Abstract
Background
Matrix metalloproteinase 9 (MMP-9) plays an important role in tumor invasion and metastasis, including lung cancer. However, whether variations in serum MMP-9 levels can serve as a biomarker for monitoring chemotherapy curative effect remains unclear. This study was designed to investigate the association between variations in serum MMP-9 levels and chemotherapy curative effect in patients with lung cancer.
Patients and methods
A total of 82 patients with advanced lung cancer were included. All newly diagnosed patients were treated with platinum-based doublet chemotherapy. Serial measurements of serum MMP-9 levels were performed by enzyme-linked immunosorbent assay. In this manner, we chose four time points to examine the association, including before chemotherapy, and 3 weeks after the beginning of the first, second, and fourth cycles of chemotherapy.
Results
Compared with the serum level of MMP-9 before progressive disease, patients with progressive disease had elevated serum levels of MMP-9. Compared with the previous time point of collecting specimens, the serum levels of MMP-9 in the patients with a complete response/partial response/stable disease decreased or were maintained stable. The differences of variation in serum MMP-9 levels in patients with different chemotherapy curative effects were all statistically significant after one cycle, two cycles, and four cycles (after one cycle: P<0.001; after two cycles: P<0.001; after four cycles: P=0.01). However, patients with small-cell lung cancer did not exhibit similar test results.
Conclusion
The variation in serum MMP-9 levels in patients with non-small-cell lung cancer during chemotherapy was closely related to chemotherapy curative effect and could be useful to monitor chemotherapy curative effect for a small portion of patients.
Keywords: prognosis, lung adenocarcinoma, lung squamous carcinoma, chemotherapy, treatment monitoring
Introduction
Lung cancer is one of the worldwide leading causes of cancer-related mortality.1 More than 70% of patients are diagnosed with advanced lung cancer because of the lack of early symptoms and specific clinical manifestations. Chemotherapy remains an important modality for treatment, although the 5-year survival rate is <15%. The main cause of treatment difficulty is tumor invasion and metastasis. Matrix metalloproteinase 9 (MMP-9), a member of the MMP family, is closely correlated with tumor cell invasion and metastasis.2 Downregulation of MMP-9 expression by RNA interference or the use of DNAzymes inhibits cell migration and invasion.2–5 Previous studies have demonstrated that overexpression of MMP-9 in serum or tissue is related to an increased risk of lung cancer invasion and metastasis.6–9
Previous investigations on the use of serum MMP-9 levels as a biomarker in patients with lung cancer focused primarily on prognosis.10,11 Little is known about the usefulness of serum MMP-9 as a biomarker for chemotherapy curative effect. Yazar et al12 demonstrated that serum MMP-9 levels were not associated with chemotherapy curative effect; however, Ertan et al13 found decreased serum MMP-9 levels only in chemotherapy responders among patients with advanced non-small-cell lung cancer (NSCLC).
In the present study, we investigated the correlation between variations in serum MMP-9 levels and chemotherapy curative effect in patients with lung cancer and evaluated the potential use of variations in serum MMP-9 levels as a biomarker for monitoring chemotherapy curative effect.
Patients and methods
Patients
In total, 82 patients with advanced lung cancer registered at Beijing Chest Hospital, Capital Medical University, People’s Republic of China, from August 2013 to October 2014, were involved in this study. The patients’ diagnosis was confirmed by histological or cytological examination. None of the patients had received prior treatment. The patient sample comprised 59 males and 23 females with a median age of 60 years (range, 20–79 years). The patients were classified into those with NSCLC (n=53, including 35 adenocarcinomas and 18 squamous cell carcinomas) and those with small-cell lung cancer (SCLC; n=29). Of the 82 patients, 25 patients were classified as stage III and 57 were classified as stage IV.14 The clinical characteristics of the patients are shown in Table 1. The inclusion criteria were as follows: 1) adequate organ, bone marrow, liver, and renal function; 2) measureable lesions before treatment; 3) Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; and 4) life expectancy ≥3 months. This study was approved by the Ethics Committee of Beijing Chest Hospital, and all of the patients provided written informed consent.
Table 1.
The clinical characteristics of the patients
| Clinical characteristics | n (%) |
|---|---|
| Sex | |
| Male | 59 (72.0) |
| Female | 23 (28.0) |
| Age (range) | 60 (20–79) |
| ≤60 years | 47 (57.3) |
| >60 years | 35 (42.7) |
| Smoking status | |
| Never | 17 (20.7) |
| Smoker | 65 (79.3) |
| Baseline ECOG performance status | |
| 0–1 | 72 (87.8) |
| 2 | 10 (12.2) |
| Histological subtype | |
| Adenocarcinomas | 35 (42.7) |
| Squamous cell carcinomas | 18 (21.9) |
| SCLC | 29 (35.4) |
| T status | |
| T1 | 3 (3.7) |
| T2 | 28 (34.1) |
| T3 | 13 (15.9) |
| T4 | 38 (46.3) |
| N status | |
| N0 | 8 (9.8) |
| N1 | 3 (3.7) |
| N2 | 23 (28) |
| N3 | 48 (58.5) |
| M status | |
| M0 | 25 (30.5) |
| M1 | 57 (69.5) |
| TNM stage | |
| IIIa | 8 (9.8) |
| IIIb | 17 (20.7) |
| IV | 57 (69.5) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; SCLC, small-cell lung cancer.
Chemotherapy and response evaluation
All patients were treated with platinum-based doublets, including cisplatin/carboplatin/nedaplatin (cisplatin 75 mg/m2 on days 1 and 2; carboplatin area under the curve 5 on day 2; and nedaplatin 75 mg/m2 on day 1). Platinum was combined with the following drugs to treat different histological types of cancer: paclitaxel liposome or pemetrexed (500 mg/m2 on day 1) for adenocarcinoma, paclitaxel liposome (150–175 mg/m2 on day 1) for squamous cell carcinoma, and etoposide (100 mg once, days 1–5) for SCLC. The cycle was repeated every 3 weeks. We terminated chemotherapy in progressive disease (PD) or direct refusal, and we excluded patients with unbearable toxicity during chemotherapy.
Tumor size was determined by computed tomography scans at four time points, including before chemotherapy and 3 weeks after the beginning of the first, second, and fourth cycles of chemotherapy. Patient responses were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1): complete response (CR), partial response (PR), stable disease (SD), and PD.15
Sample collection and measurement of serum MMP-9 levels
Blood samples were collected at four time points: before chemotherapy and 3 weeks after the beginning of the first, second, and fourth cycles of chemotherapy. Serial samples were obtained from 53 NSCLC and 29 SCLC patients. We obtained all the samples at the first and second time points, from 44 and 23 patients at the third time point, and ended up with 26 and 13 patients at the last time point from NSCLC an SCLC patients respectively. Each sample was centrifuged at 3,000 rpm for 15 minutes at room temperature, separated, and stored in aliquots at −80°C until use.
The serum MMP-9 level was determined using an enzyme-linked immunosorbent assay kit for MMP-9 (USCN Life Science, Houston, TX, USA) according to the manufacturer’s instructions. In brief, 100 μL of standard dilution and blank aliquots were added, and the sera were diluted 400-fold in an appropriate well and incubated for 24 hours at 4°C. After the reaction solution was removed, 100 μL of diluted detection antibodies were added to each well and incubated for 1 hour at 37°C. The wells were washed three times, and 100 μL of diluted detection antibodies conjugated with horseradish peroxidase were added to each well and incubated for 30 minutes at 37°C. The wells were washed five times, and 90 μL of tetramethylbenzidine substrate solution was added to each well and incubated at 37°C for 15 minutes in the dark. Approximately 50 μL of stop solution was added to each well, and absorbance at 450 nm was determined. Each sample was detected in duplicate.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 (IBM Corporation, Armonk, NY, USA). Nonparametric statistical analysis was used because of the wide range of data obtained in this study. The serum MMP-9 concentrations are presented as medians and ranges. The results of the comparison between serum MMP-9 levels and tumor response were analyzed using the Mann–Whitney U-test. A chi-squared (χ2) test was performed to evaluate the associations between clinical variables. A two-sided level of 0.05 was used in the statistical analyses. A P-value of <0.05 was considered statistically significant.
Results
Correlation between prechemotherapy serum MMP-9 levels and clinical characteristics
There was no correlation between the serum level of MMP-9 in lung cancer patients and sex, age, smoking status, performance status score, histological types, primary tumor size, regional lymph node metastases, distant metastases, and TNM stages.
Further stratified analysis showed that serum MMP-9 levels before chemotherapy were not significantly associated with the clinical characteristics of the patients with NSCLC and SCLC. Specific data are shown in Table 2.
Table 2.
The correlation between serum MMP-9 levels before chemotherapy and clinical characteristics of patients with NSCLC and SCLC
| Characteristics | Serum MMP-9 level (μg/mL) | |||
|---|---|---|---|---|
|
| ||||
| NSCLC
|
SCLC
|
|||
| Median (range) | P-value | Median (range) | P-value | |
| Sex | 0.121 | 0.463 | ||
| Male | 2.80 (1.08–6.96) | 2.14 (1.01–5.54) | ||
| Female | 2.29 (0.80–3.59) | 2.38 (1.23–4.06) | ||
| Age, years | 0.536 | 0.505 | ||
| ≤60 | 2.61 (0.80–5.49) | 2.14 (1.01–5.07) | ||
| <60 | 2.79 (1.08–6.96) | 2.69 (1.27–5.54) | ||
| Smoking status | 0.965 | 0.484 | ||
| Never | 2.87 (1.34–4.84) | 1.95 (1.23–3.48) | ||
| Smoker | 2.74 (0.80–6.96) | 2.59 (1.01–5.54) | ||
| Baseline ECOG performance status | 0.301 | 0.773 | ||
| 0–1 | 2.74 (0.80–6.96) | 2.37 (1.01–5.54) | ||
| 2 | 3.66 (1.49–6.00) | 2.14 (1.55–3.48) | ||
| Histological subtype | 0.499 | NA | ||
| Adenocarcinomas | 2.81 (1.08–6.00) | NA | ||
| Squamous cell carcinomas | 2.63 (0.80–6.96) | NA | ||
| T status | 0.928 | 0.984 | ||
| T1 | 2.17 (1.82–4.02) | NA | ||
| T2 | 2.72 (0.80–3.79) | 2.14 (1.01–5.07) | ||
| T3 | 2.51 (1.49–6.96) | 2.34 (1.55–3.14) | ||
| T4 | 2.82 (1.08–6.34) | 2.29 (1.06–5.54) | ||
| N status | 0.800 | 0.465 | ||
| N0 | 2.55 (1.82–4.20) | NA | ||
| N1 | 2.82 (2.49–3.16) | NA | ||
| N2 | 2.83 (1.49–4.84) | 2.03 (1.38–3.25) | ||
| N3 | 2.71 (0.80–6.96) | 2.61 (1.01–5.54) | ||
| M status | 0.497 | 0.983 | ||
| M0 | 2.72 (0.80–6.96) | 2.37 (1.06–5.54) | ||
| M1 | 2.78 (1.08–6.34) | 2.14 (1.01–5.07) | ||
| TNM stage | 0.497 | 0.965 | ||
| III | 2.72 (0.80–6.96) | 2.38 (1.06–5.54) | ||
| IV | 2.78 (1.08–6.34) | 2.14 (1.01–5.07) | ||
Abbreviations: MMP-9, matrix metalloproteinase 9; NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer; ECOG, Eastern Cooperative Oncology Group; NA, not applicable.
Tumor responses to chemotherapy after the various cycles of chemotherapy
Tumor responses were divided into controlled disease (CR/PR/SD) and PD. The tumor responses of the patients with NSCLC and SCLC during the various cycles of chemotherapy are shown in Table 3.
Table 3.
Tumor responses to chemotherapy after the various cycles of chemotherapy
| Group | After one cycle
|
After two cycles
|
After four cycles
|
|||
|---|---|---|---|---|---|---|
| CR/PR/SD | PD | CR/PR/SD | PD | CR/PR/SD | PD | |
| NSCLC (n) | 47 | 6 | 37 | 13 | 14 | 25 |
| SCLC (n) | 29 | 0 | 22 | 1 | 10 | 4 |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer.
Correlation between variation in serum MMP-9 levels and tumor responses in patients with NSCLC
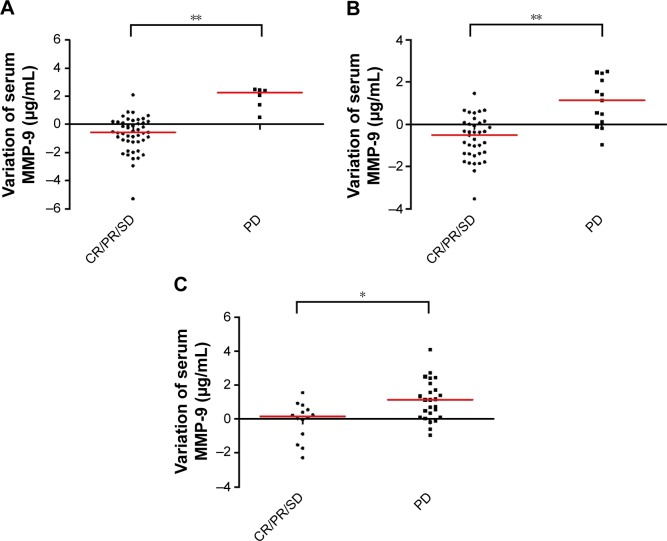
According to the response evaluation, we analyzed the difference of variation in serum levels of MMP-9 in patients with different chemotherapy curative effects evaluated after one cycle, two cycles, and four cycles of chemotherapy. We analyzed the difference of the variation in serum MMP-9 levels between the PD patients and the CR/PR/SD patients. In PD patients, the serum level of MMP-9 increased before PD, and in CR/PR/SD patients, serum level of MMP-9 was decreased or stable. The difference of variation in serum MMP-9 levels in patients with different chemotherapy curative effects was statistically significant after one cycle, two cycles, and four cycles (after one cycle: P<0.001, Figure 1A; after two cycles: P<0.001, Figure 1B; and after four cycles: P=0.01, Figure 1C). The results showed serum MMP-9 level was associated with patients’ chemotherapy response.
Figure 1.
The correlation of variation in serum MMP-9 levels and response to chemotherapy in patients with advanced NSCLC.
Notes: Serum MMP-9 levels increased in patients with NSCLC who developed PD and decreased in patients who developed CR/PR/SD. The difference was statistically significant. (A) The variation in serum MMP-9 levels in patients with different curative effects after one cycle. (B) The variation in serum MMP-9 levels in patients with different curative effects after two cycles. (C) The variation in serum MMP-9 levels in patients with different curative effects after four cycles. *P<0.05 and **P<0.01. Each dot represents a patient and the red lines represent the median values.
Abbreviations: MMP-9, matrix metalloproteinase 9; NSCLC, non-small-cell lung cancer; PD, progressive disease; CR, complete response; PR, partial response; SD, stable disease.
Variation in serum MMP-9 levels and tumor responses in patients with SCLC
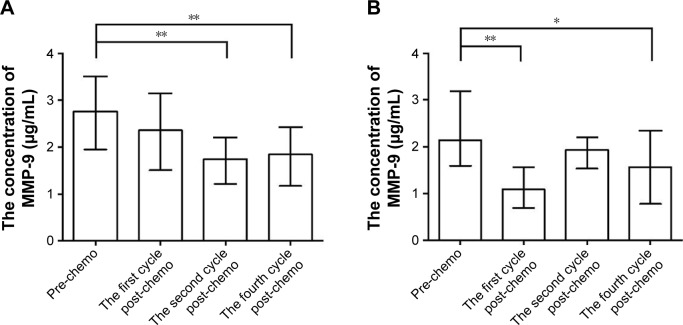
Among patients with NSCLC, the serum MMP-9 levels gradually decreased after the first and second cycles of chemotherapy, and then stabilized after the fourth cycle (baseline vs first, second, and fourth cycles: P=0.126, P=0.001, and P<0.001, respectively; Figure 2A). The serum MMP-9 levels decreased rapidly after the first cycle of chemotherapy in patients with SCLC but increased after the second cycle and then decreased again after the fourth cycle (baseline vs first, second, and fourth cycles: P<0.001, P=0.224, and P=0.049, respectively; Figure 2B). These results differed from those observed in patients with NSCLC.
Figure 2.
The variation in serum MMP-9 levels during chemotherapy.
Notes: (A) Compared with baseline, serum MMP-9 levels gradually decreased after the first and second cycles of chemotherapy, then stabilized after the fourth cycle in patients with NSCLC. (B) Serum MMP-9 levels decreased rapidly after the first cycle of chemotherapy in patients with SCLC but increased after the second cycle and then decreased again after the fourth cycle. *P<0.05 and **P<0.01. Columns represent the median values and bars represent the interquartile ranges.
Abbreviations: MMP-9, matrix metalloproteinase 9; NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer; pre-chemo, before chemotherapy; the first post-chemo, after the first cycle of chemotherapy; the second cycle post-chemo, after the second cycle of chemotherapy; the fourth cycle post-chemo, after the fourth cycle of chemotherapy.
None of the patients with SCLC developed PD after one cycle of chemotherapy, and only one patient developed PD after two cycles of chemotherapy; thus, we did not perform further analysis. As to patients after four cycles of chemotherapy, the difference of variation in serum MMP-9 levels was between the PD and before PD; similarly, the difference was between the CR/PR/SD and before specimens collected. Serum MMP-9 levels decreased in either CR/PR/SD or PD groups. The decrease in the CR/PR/SD group was greater than that in the PD group; however, the difference was not statistically significant (P=0.839).
Discussion
Chemotherapy is the main treatment for advanced-stage lung cancer. It is very important to adjust the treatment regimen according to the patient’s response. Imaging examination is currently the primary means of assessing efficacy. However, potential and early metastases cannot be easily detected by imaging data.16 Consequently, economic and convenient serological markers play an important role in the evaluation of chemotherapy responses.
MMP-9, also known as type IV collagenase, is involved in the degradation of the main constituents of the extracellular matrix and basement membrane. Overexpression of MMP-9 facilitates metastasis. Several studies have examined the association between variations in serum MMP-9 levels and tumor responses in ovarian cancer,17 cervical cancer,18 and breast cancer;19 however, the conclusion is inconsistent. Yoshimoto et al20 investigated the association between variations in serum MMP-9 levels before and after gefitinib treatment and the response to gefitinib treatment in patients with NSCLC. The result showed that serum MMP-9 levels did not change significantly after gefitinib treatment and were not associated with the response to gefitinib treatment. Reckamp et al21 demonstrated that serum MMP-9 levels decreased in patients with a PR to combination therapy with erlotinib and celecoxib; however, the difference was not statistically significant. It remained unclear whether variations in serum MMP-9 levels can be used to evaluate the response to combination therapy with erlotinib and celecoxib. Ertan et al13 only demonstrated that serum MMP-9 levels were significantly lower when compared to before chemotherapy in responders with advanced-stage NSCLC (P=0.005). The association between variations in serum MMP-9 levels and chemotherapy curative effect in patients with NSCLC needed to be further studied. To the best of our knowledge, the present study is the first to investigate the association between variations in serum MMP-9 levels and chemotherapy curative effect in patients with lung cancer.
We conducted sequential measurements of serum MMP-9 levels in patients with lung cancer in parallel with response evaluation. Our results showed that serum MMP-9 levels increased in patients with NSCLC who developed PD and, on the contrary, decreased in patients who developed CR/PR/SD. The difference was statistically significant. These results suggest that serial measurement of serum MMP-9 levels could be useful for monitoring chemotherapy curative effect in patients with NSCLC. Our study showed that this sequential variation in serum MMP-9 levels could be evaluated as an indicator of response categorized as either controlled disease (CR/PR/SD) or PD in patients with NSCLC. The serum MMP-9 levels that steadily decline or decline and then stabilize indicate CR/PR/SD, whereas increasing levels indicate PD.
We also analyzed the variations in serum MMP-9 levels and chemotherapy curative effect in patients with SCLC. The variations in serum MMP-9 levels in patients with SCLC differed from those in patients with NSCLC. The exact reason is not clear, but may be associated with different biological characteristics of NSCLC and SCLC. It may also be related to our small sample size of SCLC patients and will be further studied in the future. We did not find an association between variations in serum MMP-9 levels and chemotherapy curative effect. The use of variations in serum MMP-9 levels for monitoring chemotherapy curative effect should also be studied in depth.
Our study showed that serum MMP-9 levels before chemotherapy in lung cancer patients were not associated with sex, age, smoking status, performance status score, histological types, primary tumor size, regional lymph node metastases, distant metastases, and TNM stages. El-Badrawy et al22 demonstrated that serum MMP-9 levels in patients with stages III and IV lung cancer were higher than those with stages I and II cancer. The patients involved in our study were diagnosed at the advanced stage of the disease. Our results showed that serum MMP-9 levels were not different between stages III and IV lung cancer. It is necessary to analyze thoroughly the serum MMP-9 levels of different stages. Jumper et al23 found no difference in serum MMP-9 levels before chemotherapy between patients with NSCLC and those with SCLC. Our study showed that serum MMP-9 levels before chemotherapy in lung cancer patients were not associated with histological types, which is in accord with the study of Jumper et al. In the further stratified analysis, serum MMP-9 levels before chemotherapy were also not significantly associated with the clinical characteristics of patients with NSCLC and SCLC.
Our study showed that the variation in serum MMP-9 levels in patients with NSCLC during chemotherapy was closely related to chemotherapy curative effect. The serum MMP-9 levels that steadily decline or decline and then stabilize indicate CR/PR/SD, whereas increasing levels indicate PD. The variation in serum MMP-9 levels could be used as a biomarker for monitoring chemotherapy curative effect and laid certain foundation for further clinical research.
Conclusion
The variation in serum MMP-9 levels in patients with NSCLC during chemotherapy was closely related to chemotherapy curative effect and could be useful to monitor chemotherapy curative effect for a small portion of patients. The variation in serum MMP-9 level could be evaluated as an indicator of treatment response categorized as either controlled disease (CR/PR/SD) or PD.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25(1):9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 3.Rao JS, Gondi C, Chetty C, Chittivelu S, Joseph PA, Lakka SS. Inhibition of invasion, angiogenesis, tumor growth, and metastasis by adenovirus-mediated transfer of antisense uPAR and MMP-9 in non-small cell lung cancer cells. Mol Cancer Ther. 2005;4(9):1399–1408. doi: 10.1158/1535-7163.MCT-05-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tummalapalli P, Spomar D, Gondi CS, et al. RNAi-mediated abrogation of cathepsin B and MMP-9 gene expression in a malignant meningioma cell line leads to decreased tumor growth, invasion and angiogenesis. Int J Oncol. 2007;31(5):1039–1050. [PMC free article] [PubMed] [Google Scholar]
- 5.Mehner C, Hockla A, Miller E, Ran S, Radisky DC, Radisky ES. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget. 2014;5(9):2736–2749. doi: 10.18632/oncotarget.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin Y, Li JP, Tang LY, et al. Protein expression and significance of VEGF, EGFR and MMP-9 in non-small cell lung carcinomas. Asian Pac J Cancer Prev. 2011;12(6):1473–1476. [PubMed] [Google Scholar]
- 7.Zheng S, Chang Y, Hodges KB, et al. Expression of KISS1 and MMP-9 in non-small cell lung cancer and their relations to metastasis and survival. Anticancer Res. 2010;30(3):713–718. [PubMed] [Google Scholar]
- 8.Koc M, Ediger D, Budak F, et al. Matrix metalloproteinase-9 (MMP-9) elevated in serum but not in bronchial lavage fluid in patients with lung cancer. Tumori. 2006;92(2):149–154. doi: 10.1177/030089160609200211. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Wu JZ, Zhang JY, et al. Detection of circulating vascular endothelial growth factor and matrix metalloproteinase-9 in non-small cell lung cancer using Luminex multiplex technology. Oncol Lett. 2014;7(2):499–506. doi: 10.3892/ol.2013.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laack E, Kohler A, Kugler C, et al. Pretreatment serum levels of matrix metalloproteinase-9 and vascular endothelial growth factor in non-small-cell lung cancer. Ann Oncol. 2002;13(10):1550–1557. doi: 10.1093/annonc/mdf270. [DOI] [PubMed] [Google Scholar]
- 11.Shimanuki Y, Takahashi K, Cui R, et al. Role of serum vascular endothe-lial growth factor in the prediction of angiogenesis and prognosis for non-small cell lung cancer. Lung. 2005;183(1):29–42. doi: 10.1007/s00408-004-2521-4. [DOI] [PubMed] [Google Scholar]
- 12.Yazar A, Soydinc H, Ertan E, Yasasever V, Tas F. The role of serum vascular endothelial growth factor and matrix metalloproteinase-9 in predicting response to chemotherapy in patients with advanced nonsmall cell lung cancer. South Med J. 2008;101(3):327–328. doi: 10.1097/SMJ.0b013e318164e432. [DOI] [PubMed] [Google Scholar]
- 13.Ertan E, Soydinc H, Yazar A, Ustuner Z, Tas F, Yasasever V. Matrix metalloproteinase-9 decreased after chemotherapy in patients with non-small cell lung cancer. Tumori. 2011;97(3):286–289. doi: 10.1177/030089161109700305. [DOI] [PubMed] [Google Scholar]
- 14.Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009;15(1):4–9. [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Yaghmai V, Miller FH, Rezai P, Benson AB, 3rd, Salem R. Response to treatment series: part 2, tumor response assessment – using new and conventional criteria. AJR Am J Roentgenol. 2011;197(1):18–27. doi: 10.2214/AJR.11.6581. [DOI] [PubMed] [Google Scholar]
- 17.Rauvala M, Turpeenniemi-Hujanen T, Puistola U. The value of sequential serum measurements of gelatinases and tissue inhibitors during chemotherapy in ovarian cancer. Anticancer Res. 2006;26(6C):4779–4784. [PubMed] [Google Scholar]
- 18.Braicu EI, Gasimli K, Richter R, et al. Role of serum VEGFA, TIMP2, MMP2 and MMP9 in monitoring response to adjuvant radiochemo-therapy in patients with primary cervical cancer – results of a companion protocol of the randomized NOGGO-AGO phase III clinical trial. Anticancer Res. 2014;34(1):385–391. [PubMed] [Google Scholar]
- 19.Makhoul I, Griffin RJ, Siegel E, et al. High-circulating Tie2 is associated with pathologic complete response to chemotherapy and antiangiogenic therapy in breast cancer. Am J Clin Oncol. 2014 Feb 26; doi: 10.1097/COC.0000000000000046. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshimoto A, Kasahara K, Nishio M, et al. Changes in angiogenic growth factor levels after gefitinib treatment in non-small cell lung cancer. Jpn J Clin Oncol. 2005;35(5):233–238. doi: 10.1093/jjco/hyi074. [DOI] [PubMed] [Google Scholar]
- 21.Reckamp KL, Gardner BK, Figlin RA, et al. Tumor response to combination celecoxib and erlotinib therapy in non-small cell lung cancer is associated with a low baseline matrix metalloproteinase-9 and a decline in serum-soluble E-cadherin. J Thorac Oncol. 2008;3(2):117–124. doi: 10.1097/JTO.0b013e3181622bef. [DOI] [PubMed] [Google Scholar]
- 22.El-Badrawy MK, Yousef AM, Shaalan D, Elsamanoudy AZ. Matrix metalloproteinase-9 expression in lung cancer patients and its relation to serum mmp-9 activity, pathologic type, and prognosis. J Bronchology Interv Pulmonol. 2014;21(4):327–334. doi: 10.1097/LBR.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 23.Jumper C, Cobos E, Lox C. Determination of the serum matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of matrix metallo-proteinase-1 (TIMP-1) in patients with either advanced small-cell lung cancer or non-small-cell lung cancer prior to treatment. Respir Med. 2004;98(2):173–177. doi: 10.1016/j.rmed.2003.08.014. [DOI] [PubMed] [Google Scholar]