Abstract
Background
Individuals at high risk to develop alcoholism often manifest neurocognitive deficits as well as increased impulsivity. The goal of the present study is to elucidate reward processing deficits, externalizing disorders, and impulsivity as elicited by electrophysiological, clinical and behavioral measures in subjects at high risk for alcoholism from families densely affected by alcoholism in the context of brain maturation across age groups and gender.
Methods
Event-related potentials (ERPs) and current source density (CSD) during a monetary gambling task (MGT) were measured in 12–25 year old offspring (N = 1864) of families in the Collaborative Study on the Genetics of Alcoholism (COGA) Prospective study; the high risk (HR, N = 1569) subjects were from families densely affected with alcoholism and the low risk (LR, N = 295) subjects were from community families. Externalizing disorders and impulsivity scores were also compared between LR and HR groups.
Results
HR offspring from older (16–25 years) male and younger (12–15 years) female subgroups showed lower P3 amplitude than LR subjects. The amplitude decrement was most prominent in HR males during the loss condition. Overall, P3 amplitude increase at anterior sites and decrease at posterior areas were seen in older compared to younger subjects, suggesting frontalization during brain maturation. The HR subgroups also exhibited hypofrontality manifested as weaker CSD activity during both loss and gain conditions at frontal regions. Further, the HR subjects had higher impulsivity scores and increased prevalence of externalizing disorders. P3 amplitudes during the gain condition were negatively correlated with impulsivity scores.
Conclusions
Older male and younger female HR offspring, compared to their LR counterparts, manifested reward processing deficits as indexed by lower P3 amplitude and weaker CSD activity, along with higher prevalence of externalizing disorders and higher impulsivity scores.
Significance
Reward related P3 is a valuable measure reflecting neurocognitive dysfunction in subjects at risk for alcoholism, as well as to characterize reward processing and brain maturation across gender and age group.
Keywords: Alcohol use disorders, family history of alcoholism, P3, current source density, reward processing, impulsivity, endophenotype, brain maturation, hypofrontality, frontalization
1. Introduction
Alcoholism is a complex disorder with multiple etiological pathways involving a host of genetic and environmental factors along with their interactions in its onset, manifestations, course, and treatment outcome. Converging evidence supports the notion that there may be a wide range of genetic, biological, neurocognitive and environmental factors involved in the causal pathways to develop alcoholism. Electrophysiological measures, such as electroencephalogram (EEG), event-related potentials (ERPs), and event-related oscillations (EROs) have played a vital role as biological markers or endophenotypes to understand neurocognitive mechanisms involved in alcohol use and related disorders (see Porjesz et al., 2005, for a review). These electrophysiological methods provide a direct measure of brain activity with high temporal sensitivity to understand neurocognitive processes, while being non-invasive and inexpensive for its applications. Specifically, ERPs can measure dynamically changing brain activity in real time during perceptual, motor, and cognitive processing while performing a task (Picton and Hillyard, 1988). ERPs have been widely and successfully used to examine neurocognitive processing during various experimental tasks in normal populations as well as in a range of clinical conditions including alcoholism (Porjesz and Begleiter, 1985; Begleiter and Porjesz, 1990a; Porjesz et al., 1996; Porjesz and Begleiter, 1997; Porjesz et al., 2005).
A landmark finding in the electrophysiology of human alcoholism is that individuals with alcohol dependence as well as their high risk offspring show low voltage P3(00) amplitude (for reviews, see Begleiter and Porjesz, 1990b; Porjesz and Begleiter, 1990, 1991; Polich et al., 1994; Porjesz and Begleiter, 1997; Porjesz et al., 2005). P3 is a robust, positive going ERP wave occurring around 300–700 ms following the onset of a stimulus, indicative of its context (Donchin and Coles, 1988) or importance (Sutton et al., 1978; Begleiter et al., 1983) during signal/cognitive processing. Since the first report by Begleiter et al. (1984) of low P3 amplitude in the sons of alcoholic fathers (in a study without any alcohol challenge), this finding has been replicated across many different experimental paradigms in male as well as female high risk subjects (i.e., offspring of alcoholics) in diverse samples (for reviews, see Porjesz et al., 2005; Rangaswamy and Porjesz, 2014).
It was a turning point in alcoholism research that lower P3 amplitude, observed in alcoholic individuals (Porjesz and Begleiter, 1981; Oscar-Berman, 1987; Pfefferbaum et al., 1987; Porjesz et al., 1987; Cohen et al., 1995; Cohen et al., 1997b; Rodriguez Holguin et al., 1999a; Hada et al., 2000; Prabhu et al., 2001; Cohen et al., 2002; Suresh et al., 2003; Kamarajan et al., 2005a; Fein and Chang, 2006; Kamarajan et al., 2010; Fein and Andrew, 2011), was also found in individuals with a family history of alcoholism who were considered to be genetically vulnerable but had not yet developed alcoholism (Elmasian et al., 1982; O’Connor et al., 1987; Porjesz and Begleiter, 1990; Benegal et al., 1995; Porjesz et al., 1996; Ramachandran et al., 1996; Kamarajan et al., 2005b) and had never or only rarely been exposed to alcohol (Begleiter et al., 1984; Begleiter et al., 1987; Whipple et al., 1988; Whipple et al., 1991; Hill and Steinhauer, 1993; Steinhauer and Hill, 1993; Hill et al., 1995). However, it must be stated that P3 reduction in high risk subjects, as a phenomenon, is not consistent or ubiquitous in the literature, but often with equivocal as well as subgroup-specific findings, and has been found to be strongest in younger males (for a meta-analysis, see Polich et al., 1994). For example, some studies reported that P3 reductions were observed only in boys of alcoholic parents (e.g., Hill and Steinhauer, 1993), while other studies found the effect in both genders (e.g., Porjesz et al., 1996). Similarly, this effect has been found to be stronger in younger subjects (e.g., Begleiter et al., 1987; Polich et al., 1994) but still robust in adolescents/young adults (O’Connor et al., 1987; Porjesz and Begleiter, 1990; Porjesz et al., 1996; Ramachandran et al., 1996; Van der Stelt et al., 1998; Kamarajan et al., 2005b). Possible reasons for these inconsistent and/or subgroup specific findings may include the following: (i) the studies may differ methodologically (in terms of sample characteristics, task paradigms, ERP recording and signal processing, statistical techniques, etc.); (ii) definition of “risk” may differ across studies; and (iii) P3 reduction may in fact be a function of age, gender, and task paradigms and may be moderated by several confounding (or unmeasured) factors such as personality traits, situational/familial/sociocultural factors, and other variations due to genetic and epigenetic factors.
It is also important to note that low P3 is not unique to alcoholics and their high risk relatives, but is also found in individuals with one or more externalizing disorders or disinhibitory conditions (Carlson et al., 1999; Hill and Shen, 2002; Iacono et al., 2002; Iacono et al., 2003; Iacono and McGue, 2006; Patrick et al., 2006; Carlson et al., 2007; Hicks et al., 2007; Iacono et al., 2008; Patrick, 2008; Gilmore et al., 2010a; Gilmore et al., 2010b, 2012). As reported by several studies, an underlying feature among risk propensity, externalizing disorders and alcoholism is the concept of “impulsivity”, which is a conglomerate of personality traits that can result in premature, unduly risky and poorly conceived actions, and is known to be closely related to disinhibitory traits and clinical vulnerability (Gorenstein and Newman, 1980; Martin et al., 1994; Olson et al., 1999; Krueger and Piasecki, 2002; Hall et al., 2007; Kamarajan et al., 2007; Crews and Boettiger, 2009; Romer et al., 2009). Interestingly, P3 amplitude has been found to be either negatively correlated with impulsivity or lower in high impulsive subjects regardless of having a diagnosis of alcoholism and/or related disorders (Justus et al., 2001; Moeller et al., 2004; Chen et al., 2007; Ruchsow et al., 2008; Kamarajan et al., 2010).
Alcoholism has often been characterized as a reward deficit disorder (Koob, 2013; Forbes et al., 2014), and several studies have successfully used ERPs to examine reward processing in healthy individuals (Homberg et al., 1980, 1981; Begleiter et al., 1983; Ivanitsky et al., 1986; Gehring and Willoughby, 2002; Yeung and Sanfey, 2004; Hajcak et al., 2005; Nieuwenhuis et al., 2005; Toyomaki and Murohashi, 2005a, b; Hajcak et al., 2006; Holroyd et al., 2006; Yu and Zhou, 2006; Hajcak et al., 2007; Kamarajan et al., 2009), as well as in alcoholic and HR offspring (Porjesz et al., 1987; Ramsey and Finn, 1997; Fein and Chang, 2008; Kamarajan et al., 2010). Major ERP components studied during outcome/feedback processing during monetary gambling tasks (MGT) are the outcome-related negativity (ORN) or N2 (between 200 ms and 300 ms) and the outcome-related positivity (ORP) or P3 (between 300 ms and 600 ms) (Gehring and Willoughby, 2002; Yeung and Sanfey, 2004; Hajcak et al., 2005; Yeung et al., 2005; Hajcak et al., 2006; Holroyd et al., 2006; Cohen and Ranganath, 2007; Kamarajan et al., 2009). In our previous ERP study of reward processing using a MGT in alcoholics, we found that alcoholics showed significantly lower amplitudes of N2/ORN and P3/ORP components and decreased current density in cingulate gyrus, along with higher levels of impulsivity and risk-taking features than controls (Kamarajan et al., 2010). While FRN/N2 is another important component of feedback processing, the current study focuses solely on the P3 component for the following reasons: 1) dealing with both components (P3 and N2) in a single study with multiple factors (risk group, gender, age group) may render the study too complex; 2) with regard to alcoholism and risk, P3 is considered to be the most robust ERP component and a sensitive biomarker, and therefore the analysis of P3 has assumed its precedence in the current study; 3) FRN/N2 is a relatively subtle component and more prone to artifact distortions, rendering it more difficult to measure (especially in such a large sample of adolescents and young adults) compared to the large P3 component; and 4) implementation of a more sophisticated source localization method (e.g., sLORETA) may be essential to examine the key brain sources (e.g., anterior cingulate region) attributed to the FRN (Crowley et al., 2013). For these reasons, only the P3 component has been dealt with in the current study. As current source density (CSD), a source derivation method of electrophysiological activity, has been successfully used in several neuropsychiatric disorders including alcoholism (for a review, see Kamarajan et al., 2015), we have also compared CSD topography across the groups (see Section 2.5 for more information on the CSD method).
The overarching aim of the present study is to examine reward processing (as indexed by P3 amplitude and CSD), and externalizing features in HR offspring recruited from high density alcoholism families in comparison with LR (comparison) subjects recruited from a community sample in the COGA Prospective Study, in the context of brain maturation across age groups and gender. This is the first ERP study using a monetary gambling paradigm to study HR offspring, and has been designed to examine the following hypotheses: (1) HR offspring will show lower P3 amplitude during reward processing than low-risk (LR) individuals from the comparison families; 2) HR group will show current density differences in both magnitude and topography as compared to the LR group; and (3) HR group will have higher impulsivity scores than the LR group. As the literature regarding P3 reduction in high risk subjects, as mentioned earlier, has often been equivocal with regard to gender- and age-based subgroups, the current study also investigates the effects of age and gender on P3 amplitudes in specific subgroups. It is expected that findings of the present study may shed further light on the complex relationship among reward processing deficits (indexed by P3 amplitude and CSD), impulsivity, and externalizing disorders involved in the development of alcoholism.
2. Materials and Methods
2.1. Participants
The sample included 1864 adolescents and young adults (903 males and 961 females) between 12 to 25 years of age (see Table 1) and was derived from the prospective study of the Collaborative Study on the Genetics of Alcoholism (COGA) (Begleiter et al., 1995; Edenberg et al., 2005). The prospective study began in 2004, focusing on participants who were relatives (e.g., offspring) of the high-risk and comparison (comparison) families ascertained in previous phases of COGA. Participants are reassessed every two years with clinical, behavioral and neurophysiological assessments. Data from six collection sites have been included in this study: SUNY Downstate Medical Center at Brooklyn, New York; University of Connecticut Health Science Center; Washington University School of Medicine in St. Louis; University of California at San Diego; University of Iowa, and Indiana University School of Medicine. Recruitment and assessment procedures have been described elsewhere (Begleiter et al., 1995; Reich et al., 1998; Foroud et al., 2000; Nurnberger et al., 2004), and are also available at this website: https://zork5.wustl.edu/coganew/data/instruments.html. The experimental protocols were approved by each site’s institutional review board, and informed consent was obtained from all participants.
Table 1.
Number of participants categorized by age group, gender, and risk group.
| Age Group | Gender | Risk Group | Total | |
|---|---|---|---|---|
| Low-risk (LR) | High-risk (HR) | |||
| 12–15 years | Male | 70 | 317 | 387 |
| Female | 96 | 329 | 425 | |
| Total | 166 | 646 | 812 | |
| 16–25 years | Male | 64 | 452 | 516 |
| Female | 65 | 471 | 536 | |
| Total | 129 | 923 | 1052 | |
| Total | Male | 134 | 769 | 903 |
| Female | 161 | 800 | 961 | |
| Total | 295 | 1569 | 1864 | |
Subjects were excluded from neurophysiological assessment if they had any of the following: (1) recent substance or alcohol use (i.e., positive breath-analyzer test and/or urine screen), (2) hepatic encephalopathy/cirrhosis of the liver, (3) history of head injury, seizures or neurosurgery, (4) uncorrected sensory deficits, (5) history/symptoms of psychoses, (6) self-reported positive test for human immunodeficiency virus, and (7) other acute/chronic medical illnesses that affects brain function. The HR group consisted of individuals from the COGA high density alcoholism families who had at least one parent with DSM-IV alcohol dependence, while the LR group consisted of individuals from the community families without any parental history of alcohol dependence. The groups were further subdivided based on gender and age group (12–15 and 16–25 years old) (see Table 1). As the age distribution was skewed in the overall sample (i.e., relatively more subjects were represented at younger ages), the sample was subdivided into these age groups, in order to provide adequate sample sizes for each age group and ensure good pubertal and post-pubertal representation. Table 2 shows lifetime prevalence rates of externalizing disorders for HR and LR groups. These externalizing scores were higher in HR offspring compared to LR individuals in each gender as well in the total sample (see Table 2).
Table 2.
Prevalence rates in counts and percentage (in parentheses) for the diagnoses of externalizing disorders (EXT) in LR and HR groups in the total sample and within gender. Note that the HR group had higher prevalence rates in all the diagnoses than the LR group. Significance levels based on Chi-square test have been marked with asterisks (in HR columns). Diagnosis of SUD is based on DSM-IV criteria for dependence or abuse. Empty cell (with a dash) represents the count of zero.
| Diagnosis | Male (affected cases) | Female (affected cases) | Total (affected cases) | |||
|---|---|---|---|---|---|---|
| LR | HR | LR | HR | LR | HR | |
| Alcohol | 9 (6.98) | 112 (14.95)** | 5 (3.16) | 90 (11.49)*** | 14 (4.88) | 202 (13.19)*** |
| Tobacco | 5 (3.88) | 64 (8.54)* | 2 (1.27) | 62 (7.92)*** | 7 (2.44) | 126 (8.22)*** |
| Marijuana | 9 (6.98) | 123 (16.55)** | 2 (1.27) | 75 (9.59)*** | 11 (3.85) | 198 (12.98)*** |
| Cocaine | - | 9 (1.20) | - | 8 (1.02) | - | 17 (1.11)* |
| Stimulant | - | 6 (0.80) | - | 13 (1.66)* | - | 19 (1.24)* |
| Sedative | - | 3 (0.40) | - | 4 (0.51) | - | 7 (0.46) |
| Opiate | - | 5 (0.67) | - | 9 (1.15) | - | 14 (0.92)* |
| Other Drugs | - | 8 (1.07) | - | 12 (1.53)* | - | 20 (1.30)** |
| ASPD | 3 (8.33) | 40 (15.56) | 1 (2.56) | 25 (8.31) | 4 (5.33) | 65 (11.65) |
| CD | 5 (3.88) | 81 (10.81)** | 5 (3.16) | 45 (5.76) | 10 (3.48) | 126 (8.24)** |
| ADHD | 3 (2.33) | 27 (3.63) | 2 (1.27) | 13 (1.66) | 5 (1.75) | 40 (2.62) |
| ODD | 1 (0.78) | 21 (2.94) | 1 (0.64) | 13 (1.70) | 2 (0.70) | 34 (2.30)* |
| Any SUD | 15 (11.63) | 189 (25.17)*** | 8 (5.06) | 154 (19.52)*** | 23 (8.01) | 343 (22.27)*** |
| Any EXT | 20 (15.50) | 239 (31.82)*** | 14 (8.86) | 186 (23.57)*** | 34 (11.85) | 425 (27.60)*** |
p < 0.05;
p < 0.01;
p < 0.001
2.2. Assessment Tools
The clinical and impulsivity data of the sample were collected with three instruments: (1) SSAGA – The Semi Structured Assessment for the Genetics of Alcoholism (Bucholz et al., 1994) evaluated clinical diagnoses and symptoms; (2) FHAM The Family History Assessment Module (Rice et al., 1995) assessed major psychiatric disorders among relatives of the participants; and (3) BIS – The Barratt Impulsiveness Scale (BIS) (Patton et al., 1995) provided ratings of impulsivity in three categories viz., attentional, motor, and non-planning, yielding individual as well as total scores.
2.3. Monetary Gambling Task
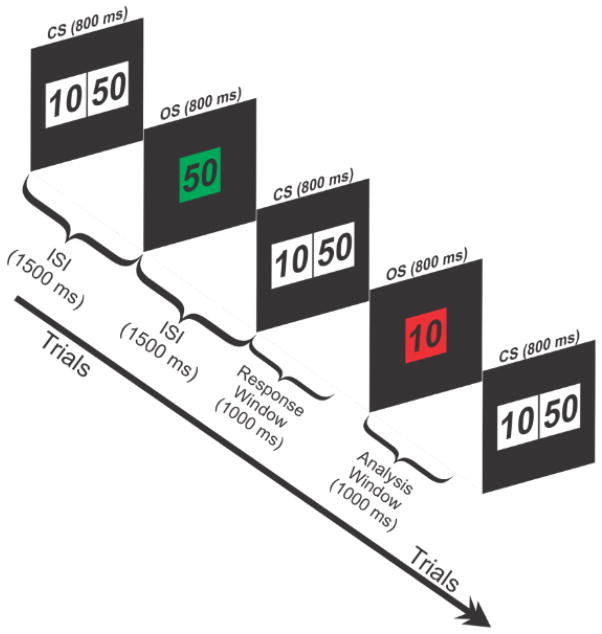
The monetary gambling task (MGT) used in this study is illustrated in Fig. 1. Each trial began with a choice stimulus (CS), with two numbers 10 (left box) and 50 (right box), with a monetary value in US cents, displayed for 800 ms. The participants select a bet of either 50¢ or 10¢, and receive feedback of either loss or gain for the selected amount (outcome stimulus, OS). The task details have been described in our previous publications (Kamarajan et al., 2008; Kamarajan et al., 2009; Kamarajan et al., 2010; Kamarajan et al., 2012). The inter-stimulus interval between a CS and OS, and between an OS and next CS is 1500 ms. The task involved a total of 172 trials, each with one of four possible outcomes: Loss 50, Loss 10, Gain 50, and Gain 10. P3 amplitudes were measured during the ERP epochs of outcome stimuli (1000 ms poststimulus) which contained the feedback of either loss or gain for each trial (i.e., the epochs following colored frames in Fig. 1).
Fig. 1.
Schematic illustration of the monetary gambling task. Each trial starts with a choice stimulus (CS) which lasts for 800 ms and displays two amounts (10¢ or 50¢) to bet with. The participant selects one of the amounts and receives an outcome of either gain (green box) or loss (red box) for the selected amount as shown by the outcome stimulus (OS). A trial with a gain of 50¢ and the next trial with a loss of 10¢ are illustrated.
2.4. EEG Data Acquisition and Signal Analysis
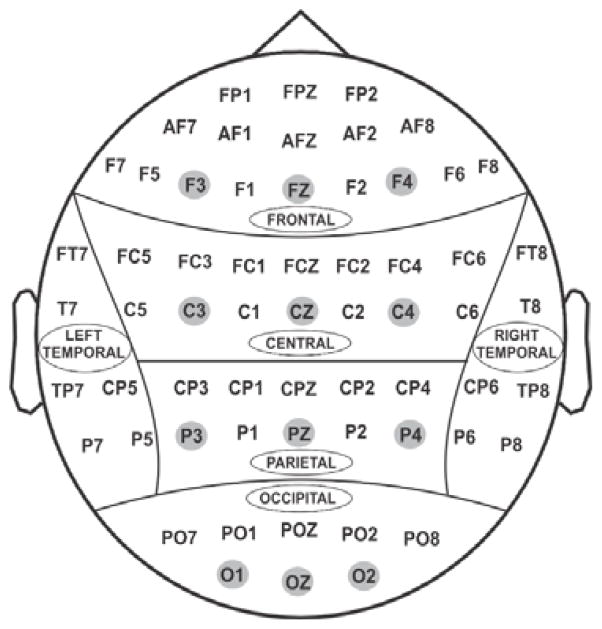
Identical experimental procedures and EEG acquisition systems were used at all neurophysiology collection sites of COGA (Begleiter et al., 1995; Porjesz and Begleiter, 1995) with inter-laboratory consistency in recordings (Alexander et al., 1994; Cohen et al., 1994; Kuperman et al., 1995; Rohrbaugh et al., 1997). Subjects were seated comfortably 1 m from a monitor in a dimly lit sound-attenuated RF-shielded booth (Industrial Acoustics, Inc., Bronx, NY, USA), and wore a 61-channel electrode cap (Electro-Cap International, Inc., Eaton, OH, USA) based on the Extended 10–20 Systems (Chatrian et al., 1985; Chatrian et al., 1988; Oostenveld and Praamstra, 2001) (Fig. 2), with a reference electrode at the tip of the nose and with a ground electrode at the forehead. The electrooculogram (EOG) was recorded by a supraorbital vertical electrode and by a horizontal electrode on the external canthus of the left eye. Electrode impedances were maintained below 5 kΩ. Electrical activity was amplified 10,000 times using SynAmps2 amplifiers (Compumedics USA, Charlotte, NC) and was recorded continuously over a bandwidth of DC–100.0 Hz on a Neuroscan system (Versions 4.3–4.5; Compumedics USA, Charlotte, NC) at sampling rate of 500 Hz. All EEG data were resampled offline at 256 Hz for the analyses. Artifact rejection threshold during preprocessing was set at ±100 μV.
Fig. 2.
Sixty-one electrodes as recorded in the current study from the surface of the scalp. Twelve electrodes (F3, FZ, F4, C3, CZ, C4, P3, PZ, P4, O1, OZ, O2) representing frontal, central, parietal and occipital regions were selected for statistical analyses.
ERP waveforms were filtered at 0.03–16.0 Hz and the P3 amplitude was measured as the voltage difference from the pre-stimulus baseline window (0–100 ms) to the largest positive going peak within the post-stimulus time window (275–600 ms) after the onset of an outcome stimulus. A semi-automatic computerized algorithm developed in our laboratory identified the P3 peak as a time point on the P3 wave with maximum amplitude within the 275–600 ms post-stimulus time window (Cohen et al., 2002; Kamarajan et al., 2009). Further, ERP waveforms were visually analyzed for the correctness of peak identification and morphology of the waveforms. Since the P3 topography for smaller and larger amounts (10¢ and 50¢) were similar (Kamarajan et al., 2009; Kamarajan et al., 2010), trials for the two amounts (10¢ and 50¢) were combined for the loss condition (loss 10¢ + loss 50¢) as well as for the gain condition (gain 10¢ + gain 50¢) in order to have more trials per subject and more subjects per group. Mean trial numbers across the risk groups in each condition are: LR = 39.12 and HR = 36.51 for the loss condition; LR = 42.24 and HR = 40.53 for the gain condition. Although signal-to-noise-ratio (SNR) and minimum number of trials needed for the stability P3 in gambling paradigm have not been determined in the current study, trial numbers for each condition seem to be adequate. In view of the findings that the SNR did not differ when the trial numbers exceeded eight for the ERN/Pe components (Olvet and Hajcak, 2009), and that the components can be reliably measured with 8 trials for ERN (Olvet and Hajcak, 2009) and with 20 trials for the FRN (Marco-Pallares et al., 2011), it is very unlikely that SNR-related issues may confound the results of the current study given the higher number of trials in each condition.
2.5. Current Source Density mapping
The recorded EEG potential at each electrode does not represent brain sources solely adjacent to the electrode location (Nunez, 1981), because of two limitations (Kayser and Tenke, 2015): (i) EEG is measured as a potential difference between the recorded electrode and the reference electrode, and (ii) the EEG signal is a mixture of propagated neuroelectric activity from multiple local and distal brain sources and is smeared by volume conduction. Both of these limitations can be mitigated by use of the surface Laplacian algorithm (Hjorth, 1975), which is a simple mathematical transformation applied to the EEG surface potentials (for a tutorial review on this topic, see Kayser and Tenke, 2015). The CSD provides information about local radial current density representing components of the primary neural activity in the scalp region (Nunez and Pilgreen, 1991). Thus, the CSD topographic map is a spatially enhanced representation of current generators with more sharply localized peaks than those of the scalp potential, while eliminating volume-conducted contributions from distant regions and sources (Tenke and Kayser, 2012). CSD topography thus represents reference-free estimates of radial current flow at the scalp represented in positive and negative polarities (Kayser and Tenke, 2015). The positivity or “source” represents current flow from the brain towards the scalp (i.e., outward flow), while the negativity or “sink” indicates current flow towards the brain from the scalp (i.e., inward flow) (Nunez and Srinivasan, 2006). CSD methods have been successfully implemented in several neuropsychiatric disorders (Kamarajan et al., 2015) including alcoholism (Ramachandran et al., 1996; Ji et al., 1999; Hada et al., 2000; Kamarajan et al., 2005a; Kamarajan et al., 2012) in order to understand possible brain sources underlying scalp-recorded potentials. In the present study, CSD maps were constructed from the Laplacian transformed grand averaged data of P3 amplitudes, for each group and condition, as described by Wang and Begleiter (1999). Z-scored maps for P3 amplitude as well as CSD were plotted so that topographic patterns could be compared across groups regardless of the magnitude of amplitude/CSD. Topographic activation patterns of sources and sinks between LR and HR groups were compared.
2.6. Statistical Analyses
Statistical analyses were performed using SPSS 21.0 (IBM Corporation, Armonk, NY). Prevalence rates of externalizing diagnoses were compared between HR and LR groups in each gender as well as total samples using Chi-square test with significance testing for Likelihood Ratio (see Table 2). P3 amplitudes from 12 electrodes (F3, FZ, F4, C3, CZ, C4, P3, PZ, P4, O1, OZ, O2) were analyzed using a repeated measures analysis of variance (RM-ANOVA) of the general linear model involving Risk Group (HR and LR), Age Group (12–15 and 16–25 years), and Gender (male and female) as between-subjects factors, and Task condition (Loss and Gain) and Region (frontal, central, parietal, and occipital) as within-subjects factors. Four scalp regions with three electrodes per region (Frontal: F3, FZ, F4; Central: C3, CZ, C4; Parietal: P3, PZ, P4; Occipital: O1, OZ, O2) were included in the model. Although affected status (i.e., individuals with any externalizing diagnosis as shown in Table 2) was initially used as a covariate in the RM-ANOVA model, it was removed from the later analysis which is presented here, as the covariate (i.e., externalizing factor) showed neither a main effect nor any interaction effect with any factors in the model. As the electrophysiology data for the within-subjects factors in the current study did not adhere to the sphericity assumption (i.e., the equality of the variances of the differences between levels of repeated measures factor, such as region), we used multivariate test statistics, which are not dependent on the assumption of sphericity (O’Brien and Kaiser, 1985; Field, 1988). Hence this provides an appropriate alternative method to analyze EEG data with repeated-measures multivariate analyses of variance, which does not assume sphericity and therefore does not require any corrections (cf. Bell and Cuevas, 2012). Bonferroni adjusted pairwise comparisons within each significant main and interaction effects were analyzed using estimated marginal means (EMM) and standard errors (SE). The BIS scores between LR and HR groups were analyzed using one-way ANOVA. The relationship between P3 amplitudes and BIS scores was examined using Pearson correlation coefficients.
3. Results
3.1. Prevalence Rates of Externalizing Disorders
Table 2 shows comparison of prevalence rates of externalizing (EXT) disorders, which includes DSM-IV lifetime diagnosis for SUD (dependence/abuse) and other related disorders, such as anti-social personality disorder (ASPD), conduct disorder (CD), attention deficit hyperactivity disorder (ADHD), and oppositional defiant disorder (ODD). “Other drugs” refers to the substances other than those mentioned in the list. Significant differences in the prevalence rates between LR and HR subjects were observed in several diagnoses for all three comparisons: (i) Males: alcohol, tobacco, marijuana, CD, any SUD, and any EXT; (ii) Females: alcohol, tobacco, marijuana, stimulant, other drugs, any SUD, and any EXT; and (iii) All subjects: alcohol, tobacco, marijuana, cocaine, stimulant, opiate, other drugs, CD, ODD, any SUD, and any EXT. The diagnoses that were significant in all the comparisons were: alcohol, tobacco, marijuana, any SUD, and any EXT.
3.2. Mean P3 Values across the Subgroups
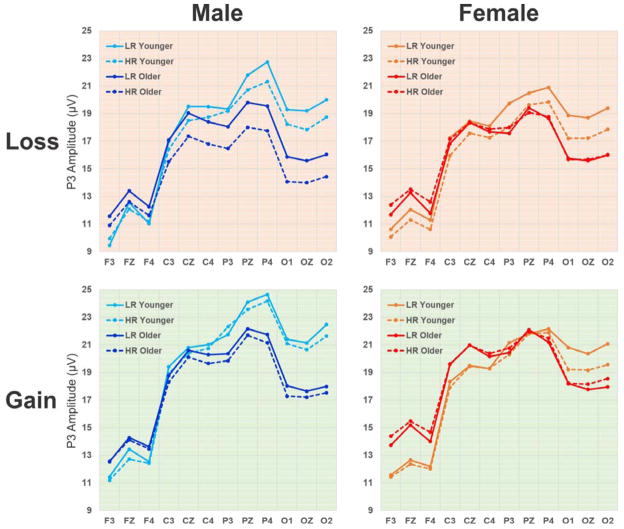
Qualitative comparison of mean P3 values for each subgroup during loss and gain conditions are illustrated in Fig. 3. Section 3.2 describes the statistical comparison of P3 amplitudes across factors with RM-ANOVA. Lower P3 amplitudes in HR compared to LR subjects (solid vs. broken lines within each panel in Fig. 3) was more evident in both younger and older male subjects only during the loss condition (top left panel), as well as in younger female subjects during both loss and gain conditions (orange lines in right-side panels). Further, a comparison between genders (left-side vs. right-side panels in Fig. 3), indicates that younger males during both loss and gain conditions showed higher P3 amplitudes than their female counterparts (cyan vs. orange lines). Furthermore, older subjects showed increased P3 amplitudes in anterior sites and a decrease in posterior regions compared to younger subjects (darker vs. lighter colored lines within each panel in Fig. 3), reflecting brain maturation during development (“frontalization”).
Fig. 3.
Qualitative comparison of mean P3 values for each subgroup during loss and gain conditions across 12 electrodes representing 4 regions (frontal: F3, FZ, F4; central: C3, CZ, C4; parietal: P3, PZ, P4; occipital: O1, OZ, O2). HR offspring display markedly lower P3 amplitude than LR subjects in both younger and older male subgroups during loss condition as well as in younger female subjects during both loss and gain conditions.
3.3. RM-ANOVA Results
Table 3 lists the main and interaction effects in the RM-ANOVA model, and Fig. 4 illustrates the pairwise comparisons of the estimated marginal means of the P3 amplitude between the levels of risk groups, gender, and age groups at each scalp region for the loss and gain conditions. P3 amplitude showed significant main effects for age group, condition, and region along with the following 2-way and 3-way interaction effects: (i) age group × gender, (ii) age group × region, (iii) gender × region, (iv) condition × region, (v) risk group × condition × region, (vi) gender × condition × region (Table 3). These results are explained for each major factor (risk group, gender, and age group) in the order of higher order (complex) to lower order (simpler) effects in the subsections below.
Table 3.
The main and interaction effects of the RM-ANOVA of P3 amplitude (dependent variable) with several between-subjects and within-subjects factors. Degrees of Freedom, F value, and p value are shown. Significant effects have been highlighted with bold font.
| Effect | df | F | p |
|---|---|---|---|
| Risk-group | 1 | 1.70 | 0.1926 |
| Gender | 1 | 0.82 | 0.3646 |
| Age-group | 1 | 7.53 | 0.0061** |
| Risk-group × Gender | 1 | 1.70 | 0.1927 |
| Risk-group × Age-group | 1 | 0.72 | 0.3973 |
| Gender × Age-group | 1 | 4.31 | 0.0381* |
| Risk-group × Gender × Age-group | 1 | 1.14 | 0.2863 |
| Condition | 1 | 234.77 | < 0.0001*** |
| Condition × Risk-group | 1 | 2.66 | 0.1034 |
| Condition × Gender | 1 | 1.53 | 0.2161 |
| Condition × Age-group | 1 | 2.73 | 0.0984 |
| Condition × Risk-group × Gender | 1 | 0.01 | 0.9302 |
| Condition × Risk-group × Age-group | 1 | 0.19 | 0.6638 |
| Condition × Gender × Age-group | 1 | 2.96 | 0.0854 |
| Condition × Risk-group × Gender × Age-group | 1 | 2.17 | 0.1407 |
| Region | 3 | 1165.91 | < 0.0001*** |
| Region × Risk-group | 3 | 2.21 | 0.0851 |
| Region × Gender | 3 | 4.33 | 0.0047** |
| Region × Age-group | 3 | 38.79 | < 0.0001*** |
| Region × Risk-group × Gender | 3 | 0.74 | 0.5263 |
| Region × Risk-group × Age-group | 3 | 0.79 | 0.5021 |
| Region × Gender × Age-group | 3 | 0.54 | 0.6560 |
| Region × Risk-group × Gender × Age-group | 3 | 0.15 | 0.9289 |
| Condition × Region | 3 | 24.34 | < 0.0001*** |
| Condition × Region × Risk-group | 3 | 2.93 | 0.0327* |
| Condition × Region × Gender | 3 | 2.99 | 0.0301* |
| Condition × Region × Age-group | 3 | 1.99 | 0.1128 |
| Condition × Region × Risk-group × Gender | 3 | 1.43 | 0.2321 |
| Condition × Region × Risk-group × Age-group | 3 | 1.24 | 0.2935 |
| Condition × Region × Gender × Age-group | 3 | 0.46 | 0.7116 |
| Condition × Region × Risk-group × Gender × Age-group | 3 | 0.87 | 0.4538 |
p < 0.05,
p < 0.01,
p < 0.001
Fig. 4.
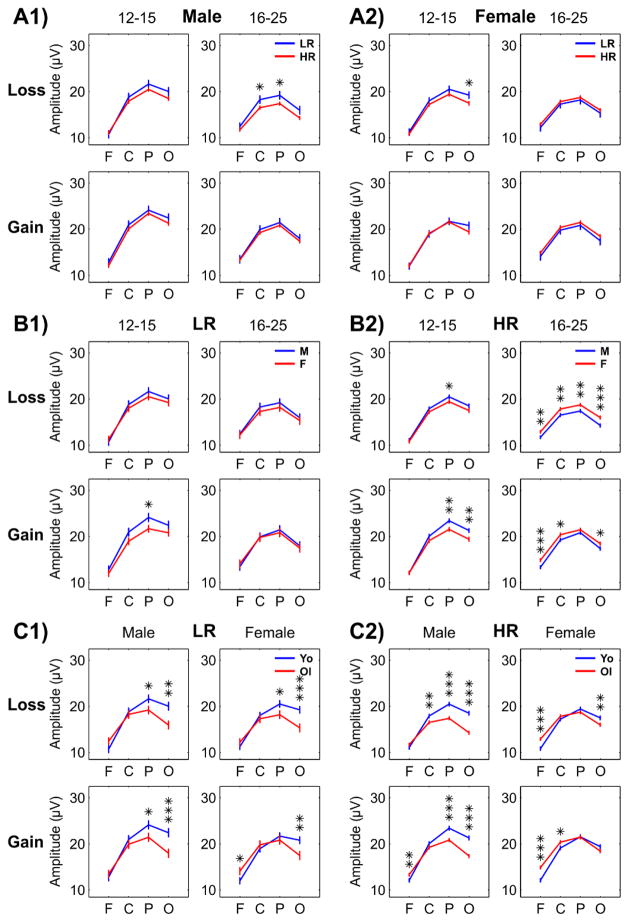
Multiple comparisons across different groups during loss and gain conditions. Estimated marginal means and standard error (±1 SE) of pairwise comparisons between the levels of risk groups (panel-sets A1 and A2), gender (panel-sets B1 and B2), and age groups (panel-sets C1 and C2) in loss and gain condition have been plotted for each scalp region (F: frontal; C: central; P: parietal; and O: occipital). Bonferroni adjusted level of significance for each comparison is shown in asterisks (*p < 0.05, **p < 0.01, ***p < 0.001).
3.3.1. Risk Group
A higher order interaction (risk group × condition × region) was found for risk group, suggesting that P3 amplitudes between risk groups varied in specific regions and conditions. Follow-up analysis revealed that HR offspring had significantly lower P3 amplitudes in parietal and occipital regions only during loss condition. Further, Bonferroni corrected multiple pairwise comparisons (see Fig. 4, panels A1–A2) revealed that risk group differences in P3 amplitudes (HR < LR) was significant in older males (in central and parietal regions) as well as in younger females in occipital region during the loss condition (see Fig. 5).
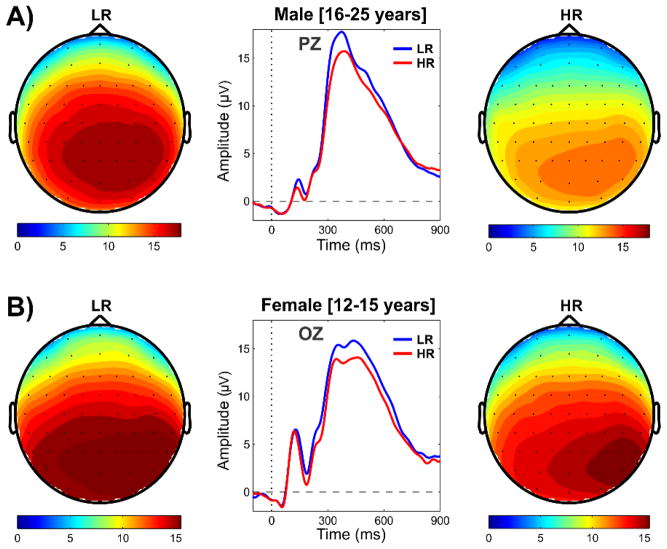
Fig. 5.
Topographic maps and waveforms showing significant difference in P3 amplitude between HR and LR subjects in older males (panel A) and younger females (panel B) during the loss condition. The vertical dotted line in the waveform plots (middle subpanels) represents the onset of outcome stimulus, and the horizontal dashed line indicates the baseline (0 μV). The color scales for the topographic maps (left and right subpanels) represent P3 amplitude in μV.
3.3.2. Gender
In terms of gender effects, the 3-way interaction gender × condition × region interaction revealed that males displayed significantly higher P3 amplitude than females only at the parietal region during the gain condition. Significant gender × age group interaction indicated that males showed higher P3 amplitude than females only in the younger age group. Gender x region effect showed that overall P3 amplitudes were higher for males (than females) in central, parietal, and occipital regions, while the frontal region showed the opposite pattern (females > males). Comparison between males and females across other factors (see Fig. 4, panels B1–B2) revealed that males displayed higher P3 amplitudes (with significant differences mainly among younger subjects) than females in all but the older HR subgroup.
3.3.3. Age Group
The age group × gender effect showed that younger male subjects manifested significantly higher P3 amplitude, but this pattern (younger > older) was not statistically significant in female subjects. In terms of gender differences across scalp regions, the younger age group showed significantly higher P3 amplitude in parietal and occipital regions while the older group showed significant increases in the frontal region as revealed by age group × region effect. Overall, younger subjects displayed significantly higher P3 amplitude than older subjects as shown by the main effect of age group. Comparisons between age groups across other factors (see Fig. 4, panels C1–C2) confirm that this pattern (younger > older) is true in several subgroups except for the anterior regions in older females, where the opposite pattern (older > younger) was observed.
3.3.4. Within-Subjects Factors
Effects of within-subjects factors (condition × region, condition, and region) were highly significant (p < 0.001). Condition × region interaction indicated that P3 was larger in the gain compared to the loss condition in all regions, and this observation was further confirmed by the condition main effect. The region main effect showed that overall P3 amplitude was highest in the parietal region, followed by central, occipital and frontal regions (i.e., parietal > central > occipital > frontal), thus indicating that P3 scalp voltage has a posterior topography.
3.4. Current Source Density
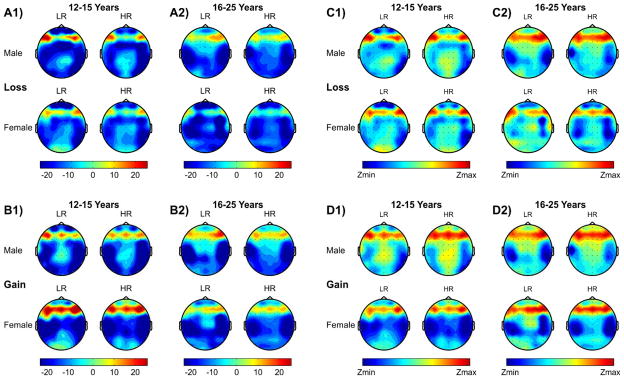
Topographic maps of CSD for each subgroup during loss and gain conditions are shown in Fig. 6 (panels A1, A2, B1, and B2). Differences across conditions and groups have been observed in sources (positivity) and sinks (negativity). Overall, the results showed that the loss condition had predominant left and right frontal sources, while the gain condition had an additional mid-frontal source as well. Younger and older male HR offspring displayed weaker source activation at the left and right frontal regions during both loss and gain conditions in comparison their LR counterparts. Among females, the right frontal source was weaker during both loss and gain conditions in younger HR subjects compared to their LR counterparts. Further, a weaker prefrontal sink activation was found in younger male HR offspring compared to male LR subjects during both loss and gain conditions and only during the loss condition in females. Central and posterior sinks were almost comparable across groups.
Fig. 6.
Topographic maps of CSD activity [panels A1, A2, B1, B2], and Z-scores [panels C1, C2, D2, D2]. Lower CSD activations of frontal sources and sinks are seen in HR offspring (more prominently among males) than LR group. Z-score maps are shown to highlight the topographic patterns more clearly across the different groups, but the magnitudes between the Z-score maps cannot be compared.
3.5. BIS Impulsivity
3.5.1. Impulsivity Scores between LR and HR Subjects
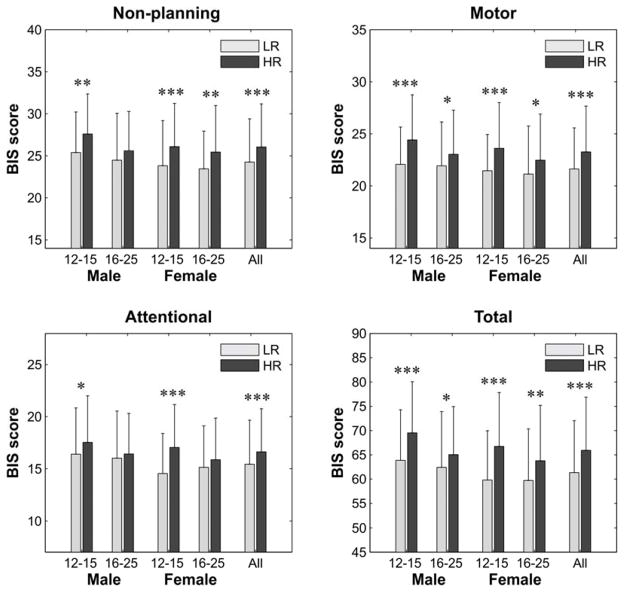
Comparisons of impulsivity scores between LR and HR groups are shown in Fig. 7. Overall, in the total sample, the HR offspring displayed significantly increased impulsivity on each of the scores: non-planning (F = 26.47; p < 0.0001), motor (F = 34.21; p < 0.0001), attentional (F = 28.47; p < 0.0001), and total (F = 45.44; p < 0.0001). Specifically, younger HR males exhibited significantly higher impulsivity in non-planning (F = 6.87; p = 0.0091), motor (F = 15.01; p = 0.0001), attentional (F = 5.35; p = 0.0213), and total impulsivity (F = 13.64; p = 0.0003) than their LR counterparts. On the other hand, older HR males showed increased impulsivity only in motor (F = 5.44; p = 0.0202) and total impulsivity (F = 5.56; p = 0.0188) compared to older LR males. Among females, younger HR offspring had significantly higher impulsivity on all the scores than the LR subjects: non-planning (F = 13.36; p = 0.0003), motor (F = 16.21; p = 0.0001), attentional (F = 28.49; p < 0.0000), and total impulsivity (F = 27.80; p < 0.0001). Similarly, older HR females showed significantly increased BIS scores for non-planning (F = 7.52; p = 0.0063), motor (F = 6.21; p = 0.0130), and total impulsivity (F = 8.85; p = 0.0031) compared to older LR females.
Fig. 7.
Comparison of BIS scores between LR and HR subjects across subgroups and all subjects. HR offspring in each subgroup showed increased impulsivity in the BIS subscales and the total score. Error bars represent 1 standard deviation. Significance level for each comparison is shown by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001).
3.5.2. Correlations between BIS scores and P3 amplitudes
The relationship between P3 amplitude and impulsivity has been summarized in Table 4. Gain-related P3 amplitudes had significant negative correlations with BIS scores, while there was no such relationship observed for the loss condition. The significant correlations during the gain condition were: FZ with non-planning, attentional, and total scores, CZ with attentional and total scores, and both PZ and OZ with attentional score. Although these correlations were statistically significant, the values of the coefficients (r) are much smaller, and therefore these results should be interpreted with caution. Correlations within the groups (risk group, gender and age group) were not significant.
Table 4.
Correlations (r) and level of significance (p) between BIS scores and P3 amplitudes are shown.
| Condition | Electrode | Non-planning | Motor | Attentional | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | ||
| Loss | FZ | −0.046 | 0.0599 | −0.029 | 0.2363 | −0.033 | 0.1859 | −0.046 | 0.0636 |
| CZ | −0.036 | 0.1386 | −0.023 | 0.3603 | −0.018 | 0.4555 | −0.033 | 0.1798 | |
| PZ | −0.033 | 0.1787 | −0.016 | 0.5163 | −0.020 | 0.4242 | −0.029 | 0.2334 | |
| OZ | −0.030 | 0.2288 | −0.023 | 0.3435 | −0.024 | 0.3242 | −0.032 | 0.1886 | |
| Gain | FZ | −0.051 | 0.0359* | −0.043 | 0.0748 | −0.066 | 0.0065** | −0.066 | 0.0066** |
| CZ | −0.043 | 0.0741 | −0.033 | 0.1747 | −0.071 | 0.0032** | −0.060 | 0.0129* | |
| PZ | −0.025 | 0.3090 | −0.014 | 0.5725 | −0.062 | 0.0103* | −0.040 | 0.0951 | |
| OZ | −0.012 | 0.6277 | −0.007 | 0.7688 | −0.048 | 0.0469* | −0.026 | 0.2741 | |
p < 0.05,
p < 0.01,
p < 0.001
4. Discussion
The goal of the present study was to elucidate reward processing deficits as indexed by P3 amplitude and CSD topography in the HR offspring from high density alcoholism families in contrast to the LR individuals from the comparison families, in the context of brain maturation across age groups and gender. Impulsivity scores were compared between the risk groups, and the relationship between P3 amplitude and impulsivity in the context of age and gender was also examined. The current study has yielded several findings:
HR offspring from older male and younger female subgroups showed significantly lower P3 amplitude than LR comparison subgroups during the loss condition;
Lower CSD activity at frontal sources in the HR older male and younger female subgroups compared to the comparison LR groups was observed;
Males had higher P3 amplitude than females in both younger and older LR groups as well in the younger HR group, while the opposite pattern (female > male) was observed in the older HR subjects;
Younger subjects produced significantly higher P3 amplitudes than older subjects during loss and gain conditions in both LR and HR groups;
HR subjects as a whole and in each subgroup showed increased BIS impulsivity scores compared to their LR counterparts;
P3 amplitudes (during gain condition) were negatively correlated with impulsivity scores.
4.1. P3 Deficits and Risk for Alcoholism
4.1.1. P3, Cognitive Function and Reward Processing
In the current study, HR older male and younger female subgroups exhibited significantly lower P3 amplitude during reward processing than the comparison LR subgroups. In stimulus discrimination paradigms, P3 amplitude has been theorized to reflect a capacity or set of brain resources related to mental processes (Kok, 1997, 2001), or subjective probability, stimulus meaning, and information transmission (Johnson, 1986). On the other hand, in the reward paradigms, P3 amplitude has been shown to index both valence and/or magnitude of the reward (e.g., Homberg et al., 1981; Begleiter et al., 1983; Otten et al., 1995; Yeung and Sanfey, 2004; Goldstein et al., 2006). Distinct topographic patterns and specific brain sources for different outcomes (i.e., loss and gain) have also been reported (Kamarajan et al., 2009; Kamarajan et al., 2010). This suggests that lower P3 amplitude in a monetary gambling paradigm may be due to dysfunctional reward processing at the neural level.
4.1.2. Lower P3 in HR offspring
The major finding of the present study was that older male HR offspring and younger female HR offspring showed significantly lower P3 amplitudes during the loss condition. Although the present study is the first to report P3 deficits during reward processing in a monetary gambling paradigm in HR offspring, similar reward-related P3 deficits have been previously reported in alcoholics (Porjesz et al., 1987; Kamarajan et al., 2010) and their offspring (Ramsey and Finn, 1997). Since, reward related P3 amplitude has been found to be sensitive to both the valence (loss/gain) and magnitude (larger/smaller) of the outcomes (Toyomaki and Murohashi, 2005a; Kamarajan et al., 2009; Kamarajan et al., 2010), it can be taken as an index of reward processing. Hence, low P3 amplitudes manifested by HR offspring may represent dysfunctional or suboptimal reward processing.
4.2. Topographic differences in P3
Although P3 differences between the risk groups were significant at central and parietal regions for the loss condition in older males and at occipital region in younger females, the source analysis using surface Laplacian (CSD maps) revealed weaker current density in HR offspring at the frontal sources. These frontal deficits or ‘hypofrontality’ of HR subjects as demonstrated in CSD maps may explain the lower P3 in these individuals (as discussed in detail in the following sections). Further, imaging studies in adolescent subjects who were vulnerable for and/or diagnosed with substance use disorders (SUDs) (Bava et al., 2009; Bava et al., 2010; Bava and Tapert, 2010; Casey and Jones, 2010; Dayan et al., 2010; Feldstein Ewing et al., 2014) and other externalizing disorders (Rubia et al., 1999; Rubia et al., 2000) have shown evidence for frontal lobe dysfunction, possibly due to a “dysmaturational pathogenesis for hypofrontality” as phrased by Rubia et al. (2000) in their work on ADHD children.
4.2.1. P3 Differences between Risk Groups in Each Gender
It should be noted that statistically significant P3 differences between HR and LR subjects were specific to age group, gender and task condition (Fig. 4, panels A1 and A2). In males, the P3 difference between the risk groups was statistically significant solely in the older age group at the central and parietal regions and only in the loss condition (Fig. 4, panel A1). In females, on the other hand, statistically significant P3 differences were observed only in the younger group at the occipital region during the loss condition (Fig. 4, panel A2). This finding suggests that although both male and female HR children/adolescents manifest lower P3 amplitudes, this deficit becomes augmented in males as they age during development while it gets attenuated in females as they age into late adolescence and young adulthood. This gender specific finding is in line with previous findings indicating that low P3 amplitudes in high risk offspring of alcoholics have been reported more often in males (Begleiter et al., 1984; O’Connor et al., 1986; Begleiter et al., 1987; O’Connor et al., 1987; Whipple et al., 1991; Berman et al., 1993; Benegal et al., 1995; Ramachandran et al., 1996; Cohen et al., 1997a; Ramsey and Finn, 1997; Rodriguez Holguin et al., 1999b) than in females (Porjesz et al., 1996; Van der Stelt et al., 1998), while there are only a few studies showing P3 differences (between risk groups) in both males and females (e.g., Van der Stelt et al., 1998). Further, lower P3 has been shown to be more robust in high risk male than female offspring (e.g., Polich et al., 1994; Hill et al., 1999). It should also be noted that P3 differences (between HR and LR subjects) in both younger and older males is in line with previous studies which have reported lower P3 amplitude in samples of ‘at risk’ children/adolescents (Begleiter et al., 1984; Begleiter et al., 1987; Berman et al., 1993) as well as in young adults (O’Connor et al., 1986; O’Connor et al., 1987; Ramachandran et al., 1996; Cohen et al., 1997a; Ramsey and Finn, 1997; Kamarajan et al., 2005b). A meta-analytic study revealed that P3 reduction was more robust in younger male subjects with family history of alcoholism and showed higher effect sizes for younger ages than for older subjects (Polich et al., 1994). There are a host of factors that can affect the trajectory of gender-specific development including sexual dimorphism of brain maturation (De Bellis et al., 2001; Lenroot et al., 2007; Lenroot and Giedd, 2010), which can be influenced by both genetic and environmental factors (Lenroot and Giedd, 2008; Lenroot et al., 2009a; Lenroot et al., 2009b).
4.2.2. P3 Differences between Male and Female Groups
Comparison between male and female subjects within age groups and risk groups (Fig. 4, panels B1 and B2) shows that the pattern of higher P3 amplitudes shown by males during 12–15 years becomes less prominent in the LR individuals as they aged into 16–25 years (Fig. 4, panel B1) while the gender difference in younger age (male > female) switched to the opposite direction in the older age group (female > male) in the HR offspring (Fig. 4, panel B1). This finding may suggest that the developmental and maturational changes in the P3 activity could be different in LR and HR subjects, although this interpretation has to be confirmed by further studies with a larger/comparable sample sizes of LR and HR subjects. Further, it is rather surprising that P3 amplitude between male and female subjects did not differ significantly in the LR group except in the gain condition in the younger group (Fig. 4, panel B1), although all comparisons of gender in HR subjects were significant. Although previous studies have shown that females produced higher amplitude than males in visual paradigms (Orozco and Ehlers, 1998; Guillem and Mograss, 2005; Proverbio et al., 2006) including the gambling paradigm (Kamarajan et al., 2009), the age range was not compatible with our current study (i.e., the sample in these studies were adults with 18–45 years of age, while our study has samples of children/adolescents and young adults with 12–25 years of age range). However, except in older females, there is a pattern in the current study that males produced higher P3 amplitudes than females.
These gender differences in P3 within age groups can be understood in the context of brain development. As noted earlier, there is evidence for sexual dimorphism of brain maturation (De Bellis et al., 2001; Lenroot et al., 2007; Lenroot and Giedd, 2010). Further, there is a substantial evidence of gender differences in brain connectivity that may account for gender-related cognitive differences (for a review, see Gong et al., 2011). For example, gender related connectivity differences within prefrontal cortex (PFC) showed that males exhibited stronger connectivity in the PFC regions with leftward dominance, while bilateral dominance was observed in females (Chuang and Sun, 2014). It is possible that individuals with familial/genetic risk for alcoholism may have a different developmental patterns within each gender than the normal/healthy individuals (Bava and Tapert, 2010), although this issue needs further exploration.
4.2.3. P3 differences between Age Groups (Younger vs. Older Group)
The P3 differences between age groups (Fig. 4, panels C1 and C2) are worth elaborating. It was observed that younger (12–15 years) subjects showed significantly higher P3 amplitudes in the posterior regions than their older (16–25 years) counterparts. Further, a reverse pattern (i.e., older > younger) was observed in the frontal region, although the posterior differences were more robust than the frontal differences. The finding of larger P3 in children (compared to adolescents/young adults) is in keeping with well-known developmental findings of P3 and EEG. For example, the amplitude of visual P3 decreased gradually from childhood to adulthood (e.g., Johnson, 1989; Courehesne, 1990), and this amplitude reduction can also be explained by the findings from other developmental studies which found a gradual reduction of slower rhythms (which constitute P3 response) in children’s EEG during development as they mature into adolescence/adulthood (Matousek and Petersen, 1973a; John et al., 1980; Gasser et al., 1988b; Wackermann and Matousek, 1998; Dustman et al., 1999; Clarke et al., 2001; Boord et al., 2007). In a developmental study of error-related negativity (ERN), a related component to feedback negativity, Davies et al. (2004) demonstrated that the amplitude of the ERN increased from 7 to 17 years of age and the waveforms did not reach optimal level to those of young adults until mid-teens. Further, similar to our finding, Ladouceur et al. (2007) found that P3 amplitude during error-processing was greater in the adolescent group compared to the adult group. These studies lend support to our finding of P3 differences between age groups.
Compared to the younger age group, the older subjects showed relatively higher P3 amplitudes at frontal regions, while the younger group showed relatively higher P3 amplitudes at parietal and occipital regions. The anterior-posterior modulation (“frontalization”) during brain development has been well-documented in the EEG literature. Maturational patterns of EEG activity follow grey matter reduction during development, as there is a redistribution of relative EEG power as a function of age with posterior regions maturing earlier than anterior regions (cf. Segalowitz et al., 2010). During development, theta-alpha maturation occurs first in occipital regions and then progresses gradually to frontal regions (Matousek and Petersen, 1973b; Gasser et al., 1988a; Dustman et al., 1999). Yordanova and Kolev (1996) demonstrated similar age effects in EROs by demonstrating that adults showed ERO alpha maxima at mid-central regions in contrast to the parietal maxima in children, reflecting perhaps a gradual “frontalization” with growth (cf. Segalowitz et al., 2010). These studies support our finding related to anterior-posterior differences in younger and older groups.
Our finding of increased anterior P3 activity coupled with decreased posterior activity from adolescence to adulthood corresponds with recent imaging data showing a posterior to frontal progression of cortical development from childhood through adolescence and adulthood (Gogtay et al., 2004; Gogtay and Thompson, 2010). During brain development with advancing age, the prefrontal activity becomes progressively more focal and specialized (Tamm et al., 2002; Brown et al., 2005; Durston et al., 2006). This phenomenon is termed “frontalization” or the maturational process of frontal lobes during development, whereby frontal lobes gradually take control of higher-order cognitive functions, such as executive functions and inhibitory control (Rubia et al., 2000; Yurgelun-Todd and Killgore, 2006; Segalowitz et al., 2010; Arain et al., 2013). Furthermore, imaging findings showing late maturation of prefrontal cortex (Fuster, 2002; Gogtay et al., 2004) may explain the higher P3 activity at frontal regions (and lower P3 activity in the posterior regions) in the older subjects compared to the younger group.
4.3. P3 Amplitude, Impulsivity, and Externalizing Disorders
Two important findings regarding impulsivity have been observed in the current study: (i) increased impulsivity in all subscales and total score of the BIS in the HR offspring compared to the LR group in each subgroup of gender and age (Fig. 7); and (ii) significant negative correlations between P3 amplitudes during gain condition and impulsivity scores, showing decreased P3 amplitude in individuals with higher impulsivity (Table 4). In light of the established view that impulsivity is a critical factor in the pathophysiology and/or risk propensity for alcoholism and related disorders (Petry, 2001; Finn et al., 2002; Moeller et al., 2002; Chen et al., 2007; Dom et al., 2007; Kamarajan et al., 2007; Rubio et al., 2007; Hanson et al., 2008; Rubio et al., 2008; Verdejo-Garcia et al., 2008; von Diemen et al., 2008; Crews and Boettiger, 2009; Rogers et al., 2010), our finding that HR offspring showed increased impulsivity may provide further evidence for the notion that etiological connections exist between impulsivity and alcoholism and other SUDs (for reviews, see Sher and Trull, 1994; Verdejo-Garcia et al., 2008; Dick et al., 2010). In a review, Kamarajan and Porjesz (2012) have outlined electrophysiological abnormalities in impulsivity spectrum disorders, which include AUD and other related disorders. Studies have also shown that psychometrically measured impulsivity, as done in our study, was associated with externalizing disorders in general and substance use disorder in particular (Saunders et al., 1973; O’Boyle and Barratt, 1993; Krueger et al., 2002; Romer et al., 2009). It may be worth noting that prevalence rates of externalizing disorders were significantly higher in the HR group than in the LR group, suggesting that the HR group is susceptible not only to alcoholism but also to other externalizing disorders (see Table 2). Further, our finding of a negative correlation between P3 amplitude and impulsivity scores suggests that individuals with lower P3 amplitudes have higher impulsivity, lending further support to the notion that P3 abnormalities are intrinsically related to impulsivity traits (Justus et al., 2001; McGue et al., 2001; Moeller et al., 2004; Potts et al., 2006; Chen et al., 2007; Kamarajan et al., 2009) as well as to externalizing conditions (Justus et al., 2001; McGue et al., 2001; Moeller et al., 2004; Chen et al., 2007; Kamarajan et al., 2010). It is possible that P3 deficits and high impulsivity may have common underlying brain mechanisms. Recent fMRI studies have found differences between high and low impulsive individuals in terms of engagement and activation patterns of frontal lobe structures (Sripada et al., 2011; Diekhof et al., 2012; Davis et al., 2013). Frontal lobe structures have also been implicated in several disorders involving impulsivity and externalizing traits (Vollm et al., 2004; Lee et al., 2005; Vollm et al., 2007; Wolf et al., 2011; Costa Dias et al., 2013; Cyders et al., 2014). Taken together, these findings add support to the view that P3 amplitude, impulsivity, and externalizing disorders are etiologically related (Iacono et al., 2003; Iacono and McGue, 2006; Chen et al., 2007; Carlson et al., 2009; Gao and Raine, 2009; Young et al., 2009; Gilmore et al., 2010a; Lejuez et al., 2010). It is important to note that BIS score differences between HR and LR groups varied according to the age group and gender (Fig. 7). Among males, the younger age group showed more robust differences (p < 0.001) between LR and HR groups in non-planning, motor, cognitive and total scores, while the older males displayed a modest level of significance (p < 0.05) only in motor and total scores. While our finding on age and gender differences in impulsivity are similar to the findings in the literature (Steinberg et al., 2008; Stoltenberg et al., 2008), impulsivity as such may mediate the association between gender and risk for alcohol problems (Stoltenberg et al., 2008). While there was a correlation between P3 amplitude and BIS scores during the gain condition, the lack of correlation during the loss condition in our study may warrant further exploration in similar samples. Interestingly, Bernat et al. (2011) reported that while delta-P3 amplitude was found to be reduced among individuals high in externalizing proneness, theta-FRN (N2) response was unrelated to externalizing. Further studies are needed to establish the relationship between various facets of impulsivity and neurophysiological measures, such as the P3.
4.4. Source activations of P3, Reward Processing, and Risk for Alcoholism
In this study, CSD topographic analysis yielded three key findings: i) loss condition showed bilateral frontal sources while the gain condition additionally had a prominent midfrontal source; ii) HR offspring (younger males and females as well as older males) displayed weaker CSD sources at the frontal regions in comparison their LR counterparts; and iii) a weaker prefrontal sink activation was found in younger HR offspring compared to the LR group during both loss and gain conditions in males and only during loss condition in females.
During the evaluation of monetary loss and gain, the common features of the CSD topography observed across groups were the sources (positivities) in left-frontal, right-frontal and mid-frontal regions (Fig. 6). Our finding that prominent CSD sources were located at the frontal regions corroborates existing evidence that cognitive tasks requiring evaluations of reward outcomes recruit the frontal lobes (Wallis and Miller, 2003; Glascher et al., 2010; Rangel and Hare, 2010). There is also a strong literature support for the major involvement of frontal lobes (connecting a network of other subcortical structures) during reward processing and decision making (Haber and Knutson, 2010; Rushworth et al., 2011; Economides et al., 2014; Forbes et al., 2014). Specifically, medial prefrontal cortex (mPFC) involving ACC has been shown to be involved in the processing of the reward values of the reinforcers (O’Doherty et al., 2001; Shidara and Richmond, 2002; Knutson et al., 2003; Rogers et al., 2004; Knutson et al., 2005; Shidara et al., 2005; Taylor et al., 2006; Sallet et al., 2007; Fujiwara et al., 2009; Xue et al., 2009; Economides et al., 2014).
HR offspring also showed weaker CSD activations (of the sources and sinks) in the frontal and prefrontal regions. Specifically, the lower activations in both P3 amplitude and CSD were found in both younger and older age groups in males but only in younger age groups in females. Neuroimaging studies have found pathophysiological alterations in the brain’s reward system (Diekhof et al., 2008; Park et al., 2010; Tomasi et al., 2010; Muller-Oehring et al., 2013) including altered white matter integrity in individuals with SUD (Bava et al., 2009; Jacobus et al., 2009; McQueeny et al., 2009; Bava et al., 2010), and deficient functional connectivity between frontal lobes and other key structures in subjects who are at high risk for alcoholism (Weiland et al., 2012; Wetherill et al., 2012; Weiland et al., 2013). Forbes et al. (2014) reported that young adults with family history of alcohol dependence exhibited lower medial PFC response. Taken together, all these findings support our hypothesis that suppressed CSD features at the frontal regions during reward processing in the HR group may represent dysfunctional reward circuitry as well as a ‘hypofrontality’, which may mediate or predispose toward risk for developing AUD and/or related disorders in these subjects.
4.5. Genetic Factors underlying P3 and Risk for Alcoholism and Related Disorders
The development of alcoholism is influenced by underlying biological susceptibility factors, environmental factors, and complex interactions among genes and environment (Porjesz et al., 2005; Dick and Kendler, 2012). There is a large literature indicating that low P3 amplitude can been considered to be an important genetic marker for the development of alcoholism (Begleiter et al., 1984; Goldman, 1993; Porjesz et al., 2005; Porjesz and Rangaswamy, 2007) and other externalizing disorders (Iacono et al., 2002; Iacono and McGue, 2006). Several genes have been found to be associated with delta and theta EROs underlying P3 (Jones et al., 2004; Jones et al., 2006; Chen et al., 2009; Chen et al., 2010; Zlojutro et al., 2011; Kang et al., 2012), and several of these genes have also been associated with alcoholism (Wang et al., 2004; Luo et al., 2005; Dick et al., 2007; Chen et al., 2009; Chen et al., 2010) and related externalizing disorders as well (Dick, 2007; Dick et al., 2008). Quantitative electrophysiological phenotypes, such as EEG, P3, and related EROs, have served as effective endophenotypes for gene identification in psychiatric genetics (for recent reviews, see Porjesz and Rangaswamy, 2007; Rangaswamy and Porjesz, 2008; Iacono and Malone, 2011; Chen et al., 2012; Euser et al., 2012; Pandey et al., 2012; Rangaswamy and Porjesz, 2014; Kamarajan and Porjesz, 2015). These heritable neurophysiological biomarkers (Begleiter and Porjesz, 2006; Anokhin, 2014) have been very successful in identifying susceptibility genes for alcohol dependence and related disorders (Rangaswamy and Porjesz, 2008; Chen et al., 2012) as they are directly associated with human information processing and cognitive functions (Porjesz and Rangaswamy, 2007). Interestingly, several of the same genes associated with electrophysiological phenotypes and alcoholism in adults have been found to be related to precursor phenotypes in children and adolescents, such as conduct disorder (Dick et al., 2004), onset of regular drinking (Chorlian et al., 2013), and trajectories of drunkenness (Dick et al., 2014). While heritability of the reward-related P3 is yet to be determined, it may be another promising endophenotype, as both alcoholics (Kamarajan et al., 2010) and HR offspring (in the current study) manifest lower P3 amplitude.
4.6. Limitations of the Present Study and Suggestions for Future Studies
The present study has extended our previous research in alcoholics, in order to confirm whether reward related ERP deficits observed in alcoholics are also found in children of alcoholics, and has successfully revealed differences in P3 amplitude and CSD across risk groups. However, there are some limitations in the current study, which could not be completely eliminated. For example, (i) the sample sizes for the LR groups are relatively smaller than the HR groups, and hence the findings have to be interpreted with caution; (ii) the trials involving both bet amounts (10¢ and 50¢) have been combined within loss and gain conditions respectively, in order to maximize trial numbers and sample size, while the analysis of each condition may have offered more detailed findings; (iii) the N2 component, which was difficult to measure and has not been analyzed in the current study, may have yielded additional information about reward processing deficits in HR offspring; (iv) although the mean trial numbers across the groups in each condition [LR = 42.24 and HR = 40.53 for the gain condition; LR = 39.12 and HR = 36.51 for the loss condition] seems to be sufficient and optimal for the reward-P3 (in view of the findings from earlier studies), further assessments regarding the SNR and minimum/optimal number of trials for component stabilization may have confirmed or improved the validity of current findings; and (v) complex, predictive analytic models, which have not been done in the current study, may be essential to assess the degree to which the factors of genetic risk, substance use, and impulsivity contribute independently as well as relatively contribute to the P3 deficits and CSD profile. Despite these limitations, the study has provided important findings that may have considerable implications for the use of reward related electrophysiological phenotypes to characterize neurocognitive dysfunction in alcoholism and risk status.
Future studies, while avoiding the limitations of the present study, may further consider examining: (i) the oscillations underlying P3 component (e.g., delta and theta bands) along with ERP measures, (ii) the trajectories of reward-related P3 amplitude comparing the LR and HR subjects; (iii) longitudinal changes associated with P3 and ERO measures in HR offspring by analyzing the data on multiple assessments over a period of time, (iv) brain circuitries underlying reward/outcome processing in high risk individuals by using coherence/synchrony measures of electrophysiology and by using functional connectivity measures of neuroimaging, (v) externalizing disorders along with substance use disorders, (vi) new strategies to statistically or methodologically disentangle the underlying genetic risk (in those at high risk for alcoholism) from the manifested symptoms or characteristics (e.g., externalizing disorders) that may influence the phenotype (e.g., P3) under study, (vii) the utility of reward related ERP/ERO measures as endophenotypes in genetic studies, and (viii) a more systematic study with multiple measures of impulsivity and externalizing disorders in AUD and HR offspring to further elucidate the complex interactions among these factors.
5. Conclusions
The present study has elucidated reward processing deficits in younger and older high risk offspring from dense alcoholism families of COGA. Older male and younger female HR subgroups have shown significantly lower P3 amplitude and reduced CSD activation at frontal sources during reward processing compared to their LR counterparts. High risk subjects were also found to be more impulsive than LR subjects. Negative correlation between P3 amplitudes and impulsivity scores suggested that reduced P3 amplitude and increased impulsivity (as manifested by HR offspring) may predispose toward risk for alcoholism and related disorders. Further, gender- and age-specific findings of the present study may have several implications for future research on alcoholism and risk. Lastly, it is suggested that using electrophysiological endophenotypes, such as P3 and its oscillatory components, to identify genes involved in risk for alcoholism and related disorders may serve as sensitive biomarkers that are physiologically closer to gene functions, that may shed light on novel prevention, diagnosis and treatment options.
Appendix I.
Mean (M) and standard deviation (SD) of P3 amplitudes for each subgroup
| Condition | Electrode | LR | HR | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | ||||||||||||||
| Younger | Older | Younger | Older | Younger | Older | Younger | Older | ||||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | ||
| Loss | F3 | 9.45 | 6.38 | 11.56 | 5.30 | 10.62 | 6.03 | 11.68 | 6.19 | 9.94 | 6.01 | 10.90 | 5.85 | 10.07 | 5.99 | 12.39 | 5.69 |
| FZ | 12.56 | 6.64 | 13.40 | 5.56 | 12.05 | 6.96 | 13.26 | 6.80 | 12.08 | 6.33 | 12.61 | 6.19 | 11.30 | 6.48 | 13.52 | 6.11 | |
| F4 | 11.02 | 6.53 | 12.25 | 5.25 | 11.29 | 7.14 | 11.78 | 5.86 | 11.19 | 6.11 | 11.63 | 5.67 | 10.61 | 6.16 | 12.59 | 5.73 | |
| C3 | 16.98 | 6.86 | 17.10 | 5.99 | 17.25 | 7.04 | 16.82 | 6.68 | 16.43 | 6.62 | 15.52 | 6.10 | 15.96 | 6.58 | 17.15 | 6.19 | |
| CZ | 19.52 | 7.82 | 19.04 | 6.83 | 18.45 | 7.29 | 18.36 | 7.14 | 18.50 | 7.32 | 17.36 | 6.53 | 17.57 | 7.13 | 18.32 | 6.73 | |
| C4 | 19.51 | 7.68 | 18.40 | 6.49 | 18.11 | 7.46 | 17.68 | 6.57 | 18.75 | 6.86 | 16.80 | 6.26 | 17.26 | 6.80 | 17.87 | 6.25 | |
| P3 | 19.33 | 6.16 | 18.05 | 6.89 | 19.73 | 6.61 | 17.57 | 7.25 | 19.19 | 6.98 | 16.47 | 6.12 | 18.02 | 6.60 | 18.00 | 6.18 | |
| PZ | 21.79 | 6.98 | 19.80 | 7.26 | 20.48 | 7.03 | 19.42 | 7.54 | 20.71 | 7.13 | 18.01 | 6.37 | 19.64 | 6.84 | 19.07 | 6.48 | |
| P4 | 22.73 | 7.28 | 19.55 | 7.00 | 20.89 | 7.08 | 18.65 | 7.04 | 21.31 | 7.27 | 17.76 | 6.52 | 19.84 | 6.86 | 18.78 | 6.31 | |
| O1 | 19.29 | 7.42 | 15.87 | 7.67 | 18.87 | 6.22 | 15.76 | 7.45 | 18.23 | 7.12 | 14.07 | 6.20 | 17.21 | 6.62 | 15.67 | 6.27 | |
| OZ | 19.20 | 7.25 | 15.59 | 7.89 | 18.70 | 6.13 | 15.59 | 8.18 | 17.84 | 7.30 | 13.99 | 6.26 | 17.22 | 6.62 | 15.68 | 6.31 | |
| O2 | 19.99 | 7.65 | 16.04 | 8.08 | 19.39 | 6.33 | 15.99 | 7.74 | 18.74 | 7.77 | 14.44 | 6.47 | 17.86 | 6.69 | 16.02 | 6.51 | |
| Gain | F3 | 11.43 | 6.26 | 12.53 | 5.42 | 11.56 | 6.09 | 13.72 | 6.66 | 11.18 | 6.24 | 12.57 | 6.17 | 11.42 | 5.96 | 14.38 | 6.12 |
| FZ | 13.43 | 5.90 | 14.28 | 6.01 | 12.65 | 6.53 | 15.19 | 7.46 | 12.72 | 6.63 | 14.11 | 6.56 | 12.35 | 6.71 | 15.47 | 6.76 | |
| F4 | 12.53 | 5.93 | 13.63 | 5.81 | 12.19 | 6.74 | 13.99 | 6.53 | 12.42 | 6.61 | 13.46 | 6.07 | 12.01 | 6.70 | 14.68 | 6.24 | |
| C3 | 19.41 | 7.36 | 18.78 | 5.83 | 18.33 | 6.57 | 19.59 | 7.09 | 18.92 | 6.74 | 18.30 | 6.75 | 17.92 | 6.31 | 19.62 | 6.79 | |
| CZ | 20.80 | 7.63 | 20.60 | 6.99 | 19.50 | 7.39 | 20.99 | 8.18 | 20.39 | 7.42 | 20.11 | 7.18 | 19.44 | 7.17 | 20.97 | 7.58 | |
| C4 | 21.02 | 7.37 | 20.29 | 6.64 | 19.28 | 6.87 | 20.15 | 7.26 | 20.74 | 6.90 | 19.66 | 6.92 | 19.29 | 7.03 | 20.38 | 6.91 | |
| P3 | 21.75 | 7.54 | 20.36 | 6.73 | 21.15 | 6.73 | 20.44 | 7.10 | 22.32 | 7.24 | 19.85 | 6.62 | 20.30 | 6.77 | 20.76 | 6.67 | |
| PZ | 24.10 | 7.86 | 22.17 | 7.33 | 21.82 | 7.09 | 22.10 | 8.09 | 23.57 | 7.63 | 21.70 | 7.00 | 21.81 | 7.05 | 21.95 | 7.20 | |
| P4 | 24.64 | 7.57 | 21.75 | 7.40 | 22.15 | 7.03 | 21.20 | 7.40 | 24.18 | 7.66 | 21.16 | 6.90 | 21.88 | 7.04 | 21.49 | 6.92 | |
| O1 | 21.41 | 8.17 | 18.03 | 7.77 | 20.80 | 7.41 | 18.19 | 7.32 | 21.09 | 7.70 | 17.28 | 6.70 | 19.21 | 6.84 | 18.22 | 6.48 | |
| OZ | 21.14 | 7.85 | 17.64 | 7.86 | 20.36 | 7.43 | 17.77 | 7.81 | 20.65 | 7.63 | 17.21 | 6.56 | 19.16 | 6.85 | 18.15 | 6.64 | |
| O2 | 22.47 | 8.36 | 17.98 | 8.05 | 21.08 | 7.12 | 17.95 | 7.59 | 21.63 | 8.02 | 17.53 | 6.66 | 19.56 | 6.86 | 18.55 | 6.78 | |
Acknowledgments
The Collaborative Study on the Genetics of Alcoholism (COGA), Principal Investigators B. Porjesz, V. Hesselbrock, H. Edenberg, L. Bierut, includes eleven different centers: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, J. Nurnberger Jr., T. Foroud); University of Iowa (S. Kuperman, J. Kramer); SUNY Downstate (B. Porjesz); Washington University in St. Louis (L. Bierut, J. Rice, K. Bucholz, A. Agrawal); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield, A. Brooks); University of Texas Health Science Center at San Antonio (L. Almasy), Virginia Commonwealth University (D. Dick), Icahn School of Medicine at Mount Sinai (A. Goate), and Howard University (R. Taylor). Other COGA collaborators include: L. Bauer (University of Connecticut); D. Koller, J. McClintick, S. O’Connor, L. Wetherill, X. Xuei, Y. Liu (Indiana University); G. Chan (University of Iowa; University of Connecticut); D. Chorlian, N. Manz, C. Kamarajan, A. Pandey (SUNY Downstate); J.-C. Wang, M. Kapoor (Icahn School of Medicine at Mount Sinai) and F. Aliev (Virginia Commonwealth University). A. Parsian and M. Reilly are the NIAAA Staff Collaborators. We continue to be inspired by our memories of Henri Begleiter and Theodore Reich, founding PI and Co-PI of COGA, and also owe a debt of gratitude to other past organizers of COGA, including Ting-Kai Li, currently a consultant with COGA, P. Michael Conneally, Raymond Crowe, and Wendy Reich, for their critical contributions. This study is supported by NIH grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA), and by AA05524 (PI: Bernice Porjesz) from the NIAAA.
Footnotes
Conflict of Interest
None of the authors have potential conflicts of interest to be disclosed.
References
- Alexander JE, Polich J, Bloom FE, Bauer LO, Kuperman S, Rohrbaugh J, Morzorati S, O’Connor SJ, Porjesz B, Begleiter H. P300 from an auditory oddball task: inter-laboratory consistency. Int J Psychophysiol. 1994;17:35–46. doi: 10.1016/0167-8760(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Anokhin AP. Genetic psychophysiology: advances, problems, and future directions. Int J Psychophysiol. 2014;93:173–197. doi: 10.1016/j.ijpsycho.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, Sandhu R, Sharma S. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. 2013;9:449–461. doi: 10.2147/NDT.S39776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Res. 2009;173:228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Mahmood O, Yang TT, Tapert SF. Neurocognitive correlates of white matter quality in adolescent substance users. Brain Cogn. 2010;72:347–354. doi: 10.1016/j.bandc.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychol Rev. 2010;20:398–413. doi: 10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Event-related potentials in populations at risk for alcoholism. Electroencephalogr Clin Neurophysiol Suppl. 1990a;41:177–182. doi: 10.1016/b978-0-444-81352-7.50023-6. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Neuroelectric processes in individuals at risk for alcoholism. Alcohol Alcohol. 1990b;25:251–256. doi: 10.1093/oxfordjournals.alcalc.a044998. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Genetics of human brain oscillations. Int J Psychophysiol. 2006;60:162–171. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Chou CL, Aunon JI. P3 and stimulus incentive value. Psychophysiology. 1983;20:95–101. doi: 10.1111/j.1469-8986.1983.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Rawlings R, Eckardt M. Auditory recovery function and P3 in boys at high risk for alcoholism. Alcohol. 1987;4:315–321. doi: 10.1016/0741-8329(87)90029-2. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Reich T, Hesselbrock VM, Porjesz B, Li TK, Schuckit MA, Edenberg HJ, Rice JP. The Collaborative Study on the Genetics of Alcoholism. Alcohol Health Res World. 1995;19:228–236. [PMC free article] [PubMed] [Google Scholar]
- Bell MA, Cuevas K. Using EEG to Study Cognitive Development: Issues and Practices. J Cogn Dev. 2012;13:281–294. doi: 10.1080/15248372.2012.691143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benegal V, Jain S, Subbukrishna DK, Channabasavanna SM. P300 amplitudes vary inversely with continuum of risk in first degree male relatives of alcoholics. Psychiatr Genet. 1995;5:149–156. doi: 10.1097/00041444-199524000-00001. [DOI] [PubMed] [Google Scholar]
- Berman SM, Whipple SC, Fitch RJ, Noble EP. P3 in young boys as a predictor of adolescent substance use. Alcohol. 1993;10:69–76. doi: 10.1016/0741-8329(93)90055-s. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Nelson LD, Steele VR, Gehring WJ, Patrick CJ. Externalizing psychopathology and gain-loss feedback in a simulated gambling task: dissociable components of brain response revealed by time-frequency analysis. J Abnorm Psychol. 2011;120:352–364. doi: 10.1037/a0022124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boord PR, Rennie CJ, Williams LM. Integrating “brain” and “body” measures: correlations between EEG and metabolic changes over the human lifespan. J Integr Neurosci. 2007;6:205–218. doi: 10.1142/s0219635207001416. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Katsanis J, Iacono WG, Mertz AK. Substance dependence and externalizing psychopathology in adolescent boys with small, average, or large P300 event-related potential amplitude. Psychophysiology. 1999;36:583–590. [PubMed] [Google Scholar]
- Carlson SR, McLarnon ME, Iacono WG. P300 amplitude, externalizing psychopathology, and earlier- versus later-onset substance-use disorder. J Abnorm Psychol. 2007;116:565–577. doi: 10.1037/0021-843X.116.3.565. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Thái S, McLarnon ME. Visual P3 amplitude and self-reported psychopathic personality traits: Frontal reduction is associated with self-centered impulsivity. Psychophysiology. 2009;46:100–113. doi: 10.1111/j.1469-8986.2008.00756.x. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatrian GE, Lettich E, Nelson PL. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activities. Am J EEG Technol. 1985;25:83–92. [Google Scholar]
- Chatrian GE, Lettich E, Nelson PL. Modified nomenclature for the “10%” electrode system. J Clin Neurophysiol. 1988;5:183–186. [PubMed] [Google Scholar]
- Chen AC, Manz N, Tang Y, Rangaswamy M, Almasy L, Kuperman S, Nurnberger J, Jr, O’Connor SJ, Edenberg HJ, Schuckit MA, Tischfield J, Foroud T, Bierut LJ, Rohrbaugh J, Rice JP, Goate A, Hesselbrock V, Porjesz B. Single-nucleotide polymorphisms in corticotropin releasing hormone receptor 1 gene (CRHR1) are associated with quantitative trait of event-related potential and alcohol dependence. Alcohol Clin Exp Res. 2010;34:988–996. doi: 10.1111/j.1530-0277.2010.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Porjesz B, Rangaswamy M, Kamarajan C, Tang Y, Jones KA, Chorlian DB, Stimus AT, Begleiter H. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcohol Clin Exp Res. 2007;31:156–165. doi: 10.1111/j.1530-0277.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- Chen AC, Rangaswamy M, Porjesz B. Endophenotypes in Psychiatric Genetics. In: Nurnberger JJ, Berrettini W, editors. Principles of Psychiatric Genetics. Cambridge University Press; New York: 2012. [Google Scholar]
- Chen AC, Tang Y, Rangaswamy M, Wang JC, Almasy L, Foroud T, Edenberg HJ, Hesselbrock V, Nurnberger J, Jr, Kuperman S, O’Connor SJ, Schuckit MA, Bauer LO, Tischfield J, Rice JP, Bierut L, Goate A, Porjesz B. Association of single nucleotide polymorphisms in a glutamate receptor gene (GRM8) with theta power of event-related oscillations and alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:359–368. doi: 10.1002/ajmg.b.30818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorlian DB, Rangaswamy M, Manz N, Wang JC, Dick D, Almasy L, Bauer L, Bucholz K, Foroud T, Hesselbrock V, Kang SJ, Kramer J, Kuperman S, Nurnberger J, Jr, Rice J, Schuckit M, Tischfield J, Edenberg HJ, Goate A, Bierut L, Porjesz B. Genetic and neurophysiological correlates of the age of onset of alcohol use disorders in adolescents and young adults. Behav Genet. 2013;43:386–401. doi: 10.1007/s10519-013-9604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CC, Sun CW. Gender-related effects of prefrontal cortex connectivity: a resting-state functional optical tomography study. Biomed Opt Express. 2014;5:2503–2516. doi: 10.1364/BOE.5.002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: development of the normal child. Clin Neurophysiol. 2001;112:806–814. doi: 10.1016/s1388-2457(01)00488-6. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Ji J, Chorlian DB, Begleiter H, Porjesz B. Alcohol-related ERP changes recorded from different modalities: a topographic analysis. Alcohol Clin Exp Res. 2002;26:303–317. [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neuroelectric correlates of response production and inhibition in individuals at risk to develop alcoholism. Biol Psychiatry. 1997a;42:57–67. doi: 10.1016/S0006-3223(96)00221-1. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neurophysiological correlates of response production and inhibition in alcoholics. Alcohol Clin Exp Res. 1997b;21:1398–1406. [PubMed] [Google Scholar]
- Cohen HL, Wang W, Porjesz B, Bauer L, Kuperman S, O’Connor SJ, Rohrbaugh J, Begleiter H. Visual P300: an interlaboratory consistency study. Alcohol. 1994;11:583–587. doi: 10.1016/0741-8329(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Wang W, Porjesz B, Begleiter H. Auditory P300 in young alcoholics: regional response characteristics. Alcohol Clin Exp Res. 1995;19:469–475. doi: 10.1111/j.1530-0277.1995.tb01533.x. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Ranganath C. Reinforcement learning signals predict future decisions. J Neurosci. 2007;27:371–378. doi: 10.1523/JNEUROSCI.4421-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Dias TG, Wilson VB, Bathula DR, Iyer SP, Mills KL, Thurlow BL, Stevens CA, Musser ED, Carpenter SD, Grayson DS, Mitchell SH, Nigg JT, Fair DA. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013;23:33–45. doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courehesne E. Chronology of postnatal human brain development: ERP, PET, myelinogenesis, and synaptogenesis studies. In: Rohrbaugh J, Parasuraman R, Johnson R Jr, editors. Issues in event-related potential research: Tutorials and interdisciplinary vantages. Oxford University Press; Oxford: 1990. [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, Hommer RE, South M, Molfese PJ, Fearon RM, Mayes LC. A developmental study of the feedback-related negativity from 10–17 years: age and sex effects for reward versus non-reward. Dev Neuropsychol. 2013;38:595–612. doi: 10.1080/87565641.2012.694512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Dzemidzic M, Eiler WJ, Coskunpinar A, Karyadi K, Kareken DA. Negative urgency and ventromedial prefrontal cortex responses to alcohol cues: FMRI evidence of emotion-based impulsivity. Alcohol Clin Exp Res. 2014;38:409–417. doi: 10.1111/acer.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Dev Neuropsychol. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Davis FC, Knodt AR, Sporns O, Lahey BB, Zald DH, Brigidi BD, Hariri AR. Impulsivity and the modular organization of resting-state neural networks. Cereb Cortex. 2013;23:1444–1452. doi: 10.1093/cercor/bhs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan J, Bernard A, Olliac B, Mailhes AS, Kermarrec S. Adolescent brain development, risk-taking and vulnerability to addiction. Journal of Physiology-Paris. 2010;104:279–286. doi: 10.1016/j.jphysparis.2010.08.007. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Dick DM. Identification of genes influencing a spectrum of externalizing psychopathology. Curr Dir Psychol Sci. 2007;16:331–335. [Google Scholar]
- Dick DM, Agrawal A, Wang JC, Hinrichs A, Bertelsen S, Bucholz KK, Schuckit M, Kramer J, Nurnberger J, Jr, Tischfield J, Edenberg HJ, Goate A, Bierut LJ. Alcohol dependence with comorbid drug dependence: genetic and phenotypic associations suggest a more severe form of the disorder with stronger genetic contribution to risk. Addiction. 2007;102:1131–1139. doi: 10.1111/j.1360-0443.2007.01871.x. [DOI] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Wang JC, Grucza RA, Schuckit M, Kuperman S, Kramer J, Hinrichs A, Bertelsen S, Budde JP, Hesselbrock V, Porjesz B, Edenberg HJ, Bierut LJ, Goate A. Using dimensional models of externalizing psychopathology to aid in gene identification. Arch Gen Psychiatry. 2008;65:310–318. doi: 10.1001/archpsyc.65.3.310. [DOI] [PubMed] [Google Scholar]
- Dick DM, Cho SB, Latendresse SJ, Aliev F, Nurnberger JI, Jr, Edenberg HJ, Schuckit M, Hesselbrock VM, Porjesz B, Bucholz K, Wang JC, Goate A, Kramer JR, Kuperman S. Genetic influences on alcohol use across stages of development: GABRA2 and longitudinal trajectories of drunkenness from adolescence to young adulthood. Addict Biol. 2014;19:1055–1064. doi: 10.1111/adb.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Kendler KS. The impact of gene-environment interaction on alcohol use disorders. Alcohol Res. 2012;34:318–324. doi: 10.35946/arcr.v34.3.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Li TK, Edenberg HJ, Hesselbrock V, Kramer J, Kuperman S, Porjesz B, Bucholz K, Goate A, Nurnberger J, Foroud T. A genome-wide screen for genes influencing conduct disorder. Mol Psychiatry. 2004;9:81–86. doi: 10.1038/sj.mp.4001368. [DOI] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Falkai P, Gruber O. Functional neuroimaging of reward processing and decision-making: a review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res Rev. 2008;59:164–184. doi: 10.1016/j.brainresrev.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Nerenberg L, Falkai P, Dechent P, Baudewig J, Gruber O. Impulsive personality and the ability to resist immediate reward: an fMRI study examining interindividual differences in the neural mechanisms underlying self-control. Hum Brain Mapp. 2012;33:2768–2784. doi: 10.1002/hbm.21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dom G, De Wilde B, Hulstijn W, Sabbe B. Dimensions of impulsive behaviour in abstinent alcoholics. Pers Individ Dif. 2007;42:465–476. [Google Scholar]
- Donchin E, Coles GH. Is the P300 component a manifestation of context updating? Behav Brain Sci. 1988;11:357–374. [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Shearer DE, Emmerson RY. Life-span changes in EEG spectral amplitude, amplitude variability and mean frequency. Clin Neurophysiol. 1999;110:1399–1409. doi: 10.1016/s1388-2457(99)00102-9. [DOI] [PubMed] [Google Scholar]
- Economides M, Guitart-Masip M, Kurth-Nelson Z, Dolan RJ. Anterior cingulate cortex instigates adaptive switches in choice by integrating immediate and delayed components of value in ventromedial prefrontal cortex. J Neurosci. 2014;34:3340–3349. doi: 10.1523/JNEUROSCI.4313-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Bierut LJ, Boyce P, Cao M, Cawley S, Chiles R, Doheny KF, Hansen M, Hinrichs T, Jones K, Kelleher M, Kennedy GC, Liu G, Marcus G, McBride C, Murray SS, Oliphant A, Pettengill J, Porjesz B, Pugh EW, Rice JP, Rubano T, Shannon S, Steeke R, Tischfield JA, Tsai YY, Zhang C, Begleiter H. Description of the data from the Collaborative Study on the Genetics of Alcoholism (COGA) and single-nucleotide polymorphism genotyping for Genetic Analysis Workshop 14. BMC Genet. 2005;6(Suppl 1):S2. doi: 10.1186/1471-2156-6-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmasian R, Neville H, Woods D, Schuckit M, Bloom F. Event-related brain potentials are different in individuals at high and low risk for developing alcoholism. Proc Natl Acad Sci U S A. 1982;79:7900–7903. doi: 10.1073/pnas.79.24.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euser AS, Arends LR, Evans BE, Greaves-Lord K, Huizink AC, Franken IH. The P300 event-related brain potential as a neurobiological endophenotype for substance use disorders: a meta-analytic investigation. Neurosci Biobehav Rev. 2012;36:572–603. doi: 10.1016/j.neubiorev.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Fein G, Andrew C. Event-related potentials during visual target detection in treatment-naive active alcoholics. Alcohol Clin Exp Res. 2011;35:1171–1179. doi: 10.1111/j.1530-0277.2011.01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Chang M. Visual P300s in long-term abstinent chronic alcoholics. Alcohol Clin Exp Res. 2006;30:2000–2007. doi: 10.1111/j.1530-0277.2006.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Chang M. Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naive alcoholics. Drug Alcohol Depend. 2008;92:141–148. doi: 10.1016/j.drugalcdep.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Sakhardande A, Blakemore SJ. The effect of alcohol consumption on the adolescent brain: A systematic review of MRI and fMRI studies of alcohol-using youth. NeuroImage: Clinical. 2014;5:420–437. doi: 10.1016/j.nicl.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AP. A bluffer’s guide to sphericity. Newsletter of the Mathematical, Statistical and Computing Section of the British Psychological Society. 1988;6:13–22. [Google Scholar]
- Finn PR, Mazas CA, Justus AN, Steinmetz J. Early-onset alcoholism with conduct disorder: go/no go learning deficits, working memory capacity, and personality. Alcohol Clin Exp Res. 2002;26:186–206. [PubMed] [Google Scholar]
- Forbes EE, Rodriguez EE, Musselman S, Narendran R. Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. PLoS One. 2014;9:e94640. doi: 10.1371/journal.pone.0094640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroud T, Edenberg HJ, Goate A, Rice J, Flury L, Koller DL, Bierut LJ, Conneally PM, Nurnberger JI, Bucholz KK, Li TK, Hesselbrock V, Crowe R, Schuckit M, Porjesz B, Begleiter H, Reich T. Alcoholism susceptibility loci: confirmation studies in a replicate sample and further mapping. Alcohol Clin Exp Res. 2000;24:933–945. [PubMed] [Google Scholar]
- Fujiwara J, Tobler PN, Taira M, Iijima T, Tsutsui K. Segregated and integrated coding of reward and punishment in the cingulate cortex. J Neurophysiol. 2009;101:3284–3293. doi: 10.1152/jn.90909.2008. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Frontal lobe and cognitive development. J Neurocytol. 2002;31:373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- Gao Y, Raine A. P3 event-related potential impairments in antisocial and psychopathic individuals: a meta-analysis. Biol Psychol. 2009;82:199–210. doi: 10.1016/j.biopsycho.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Gasser T, Jennen-Steinmetz C, Sroka L, Verleger R, Mocks J. Development of the EEG of school-age children and adolescents. II Topography. Electroencephalogr Clin Neurophysiol. 1988a;69:100–109. doi: 10.1016/0013-4694(88)90205-2. [DOI] [PubMed] [Google Scholar]
- Gasser T, Verleger R, Bacher P, Sroka L. Development of the EEG of school-age children and adolescents. I Analysis of band power. Electroencephalogr Clin Neurophysiol. 1988b;69:91–99. doi: 10.1016/0013-4694(88)90204-0. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, Bernat EM, Iacono WG. Relationship between the P3 event-related potential, its associated time-frequency components, and externalizing psychopathology. Psychophysiology. 2010a;47:123–132. doi: 10.1111/j.1469-8986.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, Iacono WG. Brain electrophysiological endophenotypes for externalizing psychopathology: a multivariate approach. Behav Genet. 2010b;40:186–200. doi: 10.1007/s10519-010-9343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, Iacono WG. Is the P3 amplitude reduction seen in externalizing psychopathology attributable to stimulus sequence effects? Psychophysiology. 2012;49:248–251. doi: 10.1111/j.1469-8986.2011.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Daw N, Dayan P, O’Doherty JP. States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron. 2010;66:585–595. doi: 10.1016/j.neuron.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain Cogn. 2010;72:6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. Recent developments in alcoholism:genetic transmission. Recent Dev Alcohol. 1993;11:231–248. [PubMed] [Google Scholar]
- Goldstein RZ, Cottone LA, Jia Z, Maloney T, Volkow ND, Squires NK. The effect of graded monetary reward on cognitive event-related potentials and behavior in young healthy adults. Int J Psychophysiol. 2006;62:272–279. doi: 10.1016/j.ijpsycho.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, He Y, Evans AC. Brain connectivity: gender makes a difference. Neuroscientist. 2011;17:575–591. doi: 10.1177/1073858410386492. [DOI] [PubMed] [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: a new perspective and a model for research. Psychol Rev. 1980;87:301–315. [PubMed] [Google Scholar]
- Guillem F, Mograss M. Gender differences in memory processing: evidence from event-related potentials to faces. Brain Cogn. 2005;57:84–92. doi: 10.1016/j.bandc.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hada M, Porjesz B, Begleiter H, Polich J. Auditory P3a assessment of male alcoholics. Biol Psychiatry. 2000;48:276–286. doi: 10.1016/s0006-3223(00)00236-5. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Holroyd CB, Moser JS, Simons RF. Brain potentials associated with expected and unexpected good and bad outcomes. Psychophysiology. 2005;42:161–170. doi: 10.1111/j.1469-8986.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol Psychol. 2006;71:148–154. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. It’s worse than you thought: the feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology. 2007;44:905–912. doi: 10.1111/j.1469-8986.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychol Sci. 2007;18:326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Luciana M, Sullwold K. Reward-related decision-making deficits and elevated impulsivity among MDMA and other drug users. Drug Alcohol Depend. 2008;96:99–110. doi: 10.1016/j.drugalcdep.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Bernat E, Malone SM, Iacono WG, Patrick CJ, Krueger RF, McGue M. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology. 2007;44:98–105. doi: 10.1111/j.1469-8986.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muka D, Steinhauer S, Locke J. P300 amplitude decrements in children from families of alcoholic female probands. Biol Psychiatry. 1995;38:622–632. doi: 10.1016/0006-3223(94)00384-7. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S. Neurodevelopmental patterns of visual P3b in association with familial risk for alcohol dependence and childhood diagnosis. Biol Psychiatry. 2002;51:621–631. doi: 10.1016/s0006-3223(01)01301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, Connolly J. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biol Psychiatry. 1999;46:970–981. doi: 10.1016/s0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR. Assessment of prepubertal and postpubertal boys and girls at risk for developing alcoholism with P300 from a visual discrimination task. J Stud Alcohol. 1993;54:350–358. doi: 10.15288/jsa.1993.54.350. [DOI] [PubMed] [Google Scholar]
- Hjorth B. An on-line transformation of EEG scalp potentials into orthogonal source derivations. Electroencephalogr Clin Neurophysiol. 1975;39:526–530. doi: 10.1016/0013-4694(75)90056-5. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Hajcak G, Larsen JT. The good, the bad and the neutral: electrophysiological responses to feedback stimuli. Brain Res. 2006;1105:93–101. doi: 10.1016/j.brainres.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Homberg V, Grunewald G, Grunewald-Zuberbier E. The incentive value of stimuli and the P300 component of cerebral evoked potentials. Prog Brain Res. 1980;54:629–633. doi: 10.1016/s0079-6123(08)61682-9. [DOI] [PubMed] [Google Scholar]
- Homberg V, Grunewald G, Grunewald-Zuberbier E. The variation of p300 amplitude in a money-winning paradigm in children. Psychophysiology. 1981;18:258–262. doi: 10.1111/j.1469-8986.1981.tb03030.x. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch Gen Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM. Developmental Endophenotypes: Indexing Genetic Risk for Substance Abuse with the P300 Brain Event-Related Potential. Child Dev Perspect. 2011;5:239–247. doi: 10.1111/j.1750-8606.2011.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. Int J Psychophysiol. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Iacono WG, McGue M. Association between P3 event-related brain potential amplitude and adolescent problem behavior. Psychophysiology. 2006;43:465–469. doi: 10.1111/j.1469-8986.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- Ivanitsky AM, Kurnitskaya IV, Sobotka S. Cortical topography of event-related potentials to winning and losing in a video tennis game. Int J Psychophysiol. 1986;4:149–155. doi: 10.1016/0167-8760(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol. 2009;31:349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Porjesz B, Begleiter H. Event-related potential index of semantic mnemonic dysfunction in abstinent alcoholics. Biol Psychiatry. 1999;45:494–507. doi: 10.1016/s0006-3223(98)00062-6. [DOI] [PubMed] [Google Scholar]
- John ER, Ahn H, Prichep L, Trepetin M, Brown D, Kaye H. Developmental equations for the electroencephalogram. Science. 1980;210:1255–1258. doi: 10.1126/science.7434026. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr A triarchic model of P300 amplitude. Psychophysiology. 1986;23:367–384. doi: 10.1111/j.1469-8986.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr Developmental evidence for modality-dependent P300 generators: a normative study. Psychophysiology. 1989;26:651–667. doi: 10.1111/j.1469-8986.1989.tb03167.x. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Dick D, Goate A, Hinrichs A, Rice JP, Wang JC, Bauer LO, Crowe R, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O’Connor SJ, Rohrbaugh J, Schuckit MA, Tischfield J, Edenberg HJ, Begleiter H. A cholinergic receptor gene (CHRM2) affects event-related oscillations. Behav Genet. 2006;36:627–639. doi: 10.1007/s10519-006-9075-6. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Goate A, Wang JC, Dick DM, Hinrichs A, Kwon J, Rice JP, Rohrbaugh J, Stock H, Wu W, Bauer LO, Chorlian DB, Crowe RR, Edenberg HJ, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O’Connor SJ, Schuckit MA, Stimus AT, Tischfield JA, Reich T, Begleiter H. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. Int J Psychophysiol. 2004;53:75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Justus AN, Finn PR, Steinmetz JE. P300, disinhibited personality, and early-onset alcohol problems. Alcohol Clin Exp Res. 2001;25:1457–1466. doi: 10.1097/00000374-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Pandey AK, Chorlian DB, Porjesz B. The use of current source density as electrophysiological correlates in neuropsychiatric disorders: A review of human studies. Int J Psychophysiol. 2015 doi: 10.1016/j.ijpsycho.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B. Brain waves in Impulsivity Spectrum Disorders. In: Cyders MA, editor. Psychology of Impulsivity. Nova Science Publishers; Hauppauge, NY: 2012. pp. 20–93. [Google Scholar]
- Kamarajan C, Porjesz B. Advances in Electrophysiological Research. Alcohol Research: Current Reviews. 2015;37:53–87. doi: 10.35946/arcr.v37.1.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biol Psychol. 2005a;69:353–373. doi: 10.1016/j.biopsycho.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. Spatial-anatomical mapping of NoGo-P3 in the offspring of alcoholics: evidence of cognitive and neural disinhibition as a risk for alcoholism. Clin Neurophysiol. 2005b;116:1049–1061. doi: 10.1016/j.clinph.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Rangaswamy M, Tang Y, Chorlian DB, Padmanabhapillai A, Saunders R, Pandey AK, Roopesh BN, Manz N, Stimus AT, Begleiter H. Brain signatures of monetary loss and gain: outcome-related potentials in a single outcome gambling task. Behav Brain Res. 2009;197:62–76. doi: 10.1016/j.bbr.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Rangaswamy M, Chorlian DB, Manz N, Tang Y, Pandey AK, Roopesh BN, Stimus AT, Porjesz B. Theta oscillations during the processing of monetary loss and gain: a perspective on gender and impulsivity. Brain Res. 2008;1235:45–62. doi: 10.1016/j.brainres.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Rangaswamy M, Chorlian DB, Tang Y, Manz N, Saunders R, Pandey AK, Roopesh BN, Stimus AT, Porjesz B. Neurocognitive correlates of impulsivity and alcoholism: theta oscillations during a gambling task. Annual Meeting of the Cognitive Neuroscience Society; New York, USA. 2007. [Google Scholar]
- Kamarajan C, Rangaswamy M, Manz N, Chorlian DB, Pandey AK, Roopesh BN, Porjesz B. Topography, power, and current source density of theta oscillations during reward processing as markers for alcohol dependence. Hum Brain Mapp. 2012;33:1019–1039. doi: 10.1002/hbm.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Rangaswamy M, Tang Y, Chorlian DB, Pandey AK, Roopesh BN, Manz N, Saunders R, Stimus AT, Porjesz B. Dysfunctional reward processing in male alcoholics: an ERP study during a gambling task. J Psychiatr Res. 2010;44:576–590. doi: 10.1016/j.jpsychires.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, Rangaswamy M, Manz N, Wang JC, Wetherill L, Hinrichs T, Almasy L, Brooks A, Chorlian DB, Dick D, Hesselbrock V, Kramer J, Kuperman S, Nurnberger J, Jr, Rice J, Schuckit M, Tischfield J, Bierut LJ, Edenberg HJ, Goate A, Foroud T, Porjesz B. Family-based genome-wide association study of frontal theta oscillations identifies potassium channel gene KCNJ6. Genes Brain Behav. 2012;11:712–719. doi: 10.1111/j.1601-183X.2012.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Issues and considerations for using the scalp surface Laplacian in EEG/ERP research: A tutorial review. Int J Psychophysiol. 2015 doi: 10.1016/j.ijpsycho.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok A. Event-related-potential (ERP) reflections of mental resources: a review and synthesis. Biol Psychol. 1997;45:19–56. doi: 10.1016/s0301-0511(96)05221-0. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Piasecki TM. Toward a dimensional and psychometrically-informed approach to conceptualizing psychopathology. Behav Res Ther. 2002;40:485–499. doi: 10.1016/s0005-7967(02)00016-5. [DOI] [PubMed] [Google Scholar]
- Kuperman S, Porjesz B, Arndt S, Bauer L, Begleiter H, Cizadlo T, O’Connor S, Rohrbaugh J. Multi-center N400 ERP consistency using a primed and unprimed word paradigm. Electroencephalogr Clin Neurophysiol. 1995;94:462–470. doi: 10.1016/0013-4694(94)00312-9. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. Development of action monitoring through adolescence into adulthood: ERP and source localization. Dev Sci. 2007;10:874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Lee TM, Zhou WH, Luo XJ, Yuen KS, Ruan XZ, Weng XC. Neural activity associated with cognitive regulation in heroin users: A fMRI study. Neurosci Lett. 2005;382:211–216. doi: 10.1016/j.neulet.2005.03.053. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Magidson JF, Mitchell SH, Sinha R, Stevens MC, de Wit H. Behavioral and Biological Indicators of Impulsivity in the Development of Alcohol Use, Problems, and Disorders. Alcoholism-Clinical and Experimental Research. 2010;34:1334–1345. doi: 10.1111/j.1530-0277.2010.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. The changing impact of genes and environment on brain development during childhood and adolescence: initial findings from a neuroimaging study of pediatric twins. Dev Psychopathol. 2008;20:1161–1175. doi: 10.1017/S0954579408000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. 2010;72:46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Lee NR, Giedd JN. Effects of sex chromosome aneuploidies on brain development: evidence from neuroimaging studies. Dev Disabil Res Rev. 2009a;15:318–327. doi: 10.1002/ddrr.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, Kendler KS, Evans AC, Giedd JN. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum Brain Mapp. 2009b;30:163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Kranzler HR, Zuo L, Wang S, Blumberg HP, Gelernter J. CHRM2 gene predisposes to alcohol dependence, drug dependence and affective disorders: results from an extended case-control structured association study. Hum Mol Genet. 2005;14:2421–2434. doi: 10.1093/hmg/ddi244. [DOI] [PubMed] [Google Scholar]
- Marco-Pallares J, Cucurell D, Munte TF, Strien N, Rodriguez-Fornells A. On the number of trials needed for a stable feedback-related negativity. Psychophysiology. 2011;48:852–860. doi: 10.1111/j.1469-8986.2010.01152.x. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Blackson TC, Vanyukov MM, Moss HB, Tarter RE. Aggressivity, inattention, hyperactivity, and impulsivity in boys at high and low risk for substance abuse. J Abnorm Child Psychol. 1994;22:177–203. doi: 10.1007/BF02167899. [DOI] [PubMed] [Google Scholar]
- Matousek M, Petersen I. Automatic evaluation of EEG background activity by means of age-dependent EEG quotients. Electroencephalogr Clin Neurophysiol. 1973a;35:603–612. doi: 10.1016/0013-4694(73)90213-7. [DOI] [PubMed] [Google Scholar]
- Matousek M, Petersen I. Frequency analysis of the EEG in normal children and adolescents. In: Kellaway P, Petersen I, editors. Automation of clinical electroencephalography. Raven Press; New York: 1973b. pp. 75–102. [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001;25:1156–1165. [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Fischer CJ, Dougherty DM, Reilly EL, Mathias CW, Swann AC. P300 event-related potential amplitude and impulsivity in cocaine-dependent subjects. Neuropsychobiology. 2004;50:167–173. doi: 10.1159/000079110. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Oderinde V, Mathias CW, Harper RA, Swann AC. Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug Alcohol Depend. 2002;68:105–111. doi: 10.1016/s0376-8716(02)00106-0. [DOI] [PubMed] [Google Scholar]
- Muller-Oehring EM, Jung YC, Sullivan EV, Hawkes WC, Pfefferbaum A, Schulte T. Midbrain-Driven Emotion and Reward Processing in Alcoholism. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Heslenfeld DJ, von Geusau NJ, Mars RB, Holroyd CB, Yeung N. Activity in human reward-sensitive brain areas is strongly context dependent. Neuroimage. 2005;25:1302–1309. doi: 10.1016/j.neuroimage.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Nunez PL. Electric fields of the brain: the neurophysics of EEG. Oxford University Press; New York: 1981. [Google Scholar]
- Nunez PL, Pilgreen KL. The spline-Laplacian in clinical neurophysiology: a method to improve EEG spatial resolution. J Clin Neurophysiol. 1991;8:397–413. [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric Fields of the Brain: The Neurophysics of EEG. 2. Oxford University Press; New York: 2006. [Google Scholar]
- Nurnberger JI, Jr, Wiegand R, Bucholz K, O’Connor S, Meyer ET, Reich T, Rice J, Schuckit M, King L, Petti T, Bierut L, Hinrichs AL, Kuperman S, Hesselbrock V, Porjesz B. A family study of alcohol dependence: coaggregation of multiple disorders in relatives of alcohol-dependent probands. Arch Gen Psychiatry. 2004;61:1246–1256. doi: 10.1001/archpsyc.61.12.1246. [DOI] [PubMed] [Google Scholar]
- O’Boyle M, Barratt E. Impulsivity and DSM-III-R personality disorders. Pers Individ Dif. 1993;14:609–611. [Google Scholar]
- O’Brien RG, Kaiser MK. MANOVA method for analyzing repeated measures designs: an extensive primer. Psychol Bull. 1985;97:316–333. [PubMed] [Google Scholar]
- O’Connor S, Hesselbrock V, Tasman A. Correlates of increased risk for alcoholism in young men. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:211–218. doi: 10.1016/0278-5846(86)90075-8. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Hesselbrock V, Tasman A, DePalma N. P3 amplitudes in two distinct tasks are decreased in young men with a history of paternal alcoholism. Alcohol. 1987;4:323–330. doi: 10.1016/0741-8329(87)90030-9. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- Olson SL, Schilling EM, Bates JE. Measurement of impulsivity: construct coherence, longitudinal stability, and relationship with externalizing problems in middle childhood and adolescence. J Abnorm Child Psychol. 1999;27:151–165. doi: 10.1023/a:1021915615677. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46:957–961. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Praamstra P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin Neurophysiol. 2001;112:713–719. doi: 10.1016/s1388-2457(00)00527-7. [DOI] [PubMed] [Google Scholar]
- Orozco S, Ehlers CL. Gender differences in electrophysiological responses to facial stimuli. Biol Psychiatry. 1998;44:281–289. doi: 10.1016/s0006-3223(97)00487-3. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Alcohol-related ERP changes in cognition. Alcohol. 1987;4:289–292. doi: 10.1016/0741-8329(87)90025-5. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Gaillard AW, Wientjes CJ. The relation between event-related brain potential, heart rate, and blood pressure responses in an S1–S2 paradigm. Biol Psychol. 1995;39:81–102. doi: 10.1016/0301-0511(94)00969-5. [DOI] [PubMed] [Google Scholar]
- Pandey AK, Kamarajan C, Rangaswamy M, Porjesz B. Event-Related Oscillations in Alcoholism Research: A Review. J Addict Res Ther Suppl. 2012;7:1–13. doi: 10.4172/2155-6105.S7-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, Heinz A. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci. 2010;30:7749–7753. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ. Psychophysiological correlates of aggression and violence: an integrative review. Philos Trans R Soc Lond B Biol Sci. 2008;363:2543–2555. doi: 10.1098/rstb.2008.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Petry NM. Substance abuse, pathological gambling, and impulsiveness. Drug Alcohol Depend. 2001;63:29–38. doi: 10.1016/s0376-8716(00)00188-5. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Ford JM. Late event-related potential changes in alcoholics. Alcohol. 1987;4:275–281. doi: 10.1016/0741-8329(87)90023-1. [DOI] [PubMed] [Google Scholar]
- Picton TW, Hillyard SA. Endogenous event-related potentials. In: Picton TW, editor. Handbook of electroencephalography and clinical neurophysiology. Human event-related potentials. Elsevier; Amsterdam: 1988. pp. 361–426. [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Human evoked brain potentials and alcohol. Alcohol Clin Exp Res. 1981;5:304–317. doi: 10.1111/j.1530-0277.1981.tb04904.x. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. The use of event-related potentials in the study of alcoholism: implications for the study of drugs of abuse. NIDA Res Monogr. 1985;62:77–99. [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Event-related potentials in individuals at risk for alcoholism. Alcohol. 1990;7:465–469. doi: 10.1016/0741-8329(90)90033-9. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Neurophysiological factors in individuals at risk for alcoholism. Recent Dev Alcohol. 1991;9:53–67. [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. EventRelated Potentials and Cognitive Function in Alcoholism. Alcohol Health Res World. 1995;19:108–112. [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Event-related potentials in COA’s. Alcohol Health Res World. 1997;21:236–240. [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Bihari B, Kissin B. Event-related brain potentials to high incentive stimuli in abstinent alcoholics. Alcohol. 1987;4:283–287. doi: 10.1016/0741-8329(87)90024-3. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Litke A, Bauer LO, Kuperman S, O’Connor SJ, Rohrbaugh J. Visual P3 as a potential phenotypic marker for alcoholism: Evidence from the COGA national project. In: Ogura C, Koga Y, Shimokochi M, editors. Recent Advances in Event-Related Brain Potential Research. Elsevier Science; Holland: 1996. pp. 539–549. [Google Scholar]
- Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. ScientificWorldJournal. 2007;7:131–141. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Potts GF, George MR, Martin LE, Barratt ES. Reduced punishment sensitivity in neural systems of behavior monitoring in impulsive individuals. Neurosci Lett. 2006;397:130–134. doi: 10.1016/j.neulet.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Prabhu VR, Porjesz B, Chorlian DB, Wang K, Stimus A, Begleiter H. Visual P3 in female alcoholics. Alcohol Clin Exp Res. 2001;25:531–539. [PubMed] [Google Scholar]
- Proverbio AM, Brignone V, Matarazzo S, Del Zotto M, Zani A. Gender and parental status affect the visual cortical response to infant facial expression. Neuropsychologia. 2006;44:2987–2999. doi: 10.1016/j.neuropsychologia.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Ramachandran G, Porjesz B, Begleiter H, Litke A. A simple auditory oddball task in young adult males at high risk for alcoholism. Alcohol Clin Exp Res. 1996;20:9–15. doi: 10.1111/j.1530-0277.1996.tb01035.x. [DOI] [PubMed] [Google Scholar]
- Ramsey SE, Finn PR. P300 from men with a family history of alcoholism under different incentive conditions. J Stud Alcohol. 1997;58:606–616. doi: 10.15288/jsa.1997.58.606. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. Uncovering genes for cognitive (dys)function and predisposition for alcoholism spectrum disorders: a review of human brain oscillations as effective endophenotypes. Brain Res. 2008;1235:153–171. doi: 10.1016/j.brainres.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. Understanding alcohol use disorders with neuroelectrophysiology. In: Pfefferbaum A, Sullivan EV, editors. Handbook of Clinical Neurology. Elsevier; New York: 2014. pp. 383–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Hare T. Neural computations associated with goal-directed choice. Curr Opin Neurobiol. 2010;20:262–270. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li TK, Conneally PM, Nurnberger JI, Jr, Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, Carr K, Crose C, Willig C, Begleiter H. Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet. 1998;81:207–215. [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez Holguin S, Porjesz B, Chorlian DB, Polich J, Begleiter H. Visual P3a in male alcoholics and controls. Alcohol Clin Exp Res. 1999a;23:582–591. [PubMed] [Google Scholar]
- Rodriguez Holguin S, Porjesz B, Chorlian DB, Polich J, Begleiter H. Visual P3a in male subjects at high risk for alcoholism. Biol Psychiatry. 1999b;46:281–291. doi: 10.1016/s0006-3223(98)00247-9. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Moeller FG, Swann AC, Clark L. Recent research on impulsivity in individuals with drug use and mental health disorders: implications for alcoholism. Alcohol Clin Exp Res. 2010;34:1319–1333. doi: 10.1111/j.1530-0277.2010.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rohrbaugh JW, Dunham DN, Stewart PA, Bauer LO, Kuperman S, O’Connor SJ, Porjesz B, Begleiter H. Slow brain potentials in a visual-spatial memory task: topographic distribution and inter-laboratory consistency. Int J Psychophysiol. 1997;25:111–122. doi: 10.1016/s0167-8760(96)00714-3. [DOI] [PubMed] [Google Scholar]
- Romer D, Betancourt L, Giannetta JM, Brodsky NL, Farah M, Hurt H. Executive cognitive functions and impulsivity as correlates of risk taking and problem behavior in preadolescents. Neuropsychologia. 2009;47:2916–2926. doi: 10.1016/j.neuropsychologia.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Bullmore ET. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am J Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rubio G, Jimenez M, Rodriguez-Jimenez R, Martinez I, Avila C, Ferre F, Jimenez-Arriero MA, Ponce G, Palomo T. The role of behavioral impulsivity in the development of alcohol dependence: a 4-year follow-up study. Alcohol Clin Exp Res. 2008;32:1681–1687. doi: 10.1111/j.1530-0277.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- Rubio G, Jimenez M, Rodriguez-Jimenez R, Martinez I, Iribarren MM, Jimenez-Arriero MA, Ponce G, Avila C. Varieties of impulsivity in males with alcohol dependence: the role of Cluster-B personality disorder. Alcohol Clin Exp Res. 2007;31:1826–1832. doi: 10.1111/j.1530-0277.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Groen G, Kiefer M, Buchheim A, Walter H, Martius P, Reiter M, Hermle L, Spitzer M, Ebert D, Falkenstein M. Response inhibition in borderline personality disorder: event-related potentials in a Go/Nogo task. J Neural Transm. 2008;115:127–133. doi: 10.1007/s00702-007-0819-0. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Sallet J, Quilodran R, Rothe M, Vezoli J, Joseph JP, Procyk E. Expectations, gains, and losses in the anterior cingulate cortex. Cogn Affect Behav Neurosci. 2007;7:327–336. doi: 10.3758/cabn.7.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JT, Reppucci ND, Sarata BP. An examination of impulsivity as a trait characterizing delinquent youth. Am J Orthopsychiatry. 1973;43:789–795. doi: 10.1111/j.1939-0025.1973.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Santesso DL, Jetha MK. Electrophysiological changes during adolescence: a review. Brain Cogn. 2010;72:86–100. doi: 10.1016/j.bandc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. J Abnorm Psychol. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Shidara M, Mizuhiki T, Richmond BJ. Neuronal firing in anterior cingulate neurons changes modes across trials in single states of multitrial reward schedules. Exp Brain Res. 2005;163:242–245. doi: 10.1007/s00221-005-2232-y. [DOI] [PubMed] [Google Scholar]
- Shidara M, Richmond BJ. Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science. 2002;296:1709–1711. doi: 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- Sripada CS, Gonzalez R, Phan KL, Liberzon I. The neural correlates of intertemporal decision-making: contributions of subjective value, stimulus type, and trait impulsivity. Hum Brain Mapp. 2011;32:1637–1648. doi: 10.1002/hbm.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev Psychol. 2008;44:1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Hill SY. Auditory event-related potentials in children at high risk for alcoholism. J Stud Alcohol. 1993;54:408–421. doi: 10.15288/jsa.1993.54.408. [DOI] [PubMed] [Google Scholar]
- Stoltenberg SF, Batien BD, Birgenheir DG. Does gender moderate associations among impulsivity and health-risk behaviors? Addict Behav. 2008;33:252–265. doi: 10.1016/j.addbeh.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh S, Porjesz B, Chorlian DB, Choi K, Jones KA, Wang K, Stimus A, Begleiter H. Auditory P3 in female alcoholics. Alcohol Clin Exp Res. 2003;27:1064–1074. doi: 10.1097/01.ALC.0000075549.49800.A0. [DOI] [PubMed] [Google Scholar]
- Sutton S, Tueting P, Hammer M. Evoked potentials and feedback. In: Otto DA, editor. Multidisciplinary perspectives in event related brain potential research. U.S. Government Printing Office; Washington, DC: 1978. pp. 184–188. [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Martis B, Fitzgerald KD, Welsh RC, Abelson JL, Liberzon I, Himle JA, Gehring WJ. Medial frontal cortex activity and loss-related responses to errors. J Neurosci. 2006;26:4063–4070. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. Generator localization by current source density (CSD): implications of volume conduction and field closure at intracranial and scalp resolutions. Clin Neurophysiol. 2012;123:2328–2345. doi: 10.1016/j.clinph.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, Woicik PA, Telang F, Goldstein RZ. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010;5:e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomaki A, Murohashi H. Discrepancy between feedback negativity and subjective evaluation in gambling. Neuroreport. 2005a;16:1865–1868. doi: 10.1097/01.wnr.0000185962.96217.36. [DOI] [PubMed] [Google Scholar]
- Toyomaki A, Murohashi H. The ERPs to feedback indicating monetary loss and gain on the game of modified “rock-paper-scissors”. Int Congr Ser. 2005b;1278:381–384. [Google Scholar]
- Van der Stelt O, Geesken R, Gunning WB, Snel J, Kok A. P3 scalp topography to target and novel visual stimuli in children of alcoholics. Alcohol. 1998;15:119–136. doi: 10.1016/s0741-8329(97)00106-7. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Vollm B, Richardson P, McKie S, Elliott R, Dolan M, Deakin B. Neuronal correlates of reward and loss in Cluster B personality disorders: a functional magnetic resonance imaging study. Psychiatry Res. 2007;156:151–167. doi: 10.1016/j.pscychresns.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Vollm B, Richardson P, Stirling J, Elliott R, Dolan M, Chaudhry I, Del Ben C, McKie S, Anderson I, Deakin B. Neurobiological substrates of antisocial and borderline personality disorder: preliminary results of a functional fMRI study. Crim Behav Ment Health. 2004;14:39–54. doi: 10.1002/cbm.559. [DOI] [PubMed] [Google Scholar]
- von Diemen L, Bassani DG, Fuchs SC, Szobot CM, Pechansky F. Impulsivity, age of first alcohol use and substance use disorders among male adolescents: a population based case-control study. Addiction. 2008;103:1198–1205. doi: 10.1111/j.1360-0443.2008.02223.x. [DOI] [PubMed] [Google Scholar]
- Wackermann J, Matousek M. From the ‘EEG age’ to a rational scale of brain electric maturation. Electroencephalogr Clin Neurophysiol. 1998;107:415–421. doi: 10.1016/s0013-4694(98)00090-x. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18:2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, Kwon JM, Wu W, Dick DM, Rice J, Jones K, Nurnberger JI, Jr, Tischfield J, Porjesz B, Edenberg HJ, Hesselbrock V, Crowe R, Schuckit M, Begleiter H, Reich T, Goate AM, Bierut LJ. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13:1903–1911. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- Wang K, Begleiter H. Local polynomial estimate of surface Laplacian. Brain Topogr. 1999;12:19–29. doi: 10.1023/a:1022225522447. [DOI] [PubMed] [Google Scholar]
- Weiland BJ, Nigg JT, Welsh RC, Yau WY, Zubieta JK, Zucker RA, Heitzeg MM. Resiliency in adolescents at high risk for substance abuse: flexible adaptation via subthalamic nucleus and linkage to drinking and drug use in early adulthood. Alcohol Clin Exp Res. 2012;36:1355–1364. doi: 10.1111/j.1530-0277.2012.01741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Welsh RC, Yau WY, Zucker RA, Zubieta JK, Heitzeg MM. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug Alcohol Depend. 2013;128:130–139. doi: 10.1016/j.drugalcdep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Bava S, Thompson WK, Boucquey V, Pulido C, Yang TT, Tapert SF. Frontoparietal connectivity in substance-naive youth with and without a family history of alcoholism. Brain Res. 2012;1432:66–73. doi: 10.1016/j.brainres.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipple SC, Berman SM, Noble EP. Event-related potentials in alcoholic fathers and their sons. Alcohol. 1991;8:321–327. doi: 10.1016/0741-8329(91)90497-k. [DOI] [PubMed] [Google Scholar]
- Whipple SC, Parker ES, Noble EP. An atypical neurocognitive profile in alcoholic fathers and their sons. J Stud Alcohol. 1988;49:240–244. doi: 10.15288/jsa.1988.49.240. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Sambataro F, Vasic N, Schmid M, Thomann PA, Bienentreu SD, Wolf ND. Aberrant connectivity of resting-state networks in borderline personality disorder. J Psychiatry Neurosci. 2011;36:402–411. doi: 10.1503/jpn.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A. Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cereb Cortex. 2009;19:1019–1027. doi: 10.1093/cercor/bhn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Holroyd CB, Cohen JD. ERP correlates of feedback and reward processing in the presence and absence of response choice. Cereb Cortex. 2005;15:535–544. doi: 10.1093/cercor/bhh153. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. J Neurosci. 2004;24:6258–6264. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova JY, Kolev VN. Developmental changes in the alpha response system. Electroencephalogr Clin Neurophysiol. 1996;99:527–538. doi: 10.1016/s0013-4694(96)95562-5. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J Abnorm Psychol. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Zhou X. Brain potentials associated with outcome expectation and outcome evaluation. Neuroreport. 2006;17:1649–1653. doi: 10.1097/01.wnr.0000236866.39328.1d. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Killgore WD. Fear-related activity in the prefrontal cortex increases with age during adolescence: a preliminary fMRI study. Neurosci Lett. 2006;406:194–199. doi: 10.1016/j.neulet.2006.07.046. [DOI] [PubMed] [Google Scholar]
- Zlojutro M, Manz N, Rangaswamy M, Xuei X, Flury-Wetherill L, Koller D, Bierut LJ, Goate A, Hesselbrock V, Kuperman S, Nurnberger J, Jr, Rice JP, Schuckit MA, Foroud T, Edenberg HJ, Porjesz B, Almasy L. Genome-wide association study of theta band event-related oscillations identifies serotonin receptor gene HTR7 influencing risk of alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:44–58. doi: 10.1002/ajmg.b.31136. [DOI] [PMC free article] [PubMed] [Google Scholar]