Abstract
Objective
To clarify the differences in the baseline characteristics, prevalence and incidence of atherothrombosis in patients recruited from Asia versus non-Asian regions.
Design
International Prospective Cohort Study.
Setting
Region focused substudy.
Patients
The Reduction of Atherothrombosis for Continued Health (REACH) Registry recruited 68 236 stable outpatients with established atherothrombosis or ≥3 atherothrombotic risk factors from 44 countries.
Interventions
No intervention.
Main outcome measures
Risk factors, use of medications, vascular disease bed location, and 1-year cardiovascular (CV) outcomes (CV death, myocardial infarction, stroke).
Results
The percentages of patients recruited with CVD (Cerebrovascular Disease) were higher in Asia (41.0%) than in non-Asian regions (25.1%) (p<0.0001). The prevalence of diabetes mellitus was higher in Asia (46.6%) than in non-Asian regions (43.3%) (p<0.0001) despite the former having a lower body mass index (BMI) (24.4±3.9 vs 28.8±5.6) (p<0.0001). The combined endpoint of CV death/myocardial infarction/stroke of patients recruited from non-Asian regions of 4.38% (95% CI 4.20 to 4.56) is equivalent to those from the Asian region excluding Japan of 4.65% (95% CI 4.04 to 5.25), but that is significantly lower in patients recruited from Japan of 3.40% (95% CI 2.76 to 4.04, p<0.05).
Conclusions
There is a higher prevalence of CVD and higher prevalence of diabetus mellitus with lower body mass index in patients recruited from the Asian region as compared those recruited from non-Asian regions. The CV event rate in patients recruited from non-Asian regions is equivalent to that of patients recruited from the Asian region excluding Japan, but significantly lower in patients recruited from Japan.
Keywords: Atherothrombosis, geographic variation, mortality, outcomes, risk factor, atherosclerosis, epidemiology, platelets, thrombosis
Introduction
The prevalence and incidence of atherothrombotic diseases including myocardial infarction (MI) and ischaemic stroke along with the risk-factor profile of these diseases vary greatly across the regions of the world.1–13 Regional differences in the prevalence and incidence of atherothrombotic disease may depend upon the genetic variability,14 15 lifestyle difference16 and regional differences in the medical care system, among others.
In the present study, we focus on risk-factor profiles, medication use and 1-year outcomes in patients with or at risk of atherothrombosis recruited from countries regionally located in Asia and those recruited from regions outside Asia. We used data from the Reduction of Atherothrombosis for Continued Health (REACH) Registry, which enrolled an international, prospective cohort of patients with established atherothrombotic disease (coronary artery disease (CAD), cerebrovascular disease (CVD) and peripheral artery disease (PAD)) and patients who were at high risk of atherothrombosis.17 The baseline characteristics of more than 68 000 patients (including >10 000 patients recruited from Asian countries),18 their 1, 3 and 4-year cardiovascular (CV) outcomes,19–21 and several substudy analyses have been published.22–26 Here, we present a detailed analysis of the descriptive differences between patients recruited from Asia and those patients recruited from non-Asian regions using the same inclusion and exclusion criteria.
Methods
The methods and rationale for the REACH Registry have been published previously.17 Briefly, the REACH Registry recruited stable outpatients aged ≥45 years with either established atherothrombotic disease (CAD, CVD and/or PAD) or three or more risk factors for atherothrombosis (risk factors only (RFO)). The enrolment and exclusion criteria were predefined and have been published elsewhere.17 Patients were recruited after study approval by the institutional review board in each country or hospital according to local requirements, and written informed consent was obtained.
We analysed the risk factors (age, gender; hypertension, hypercholesterolaemia, diabetus mellitus (DM) defined by local practice guidelines, obesity assessed by both body mass index (BMI) and waist circumference, and smoking status) and usage of drugs for antithrombotic and correction for risk factors from the baseline database and the incidence of the primary endpoint of CV death, non-fatal MI and non-fatal stroke as well as major bleeding endpoints using the database of 1-year outcome of 10 692 patients (9122 symptomatic, 1570 asymptomatic) recruited from Asia compared with 54 285 patients recruited from 34 other countries outside Asia. Those patients from non-Asian regions were recruited primarily (>98%) from North and South America, Europe and Australia, and an additional 846 patients were recruited from the Middle East (44 089 symptomatic, 10 196 asymptomatic).
Statistical analysis
One-year event rates are expressed primarily as crude annualised event rates and percentages. One-year CV outcomes were adjusted for age, sex, key risk factors (15.2% smokers, 81.7% hypertension, 43.9% diabetes, 72.1% hypercholesterolaemia) and region of vascular disease (CAD, CVD, PAD). As we have already demonstrated in the REACH Registry that 1-, 3- and 4-year CV event rates in patients recruited from Japan differ substantially from those recruited from other regions,19–21 a subanalysis was conducted, comparing patients recruited from Japan with patients recruited from the Asian region excluding Japan.
Comparisons between patients recruited from Asia (including Japan) and non-Asian regions, and between those recruited from Asia (excluding Japan) and Japan, were performed using the Pearson χ2 test for qualitative variables, Student t test for quantitative variables and adjusted logrank test for event rates. Comparisons between patients recruited from Asia (excluding Japan), Japan and non-Asian regions were performed using an analysis of variance. The statistical analysis was performed using SAS v9 software.
Role of the funding source
All manuscripts in the REACH Registry are prepared by independent authors who are not governed by the funding sponsors and are reviewed by an academic publications committee before submission. The funding sponsors have the opportunity to review manuscript submissions but do not have authority to change any aspect of a manuscript.
Results
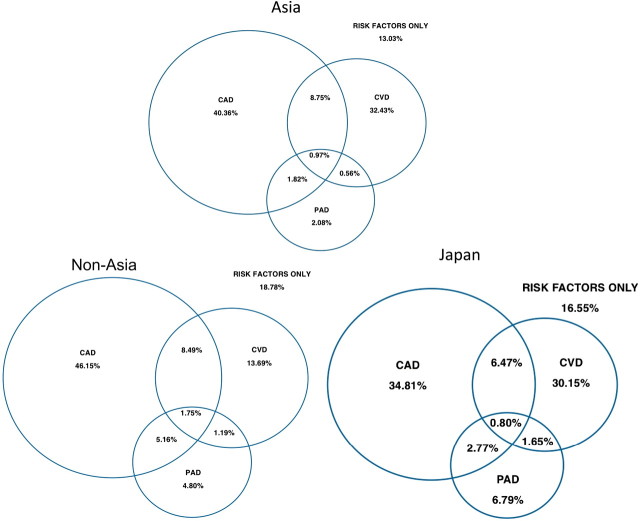
In the 54 285 patients recruited from non-Asian regions, the majority of patients with symptoms (75.8%) had CAD. The percentages of patients recruited with CVD were higher in patients recruited from Asia (41.0%) than those recruited from non-Asian regions (25.1%) (figure 1, table 1). Among patients with symptoms, the percentages of patients recruited with polyvascular disease (two or more vascular regions) were significantly lower in patients recruited from Asia (13.9%) than in non-Asian regions (20.4%) (figure 1).
Figure 1.
Proportions of patients with coronary artery disease, cardiovascular disease, peripheral artery disease and with risk factors only according to region of enrolment. CAD, coronary artery disease; CVD, cerebrovascular disease; PAD, peripheral artery disease.
Table 1.
Baseline risk-factor profile for patients recruited from Asia (including Japan) and non-Asian regions
| All patients (n=64 977) | Multiple risk factors only (n=11 766) | Coronary artery disease (n=38 602) | Cerebrovascular disease (n=18 013) | Peripheral artery disease (n=7911) | ||||||
| Asia (n=10 692) | Non-Asia (n=54 285) | Asia (n=1570) | Non-Asia (n=10 196) | Asia (n=5195) | Non-Asia (n=33 407) | Asia (n=4384) | Non-Asia (n=13 629) | Asia (n=911) | Non-Asia (n=7000) | |
| Age (years) | 67.5±9.7 | 68.8±10.2 | 68.9±9.1 | 69.2±10.0 | 66.8±9.7 | 68.6±10.2 | 67.9±9.7 | 70.0±10.2 | 70.1±9.0 | 69.2±10.0 |
| p Value | <0.0001 | 0.31 | <0.0001 | <0.0001 | 0.0032 | |||||
| Men | 67.0 | 63.1 | 49.2 | 49.5 | 73.6 | 69.3 | 65.2 | 57.8 | 78.6 | 69.9 |
| p Value | <0.0001 | 0.82 | <0.0001 | <0.0001 | <0.0001 | |||||
| Hypertension | 75.2 | 83.0 | 79.9 | 91.7 | 72.9 | 81.3 | 78.0 | 84.8 | 78.4 | 81.5 |
| p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.022 | |||||
| Hypercholesterolaemia | 54.2 | 75.6 | 69.7 | 83.7 | 61.1 | 79.1 | 42.4 | 62.6 | 44.7 | 69.3 |
| p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| Diabetes | 46.6 | 43.3 | 86.4 | 73.0 | 41.5 | 37.3 | 38.3 | 36.5 | 48.4 | 42.8 |
| p Value | <0.0001 | <0.0001 | <0.0001 | 0.039 | 0.0014 | |||||
| Waist circumference (cm) | 87.3±11.9 | 100.1±15.9 | 87.3±12.1 | 102.6±16.9 | 88.0±11.9 | 100.1±15.5 | 86.9±11.8 | 98.6±15.9 | 86.3±12.3 | 99.3±15.4 |
| p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| Waist circumference* | 49.5 | 52.8 | 50.3 | 64.4 | 51.1 | 50.3 | 48.5 | 51.5 | 46.5 | 48.5 |
| p Value | <0.0001 | <0.0001 | 0.34 | <0.001 | 0.25 | |||||
| BMI (kg/m2) | 24.4±3.9 | 28.8±5.6 | 24.9±4.0 | 30.5±6.4 | 24.7±3.9 | 28.7±5.4 | 24.2±3.8 | 28.1±5.3 | 23.2±3.5 | 27.6±5.1 |
| p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| BMI>30 (obesity) | 6.5 | 34.5 | 9.3 | 47.1 | 6.9 | 33.1 | 5.2 | 29.3 | 3.5 | 26.3 |
| p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| Current smoker | 14.7 | 15.3 | 21.6 | 18.8 | 11.8 | 13.1 | 14.0 | 14.3 | 19.4 | 25.0 |
| Never smoker | 48.7 | 42.0 | 60.5 | 51.1 | 45.8 | 39.0 | 50.4 | 46.1 | 27.6 | 24.0 |
| Former smoker | 36.6 | 42.7 | 17.9 | 30.1 | 42.4 | 47.9 | 35.6 | 39.6 | 53.0 | 51.0 |
| p Value† | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.001 | |||||
Data are mean±SD or %.
*Waist circumference in Japanese (men ≥85 cm/women ≥90 cm), Asian (men ≥90 cm/women ≥80 cm) and non-Asian (men ≥102 cm/women ≥88 cm) populations.
†Comparing smoking status: current; former; never.
Asia, patients recruited from Asian countries; BMI, body mass index; Non-Asia, patients recruited from regions other than Asia.
There were substantial differences in the risk-factor profiles of patients recruited from Asia and non-Asian regions (table 1). Patients recruited from Asia were younger and more predominantly male, with a lower prevalence of hypertension and hypercholesterolaemia but a higher prevalence of diabetes mellitus despite having a smaller waist circumference and a lower BMI (all p<0.0001). Details of the results in each vascular disease category (ie, RFO, CAD, CVD and PAD) are also summarised in table 1. The differences between patients recruited from Asia and non-Asian regions described above were consistent across all subcategories, except for no difference in age and sex in RFO patients and no difference in waist circumference in CAD and PAD patients.
Intraregional comparative analysis demonstrated that patients recruited from Japan were older and more predominantly male, with a lower prevalence of hypertension and hypercholesterolaemia, a lower waist circumference and BMI, and a higher prevalence of former and current smokers than patients recruited from Asian countries excluding Japan (all p<0.0001) (table 2).
Table 2.
Baseline risk-factor profile for patients recruited from Asia (excluding Japan) and Japan
| Asia (ex. Japan) (n=5671) | Japan (n=5021) | p Value | |
| Age (years) | 65.1±9.8 | 70.3±8.7 | <0.0001 |
| Men | 3689 (65.1%) | 3477 (69.3%) | <0.0001 |
| Diabetes | 2685 (47.4%) | 2298 (45.8%) | 0.10 |
| Hypertension | 4479 (79.0%) | 3559 (70.9%) | <0.0001 |
| Treated hypercholesterolaemia | 3460 (61.0%) | 2331 (46.4%) | <0.0001 |
| Waist circumference (cm) | 89.6±13.2 | 84.9±9.7 | <0.0001 |
| Body mass index | |||
| Overall (kg/m2) | 25.0±4.2 | 23.9±3.3 | <0.0001 |
| 25 to <30 (overweight) | 2098 (37.5%) | 1474 (29.4%) | <0.0001 |
| 30 to <35 (class I) | 404 (7.2%) | 185 (3.7%) | |
| 35 to <40 (class II) | 60 (1.1%) | 12 (0.2%) | |
| ≥40 (class III) | 30 (0.5%) | 4 (0.1%) | |
| >30 (obese) | 494 (8.8%) | 201 (4.0) | <0.0001 |
| Smoking status | |||
| Current smoker | 713 (12.8%) | 804 (16.9%) | |
| Former smoker | 1631 (29.3%) | 2155 (45.2%) | <0.0001 |
| Never smoked | 3225 (57.9%) | 1811 (38.0%) | |
Data are mean±SD or number (%).
Pharmacological medications and control of risk factors
The use of chronic antiplatelet and anticoagulant therapy in patients recruited from Asia (excluding Japan), Japan and the non-Asian regions is shown in table 3. Use of antiplatelet in patients recruited from Asia excluding Japan is higher than those patients recruited from Japan and the non-Asian regions, but the use and dosage of aspirin in patients recruited from Asia or Japan were lower than those patients recruited from non-Asian regions. Moreover, the lower use of aspirin in patients recruited from Japan is notable. The use of dual antiplatelet therapy was similar in patients recruited from Asia and non-Asian regions. The use of anticoagulants was lower in Asia excluding Japan, even in patients who had atrial fibrillation (AF). The use of antiplatelet agent (and aspirin) plus an anticoagulant was lower in patients recruited from Asia excluding Japan as compared with those patients recruited from Japan and from non-Asian regions.
Table 3.
Medications used at baseline in patients recruited from Asia (excluding Japan), Japan and non-Asian regions
| Non-Asia (n=54 285) | Asia (excluding Japan) (n=5671) | Japan (n=5021) | p Value* | p Value† | |
| Antithrombotic agents | |||||
| ≥1 Antiplatelet agent | 42 718 (78.8%) | 4628 (81.6%) | 3711 (73.9%) | <0.0001 | <0.0001 |
| Aspirin | 37 282 (68.9%) | 3606 (63.6%) | 2737 (54.5%) | <0.0001 | <0.0001 |
| Aspirin dose (mg/day) | 148.7±109.4 | 108.0±56.7 | 99.2±22.7 | <0.0001 | <0.0001 |
| Other antiplatelet agent | 12 579 (23.3%) | 1745 (30.8%) | 1589 (31.7%) | 0.34 | <0.0001 |
| Any two antiplatelet agents | 7143 (13.3%) | 723 (12.8%) | 615 (12.3%) | 0.43 | 0.08 |
| Oral anticoagulants | 6749 (12.9%) | 365 (6.5%) | 625 (12.5%) | <0.0001 | <0.0001 |
| Oral anticoagulants and aspirin | 2039 (3.9%) | 131 (2.3%) | 285 (5.9%) | <0.0001 | <0.0001 |
| Oral anticoagulants and antiplatelet agents | 2404 (4.6%) | 179 (3.2%) | 440 (8.8%) | <0.0001 | <0.0001 |
| Antithrombotic agents | 47 063 (87.2%) | 4814 (85.0%) | 3896 (77.6%) | <0.0001 | <0.0001 |
| Antiplatelet agents only | 38 610 (71.2%) | 4387 (77.4%) | 3271 (65.2%) | <0.0001 | <0.0001 |
| Oral anticoagulants only | 4334 (8.0%) | 186 (3.3%) | 185 (3.7%) | 0.25 | <0.0001 |
| Oral anticoagulant in AF patients | 3302 (54.8%) | 112 (36.4%) | 189 (53.5%) | <0.0001 | <0.0001 |
| Non-steroidal anti-inflammatory drugs | 6918 (13.2%) | 232 (4.1%) | 147 (2.9%) | <0.001 | <0.0001 |
| Use of lipid-lowering agents in patients with hypercholesterolaemia | |||||
| ≥1 Lipid-lowering agent | 40 742 (90.8%) | 3524 (88.5%) | 2379 (74.2%) | <0.0001 | <0.0001 |
| Statins | 37 831 (84.4%) | 3219 (80.8%) | 2127 (66.4%) | <0.0001 | <0.0001 |
| Other lipid-lowering agents | 6454 (14.4%) | 477 (12.0%) | 399 (12.5%) | 0.55 | <0.0001 |
| Serum cholesterol (mg/dl) | 195.5±48.8 | 206.6±49.9 | 205.1±34.5 | 0.19 | <0.0001 |
| ≥1 Antihypertensive agent in patients with hypertension | 45 854 (96.4%) | 4614 (95.7%) | 3509 (89.1%) | <0.0001 | <0.0001 |
| β-blockers | 24 618 (52.0%) | 2018 (41.9%) | 772 (19.6%) | <0.0001 | <0.0001 |
| ACE inhibitors | 24 530 (51.9%) | 1661 (34.5%) | 794 (20.2%) | <0.0001 | <0.0001 |
| Angiotensin-receptor blockers | 11 154 (23.7%) | 1493 (31.0%) | 1491 (37.9%) | <0.0001 | <0.0001 |
| Diuretics | 22 872 (48.3%) | 1233 (25.6%) | 543 (13.8%) | <0.0001 | <0.0001 |
| Calcium-channel blockers | 16 188 (34.3%) | 2156 (44.7%) | 2568 (65.2%) | <0.0001 | <0.0001 |
| Nitrates | 11 133 (23.9%) | 1306 (27.3%) | 1045 (26.5%) | 0.40 | <0.0001 |
| Other antihypertensives | 5188 (11.0%) | 462 (9.6%) | 237 (6.0%) | <0.0001 | <0.0001 |
| Peripheral arterial claudication medications | 3235 (7.0%) | 250 (5.3%) | 235 (6.0%) | 0.15 | <0.0001 |
| Systolic blood pressure (mm Hg) | 140.4±19.3 | 140.9±19.6 | 140.6±16.9 | 0.37 | 0.27 |
| Diastolic blood pressure (mm Hg) | 79.3±11.3 | 81.7±11.3 | 77.6±10.8 | <0.0001 | 0.046 |
| Use of antidiabetic agents in patients with diabetes mellitus | |||||
| ≥1 Antidiabetic agent | 21 238 (86.8) | 2543 (88.7%) | 1901 (78.7%) | <0.0001 | <0.0001 |
| Sulfonylurease | 10 049 (41.4%) | 1617 (56.8%) | 1071 (44.3%) | <0.0001 | <0.0001 |
| Biguanides | 10 166 (41.8%) | 1441 (50.5%) | 309 (12.8%) | <0.0001 | <0.0001 |
| Insulin | 6595 (27.1%) | 446 (15.6%) | 565 (23.4%) | <0.0001 | <0.0001 |
| Thiazolidinediones | 4519 (18.7%) | 304 (10.7%) | 153 (6.3%) | <0.0001 | <0.0001 |
| Other antidiabetic agents | 2093 (8.8%) | 399 (14.1%) | 676 (28.0%) | <0.0001 | <0.0001 |
| Fasting blood glucose (mg/dl) | 144.9±52.2 | 153.1±59.1 | 145.8±47.2 | <0.0001 | 0.0002 |
Data are mean±SD or number (%).
*Japan versus Asia (excluding Japan).
†Non-Asia versus Asia versus Japan.
The use of lipid-lowering agents in patients with hypercholesterolaemia recruited from Japan was markedly lower than those patients recruited from Asia excluding Japan or patients recruited from non-Asian regions. Serum cholesterol concentrations in patients recruited from Asia were higher than those patients recruited from the non-Asian regions (table 3). The use of β-blockers, ACE inhibitors and diuretics in hypertensive patients was less frequent in patients recruited from Asia than those who were recruited from non-Asian regions, whereas the use of angiotensin-receptor blockers and calcium-channel blockers was much higher (table 3). Despite these different treatment strategies, systolic blood pressure was not different in patients recruited from Asia and non-Asian regions, while diastolic blood pressure was lower in patients recruited from Asia, especially from Japan. There was also a marked difference in the use of antidiabetic treatments: the use of sulfonylureas in patient with diabetes recruited from Asia excluding Japan is highest, followed by patients recruited from Japan and from non-Asian regions. The use of biguanides and of thiazolidinediones in patients recruited from Japan was markedly lower than those patients recruited from Asia excluding Japan and from non-Asian regions. Fasting blood-sugar concentration was lowest in patients recruited from non-Asian regions followed by patients recruited from Japan and those who were recruited from Asia excluding Japan.
One-year outcomes
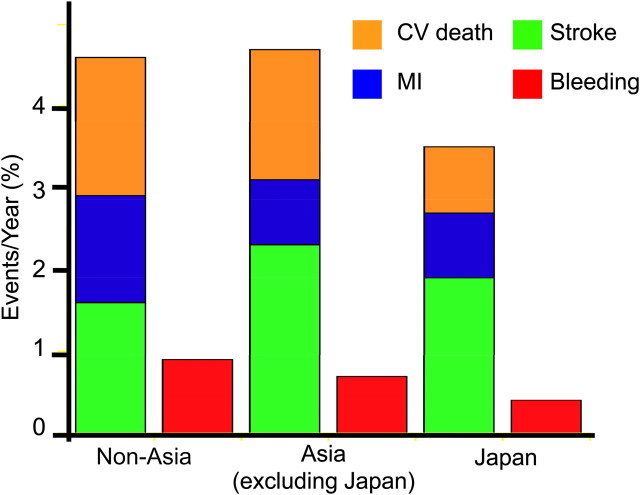
The all-cause and CV mortality at 1 year of 2.69% and 1.72% in patients recruited from non-Asian regions are equivalent to those for patients recruited from Asia excluding Japan of 2.34% to 1.62% but lower in patients recruited from Japan of 1.60% to 0.81%, respectively (p<0.01 in both comparison). As shown in figure 2, the CV death rate of patients recruited from Japan was approximately half that in patients recruited from Asia (excluding Japan) and non-Asian regions. The rates of non-fatal MI in Japan and in Asia (excluding Japan) were approximately 80% of the rate in patients recruited from non-Asian regions, while the rate of non-fatal stroke was higher in patients recruited from Asia than those patients recruited from Japan or the non-Asian regions. The combined endpoint of CV death/MI/stroke/in patients recruited from Japan of 3.40% (95% CI 2.76 to 4.04, p<0.05) was significantly lower than that of patients recruited from Asia excluding Japan of 4.65% (95% CI 4.04 to 5.25) and from non-Asian regions of 4.38% (95% CI 4.20 to 4.56), respectively (p<0.05 for both comparisons). The rate of serious bleeding events requiring both hospitalisation and transfusion in patients recruited from Japan of 0.42% was lower than that of patients recruited from Asia excluding Japan of 0.71% and from non-Asian regions of 0.90%, respectively (p<0.01 for both comparison).
Figure 2.
One-year cardiovascular and bleeding outcomes in patients recruited from Japan, Asia (excluding Japan) and from other regions of the world. CV, cardiovascular; MI, myocardial infarction.
Discussion
Our study is the first detailed Asian-focused substudy arising from the vast, international, contemporary REACH database of patients with, or at high risk of, atherothrombosis. Our results have confirmed the finding of previous comparative, nation-specific cohort studies7 13 27–29 and international case–control studies including the INTERHEART study,2 8 Seven Country Study10 and WHO Monitoring Trends and Determinants in Cardiovascular Disease (WHO MONICA) project4 12 that the prevalence of CVD is higher in patients recruited from Asian countries at baseline than non-Asian regions. In accordance with the higher prevalence of CVD and lower prevalence of CAD in patients recruited from Asia, the annual incidence of CV death and non-fatal MI was lower in these patients than in those recruited from non-Asian regions. Of note, the rate of non-fatal stroke at 1 year in Japan was substantially lower than in the other Asian regions, although the prevalence of CVD was similar. This difference may be partly explained by the lower prevalence of DM, hypertension, hypercholesterolaemia and obesity, but not by use of drugs known to reduce the risk of atherothrombotic events such as antiplatelet agents or statins because their use in Japan is lower than Asia excluding Japan or in non-Asian regions. However, as shown in the previous WHO MONICA project,12 further studies are necessary to clarify the quantitative contributions of the classical risk factors for future onset of stroke.
In the classical risk-factor profile, the markedly lower BMI and smaller waist circumference in patients recruited from Asia with higher prevalence of DM are notable. These results confirm previous findings that the reserve of insulin secretion in Asian patients (especially South Asian patients) is lower than in non-Asian patients.30 31 As metabolic syndrome is a known risk factor for type II diabetes, these data support the need for region-specific criteria for this syndrome.32 33
There were substantial differences in medication use in patients recruited from Asia (excluding Japan), Japan and non-Asian regions. In addition to lower usage of aspirin, the dose of aspirin chosen in Japan is lowest followed by Asia (excluding Japan) and non-Asian regions. One possible explanation for the less frequent use of aspirin in Asia is the relatively lower prevalence of CAD (the disease category in which the clinical benefit for aspirin is most evident).34 The lower use of oral anticoagulants in patients with a history of atrial fibrillation23 in patients recruited from Asia (excluding Japan) is notable, but the reason remains to be elucidated. Less frequent use of lipid-lowering agents and statins with a lower rate of MI and CV death in Asia despite higher serum cholesterol levels (especially in Japan) might also be explained by a lower prevalence of CAD in Asia. There are substantial differences in the use of antihypertensive agents, especially in Japan, such that the use of calcium-channel blockers was markedly higher, possibly because of a genetic deficiency in endothelial NO synthase35 and a concomitant higher prevalence of vasospastic angina in Asia (especially Japan).36
The markedly lower rates of CV death, non-fatal stroke, the combined endpoint of CV death/MI/stroke and bleeding in Japan compared with Asia (excluding Japan) were notable, but the reason for these differences cannot be clarified in this study. These intraregional differences cannot be explained by either a predominance of CVD or extent of risk-factor control. The nationwide, homogeneous medical insurance system, patients' free access to experts without the need for referrals from general practitioners37 and specific lifestyle factors such as the eating of raw fish containing omega-3 fatty acid,38 may be contributory factors. The identification of factors that contribute to lower CV and bleeding events in Japan may be important for other regions of the world in the future.
We attempted to reduce inclusion bias by selecting physicians (comprising cardiologists, neurologists, general practitioners, etc) who reflected real-world practice in each country, but there remains the possibility of recruitment bias.
In conclusion, this substudy of the large, international REACH Registry provides specific characteristics of risk-factor profiles, medication use, risk-factor control, and CV and bleeding outcomes in patients recruited from Asian countries as compared with those recruited from non-Asian regions. This information is important in designing future international clinical trials that include substantial numbers of patients from Asian countries.
Acknowledgments
We thank S Rushton-Smith, for her assistance with coordinating revisions and providing editorial help in preparing this manuscript including editing, checking content and language, formatting, referencing, and preparing tables and figures.
Appendix 1.
REACH registry executive committee: DL Bhatt, VA Boston Healthcare System and Brigham and Women's Hospital, Boston, Massachusetts, USA (chair); PG Steg, Hôpital Bichat-Claude Bernard, Paris, France (chair); EM Ohman, Duke University Medical Center, Durham, North Carolina, USA; J Röther, Klinikum Minden, Minden, Germany; PW Wilson, Emory University School of Medicine, Atlanta, Georgia, USA.
REACH registry global publication committee: MJ Alberts, Northwestern University Medical School, Chicago, USA; DL Bhatt, VA Boston Healthcare System and Brigham and Women's Hospital, Boston, Massachusetts, USA (chair); R D'Agostino, Boston University, Boston, Massachusetts, USA; K Eagle, University of Michigan, Ann Arbor, Michigan, USA; S Goto, Tokai University School of Medicine, Isehara, Kanagawa, Japan; AT Hirsch, University of Minnesota School of Public Health and Minneapolis Heart Institute Foundation, Minneapolis, Minnesota, USA; C-S Liau, Taiwan University Hospital and College of Medicine, Taipei, Taiwan; J-L Mas, Centre Raymond Garcin, Paris, France; EM Ohman, Duke University Medical Center, Durham, North Carolina, USA; J Röther, Klinikum Minden, Minden, Germany; SC Smith, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA; PG Steg, Hôpital Bichat-Claude Bernard, Paris, France (chair); PWF Wilson, Emory University School of Medicine, Atlanta, Georgia, USA.
Footnotes
Funding: The REACH Registry is sponsored by Sanofi-Aventis, Bristol-Myers Squibb and the Waksman Foundation (Tokyo). The REACH Registry is endorsed by the World Heart Federation. A complete list of REACH investigators is accessible online at http://www.reachregistry.org. The REACH Registry enforces a no ghost-writing policy.
Competing interests: SG has received honoraria and consulting fees from Eisai, Sanofi-Aventis, Daiichi-Sankyo, GlaxoSmithKline, Bristol-Myers Squibb, Otsuka, Bayer, Schering-Plough, Takeda, Astellas, AstraZeneca, Novartis and Kowa. SG also received research grants from Pfizer, Ono, Eisai, Otsuka, Daiichi-Sankyo, Sanofi-Aventis, Takeda and Astellas within the last 3 years. YI has received honoraria or donations from Sanofi-Aventis, Daiichi-Sankyo and Bayer. JCNC has received consultancy fees or research grants or donations from Sanofi-Aventis, Bayer, MSD, Lilly, Pfizer, AstraZeneca, Bristol-Myers Squibb and GlaxoSmithKline. All proceeds go to The Chinese University of Hong Kong to support ongoing research and education programmes in diabetes and related disease. PWFW has received grant support from Sanofi-Aventis. TCY has received an honorarium from Sanofi-Aventis. MTBA has received honoraria from Sanofi-Aventis, Pfizer, Otsuka and Bayer, is a member of an advisory board sponsored by Sanofi-Aventis and has received study grants from AstraZeneca, Boehringer-Ingelheim, Mayer, MSD, Otsuka, Pfizer, Sanofi-Aventis and Servier. PGS discloses the following relationships: Research Grant: Sanofi-Aventis (significant); speakers of bureau (all modest): Boehringer-Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Nycomed, Sanofi-Aventis, Servier, The Medicines Company; Consulting/advisory board (all modest): Astellas, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Endotis, GlaxoSmithKline, Medtronic, MSD, Nycomed, Sanofi-Aventis, Servier, The Medicines Company. Stockholding: none. G Salette is an employee of Sanofi-Aventis. DLB discloses the following relationships: honoraria (donated to non-profits for >2 years) AstraZeneca, Bristol Myers Squibb, Centocor, Daiichi-Sankyo, Eisai, Eli Lilly, GlaxoSmithKline, Millennium, Paringenix, PDL, Sanofi-Aventis, Schering Plough, The Medicines Company, TNS Healthcare; speakers of bureau (>2 years ago) Bristol Myers Squibb, Sanofi-Aventis, The Medicines Company; Consultant/Advisory Board (any honoraria donated to non-profits) AstraZeneca, Bristol Myers Squibb, Cardax, Centocor, Cogentus, Daiichi-Sankyo, Eisai, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, McNeil, Medtronic, Millennium, Otsuka, Paringenix, PDL, Philips, Portola, Sanofi-Aventis, Schering Plough, The Medicines Company, TNS Healthcare, Vertex; expert testimony regarding clopidogrel (the compensation was donated to a non-profit organisation).
Patient consent: Obtained.
Ethics approval: Ethics approval was provided by the approval of the study by the institutional review board in each 44 country or hospital according to local requirements.
Contributors: The first draft was written by SG. SG had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. SG, YI, JCNC, TCY, CSL and MTBA were responsible for the study concept and design, and acquisition of data. PWFW was responsible for the analysis and interpretation of data. SG, YI, PWFW, PGS and DLB were responsible for the drafting of the manuscript. All authors were involved with the critical revision of the manuscript for important intellectual content. G Salette was responsible for the statistical analysis. PGS and DLB obtained funding for the study and provided administrative, technical or material support, and study supervision.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Parikh NI, Pencina MJ, Wang TJ, et al. A risk score for predicting near-term incidence of hypertension: the Framingham Heart Study. Ann Intern Med 2008;148:102–10. [DOI] [PubMed] [Google Scholar]
- 2.Iqbal R, Anand S, Ounpuu S, et al. Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study. Circulation 2008;118:1929–37. [DOI] [PubMed] [Google Scholar]
- 3.McGorrian C, Yusuf S, Islam S, et al. Estimating modifiable coronary heart disease risk in multiple regions of the world: the INTERHEART Modifiable Risk Score. Eur Heart J 2010;32:581–9. [DOI] [PubMed] [Google Scholar]
- 4.Tunstall-Pedoe H, Kuulasmaa K, Mahonen M, et al. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA project populations. Monitoring trends and determinants in cardiovascular disease. Lancet 1999;353:1547–57. [DOI] [PubMed] [Google Scholar]
- 5.McQueen MJ, Hawken S, Wang X, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case–control study. Lancet 2008;372:224–33. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet 2004;364:937–52. [DOI] [PubMed] [Google Scholar]
- 7.Kiyohara Y, Kubo M, Kato I, et al. Ten-year prognosis of stroke and risk factors for death in a Japanese community: the Hisayama study. Stroke 2003;34:2343–7. [DOI] [PubMed] [Google Scholar]
- 8.Rosengren A, Hawken S, Ounpuu S, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case–control study. Lancet 2004;364:953–62. [DOI] [PubMed] [Google Scholar]
- 9.Teo KK, Ounpuu S, Hawken S, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case–control study. Lancet 2006;368:647–58. [DOI] [PubMed] [Google Scholar]
- 10.Verschuren WM, Jacobs DR, Bloemberg BP, et al. Serum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries study. JAMA 1995;274:131–6. [PubMed] [Google Scholar]
- 11.Kubo M, Kiyohara Y, Kato I, et al. Trends in the incidence, mortality, and survival rate of cardiovascular disease in a Japanese community: the Hisayama study. Stroke 2003;34:2349–54. [DOI] [PubMed] [Google Scholar]
- 12.Stegmayr B, Asplund K, Kuulasmaa K, et al. Stroke incidence and mortality correlated to stroke risk factors in the WHO MONICA Project. An ecological study of 18 populations. Stroke 1997;28:1367–74. [DOI] [PubMed] [Google Scholar]
- 13.Goto S. Cardiovascular risk factors in patients at high risk of atherothrombosis: what can be learned from registries? Thromb Haemost 2008;100:611–13. [PubMed] [Google Scholar]
- 14.Jun ZJ, Ping T, Lei Y, et al. Prevalence of factor V Leiden and prothrombin G20210A mutations in Chinese patients with deep venous thrombosis and pulmonary embolism. Clin Lab Haematol 2006;28:111–16. [DOI] [PubMed] [Google Scholar]
- 15.Ozawa T, Niiya K, Sakuragawa N. Absence of factor V Leiden in the Japanese. Thromb Res 1996;81:595–6. [DOI] [PubMed] [Google Scholar]
- 16.Robertson TL, Kato H, Rhoads GG, et al. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California. Incidence of myocardial infarction and death from coronary heart disease. Am J Cardiol 1977;39:239–43. [DOI] [PubMed] [Google Scholar]
- 17.Ohman EM, Bhatt DL, Steg PG, et al. The REduction of Atherothrombosis for Continued Health (REACH) Registry: an international, prospective, observational investigation in subjects at risk for atherothrombotic events—study design. Am Heart J 2006;151:786.e1–10. [DOI] [PubMed] [Google Scholar]
- 18.Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006;295:180–9. [DOI] [PubMed] [Google Scholar]
- 19.Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007;297:1197–206. [DOI] [PubMed] [Google Scholar]
- 20.Alberts MJ, Bhatt DL, Mas JL, et al. Three-year follow-up and event rates in the international REduction of Atherothrombosis for Continued Health Registry. Eur Heart J 2009;30:2318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010;304:1350–7. [DOI] [PubMed] [Google Scholar]
- 22.Cacoub PP, Abola MT, Baumgartner I, et al. Cardiovascular risk factor control and outcomes in peripheral artery disease patients in the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Atherosclerosis 2009;204:e86–92. [DOI] [PubMed] [Google Scholar]
- 23.Goto S, Bhatt DL, Rother J, et al. Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J 2008;156:855–63, 63 e2. [DOI] [PubMed] [Google Scholar]
- 24.Mehta RH, Bhatt DL, Steg PG, et al. Modifiable risk factors control and its relationship with 1 year outcomes after coronary artery bypass surgery: insights from the REACH registry. Eur Heart J 2008;29:3052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rother J, Alberts MJ, Touze E, et al. Risk factor profile and management of cerebrovascular patients in the REACH Registry. Cerebrovasc Dis 2008;25:366–74. [DOI] [PubMed] [Google Scholar]
- 26.Wang TD, Goto S, Bhatt DL, et al. Ethnic differences in the relationships of anthropometric measures to metabolic risk factors in Asian patients at risk of atherothrombosis: results from the REduction of Atherothrombosis for Continued Health (REACH) Registry. Metabolism 2010;59:400–8. [DOI] [PubMed] [Google Scholar]
- 27.Ueda K, Hasuo Y, Kiyohara Y, et al. Intracerebral hemorrhage in a Japanese community, Hisayama: incidence, changing pattern during long-term follow-up, and related factors. Stroke 1988;19:48–52. [DOI] [PubMed] [Google Scholar]
- 28.Scheltens T, Verschuren WM, Boshuizen HC, et al. Estimation of cardiovascular risk: a comparison between the Framingham and the SCORE model in people under 60 years of age. Eur J Cardiovasc Prev Rehabil 2008;15:562–6. [DOI] [PubMed] [Google Scholar]
- 29.Suka M, Sugimori H, Yoshida K. Validity of the Framingham risk model applied to Japanese men. Methods Inf Med 2002;41:213–15. [PubMed] [Google Scholar]
- 30.Abate N, Chandalia M. The impact of ethnicity on type 2 diabetes. J Diabetes Complications 2003;17:39–58. [DOI] [PubMed] [Google Scholar]
- 31.Chandalia M, Lin P, Seenivasan T, et al. Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS One 2007;2:e812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CM, Huxley RR, Woodward M, et al. Comparisons of metabolic syndrome definitions in four populations of the Asia-Pacific region. Metab Syndr Relat Disord 2008;6:37–46. [DOI] [PubMed] [Google Scholar]
- 33.Patel A, Huang KC, Janus ED, et al. Is a single definition of the metabolic syndrome appropriate?—a comparative study of the USA and Asia. Atherosclerosis 2006;184:225–32. [DOI] [PubMed] [Google Scholar]
- 34. Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimura M, Yasue H, Nakayama M, et al. A missense Glu298Asp variant in the endothelial nitric oxide synthase gene is associated with coronary spasm in the Japanese. Hum Genet 1998;103:65–9. [DOI] [PubMed] [Google Scholar]
- 36.Beltrame JF, Sasayama S, Maseri A. Racial heterogeneity in coronary artery vasomotor reactivity: differences between Japanese and Caucasian patients. J Am Coll Cardiol 1999;33:1442–52. [DOI] [PubMed] [Google Scholar]
- 37.Chan JC, Gagliardino JJ, Baik SH, et al. Multifaceted determinants for achieving glycemic control: the International Diabetes Management Practice Study (IDMPS). Diabetes Care 2009;32:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoy SM, Keating GM. Omega-3 ethylester concentrate: a review of its use in secondary prevention post-myocardial infarction and the treatment of hypertriglyceridaemia. Drugs 2009;69:1077–105. [DOI] [PubMed] [Google Scholar]