Abstract
Introduction
The non-thyroidal illness syndrome (NTIS) or the sick euthyroid syndrome refers to abnormal changes in circulating thyroid hormones due to systemic illnesses. Thyroid hormones are pivotal in the regulation of normal cardiac functions. However, the effects of the NTIS on the heart in acute coronary syndrome (ACS) are still unclear.
Methods
A 6-month prospective cohort study involving 85 patients admitted with ACS was carried out. TSH, FT4 and FT3 were assessed on days 1, 5 and 42. Antithyroid peroxidase antibodies, antithyroglobulin antibodies, fasting blood sugar, HbA1c and fasting serum lipid were obtained on admission. Mortality, functional status (Killip and New York Heart Association Classifications), arrhythmias and readmission rate were recorded.
Results
The prevalence of NTIS was 53%. It was seen in 48% of unstable angina (UA), 54% of non-ST elevation myocardial infarction (NSTEMI) and 56% of ST elevation myocardial infarction (STEMI) patients. NTIS is associated with cardiovascular mortality, all-cause mortality, severe heart failure and a higher readmission rate. The levels of FT3 correlate with severity of myocardial damage as measured by CK and Troponin T. Lower TSH was seen in the non-survivors and in those with ventricular arrhythmias. The most common presentation of NTIS was low FT3 (43.5%), followed by low TSH (12.9%) and FT4 (4.7%). None of the predisposing factors analysed were associated with the development of NTIS.
Conclusions
NTIS in patients with ACS is associated with increased cardiovascular mortality and morbidity, and affects UA, NSTEMI and STEMI equally.
Keywords: Non-thyroidal illness syndrome (NTIS), acute coronary syndrome
Introduction
The non-thyroidal illness syndrome (NTIS) is also known as the sick euthyroid syndrome. It refers to abnormal changes in the concentration of circulating thyroid hormones as a result of severe systemic illnesses.1 It has been documented in sepsis, burns, major surgery, myocardial infarction, bone marrow transplantation, cardiopulmonary bypass surgery and physical trauma.2–12 These changes are similar to that observed during starvation and physiological stress.13
Several thyroid hormone profiles describe the NTIS. These include low T3, low T4, low TSH, high T4 and other abnormalities.14 Reductions in hormones levels occur within hours of the onset of the underlying disease.9 15 16
Patients with NTIS do not exhibit the clinical symptoms and signs of hypothyroidism despite the low circulating hormone levels. This reflects the acute nature of the hormonal disturbances as signs and symptoms require weeks to manifest. The patients' actual thyroid metabolic status is debatable, and it is also arguable whether this state is a physiologically adaptive energy conserving mechanism or an adverse maladaptive response.17–19
Mechanisms postulated for these hormonal changes include alterations in hypothalamic–pituitary axis regulation of TSH secretion, thyroid hormones peripheral transport and tissue uptake, presence of inhibitors and altered hormone metabolism.
Previous studies primarily focused on NTIS in acute ST elevation myocardial infarction (STEMI); only one study included UA patients.8 In this study, patients with acute coronary syndrome (ACS) which included UA, non-ST elevation myocardial infarction (NSTEMI) and STEMI were assessed for NTIS, and the various predisposing factors were also examined. Patients who developed the NTIS in acute myocardial infarction have been found to have a higher risk of subsequent mortality.6 However, the relationship between the NTIS in ACS and subsequent morbidity has not been studied. The association of NTIS with heart failure, arrhythmias and hospital readmissions were also evaluated.
Study objectives and end points
The primary objectives of this study were to determine the prevalence of NTIS in patients admitted with ACS and its association with cardiovascular morbidity and mortality. Factors that may predispose to the development of NTIS were evaluated.
Morbidities were assessed by the occurrence of arrhythmias (excluding sinus bradycardia, sinus tachycardia and extrasystolic beats), cardiac failure (using the Killip and New York Heart Association (NYHA) classifications) and the number of readmissions following the initial hospitalisation.
Methodology
This is a 6-month prospective cohort study conducted from January to July 2006 in our instituition, which is a tertiary referral centre. Patients admitted with ACS to Coronary Care Unit (CCU) or Coronary Rehabilitation Ward (CRW) were screened after informed consent had been obtained. Diagnosis of ACS was made by clinical and laboratory evidence. Exclusion criteria were patients with known thyroid disease (active thyroid disease or previous history of thyroid disease) or any autoimmune disease, patients on medications which can affect the thyroid hormone levels (thyroxine, glucocorticoids, amiodarone, heparin, oral anticoagulants, contraceptive pills, interferon, lithium and hormone replacement therapy), markedly abnormal TFT values that could not be attributed to the NTIS, patients with concomitant infection, cerebrovascular accident or any form of inflammation other than ACS, and patients with known renal or liver impairment.
On day 1, blood specimens for TSH, FT3, FT4, antithyroperoxidase antibodies, antithyroglobulin antibodies, urea and electrolytes were collected from the study subjects. Blood for creatinine kinase and troponin-T was taken three times at 6–8 h apart and the peak value recorded. Blood pressure, heart rate, symptoms and signs of cardiac failure based on the Killip and NYHA Classifications were assessed. Arrhythmias were observed by continuous cardiac monitoring for a minimum of 24 h. Twelve-lead electrocardiogram recording was conducted daily for 3 days of hospital stay.
Blood collection for TSH, FT3, FT4, urea and electrolytes, electrocardiogram, clinical assessment for symptoms and signs of cardiac failure, blood pressure and heart rate were repeated on days 5 and 42. All readmissions, procedures, interventions, use of inotropic support, complications and mortality which occurred during the study period were recorded. The diagnosis of NTIS was made if the subject's thyroid function tests fell into any of the following profiles: low FT3, low FT4, low TSH or any combinations of these abnormalities.9
Statistical analysis
Data were analysed using Statistical Product and Services (SPSS version 11.5) statistical software (SPSS, Chicago, Illinois). Non-parametric tests used were χ2 test for independence and Mann–Whitney U test. Values were expressed as median and interquartile range (IQR). Data were examined in totality or divided into groups according to cardiac diagnosis or the occurrence of the NTIS for comparative purposes. A two-tailed p value of <0.05 was considered as statistically significant.
The study was approved by the Research and Ethics Committee of the Faculty of Medicine, University Kebangsaan Malaysia, and funded by a research grant from the same institution.
Results
Out of 215 cases diagnosed as having ACS, 113 patients were excluded mainly due to chronic renal disease with GFR <30 ml/min and concurrent infection. One hundred and two patients were recruited, and the number of subjects who completed follow-up was 92. Ten subjects withdrew before the last visit. Reasons for withdrawing from the study were: eight patients preferred further treatment of their cardiac condition in private centres or sought traditional/complementary medicine treatment; the other two subjects did not offer any specific reason for withdrawing.
Subclinical hyperthyroidism and secondary hypothyroidism were diagnosed in five and two subjects respectively at the end of the study. Data from 85 subjects who completed the study were used for the final analysis.
The study population consisted of 70 (82%) male and 15 (18%) female subjects. The mean age was 58.3±11.6 years. The ethnic distribution was 38 (45%) Malays, 35 (41%) Chinese and 12(14%) Indians.
There were 27 (32%) subjects with UA, 26 (30%) with NSTEMI and 32 (38%) with STEMI.
A total of 45 subjects who constituted 53% (CI 37% to 68%) of the study population developed NTIS. There was no significant difference in the rates of developing NTIS in the various subtypes of ACS.
There were no significant differences in the baseline characteristics between the two groups except for HBA1c, which was 7.5 (2.9) in the NTIS and 6.0 (1.9) in the non-NTIS (p 0.012; table 1).
Table 1.
Baseline characteristics and biochemistry for the non-thyroidal illness syndrome (NTIS) and non-NTIS groups
| Non-thyroidal illness syndrome | Non-non-thyroidal illness syndrome | ||
|---|---|---|---|
| IQR | IQR | p Value | |
| Hypertension (%) | |||
| Yes | 23 (86.7%) | 20 (50.0%) | 0.919 |
| No | 22 (48.9%) | 20 (50.0%) | |
| Diabetes mellitus (%) | |||
| Yes | 21 (46.7%) | 11 (27.5%) | 0.069 |
| No | 24 (53.3%) | 29 (72.5%) | |
| Family history of thyroid disease (%) | |||
| Yes | 1 (2.2%) | 1 (2.5%) | 1.000 |
| No | 44 (97.8%) | 39 (97.5%) | |
| Antithyroglobulin (%) | |||
| Positive | 3 (6.7) | 4 (10.0) | 0.702 |
| Negative | 42 (93.3) | 36 (90) | |
| Antithyroperoxidase (%) | |||
| Positive | 4 (8.9) | 5 (12.6) | 0.589 |
| Negative | 41 (91.1) | 35 (87.5) | |
| HbA1c | 7.5 (2.9) | 6.0 (1.9) | 0.012* |
| FBS | 6.6 (3.8) | 6.2 (2.3) | 0.115 |
| TC | 5.4 (1.2) | 5.1 (1.7) | 0.620 |
| LDL-C | 3.3 (1.1) | 3.3 (1.4) | 0.643 |
| HDL-C | 1.3 (0.4) | 1.2 (0.3) | 0.321 |
| TG | 1.5 (0.9) | 1.6 (1.1) | 0.470 |
p Value significant <0.05. None of the subjects had goitre (evaluated by physical examination). FBS, fasting blood sugar; HBA1c, hemoglobin A1c; HDL-C, high density pipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TC, total cholesterol; TG, trigliceride.
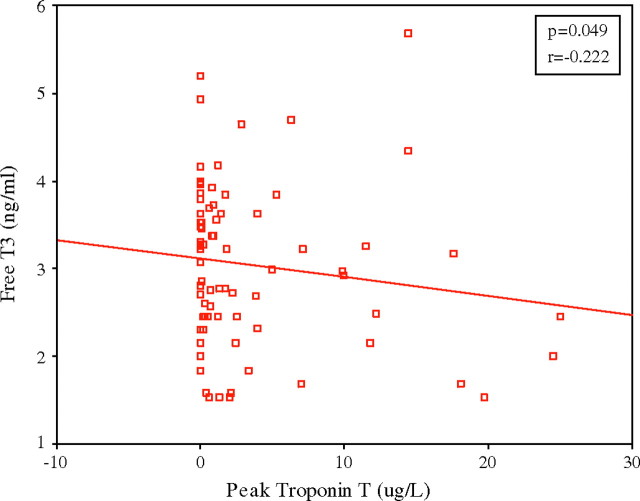
There were significant negative correlations between FT3 on day 1 and peak CK as well as peak troponion T in NTIS group (p=0.026 and p=0.049) (figure 1)
Figure 1.
Negative correlation seen in FT3 day 1 and peak troponin T with p=0.049 and r=−0.22.
There was no mortality in the non-NTIS group. All seven subjects who died had NTIS. There was a significant difference in the occurrence of NTIS between the survivors and the non-survivors with a p value of 0.013 (table 2). There was a significant difference when a further analysis was performed for cardiac mortality (p=0.046).
Table 2.
Association of non-thyroidal illness syndrome (NTIS) with all-cause mortality
| NTIS,n (%) | non NTIS,n (%) | Total,n (%) | χ2 | p Value | |
|---|---|---|---|---|---|
| Non survivors | 7 (100) | 0 (0) | 7 (100) | 4.878 | 0.013* |
| Survivors | 38 (48.7) | 40 (100) | 78 (100) | ||
| Total | 45 (52.9) | 40 (47.1) | 85 (100%) |
Test performed using χ2 test for independence. p Value significant <0.05.
There were significant differences in Killip Classification on day 1 and the number of subjects readmitted between the NTIS and non-NTIS groups. p Values were 0.030 and 0.25 respectively. More patients admitted with Killips III & IV developed NTIS compared with those without NTIS. Similarly, the patients with NTIS had a higher readmission rate (p=0.025) (table 3).
Table 3.
Comparison of morbidities between non-thyroidal illness syndrome (NTIS) and non-NTIS groups
| NTIS | Non NTIS | χ2 | p Value | |
|---|---|---|---|---|
| New York Heart Association Classification (%) | ||||
| Day 42 | ||||
| I | 30 (78.9) | 36 (90) | 1.995 | 0.369 |
| II | 5 (13.2) | 3 (7.5) | ||
| III | 3 (7.9%) | 1 (2.5) | ||
| Killips Classification (%) | ||||
| Day 1 I & II | 39 (45.9) | 40 (47) | 3.886 | 0.030* |
| III & IV | 6 (7.1) | 0 (0) | ||
| Day 5 I & II | 40 (48.8) | 40 (48.8) | 0.464 | 0.496 |
| III & IV | 2 (2.4) | 0 (0) | ||
| Arrhythmias (%) | ||||
| Day 1 | ||||
| Yes | 7 (15.6) | 2 (5) | 0.950 | 0.163 |
| No | 38 (84.4) | 38 (95) | ||
| Readmissions (%) | ||||
| Yes | 10 (22.2) | 1 (2.5) | 4.842 | 0.025* |
| No | 35 (77.8) | 39 (97.5) | ||
All tests were performed using the χ2 test for independence. p Value significant <0.05.
Discussion
The prevalence of NTIS in our study population was 53%. The UA group contributed 29%, NSTEMI 31% and STEMI 40% to the total number of NTIS diagnosed. Pavlou et al 8 reported the occurrence of NTIS in patients with UA, but the prevalence in NSTEMI has not been addressed in any previous publications. In our study, 27 (48%) of the subjects with UA had NTIS, while 14 (54%) and 18 (56%) of the subjects with NSTEMI and STEMI respectively were affected. These figures indicated that roughly equal numbers of subjects in each ACS subgroups developed NTIS. Drazner et al reported a low FT3 in 66% of patients, and a low FT3 and FT4 in 20% of patients with acute myocardial infarction.6 However, the population studied consisted of patients with MI only. No other studies have compared the incidence of the NTIS in UA, NSTEMI and STEMI. Our results generally showed that the occurrence of NTIS is common across all three subtypes of ACS.
Association of NTIS with mortality and morbidity
There were seven mortalities in our study population (table 2). All the cardiac mortalities occurred within the first 4 days of admission, except for one that occurred after 5 weeks following an angioplasty and another patient after 2 weeks. One patient died due to pneumonia at 5 weeks.
All of the non-survivors developed NTIS. They represented 15.6% of the NTIS subjects. The association of NTIS with mortality was statistically significant for both all-cause mortality and cardiac mortality.
The absence of mortality in the non-NTIS group did not permit statistical calculation on the predictive value of NTIS for mortality to be performed. Further studies involving a larger number of patients may be required to develop the predictive value of NTIS as a marker for mortality in ACS patients.
The morbidities investigated in this study were cardiac failure, arrhythmias and readmissions (table 3). Killip Classifications I & II and III & IV were divided into two groups to represent mild and severe cardiac failure. Comparisons made between the NTIS and non-NTIS group showed significant differences in Killip Classification on the day of admission and the number of subsequent readmissions.
On day 1, more subjects with NTIS were classified as Killip Classification III and IV than non-NTIS subjects. This indicated that NTIS occurred in subjects who developed complications of ACS. Correspondingly, more hospital readmissions were seen in the NTIS group.
The levels of FT3 were found to correlate with severity of myocardial damage as measured by CK and troponin T (figure 1). A negative correlation was seen in FT3 on day 1 with peak CK and FT3 day 1 with peak troponin T. The statistically non-significant correlation with troponin T may be explained by the fact that the rise of troponin T to peak value is later than the rise of CK. No correlations were found between the other thyroid hormones and CK or troponin T.
Predisposing factors for the development of NTIS
Factors that may contribute to the development of NTIS evaluated in our study were: family history of thyroid disease, presence of thyroid antibodies, goitre, consumption of traditional or complementary medicine, underlying diabetes and hypertension (table 1). No significant differences between the NTIS and non-NTIS groups were demonstrated. However, subjects with NTIS have higher HbA1c levels than those without NTIS (table 1). Though HbA1c levels are not associated with diabetes per se in this assessment, the chronic hyperglycaemic state reflected by the higher HbA1c level in the NTIS group suggests that chronic hyperglycaemia is a risk factor for developing NTIS. This is not so for acute hyperglycaemia, since the fasting blood glucose level is not associated with NTIS. Eber et al assesed the thyroid hormone levels of subjects with acute myocardial infarction with cholesterol, hypertension and smoking history but did not find any correlation.4
Conclusion
The prevalence of NTIS in our cohort of ACS patients was 53%. All the sub diagnosis of ACS namely UA, NSTEMI and STEMI were affected by NTIS. NTIS was significantly associated with all cause mortality and cardiac mortality. It was significantly associated with Killip's Classification III & IV, a higher readmission rate, and a higher level of HbA1c.
Acknowledgments
We would like to thank the Dean of the Medical Faculty Universiti Kebangsaan Malaysia (UKM) for allowing us to publish this paper.
Footnotes
Funding: Research grant of the Faculty of Medicine, National University of Malaysia (UKM), UKMMC, Jalan Yaacob Latif, 56000 Cheras, KL, Malaysia.
Competing interests: None.
Ethics approval: Ethics approval was provided by the Research and Ethics Committee of the Faculty of Medicine, University Kebangsaan Malaysia.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; not externally peer reviewed.
References
- 1.Chopra IJ, Hershman JM, Pardridge WM, et al. The thyroid function in nonthyroidal illness. Ann Intern Med 1983;98:946–57. [DOI] [PubMed] [Google Scholar]
- 2.Peeters RP, van der Geyten S, Wouters PJ, et al. Tissue thyroid hormone levels in critical illness. J Clin Endocrinol Metab 2005;90;6498–507. [DOI] [PubMed] [Google Scholar]
- 3.Cherem HJ, Nellen HH, Barabejski FG, et al. Thyroid function and abdominal surgery. A longitudinal study. Arch Med Res 1992;23:143–7. [PubMed] [Google Scholar]
- 4.Eber B, Schumaker M, Langsteger W, et al. Changes in thyroid hormone parameters after acute myocardial infarction. Cardiology 1995;86:152–6. [DOI] [PubMed] [Google Scholar]
- 5.Franklyn JA, Gammage MD, Ramsden DB, et al. Thyroid status in patients after myocardial infarction. Clin Sci 1984;67:585–90. [DOI] [PubMed] [Google Scholar]
- 6.Drazner MH, McNulty S, Califf RM, et al. The sick euthyroid syndrome in acute myocardial infarction. J Am Coll Cardiol 1995;25:105A. [Google Scholar]
- 7.Friberg L, Drvota V, Bjelak AH, et al. Association between increased levels of reverse triiodothyronine and mortality after acute myocardial infarction. Am J Med 2001;111:699–703. [DOI] [PubMed] [Google Scholar]
- 8.Pavlou HN, Kliridis PA, Panagiotopoulos AA, et al. Euthyroid sick syndrome in acute ischaemic syndromes. Angiology 2002;53:699–707. [DOI] [PubMed] [Google Scholar]
- 9.Friberg L, Werner S, Eggertsen G, et al. Rapid down-regulation of thyroid hormones in acute myocardial infarction. Is it cardio protective in patients with angina? Arch Intern Med 2002;162:1388–94. [DOI] [PubMed] [Google Scholar]
- 10.Vexiau P, Perez-Castiglioni P, Socie G, et al. The ‘euthyroid sick syndrome’: incidence, risk factors and prognostic value soon after allogeneic bone marrow transplantation. Br J Haematol 1993;85:778–82. [DOI] [PubMed] [Google Scholar]
- 11.Holland FW, Brown PS, Weintraub BD, et al. Cardiopulmonary bypass and thyroid function: a ‘sick euthyroid syndrome.’ Ann Thorac Surg 1991;52:46–50. [DOI] [PubMed] [Google Scholar]
- 12.Phillips RH, Valente WA, Caplan ES, et al. Circulating thyroid hormone changes in acute trauma: prognostic implications for clinical outcome. J Trauma 1984;24:116–19. [DOI] [PubMed] [Google Scholar]
- 13.Welle SL, Campbell RG. Decrease in resting metabolic rate during rapid weight loss is reversed by low dose thyroid hormone treatment. Metabolism 1986;35:289–91. [DOI] [PubMed] [Google Scholar]
- 14.Chopra IJ. Euthyroid sick syndrome: is it a misnomer? J Clin Endocrinol Metab 1997;82:329–34. [DOI] [PubMed] [Google Scholar]
- 15.De Grout LJ. Dangerous dogmas in medicine: the nonthyroidal illness syndrome. J Clin Endocrinol Metab 1999;84:151–62. [DOI] [PubMed] [Google Scholar]
- 16.Chopra IJ, Taing P, Mikus L. Direct determination of free triodothyronine (T3) in undiluted serum by equilibrium dialysis/radioimmunoassay. Thyroid 1996;6:255–9. [DOI] [PubMed] [Google Scholar]
- 17.Boelen A, Platvoet-ter Schiphorst MC, Wiersinga WM. Soluable cytokine receptors and the low 3,5,3ʹ-triodothyronine syndrome in patients with nonthyroidal disease. J Clin Endocrinol Metab 1995;80:971–6. [DOI] [PubMed] [Google Scholar]
- 18.Benker G, Raida M, Olbricht T, et al. TSH secretion in Cushing's syndrome: relation to glucocorticoid excess, diabetes, goiter, and the sick euthyroid syndrome. Clin Endocrinol 1990;33:777–86. [DOI] [PubMed] [Google Scholar]
- 19.Docter R, Krenning EP, de Jong M, et al. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol 1993;39:499–518. [DOI] [PubMed] [Google Scholar]