen-specific immune responses, have great implications for the treatment of cancer.5 At present, several B7 family members have been found in glioma. B7-H6 is a newly discovered member of the B7 family, which triggers the antitumor of natural killer cell cytotoxicity and cytokine secretion by binding the NKp30 receptor.6 Recent studies showed that B7-H6 mRNA and protein expressions have not been detected in most normal adult tissues, while B7-H6 cell surface expression is observed in tumor cell lines from various origins, such as lymphoma, leukemia, melanoma, and carcinoma as well as on primary tumor blood cells, which indicates that its expression may take an important part in tumor prognosis.6, 7 However, up until now, no data about the clinical significance of B7-H6 expression in patients of astrocytoma have been reported.
In this article, we investigated the B7-H6 expression in tumor specimens collected from a large cohort of astrocytoma patients. We then confirmed the correlation of intratumoral B7-H6 expression with various clinicopathological parameters and patient survival to investigate whether B7-H6 acts as a novel identified prognostic marker in astrocytoma patients.
Materials and methods
Paraffin-embedded tumor samples were obtained from 122 astrocytoma patients who underwent surgery at the Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China, between 2000 and 2008. Patients with autoimmune diseases were excluded. None of the patients had received anticancer treatments prior to surgery. The follow-up dates of the patients in this study are available and complete. Overall survival (OS), which was defined as the time from operation to patient death or the last follow-up, was used as a measure of prognosis. This study was approved by the Ethics Committee of the Sun Yat-sen University Cancer Center, and written informed consent was obtained from each patient.
Immunohistochemical staining
Immunohistochemical staining was performed using a two-step method (Envision™). Paraffin-embedded tissues were cut into 5 μm serial sections, transferred onto adhesive slides, and dried at 65°C for 30 minutes. The sections were deparaffinized with xylene and rehydrated through graded alcohols. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide solution for 30 minutes, and antigen retrieval was performed at 100°C for 30 minutes in a citrate buffer (10 mmol/L, pH 6.0). After being washed three times with phosphate-buffered saline (PBS) for 5 minutes each, the sections were incubated with 10% normal goat serum to block nonspecific binding. The slides were then incubated overnight at 4°C with rabbit antihuman B7-H6 (Abcam, Cambridge, MA, USA; dilution 1/100). The slides were incubated with horseradish peroxidase (ChemMate™, DAKO Envision™ Detection Kit, Dako, Glostrup, Denmark) at room temperature for 30 minutes. After the slides were washed in PBS, the visualization signal was developed with 3,3′-diaminobenzidine solution, and all slides were counterstained with hematoxylin, dehydrated in graded alcohol, and mounted with a neutral resin. Negative controls were prepared by replacing the primary antibody with PBS. Human gallbladder tissue was used as a positive control.
The B7-H6 immunostaining score was calculated as both the percentage of positively stained tumor cells and the staining intensity. The percent positivity was scored as “0” (<5%, negative), “1” (5%–25%, sporadic), “2” (25%–50%, focal), or “3” (>50%, diffuse). The staining intensity was scored as “0” (no staining), “1” (weakly stained), “2” (moderately stained), or “3” (strongly stained). Both the percentage of positive cells and the staining intensity were evaluated under double-blind conditions. Two independent pathologists examined and scored each sample without any knowledge of the patient outcome (double-blinded). The B7-H6 score was calculated as the percentage positive score × the staining intensity score and ranged from 0 to 9. Based on the B7-H6 expression levels, the astrocytoma patients were divided into two groups: the low B7-H6 expression group (score 0–4) and the high B7-H6 expression group (score 6–9).
Statistical analysis
The statistical significance of the correlations between B7-H6 expression and the clinicopathologic features was analyzed using the chi square (χ2) test. Univariate and multivariate survival analyses were performed using the Kaplan–Meier analysis and log-rank test. All statistical analyses were performed with the SPSS software (Version 19.0; StataCorp LP, College Station, TX, USA). For all tests, a P-value of ≤0.05 was considered statistically significant.
Results
Study population
The patient characteristics are presented in Table 1. Of the 122 patients examined, 91(74.6%) had died before the end of the observation period. The median age of the study population was 42 years (range: 2–75 years). The majority of patients (90/122, 73.8%) underwent total tumor resection. There were 41 cases (33.6%) of grade II, 32 cases (26.2%) of grade III, and 49 cases (40.2%) of grade IV astrocytoma, according to the World Health Organization (WHO) classification criteria. The median follow-up for the entire cohort was 35.3 months (range: 2.0–135.0 months). The 2-year survival rate for the entire study population was 43.4%.
Table 1.
Clinical characteristics of 122 astrocytoma patients
| Characteristic | Number (%) |
|---|---|
| Age (years) | |
| Median | 40 |
| Range | 2–75 |
| Sex | |
| Male | 72 (59.0) |
| Female | 50 (41.0) |
| WHO grade | |
| II | 41 (33.6) |
| III | 32 (26.2) |
| IV | 49 (40.2) |
| KPS | |
| ≥70 | 115 (94.3) |
| <70 | 7 (5.7) |
| Extent of resection | |
| Total | 90 (73.8) |
| Subtotal | 32 (26.2) |
| Location | |
| Supratentorial | 113 (92.6) |
| Infratentorial | 9 (7.4) |
| Death | |
| No | 31 (25.4) |
| Yes | 91 (74.6) |
Abbreviations: KPS, Karnofsky performance status; WHO, World Health Organization.
Immunohistochemical characteristics
B7-H6 expression was immunohistochemically assessed in 122 glioma specimens, of which 102 (83.6%) showed low B7-H6 expression and 20 (16.4%) exhibited high B7-H6 expression. In the positive specimens, immunoreactivity of B7-H6 was seen predominantly in the cytoplasm (Figure 1).
Figure 1.
Immunohistochemical analysis of B7-H6 expression in astrocytoma human brain specimens of varying World Health Organization grades.
Notes: B7-H6 immunohistochemically stained grade II, grade III, and grade IV astrocytoma specimens (magnification: left ×200, right ×400). Blue stain is for the nucleus of astrocytoma, brown stain is for B7-H6 expression in the cytoplasm, and the black arrows is for the strongly stained.
Relationships between B7-H6 and clinicopathological characteristics
Table 2 summarizes the associations between B7-H6 protein expression and the clinicopathological characteristics of human astrocytoma cases. The B7-H6 protein expression presented no significant association with patient age, sex distribution, Karnofsky performance status score, extent of resection, and tumor location (Table 2, P>0.05). However, the B7-H6 expression was significantly associated with the tumor WHO grade (Table 2, χ2=6.142, P=0.046).
Table 2.
Correlation of B7-H6 expression with clinicopathological characteristics of astrocytoma patients
| Variable | B7-H6 expression
|
P-value | χ2 value | |
|---|---|---|---|---|
| Low | High | |||
| All cases | 102 | 20 | ||
| Sex | ||||
| Male | 59 | 13 | ||
| Female | 43 | 7 | 0.552 | 0.354 |
| Age (years) | ||||
| ≥40 | 48 | 10 | ||
| <40 | 54 | 10 | 0.058 | 0.810 |
| KPS | ||||
| ≥70 | 95 | 20 | ||
| <70 | 7 | 0 | 0.496 | 0.464 |
| Extent of resection | ||||
| Total | 75 | 15 | ||
| Subtotal | 27 | 5 | 0.891 | 0.019 |
| Location | ||||
| Supratentorial | 96 | 17 | ||
| Infratentorial | 6 | 3 | 0.338 | 0.919 |
| WHO grade | ||||
| II | 37 | 4 | ||
| III | 29 | 3 | ||
| IV | 36 | 13 | 0.046a | 6.142 |
| Survive time after surgery | ||||
| ≥2 year | 44 | 9 | ||
| <2 year | 58 | 11 | 0.878 | 0.024 |
Note:
P-value <0.05 was considered significant.
Abbreviations: KPS, Karnofsky performance status; WHO, World Health Organization.
Correlation of intratumoral B7-H6 expression with patient survival
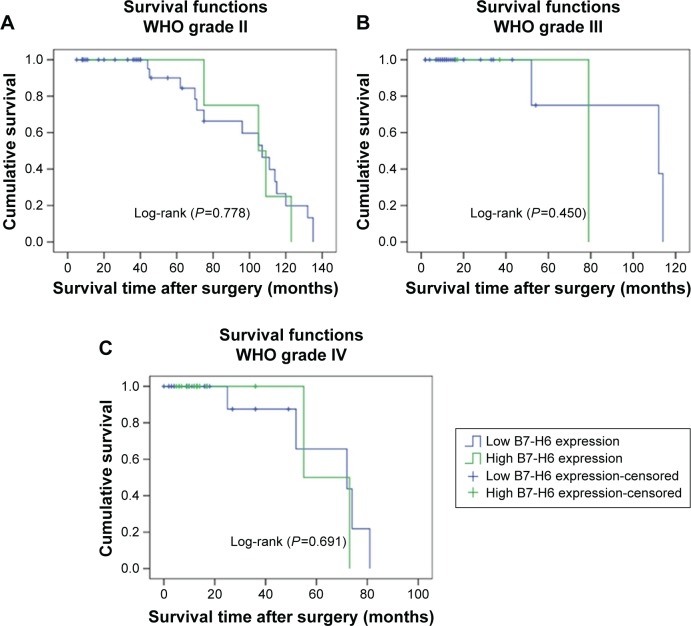
Kaplan–Meier analysis and the log-rank test were used to evaluate the effect of the B7-H6 expression on OS in astrocytoma patients. The prognostic value of B7-H6 for OS was evaluated statistical significance (Figure 2, Log-rank [P>0.05]). The separate analysis of the OS of B7-H6 expression with the different WHO grades also showed no statistical significance (Figure 3, Log-rank [P>0.05]). Univariate and multivariate analyses were conducted using Cox proportional hazards model to examine the impact of B7-H6 expression and other clinicopathological parameters in astrocytoma patients. Univariate analysis showed that the WHO grades were significant prognostic factors (Table 3, P<0.05). Multivariate Cox regression analyses showed that the WHO grade was an independent prognostic factor (Table 3, P<0.05), whereas univariate and multivariate analyses revealed that B7-H6 was not an independent prognostic factor (Table 3, P>0.05). Thus, the survival analysis did not confirm the prognostic significance of B7-H6 expression in astrocytoma.
Figure 2.
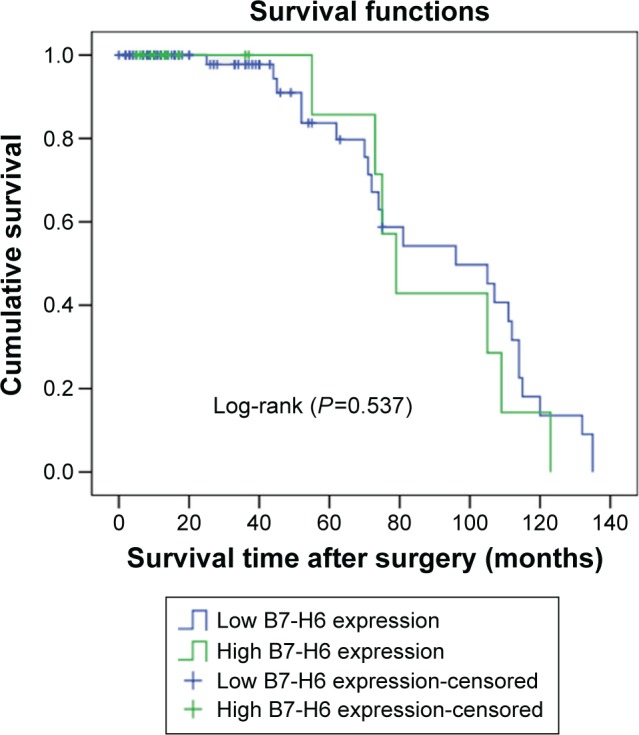
Kaplan–Meier survival curve in astrocytoma patients after surgery according to B7-H6 expression (n=122) (P=0.537).
Figure 3.
Survival curves for 122 astrocytoma patients with different WHO grades according to B7-H6 expression.
Notes: (A) Survival curves for the 39 patients with WHO grade II according to B7-H6 expression (P=0.778). (B) Survival curves for the 29 patients with WHO grade III according to B7-H6 expression (P=0.450). (C) Survival curves for the 36 patients with WHO grade IV according to B7-H6 expression (P=0.691).
Abbreviation: WHO, World Health Organization.
Table 3.
Univariate and multivariate Cox regression analyses of patient survival
| Covariate | Univariate
|
Multivariate
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Sex (male vs female) | 0.627 | 0.300–1.311 | 0.215 | 0.631 | 0.277–1.438 | 0.273 |
| Age (≥40 years vs <40 years) | 1.162 | 0.485–2.780 | 0.736 | 1.352 | 0.467–3.909 | 0.578 |
| KPS (≥70 vs <70) | 1.580 | 0.208–12.026 | 0.658 | 0.253 | 0.023–2.751 | 0.259 |
| Extent of resection (total vs subtotal) | 0.667 | 0.229–1.941 | 0.457 | 1.071 | 0.345–3.320 | 0.905 |
| Tumor location (supratentorial vs infratentorial) | 1.380 | 0.529–3.597 | 0.510 | 3.469 | 0.991–12.140 | 0.052 |
| WHO grade (T2/T3/T4) | 2.220 | 1.319–3.378 | 0.003a | 3.087 | 1.565–6.090 | 0.001a |
| B7-H6 (low vs high) | 1.308 | 0.552–3.097 | 0.542 | 0.911 | 0.352–2.360 | 0.849 |
Note:
P-value <0.05.
Abbreviations: KPS, Karnofsky performance status; WHO, World Health Organization; CI, confidence interval; HR, hazard ratio.
Discussion
Primary nervous system tumors account for 1.4% of all cancers and cause 2.4% of all cancer deaths in the US.2 Although a lot of progress has been made in reducing mortality rates owing to both earlier detection and improved therapies, including chemotherapy and radiotherapy, glioma still is a major threat to human health.8 It would therefore be valuable to develop more approaches that could improve survival rates in glioma patients. Nowadays, immunotherapy is an important and effective combination detection for malignancy. Immunotherapy such as blocking the immune checkpoint molecule that can recover the capability of T-cells to discover and attack cancer cells and promote their anticancer response is a promising strategy in cancer therapy. Antibody-based blockade of CTLA-4 ligation on T lymphocytes is associated with enhanced antitumor immunity in animal models of cancer and in patients with advanced melanoma.9 Early-stage clinical trials reported that blocking the immune checkpoint molecule programmed death 1 (PD-1) and its ligand (PD-L1) could induce impressive durable responses in patients with advanced cancer.10 The introduction of immune checkpoint inhibitors has dramatically changed the prognosis for some advanced tumors. The US Food and Drug Administration first approved ipilimumab (a checkpoint inhibitor targeted CTLA-4) to treat patients with late-stage melanoma in March 2011, and pembrolizumab and nivolumab (two checkpoint inhibitors that target PD1) for unresectable or metastatic melanoma in late 2014.11 More new agents targeting PD1 or PD-L1 have been investigated alone and in combination treatment for various cancers,12–14 including glioma.15,16 Because of the immunotherapy progress in other cancers and the current understanding of the interaction between the brain and the immune system, it provides a rationale for the exploration of immune checkpoint inhibitors in astrocytoma.
The B7 family members are transmembrane proteins which can produce a co-stimulatory signal or a co-inhibitory signal to regulate immune responses. They are widely expressed on tumor cells and the tumor cell microenvironment. The co-inhibitory B7-H ligands promote the suppression of host antitumor response, whereas the co-stimulatory molecules might affect the growth of the malignant tumor cells.5, 17 The two primary members of B7-1 and B7-2 provide a balance of positive and negative signals required for appropriate priming of naïve T-cells by interacting with CD28 and CTLA-4. The other members, such as B7-H1, B7-DC, B7-H2, B7-H3, and B7-H4, described as B7 homologs, have a less restricted distribution and their expression in cancer has been predictive of patient prognosis.18 B7-H6 is among the most recently identified members of the B7 superfamily, which is a PD-L1/B7-H3 homologue specifically binding the CTLA-4-homologous NK-effector molecule NKp30. Unlike other B7 family members, B7-H6 mRNA and protein expression are not detected in normal tissues while expressed mainly on the cell surface of various primary tumors and cell lines. B7-H6 and the expression of B7-H6 on tumor cells induces NKp30-dependent cell activation and cytotoxicity. Therefore, it seems that its expression may play an important role in tumor prognosis.7 If B7-H6 expression is specific and stable in tumor cells, immunotherapy based on the effective blockade of the tumor-associated B7-H6 could be a promising clinical strategy.
The present study provides the first investigation about the relationship between prognostic and clinical value of B7-H6 protein in astrocytoma tissues. All the astrocytoma tissues were stained for B7-H6. Immunohistochemistry stain of 122 astrocytoma samples showed that immunoreactivity of B7-H6 was seen predominantly in the cytoplasm, which is consistent with other studies.19,20 The B7-H6 expression did not show significant relevance with patient age, sex distribution, Karnofsky performance status score, extent of resection, and tumor location in astrocytoma patients. However, B7-H6 positive expression is significantly associated with WHO grade, which demonstrated that B7-H6 expression might be a marker to differentiate the WHO grade level of astrocytoma. That suggested B7-H6 expression level was significantly associated with the degree of differentiation, whereas it was not correlated with other clinical parameters. This result is similar to the study in gastric carcinoma and lung cancer.19, 20 This implied that high B7-H6 expression in astrocytoma patients may indicate more malignancy and calls for more aggressive treatment and close surveillance. However, the survival rate after an operation presented no significant difference of B7-H6 expression in astrocytoma patients. The separate analysis of the OS of B7-H6 expression with different WHO grades also showed no statistical significance. Univariate and multivariate analyses revealed that B7-H6 was not an independent prognostic factor. Thus, Kaplan–Meier analysis and the log-rank test revealed that B7-H6 expression may not be useful for predicting the OS of astrocytoma patients, which also have similar outcome in gastric carcinoma and lung cancer.19, 20 Altogether, as detected by immunohistochemistry, the survival analysis did not confirm the prognostic significance of B7-H6 expression in astrocytoma. As the number of patients we have enrolled is small and we have only detected the B7-H6 protein expression by immunohistochemistry, further studies referring to the B7-H6 expression in mRNA and gene level should be conducted to confirm the prognostic significance of B7-H6 expression in astrocytoma.
In conclusion, we have shown that B7-H6 expression occurred in astrocytoma specimens and was associated with astrocytoma grade. Since the number of astrocytoma patients we studied is small and the B7-H6 expression might be different in many other levels, more researches should be undertaken to investigate the prognostic significance expression of B7-H6 in astrocytoma.
Acknowledgments
The authors thank the patients who participated in this study. This work was supported by the Science and Technology Planning Project of Guangdong Province, People’s Republic of China (Nos Z012B031800382 and 2014A020212098); National Natural Science Foundation of China (Nos 81401908, 81201982, and 81572500); and the Doctoral Fund of Ministry of Education of China (No 20120171120110).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-oncology. 2014;16(Suppl 4):iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 5.Seliger B, Quandt D. The expression, function, and clinical relevance of B7 family members in cancer. Cancer Immunol Immunother CII. 2012;61(8):1327–1341. doi: 10.1007/s00262-012-1293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baratin M, Vivier E. B7-H6: a novel alert signal for NK cells. Med Sci M/S. 2010;26(2):119–120. doi: 10.1051/medsci/2010262119. [DOI] [PubMed] [Google Scholar]
- 7.Brandt CS, Baratin M, Yi EC, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206(7):1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang SM, Butowski NA, Sneed PK, Garner IV. Standard treatment and experimental targeted drug therapy for recurrent glioblastoma multiforme. Neurosurg Focus. 2006;20(4):E4. [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. New Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2015;27(1):39–46. doi: 10.1093/intimm/dxu095. [DOI] [PubMed] [Google Scholar]
- 11.Ito A, Kondo S, Tada K, Kitano S. Clinical development of immune checkpoint inhibitors. BioMed Res Int. 2015;2015(5):583–595. doi: 10.1155/2015/605478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azoury SC, Straughan DM, Shukla V. Immune Checkpoint Inhibitors for Cancer Therapy: Clinical Efficacy and Safety. Curr Cancer Drug Targets. 2015;15(6):452–462. doi: 10.2174/156800961506150805145120. [DOI] [PubMed] [Google Scholar]
- 13.Bedke J, Kruck S, Gakis G, Stenzl A, Goebell PJ. Checkpoint modulation – a new way to direct the immune system against renal cell carcinoma. Hum Vaccin Immunother. 2015;11(5):1201–1208. doi: 10.1080/21645515.2015.1016657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helissey C, Champiat S, Soria JC. Immune checkpoint inhibitors in advanced nonsmall cell lung cancer. Curr Opin Oncol. 2015;27(2):108–117. doi: 10.1097/CCO.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 15.Agarwalla P, Barnard Z, Fecci P, Dranoff G, Curry WT., Jr Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. J Immunother (Hagerstown, Md.: 1997) 2012;35(5):385–389. doi: 10.1097/CJI.0b013e3182562d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nature reviews. Neurology. 2015;11(9):504–514. doi: 10.1038/nrneurol.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121(5):734–744. doi: 10.1182/blood-2012-10-385591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung J, Suh WK. The CD28-B7 family in anti-tumor immunity: emerging concepts in cancer immunotherapy. Immune Netw. 2014;14(6):265–276. doi: 10.4110/in.2014.14.6.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen XJ, Shen J, Zhang GB, Chen WC. B7-H6 protein expression has no prognostic significance in human gastric carcinoma. Pathol Oncol Res. 2014;20(1):203–207. doi: 10.1007/s12253-013-9686-1. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Zhang G, Qin Y, Bai R, Huang J. B7-H6 expression in non-small cell lung cancers. Int J Clin Exp Pathol. 2014;7(10):6936–6942. [PMC free article] [PubMed] [Google Scholar]